Submitted:
26 September 2025
Posted:
29 September 2025
You are already at the latest version
Abstract
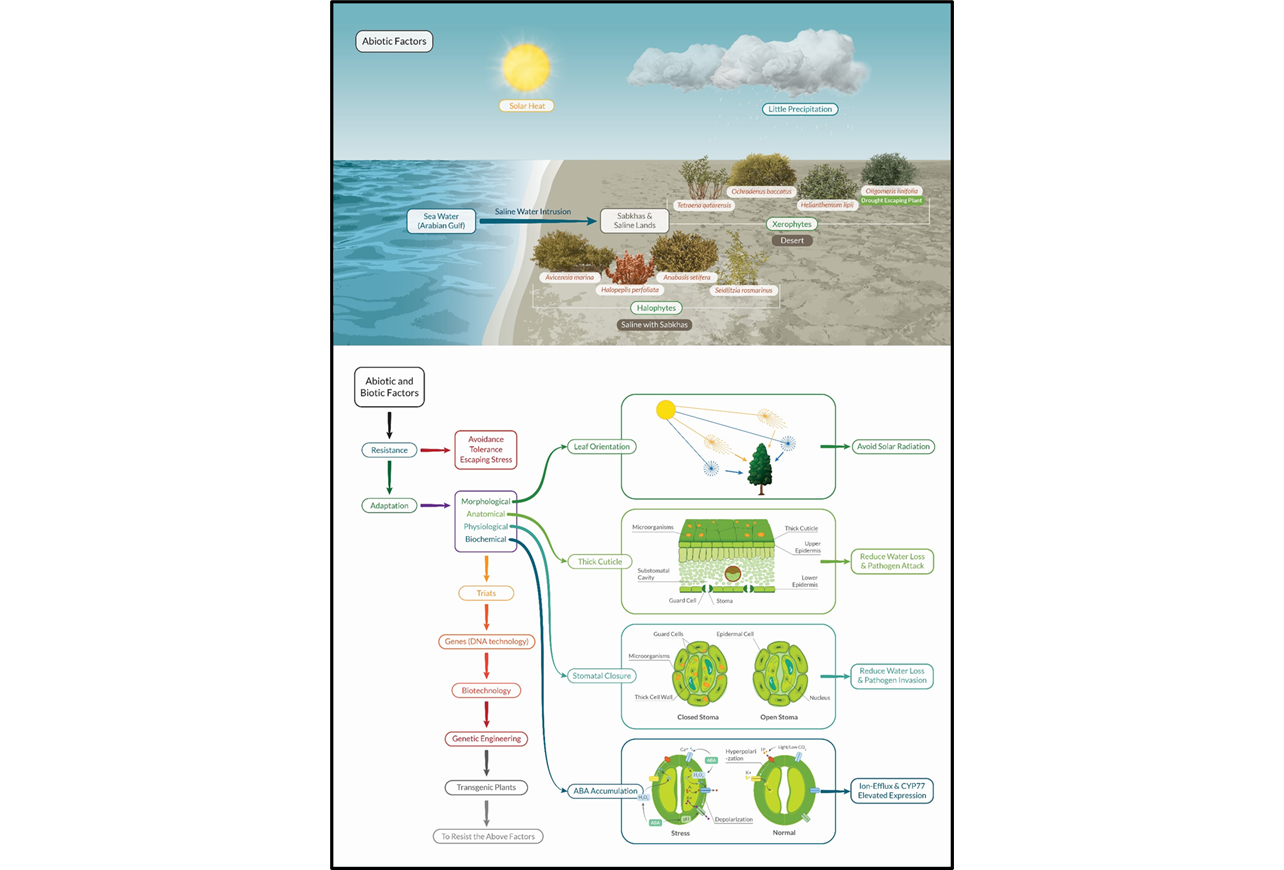
Qatar’s arid and semi-arid landscapes present extreme environmental conditions where native flora face multiple stressors, and plants must simultaneously combat abiotic pressures, including high salinity, water scarcity, intense solar radiation, and extreme temperatures, alongside biotic threats from herbivory, microbial pathogens, and interspecific competition. However, the integrated mechanisms by which these species coordinate defences against both abiotic and biotic stressors (particularly pathogenic microorganisms) remain insufficiently understood, creating a critical knowledge gap in comprehending plant persistence strategies in harsh desert environments. Therefore, this study investigates the comprehensive defence strategies that facilitate plant survival under dual pressures, examining chemical responses including antimicrobial compounds and phenolic production, structural barriers such as thick cuticles, trichomes, and reinforced cell walls, and functional trade-offs affecting water-use efficiency and gas exchange. The research assesses how abscisic acid accumulation, triggered by abiotic stress, enhances biotic resistance through specific biochemical regulatory processes, while critically evaluating the advantages and costs of structural and biochemical adaptations. These findings demonstrate that native species employ interconnected response systems to sustain themselves under simultaneous abiotic and biotic pressures, thereby supporting biodiversity, ecological resilience, and long-term stability of Qatar's fragile desert habitats through coordinated physiological and morphological strategies.
