1. Introduction
The digestive system of weaned rabbits is still not well developed and is susceptible to indigestion, diarrhea, or disturbances in the structure of the gut microbes caused by improper feeding management or pathogenic microbial infestation, which could further trigger pathological reactions or even death (Fang et al., 2020). Therefore, the improvement of weaned rabbits' intestinal health and the prevention of diarrhea have long been regarded as an important issue for the development of rabbit farming (Van Der Sluis et al., 2024). Traditional treatments of diarrhea are mostly growth-promoting additives and antibiotic drugs. However, massive supplements of antibiotics induce drug residues of animal origins, environmental pollution, and increased drug resistance and other problems are becoming increasingly serious (Oglesbee & Lord, 2020). Dandelion is a traditional Chinese medicine that clears away heat and toxic material. Its ethanol extract mainly contains phenolic acid, flavonoid, and polysaccharide (Li et al., 2022). Dandelion polysaccharides extract can resist pathogenic bacteria and improve serum immunoglobulin M (IgM), immunoglobulin G (IgG) levels and physical immunity (Mao et al., 2023), and relieve intestinal inflammation damage (Zhao et al., 2019). Flavonoids can increase the amount of antioxidant active substance dismutase (SOD) to resist oxidation (Chagas et al., 2022). However, restrained by the organism's selective metabolic absorption, the effective component in the dandelion is rarely distributed in the intestinal tract after absorption, making it less biologically active.
To improve the bioavailability of the active ingredients of dandelion, this study explored the approaches to make the active ingredients of dandelion extract more widely distributed in the intestinal tract for their action with the help of the pharmacology of traditional Chinese medicine. In traditional Chinese medicine, the theory of “Yinjing” is an important part of the theory of the nature of Chinese medicine. There are two meanings of this theory, one is that the drug itself can be concentrated in the organs and meridians to which it belongs, and the other is that the drug is directed to the appropriate part of the body. Modern medical research has found that the guiding drug probably affects the relative transfer protein and changes the structure of cell membrane and cellular pH value to advance the permeability of cell membrane and thus increases the concentration of medicine in the targeted organs or tissues (Qi et al., 2013). This “Yinjing” theory (Harrison & Churgin, 2022) provided us with an idea of combining another guiding drug with dandelion. Akebia belongs to the small intestine channel and has the medical function of inflammation and oxidation resistance (Liu et al., 2018). The β-sitosterol extracted from akebia can decrease the secretion of macrophage inflammatory factor interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α), and increase the activity of inflammatory factor interleukin 10 (IL-10) (Choi et al., 2012). Oleanolic Acid extract can reduce the concentration of mouse liver interleukin 1β(IL-1β) (Wang et al., 2013). Vanillic acid extract can reduce rat serum IL-1β, IL-6, and TNF-α levels to resist inflammation (Khoshnam et al., 2018). Based on the channel tropism theory and the medical activity of dandelion and akebia, it is assumed that, as the small intestinal tract directing medicine, akebia can lead the effective component in dandelion extract to be enriched in the specific position and strengthen the targeting effect, which could further improve the effect of dandelion in intestinal health. Studies have found that there was a close relationship between gut microbes and intestinal diseases, and intestinal flora is probably intimately connected with the physiological and biochemical functions of the body through regulating lymphocyte differentiation and inflammatory factor secretion (Dendrou et al., 2015; Grigoriadis & Van Pesch, 2015). Recent studies have pointed out the significant influence of intestinal microbiota on gut immunity through metabolism. In fecal samples from patients with inflammatory bowel disease, there is an increase in Aspergillus and Actinobacillus, and a decrease in the number of Firmicutes, which impairs the intestinal immune barrier (Kim et al., 2022). Researchers have found that modulation of the intestinal microbiota of piglets can induce both short-term and long-term effects on their intestinal health (Fang et al., 2020b; Li et al., 2022). Wen et al. (2023) found that Baicalin can moderate the intestinal micro-environmental balance. According to Sun et al. (2016), fermented Yu-Ping-Feng-San polysaccharides can maintain the intestinal barrier function and The objective of this experiment was to assess the impact of dandelion extract and akebia extract, administered both individually and in combination, on production performance, inflammatory status, and gut microbial structure in weaned rabbits.
2. Material and Methods
2.1. Ethic Statement
The animal feeding and treatment were performed in accordance with relevant requirements. Ethical approval for the experiment was obtained from the Animal Ethics Committee of Southwest University of China for animal welfare.
2.2. Experimental Animals and Feed
A total of 120 healthy, weaned rabbits (1.219 kg±0.077 kg, 35 days) were chosen and randomly allotted into four groups under two factor experimental design. Each group consisted of 10 replications, with 3 rabbits in each replication. Each factor contains 2 treatment levels, 0% and 0.5% dandelion or akebia extract in basic diet. The experiment lasted for 5 weeks including 1 week of pre-study period. Rabbits were fed with a basic diet during the pre-feeding stage, with a gradually increased feed amount from 50 g/d to 100 g/d. To avoid diarrhea caused by excessive feed intake, the feed amount during the 4 weeks of the experiment was respectively 120, 130, 140, 150 g/d, with free water access. During the formal experiment, the feed leftovers, diarrhea rate were daily recorded, while body weight was measured weekly to calculate the average daily gain.
2.3. Sample Collection and Management
After the trial, one weaned rabbit with moderate body condition in each replication was euthanized for blood, tissue and digesta sampling. Blood from anterior vein were collected in vacuum tubes without anticoagulants, and centrifugate (4 ℃, 3500rpm, 10 minutes) to obtain serum and then stored at -80℃ for further detection. Jejunal and ileal segments were longitudinally incised to harvest mucosal scrapings, which were preserved in 2 mL sterile tubes. Liver tissues were separated and placed in 1.5mL sterile centrifuge tubes. The contents of the cecum were collected in 5mL sterilized centrifuge tubes. After being frozen in liquid nitrogen, all centrifuge tubes are quickly transferred to a refrigerator at -80 ℃ for storage.
2.4. Cytokine Content Detection
The ELISA kit was used (Mlbio Co., Ltd) to measure IL-1β, IL-6, immunoglobulin A (IgA) and IgG concentration in serum, liver, and intestinal mucosa. The measurement was performed in strict adherence to the kit instructions. The assay was performed according to the procedure outlined in our previous study (Fang et al., 2020b). Specifically, samples were diluted 5 times and added into each cell wall, then filled with enzyme reagent except the blank wells. After incubation, each cell walls were washed with washing solution for 5 times and then administered color development. Determine the absorbance (OD value) of each well at 450nm with an enzyme meter.
2.5. RNA Extraction, cDNA Inversion Rate, and Real-Time PCR