Keywords:
1. Introduction
2. Mechanisms of Resistance Against Biotic Stress
2.1. Physical Defences
2.2. Chemical Defences
2.3. Symbiotic Relationships and Ecological Niches
2.4. Competition Between Native Plants in Qatar
2.4.1. Adaptive Strategies to Compete for Sunlight
2.4.2. Adaptive Strategies to Compete for Water
2.4.3. Adaptive Strategies to Compete for Nutrients
3. Native Plants of Qatar and Their Biotic Challenges
3.1. Qatari Native Plants Resist Herbivores
3.2. Qatari Native Plants Resist Pathogens
3.2.1. The Role of the Cuticle
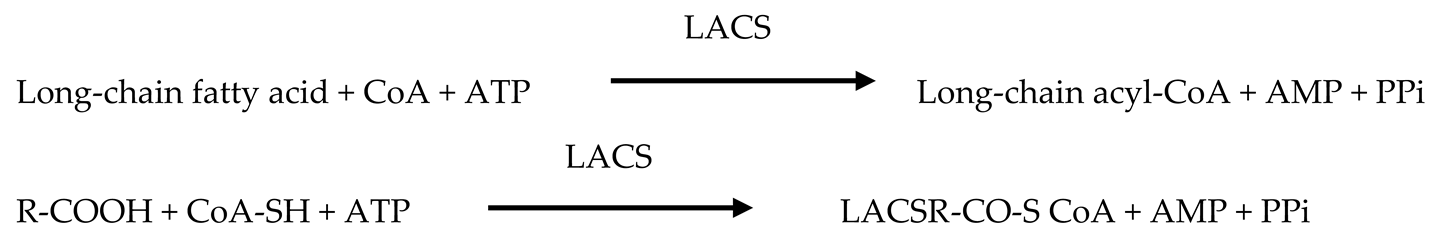
3.2.2. Key Pathways and Enzymes Involved in Cutin Biosynthesis

3.2.3. ABA Biosynthesis and Its Role as a Stress Hormone
2.2.4. The Chemical Constituents of Native Plants
4. Concluding Remarks
Author Contributions
Funding Information
Acknowledgments
Conflict of Interest
Ethics Statements
References
- Orcutt, D.M.; Nilsen, E.T. Physiology of Plants Under Stress: Soil and Biotic Factors; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Montesinos, E. Plant-associated microorganisms: A view from the scope of microbiology. Int. Microbiol. 2003, 6, 221–223. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Al-Thani, R.F. Ecophysiology of Wild Plants and Conservation Perspectives in the State of Qatar. In Agricultural Chemistry; Stoytcheva, M., Zlatev, R., Eds.; InTech: Rijeka, Croatia, 2013; pp. 37–70. [Google Scholar]
- Lu, H.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A critical review on communication mechanism within plant-endophytic fungi interactions to cope with biotic and abiotic stresses. J. Fungi 2021, 7, 719. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Tack, A.; Lobato, C.; Wassermann, B.; Berg, G. From seed to seed: The role of microbial inheritance in the assembly of the plant microbiome. Trends Microbiol. 2023, 31, 346–358. [Google Scholar] [CrossRef]
- Abulfatih, H.A.; Abdel-Bari, E.M.; Alsubaey, A.; Ibrahim, Y.M. Vegetation of Qatar; Scientific and Applied Research Center (SARC), University of Qatar: Doha, Qatar, 2001. [Google Scholar]
- Levitt, J. Responses of Plants to Environmental Stresses. Vol. II. Water, Radiation, Salt, and Other Stresses; Academic Press: New York, NY, USA; London, UK, 1980. [Google Scholar]
- Larcher, W. Physiological Plant Ecology. Eco-physiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- Abdel-Bari, E.M.; Yasseen, B.T.; Al-Thani, R.F. Halophytes in the State of Qatar; Environmental Studies Center, University of Qatar: Doha, Qatar, 2007. [Google Scholar]
- Yasseen, B.T.; Al-Thani, R.F. Endophytes, and halophytes to remediate industrial wastewater and saline soils: Perspectives from Qatar. Plants 2022, 11, 1497. [Google Scholar] [CrossRef] [PubMed]
- Batanouny, K.H. Plants in the Deserts of the Middle East; Springer: Berlin, Heidelberg, Germany, 2001. [Google Scholar]
- Mahasneh, A.M. Screening of some indigenous Qatari medicinal plants for antimicrobial activity. Phytother. Res. 2002, 16, 751–753. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Norton, J.; Abdul Majid, S.; Allan, D.; Al Safran, M.; Böer, B.; Richer, R. An Illustrated Checklist of the Flora of Qatar; UNESCO Office in Doha, Qatar Foundation, MAERSK OIL QATAR AS: Doha, Qatar, 2009. [Google Scholar]
- Al-Easa, H.S.; Rizk, A.M.; Abdel-Bari, E.M. Chemical Constituents and Nutritive Values of Range Plants in Qatar; Scientific and Applied Research Centre, University of Qatar: Doha, Qatar, 2003. [Google Scholar]
- Yasseen, B.T. An Analysis of the Effects of Salinity on Leaf Growth in Mexican Wheats. Ph.D. Thesis, University of Leeds, Leeds, UK, 1983. [Google Scholar]
- Chonan, N. Studies on the photosynthetic tissues in the leaves of cereal crops. I. The mesophyll structure of wheat leaves inserted at different levels of the shoot. Proc. Crop Sci. Soc. Jpn. 1965, 33, 388–393. [Google Scholar] [CrossRef]
- Longstreth, D.J.; Nobel, P.S. Salinity effects on leaf anatomy. Consequences for photosynthesis. Plant Physiol. 1979, 63, 700–703. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Abu-Al-Basal, M.A.; Alhadi, F.A. An Analysis of leaf growth under osmotic stress. J. Plant Sci. 2010, 5, 391–401. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Al-Thani, R.F. Wild plants in the Qatari peninsula are hidden gene bank for future research: Perspectives of desirable traits. In Cutting Edge Research in Biology; BP International: London, UK, 2023; Volume 8, pp. 207–252. [Google Scholar]
- Tripathi, G.; Gravit, P.; Stany, B.; Mishra, A.; Basu, S.; Tripathi, S. Stress Determination in Plants: Morphological, Biochemical, and Molecular Parameters. In Plant-Microbe Interactions for Environmental and Agricultural Sustainability; Pandey, A., Choure, K., El-Sheekh, M., Yadav, A.N., Eds.; Springer: Cham, Switzerland, 2025; pp. 345–378. [Google Scholar]
- Neves, J.; Sampaio, M.; Séneca, A.; Pereira, S.; Pissarra, J.; Pereira, C. Abiotic stress triggers the expression of genes involved in protein storage vacuole and exocyst-mediated routes. Int. J. Mol. Sci. 2021, 22, 10644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants' response to abiotic stress: Mechanisms and strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Abdulraheem, M.I.; Xiong, Y.; Moshood, A.Y.; Cadenas-Pliego, G.; Zhang, H.; Hu, J. Mechanisms of plant epigenetic regulation in response to plant stress: Recent discoveries and implications. Plants 2024, 13, 163. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Orcutt, D.M. The Physiology of Plants Under Stress: Abiotic Factors, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1996; Volume 1. [Google Scholar]
- Conkey, A.T.; Purchase, C.; Richer, R.; Yamaguchi, N. Terrestrial Biodiversity in Arid Environments: One Global Component of Climate Crisis Resilience. In Sustainable Qatar. Gulf Studies; Cochrane, L., Al-Hababi, R., Eds.; Springer: Singapore, 2023; Volume 9, pp. 241–265. [Google Scholar]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease resistance mechanisms in plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Gong, D.; Wang, Z.; Liu, L.; He, J.; Han, X.; Tsuda, K. How plants manage pathogen infection. EMBO Rep. 2024, 25, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kariyat, R.R.; Hardison, S.B.; De Moraes, C.M.; Mescher, M.C. Plant spines deter herbivory by restricting caterpillar movement. Biol. Lett. 2017, 13, 20170176. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Sevanto, S. Why do plants have waxy leaves? Do we know after all? Tree Physiol. 2020, 40, 823–826. [Google Scholar] [CrossRef]
- Mageroy, M.H.; Nagy, N.E.; Stefenrem, A.; Krokene, P.; Hietala, A.M. Conifer defences against pathogens and pests-mechanisms, breeding, and management. Curr. For. Rep. 2023, 9, 429–443. [Google Scholar] [CrossRef]
- Xu, H.; Lybrand, D.; Bennewitz, S.; Tissier, A.; Last, R.L.; Pichersky, E. Production of trans-chrysanthemic acid, the monoterpene acid moiety of natural pyrethrin insecticides, in tomato fruit. Metab. Eng. 2018, 47, 271–278. [Google Scholar] [CrossRef]
- Plant Disease-Edexcel, Plant Defense. Available online: https://www.bbc.co.uk/bitesize/guides/z29trwx/revision/3 (accessed on 20 July 2025).
- Kocyigit, E.; Kocaadam-Bozkurt, B.; Bozkurt, O.; Ağagündüz, D.; Capasso, R. Plant toxic proteins: Their biological activities, mechanism of action and removal strategies. Toxins 2023, 15, 356. [Google Scholar] [CrossRef]
- Jahan, T.; Nurul Huda, M.; Zhang, K.; He, Y.; Lai, D.; Dhami, N.; Quinet, M.; Ali, M.A.; Kreft, I.; Woo, S.-H.; Georgiev, M.I.; Fernie, A.R.; Zhou, M. Plant secondary metabolites against biotic stresses for sustainable crop protection. Biotechnol. Adv. 2025, 79, 108520. [Google Scholar] [CrossRef]
- Fahmy, G.M.; Al-Thani, R.F. Components of water potential and concentrations of nutrient elements in the holoparasitic angiosperm Cynomorium coccineum L. and its host. Flora 2025, 330, 152786. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Etemadi, N.; Müller, M.; Etemadi, M.; Brandón, M.G.; Ascher Jenull, J. Salt tolerance of Cressa cretica and its rhizosphere microbiota. Biologia 2020, 75, 355–366. [Google Scholar] [CrossRef]
- Ullah, A.; Mushtaq, H.; Ali, U.; Hakim; Ali, E. ; Mubeen, S. Screening, isolation, biochemical and plant growth promoting characterization of endophytic bacteria. Microbiol. Curr. Res. 2018, 2, 62–68. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Halo-thermophilic bacteria and heterocyst cyanobacteria found adjacent to halophytes at Sabkhas – Qatar: Preliminary study and possible roles. Afr. J. Microbiol. Res. 2017, 11, 1346–1354. [Google Scholar]
- Al-Thani, R.F.; Yasseen, B.T. Solutes in native plants in the Arabian Gulf region and the role of microorganisms: Future research. J. Plant Ecol. 2018, 11, 671–684. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Biological soil crusts, and extremophiles adjacent to native plants at Sabkhas and Rawdahs, Qatar: The possible roles. Front. Environ. Microbiol. 2018, 4, 55–70. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Possible future risks of pollution consequent to the expansion of oil and gas operations in Qatar. Environ. Pollut. 2023, 12, 12–52. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.K.; Sharma, J.G.; Giri, B. Insights into the multifaceted roles of soil microbes in mitigating abiotic stress in crop plants: A review. Environ. Exp. Bot. 2024, 228, 106010. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Zoukidis, K.; Vasilikiotis, C.; Apostolidis, A.; Giannakoula, A.E.; Bountla, A.; Chatziathanasiadis, A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi may improve soil fertility and the growth, nutrient uptake, and physiological performance of batavia lettuce (Lactuca sativa L. var. longifolia) plants. Horticulturae 2024, 10, 449. [Google Scholar] [CrossRef]
- Ahmed, N.; Li, J.; Li, Y.; Deng, L.; Deng, L.; Chachar, M.; Chachar, Z.; Chachar, S.; Hayat, F.; Raza, A.; Umrani, J.H.; Gong, L.; Tu, P. Symbiotic synergy: How Arbuscular Mycorrhizal Fungi enhance nutrient uptake, stress tolerance, and soil health through molecular mechanisms and hormonal regulation. IMA Fungus 2025, 16, 144989. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, L.; Chen, G.; Yao, M.; Liu, Z.; Li, X.; Yang, X.; Yang, Y.; Cai, D.; Tuerxun, Z.; Li, B.; Nie, T.; Chen, X. Arbuscular mycorrhizal fungi enhance drought resistance and alter microbial communities in maize rhizosphere soil. Environ. Technol. Innovation 2025, 37, 103947. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant growth-promoting soil bacteria: Nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Pan, L.; Cai, B. Phosphate-solubilizing bacteria: Advances in their physiology, molecular mechanisms, and microbial community effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Mahmood-Ur-Rahman; Fakhar, A. ; Rafique, M.; Chen, Y.; Yang, S.H.; Wang, X. Plant growth-promoting rhizobacteria eliminate the effect of drought stress in plants: A review. Front. Plant Sci. 2022, 13, 875774. [Google Scholar] [CrossRef]
- Yasseen, B.T.; Al-Thani, R.F.; Alhadi, F.A.; Abbas, R.A.A. Soluble sugars in plants under stress at the Arabian Gulf region: Possible roles of microorganisms. J. Plant Biochem. Physiol. 2018, 6, 224. [Google Scholar]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Gamalero, E.; Lingua, G.; Glick, B.R. Ethylene, ACC, and the plant growth-promoting enzyme ACC deaminase. Biology 2023, 12, 1043. [Google Scholar] [CrossRef]
- Hidri, R.; Metoui Ben Mahmoud, O.; Debez, A.; Zorrig, W.; Abdelly, C.; Zamarreño, A.M.; García Mina, J.M.; Azcon, R.; Aroca, R. Dual PGPR AMF inoculation offsets salinity stress impact on the fodder halophyte Sulla carnosa by concomitantly modulating plant ABA content and leaf antioxidant response. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Phytoremediation of polluted soils and waters by Native Qatari plants: Future perspectives. Environ. Pollut. 2020, 259, 113694. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, R.F.; Yasseen, B.T.; Balakrishnan, P. Microorganisms and halophytes attracted to the northeast coast of Qatar for potential phytoremediation: A case study and analysis. Int. J. Curr. Microbiol. Appl. Sci. 2025, 14, 79–102. [Google Scholar] [CrossRef]
- Abdel-Bari, E.M.M. The Flora of Qatar, The Dicotyledons (Vol. 1), The Monocotyledons (Vol. 2); Environmental Studies Center, University of Qatar: Doha, Qatar, 2012. [Google Scholar]
- Mazen, A.M.A. Crassulacean acid metabolism criteria shown by three species from flora of Qatar. QRS Repository 2011, 2011, 2427. Available online: http://www.qscience.com/doi/abs/10.5339/qnrs.2011.2427 (accessed on 28 March 2025).
- Ashore, M.M. Sabkhas in the Peninsula of Qatar– Geomorphologic and Geological and Biological Studies; Centre of Documentation and Humanitarian Studies, University of Qatar: Doha, Qatar, 1991. [Google Scholar]
- Skariah, S.; Abdul-Majid, S.; Hay, A.G.; Acharya, A.; Kano, N.; Al-Ishaq, R.K.; de Figueiredo, P.; Han, A.; Guzman, A.; Dargham, S.R.; Sameer, S.; Kim, G.E.; Khan, S.; Pillai, P.; Sultan, A.A. Soil properties correlate with microbial community structure in Qatari arid soils. Microbiol. Spectr. 2023, 11, e0346222. [Google Scholar] [CrossRef]
- Alrajhei, K.; Saleh, I.; Abu-Dieyeh, M.H. Biodiversity of arbuscular mycorrhizal fungi in plant roots and rhizosphere soil from different arid land environment of Qatar. Plant Direct 2022, 6, e369. [Google Scholar] [CrossRef]
- Umar, A.; Mwaheb, M.A.; Ameen, F.; Almomani, F.; Dufossé, L.; Gancarz, M. Role of ectomycorrhizal colonization in enhancement of nutrients for survival of plants collected from mountainous cold stress areas. BMC Microbiol. 2024, 24, 304. [Google Scholar] [CrossRef]
- Kant, M.R.; Jonckheere, W.; Knegt, B.; Lemos, F.; Liu, J.; Schimmel, B.C.; Villarroel, C.A.; Ataide, L.M.; Dermauw, W.; Glas, J.J.; Egas, M.; Janssen, A.; Van Leeuwen, T.; Schuurink, R.C.; Sabelis, M.W.; Alba, J.M. Mechanisms and ecological consequences of plant defense induction and suppression in herbivore communities. Ann. Bot. 2015, 115, 1015–1051. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Elgharib, A.; Trigo, M.M.; Moursy, M.M.; Soultan, A. Vegetation analysis and environmental relationships of Qatar's depression habitat. Plants 2025, 14, 1807. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.M.; El-Ghazaly, G.A. Medicinal and Poisonous Plants of Qatar; Scientific and Applied Research Centre, University of Qatar, The Doha Modern Printing Press, Ltd.: Doha, Qatar, 1995. [Google Scholar]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; Kumar, A.; Singh, R.P.; Meena, R.S.; Behera, T.K. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Vwioko, D.E.; Fashemi, D.S. Growth response of Ricinus communis L (Castor Oil) in spent lubricating oil polluted soil. J. Appl. Sci. Environ. Manage. 2005, 9, 73–79. [Google Scholar] [CrossRef]
- Kvesitadze, E.; Sadunishvili, T.; Kvesitadze, G. Mechanisms of organic contaminants uptake and degradation in plants. World Acad. Sci. Eng. Technol. 2009, 55, 458–468. [Google Scholar]
- Yasseen, B.T. Phytoremediation of industrial wastewater from oil and gas fields using native plants: The research perspectives in the State of Qatar. Cent. Eur. J. Exp. Biol. 2014, 3, 6–23. [Google Scholar]
- Vwioko, D.E.; Anoliefo, G.O.; Fashemi, S.D. Metal concentration in plant tissues of Ricinus communis L. (Castor Oil) grown in soil contaminated with spent lubricating oil. J. Appl. Sci. Environ. Manage. 2006, 10, 127–134. [Google Scholar] [CrossRef]
- Niu, Z.-X.; Sun, L.-N.; Sun, T.-H.; Li, Y.S.; Wang, H. Evaluation of phytoextracting cadmium and lead by sunflower, ricinus, alfalfa and mustard in hydroponic culture. J. Environ. Sci. 2007, 19, 961–967. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, K.; Singh, B.; Singh, R.P. Ricinus communis: A robust plant for bio-energy and phytoremediation of toxic metals from contaminated soil. Ecol. Eng. 2015, 84, 640–652. [Google Scholar] [CrossRef]
- Rizk, A.M. The Phytochemistry of the Flora of Qatar; Scientific and Applied Research Centre, University of Qatar, Kingprint: Richmond, UK, 1986. [Google Scholar]
- Rizk, A.M.; Al-Nowaihi, A.S. The Phytochemistry of the Horticultural Plants of Qatar; The Scientific and Applied Research Centre, University of Qatar, The Alden Press: Oxford, UK, 1989. [Google Scholar]
- Rizk, A.M.; Al-Easa, H.S.; Kornprobst, J.M. The Phytochemistry of the Macro and Blue-Green Algae of the Arabian Gulf; Faculty of Science, University of Qatar: Doha, Qatar, 1999. [Google Scholar]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: an important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Gahir, S.; Bharath, P.; Raghavendra, A.S. Stomatal closure sets in motion long-term strategies of plant defense against microbial pathogens. Front. Plant Sci. 2021, 12, 761952. [Google Scholar] [CrossRef]
- Meddya, S.; Meshram, S.; Sarkar, D.; Rakesh, S.; Datta, R.; Singh, S.; Avinash, G.; Kondeti, A.K.; Savani, A.K.; Thulasinathan, T. Plant stomata: An unrealized possibility in plant defense against invading pathogens and stress tolerance. Plants 2023, 12, 3380. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Miller, R.N.; Costa Alves, G.S.; Van Sluys, M.A. Plant immunity: unravelling the complexity of plant responses to biotic stresses. Ann. Bot. 2017, 119, 681–687. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Zhi, P.; Chang, C. Update on cuticular wax biosynthesis and its roles in plant disease resistance. Int. J. Mol. Sci. 2020, 21, 5514. [Google Scholar] [CrossRef]
- Devitt, J.K.; Chung, A.; Schenk, J.J. Inferring the genetic responses to acute drought stress across an ecological gradient. BMC Genomics 2022, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Seufert, P.; Staiger, S.; Arand, K.; Bueno, A.; Burghardt, M.; Riederer, M. Building a barrier: The influence of different wax fractions on the water transpiration barrier of leaf cuticles. Front. Plant Sci. 2022, 12, 766602. [Google Scholar] [CrossRef] [PubMed]
- Anjali; Kumar, S. ; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Dassanayake, M.; Larkin, J.C. Making plants break a sweat: The structure, function, and evolution of plant salt glands. Front. Plant Sci. 2017, 8, 406. [Google Scholar] [CrossRef]
- Al-Haliem, S.M.; Mohammed, M.J.; Hesarinejad, M.A.; Abedelmaksoud, T.G. Antimicrobial, anti-biofilm activity and antioxidants of phenolic compounds isolated from Hypericum perforatum on periodontal pathogenic oral bacteria. Food Sci. Nutr. 2025, 13, e70336. [Google Scholar] [CrossRef]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Emwas, A.H.; Khan, R.A. Salt-tolerant plants, halophytes, as renewable natural resources for cancer prevention and treatment: Roles of phenolics and flavonoids in immunomodulation and suppression of oxidative stress towards cancer management. Int. J. Mol. Sci. 2023, 24, 5171. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P.E. Biopolyester membranes of plants: cutin and suberin. Science 1980, 208, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, T.W.; Mercer, E.I. Introduction to Plant Biochemistry, 2nd ed.; Pergamon Press: Oxford, UK, 1990; pp. 312–315. [Google Scholar]
- Dennis, D.T.; Turpin, D.H. Plant Physiology, Biochemistry, and Molecular Biology; Longman Scientific & Technical: Essex, UK, 1993; pp. 339–325. [Google Scholar]
- Lea, P.J.; Leegood, R.C. Plant Biochemistry and Molecular Biology; John Wiley & Sons: Chichester, UK; New York, NY, USA, 1993; p. 312. [Google Scholar]
- Wang, X.; Chang, C. Exploring and exploiting cuticle biosynthesis for abiotic and biotic stress tolerance in wheat and barley. Front. Plant Sci. 2022, 13, 1064390. [Google Scholar] [CrossRef]
- Yang, W.; Pollard, M.; Li-Beisson, Y.; Beisson, F.; Feig, M.; Ohlrogge, J. A distinct type of glycerol-3-phosphate acyltransferase with sn-2 preference and phosphatase activity producing 2-monoacylglycerol. Plant Biol. 2010, 107, 12040–12045. [Google Scholar] [CrossRef]
- Sui, N.; Tian, S.; Wang, W.; Wang, M.; Fan, H. Overexpression of glycerol-3-phosphate acyltransferase from Suaeda salsa improves salt tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 1337. [Google Scholar] [CrossRef]
- Mao, G.; Seebeck, T.; Schrenker, D.; Yu, O. CYP709B3, a cytochrome P450 monooxygenase gene involved in salt tolerance in Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 169. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.V.; Jugulam, M. Role of cytochrome P450 enzymes in plant stress response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Chakraborty, P.; Biswas, A.; Dey, S.; Bhattacharjee, T.; Chakrabarty, S. Cytochrome P450 gene families: Role in plant secondary metabolites production and plant defense. J. Xenobiot. 2023, 13, 402–423. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Zhang, Y.; Aghaei-Dargiri, S.; Liu, G.; Zhu, S.; Yu, C.; Chen, Y.; Zhong, F.; Zhang, J. Long-chain acyl-CoA synthetases promote poplar resistance to abiotic stress by regulating long-chain fatty acid biosynthesis. Int. J. Mol. Sci. 2022, 23, 8401. [Google Scholar] [CrossRef]
- Zhao, H.; Kosma, D.K.; Lü, S. Functional role of long-chain acyl-CoA synthetases in plant development and stress responses. Front. Plant Sci. 2021, 12, 640996. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Q.; Lei, Y.; Zou, J.; Li, Q. Adaptation of cuticle metabolism to abiotic stress in plants. Crop Environ. 2025, 4, 38–44. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates, Inc., Publishers: Sunderland, MA, USA, 2010. [Google Scholar]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid, and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.K.; Khanday, D.M.; Choudhary, S.M.; Kumar, P.; Kumari, S.; Martínez-Andújar, C.; Martínez-Melgarejo, P.A.; Rai, P.K.; Pérez-Alfocea, F. Unlocking nature's stress buster: Abscisic acid's crucial role in defending plants against abiotic stress. Plant Stress 2024, 11, 100359. [Google Scholar] [CrossRef]
- Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Mo, W.; Zheng, X.; Shi, Q.; Zhao, X.; Chen, X.; Yang, Z.; Zuo, Z. Unveiling the crucial roles of abscisic acid in plant physiology: implications for enhancing stress tolerance and productivity. Front. Plant Sci. 2024, 15, 1437184. [Google Scholar] [CrossRef]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8'-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef]
- Okamoto, M.; Kuwahara, A.; Seo, M.; Kushiro, T.; Asami, T.; Hirai, N.; Kamiya, Y.; Koshiba, T.; Nambara, E. CYP707A1 and CYP707A2, which encode abscisic acid 8'-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006, 141, 97–107. [Google Scholar] [CrossRef]
- Saito, S.; Hirai, N.; Matsumoto, C.; Ohigashi, H.; Ohta, D.; Sakata, K.; Mizutani, M. Arabidopsis CYP707As encode (+)-abscisic acid 8'-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 2004, 134, 1439–1449. [Google Scholar] [CrossRef]
- Moussii, I.M.; Nayme, K.; Timinouni, M.; Jamaleddine, J.; Filali, H.; Hakkou, F. Synergistic antibacterial effects of Moroccan Artemisia herba alba, Lavandula angustifolia and Rosmarinus officinalis essential oils. Synergy 2020, 10, 100057. [Google Scholar] [CrossRef]
- Mohammed, S.; Alhusseini, L.B. Antibacterial and cytotoxic activities of different solvent extracts from Artemisia herba-alba against MCF-7 human breast cancer cells. Contemp. Oncol. 2025, 29, 159–164. [Google Scholar] [CrossRef]
- El Sahzly, A.; Abdel-All, M.; Tei, A.; Wink, M. Pyrrolizidine alkaloids from Echium rauwolfii and Echium horridum (Boraginaceae). Z. Naturforsch. C 1999, 54, 295–300. [Google Scholar] [CrossRef]
- Dawidar, A.M.; Ghani, A.; El-Shamy, M.; Tawfik, E.; Abdel-Mogib, M. Fatty Acid Pattern and Alkaloids of Echium Rauwolfii. Int. J. Sci. Eng. Appl. 2015, 4, 208–213. [Google Scholar] [CrossRef]
- Jin, J.; Boersch, M.; Nagarajan, A.; Davey, A.K.; Zunk, M. Antioxidant properties and reported ethnomedicinal use of the genus Echium (Boraginaceae). Antioxidants 2020, 9, 722. [Google Scholar] [CrossRef]
- Idrees, S.; Qureshi, R.; Bibi, Y.; Ishfaq, A.; Khalid, N.; Iftikhar, A.; Shabir, A.; Riaz, I.; Ahmad, S.N. Ethnobotanical and biological activities of Leptadenia pyrotechnica (Forssk.) Decne.: A Review. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 88–96. [Google Scholar] [CrossRef]
- El-Fitiany, R.A.; Khasawneh, M.A. Leptadenia pyrotechnica (Forsk) Decne: From edibility to drug discovery (A comparative review). Food Rev. Int. 2023, 39, 7580–7610. [Google Scholar] [CrossRef]
- Das, S.N.; Patro, V.J.; Dinda, S.C. A review: Ethnobotanical survey of genus Leucas. Pharmacogn. Rev. 2012, 6, 100–106. [Google Scholar] [CrossRef]
- Dixit, V.; Irshad, S.; Agnihotri, P.; Paliwal, A.K.; Husain, T. Evaluation of antioxidant and antimicrobial potential of Leucas urticaefolia (Lamiaceae). J. Appl. Pharm. Sci. 2015, 5, 39–45. [Google Scholar] [CrossRef]
- Nutan, R.; Veena, S. Phytochemical analysis of Leucas urticifolia (Vahl) R. Br. Ex Sm.: A traditional medicinal herb. J. Pharmacogn. Phytochem. 2019, 8, 1752–1756. [Google Scholar]
- Antil, R.; Singh, L.; Gahlawat, D.K.; Dahiya, P. Antimicrobial, phytochemical, and antioxidant potential of lamiaceae family plant: L. aspera (willd.) linn. Plant Arch. 2021, 20, 616–630. [Google Scholar]
- Abdel-Sattar, E.; Shams, M.M.; Abd-Rabo, M.M.; Mahmoud, N.; Mahrous, E.A. Chemical and biological investigations of Limonium axillare reveal mechanistic evidence for its antidiabetic activity. PLoS One 2021, 16, e0255904. [Google Scholar] [CrossRef]
- Alhaddad, F.A.; Bitaar, Z.M.; Abu-Dieyeh, M.H. Diversity, characterization, and potential applications of bacterial endophytes isolated from the halophyte Limonium axillare. J. Plant Growth Regul. 2024, 43, 2179–2196. [Google Scholar] [CrossRef]
- Alhaddad, F.; Abu-Dieyeh, M.; Jaoua, S.; Al-Ghouti, M.A.; Al-Thani, R.; Ahmed, T. Screening, diversity, and characterization of fungal endophytes isolated from the halophyte Limonium axillare and the potential of biocontrol antagonists against Fusarium oxysporum. Plant Direct 2025, 9, e70026. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, R.; Alhan, S.; Sharma, H.; Singh, N.; Yogi, R.; Chhokar, V.; Beniwal, V.; Ghosh, M.K.; Chandraker, S.K.; Rustagi, S.; Kumar, A. Lycium shawii mediated green synthesis of silver nanoparticles, characterization and assessments of their phytochemical, antioxidant, antimicrobial properties. Inorg. Chem. Commun. 2024, 159, 111735. [Google Scholar] [CrossRef]
- Al-Nemi, R.; Akkawi, M.; Sawalha, K.; Kusumastuti, S.A.; Nuralih; Kusumaningrum, S. ; Okselni, T.; Situmorang, V.C.; Septama, A.W.; Jaremko, M.; Emwas, A.H. Comprehensive metabolomics profiling and bioactivity study of Lycium shawii (Awsaj) extracts with particular emphasis on potential anti-malarial properties. Metabolites 2025, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Rajendrasozhan, S.; El Moll, H.; Snoussi, M.; Romeilah, R.M.; Shalaby, E.A.; Younes, K.M.; El-Beltagi, H.S. Phytochemical screening and antimicrobial activity of various extracts of aerial parts of Rhanterium epapposum. Processes 2021, 9, 1351. [Google Scholar] [CrossRef]
- Alanazi, A.D.; Alghabban, A.J. Antileishmanial and synergic effects of Rhanterium epapposum essential oil and its main compounds alone and combined with glucantime against Leishmania major infection. Int. J. Parasitol. Drugs Drug Resist. 2024, 26, 100571. [Google Scholar] [CrossRef] [PubMed]
- Mesmar, J.; Abdallah, R.; Badran, A.; Maresca, M.; Shaito, A.; Baydoun, E. Ziziphus nummularia: A comprehensive review of its phytochemical constituents and pharmacological properties. Molecules 2022, 27, 4240. [Google Scholar] [CrossRef] [PubMed]
- Poyil, M.M.; Alsharif, M.H.K. Phytocompounds from Saudi medicinal plant Ziziphus nummularia against vancomycin-resistant Staphylococcus aureus (VRSA) causing atopic dermatitis (AD). Int. J. Pharmacol. 2023, 19, 655–664. [Google Scholar] [CrossRef]
- Abulfatih, H.A. Ecological anatomy of xerophytic leaves from Qatar. J. King Saud Univ. Sci. 2003, 16, 19–29. [Google Scholar]
- Serrano, M.; Coluccia, F.; Torres, M.; L'Haridon, F.; Métraux, J.P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 274. [Google Scholar] [CrossRef]
- Ziv, C.; Zhao, Z.; Gao, Y.G.; Xia, Y. Multifunctional roles of plant cuticle during plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1088. [Google Scholar] [CrossRef]
- Riseh, R.S.; Fathi, F.; Gholizadeh, M.; Vazvani, M.V.; Vatankhah, M.; Kennedy, J.F. Defense-related callose deposition in plants against pathogens: A review. Int. J. Biol. Macromol. 2025, 320, 146005. [Google Scholar] [CrossRef] [PubMed]
- Khan, N. Decoding phytohormone signaling in plant stress physiology: Insights, challenges, and future directions. Environ. Exp. Bot. 2025, 231, 106099. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Kripa, K.G. Therapeutic uses of plants of genus Blepharis: A systematic review. Int. J. Pharm. Bio. Sci. 2016, 7, 236–243. [Google Scholar]
- Dirar, A.I.; Adhikari-Devkota, A.; Kunwar, R.M.; Paudel, K.R.; Belwal, T.; Gupta, G.; Chellappan, D.K.; Hansbro, P.M.; Dua, K.; Devkota, H.P. Genus Blepharis (Acanthaceae): A review of ethnomedicinally used species, and their phytochemistry and pharmacological activities. J. Ethnopharmacol. 2021, 265, 113255. [Google Scholar] [CrossRef]
- Paleg, L.G.; Aspinall, D. The Physiology and Biochemistry of Drought Resistance in Plants; Academic Press: Sydney, Australia, 1981. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Sri Laxmi, P.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake, and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Rock, C.D. Abscisic acid biosynthesis, and response. Arabidopsis Book 2002, 1, e0058. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Bastías, D.A.; Balestrini, R.; Pollmann, S.; Gundel, P.E. Environmental interference of plant-microbe interactions. Plant Cell Environ. 2022, 45, 3387–3398. [Google Scholar] [CrossRef] [PubMed]
- Mishra, U.N.; Chauhan, J.; Singhal, R.K.; Anuragi, H.; Dey, P.; Lal, D.; Pandey, S.; Gupta, N.K.; Nayak, J.K.; Tripathi, A.; Singh, M.; Yadav, M.; Sajeevan, R.S. Abiotic stress responses in forage crops and grasses: The role of secondary metabolites and biotechnological interventions. Front. Plant Sci. 2025, 16, 1542519. [Google Scholar] [CrossRef] [PubMed]

| Features | Desert plants | Mesophytes (non-stressed plants) |
Observations |
|---|---|---|---|
| Thickness of cuticle | Thick and waxy | Thin to moderate | Increase in the lipid content of the cuticle, wax biosynthesis by CER1* [85] |
| Cutin composition | Highly polymerised; includes esters of fatty acids, aliphatic polymers: cutan, stress-adapted | Primarily polyester of hydroxy and/or epoxy fatty acids, less complex and thinner | Less cutan in mesophytes unless under stress [86] |
| Wax content | High; contains long-chain hydrocarbons | Moderate; composed of simpler compounds | Little information, needs further investigation, more wax per surface area, which reduces non-stomatal water loss under stress conditions [87] |
| Antimicrobials in the cuticle | Abundant phenolics and terpenoids | Few phenolics and terpenoids (produced mainly during pathogen attack) | Secondary metabolites such as phenolics such as flavonoids, tannins), and the presence of terpenoids, as protective and signaling roles [88] |
| Salt crystals in cuticle | Present | Absent | Salt crystals might prevent microbial attack, unless adapted [89] |
| Phenolics | High concentration | Low to moderate level | Play roles as antimicrobials and antioxidants [90] |
| Stress hormones (Abscisic acid; ABA) |
Elevated | Normal physiological levels | The presence of ABA to regulate stomatal movements [91] |
| Osmo-protectants | Present such as proline and glycine-betaine, etc. | Almost absent | The presence of compatible solutes to prevent water loss [92] |
| Reactive oxygen species (ROS) | Constitutively Active | Induced only under stress | The scavenging systems include ascorbate and glutathione [93] |
| Species | General characteristics | Specific features | References |
|---|---|---|---|
| Artemisia herba-alba, Syn. Artemisia inculta* | Medicinal plant | Source of active molecules, extracts may be used to treat breast cancer, antibacterial, and possibly for other uses | [115,116] |
| Echium horridium* 67 species | Medicinal plants might contain fatty acids such as palmitic acid | Extracts show antioxidant, analgesic, anxiolytic, anti-inflammatory, antibacterial, and antiviral effects | [117,118,119] |
| Leptadenia pyrotechnica** | Medicinal plant | Produces bioactive compounds with pharmaceutical activities, exhibits antimicrobial properties, extracts can resist certain bacteria species like S. aureus, E. coli, and B. subtilis, and some fungi species such as A. flavus, and F. moniliforme | [120,121] |
| Leucas urticifolia** | Medicinal plants contain phytochemicals, such as lignans, flavonoids, coumarins, steroids, terpenes, fatty acids, and aliphatic long-chain compounds | The presence of phytochemicals with antimicrobial properties, these constituents play roles in economic, social, cultural, and ecological aspects | [122,123,124,125] |
| Limonium axillare** | A huge number of bacterial isolates were obtained from leaves; many secondary metabolites were found in plant tissues that can play roles in biocontrol of microorganisms and contribute to sustainable agriculture | Antifungal activity, hosts fungal endophytes such as Aspergillus and Cladosporium, a huge number of bacterial isolates were obtained from leaves, while root and bark are sources of antidiabetic compounds | [126,127,128] |
| Lycium shawii, Syn. Lycium arabicum** | The most common shrub in Qatar responds phenotypically to water availability, from dried twiggy bare spiny bushes to green leafy plants, medicinal plants, the presence of alkaloids and sterols and terpenes, amino acids, fatty acids, and minerals | It exhibits a wide range of pharmacological properties, including antimicrobial, antioxidant, anti-diabetic, anti-inflammatory, anti-cancer, antitrypanosomal, hepatoprotective, antiplasmodial, and cytotoxic activities, making it a potential candidate for treating malaria through its therapeutic compounds | [129,130] |
| Rhanterium epapposum* | Medicinal plants and extracts show significant activity against bacteria and fungi, and are used to cure skin infections | Extracts show antimicrobial properties and antileishmanial activity | [131,132] |
| Ziziphus nummulariais* | Medicinal plants used in traditional folk medicine, rich in phytochemical constituents with pharmacological properties. These components include alkaloids, flavonoids, saponins, glycosides, tannins, and phenolic compounds | Extracts of this plant show a great deal of antibacterial and antifungal activities, exhibit, help to resist pathogens and treat various types of diseases, including cancer, diabetes, and cardiovascular diseases | [133,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





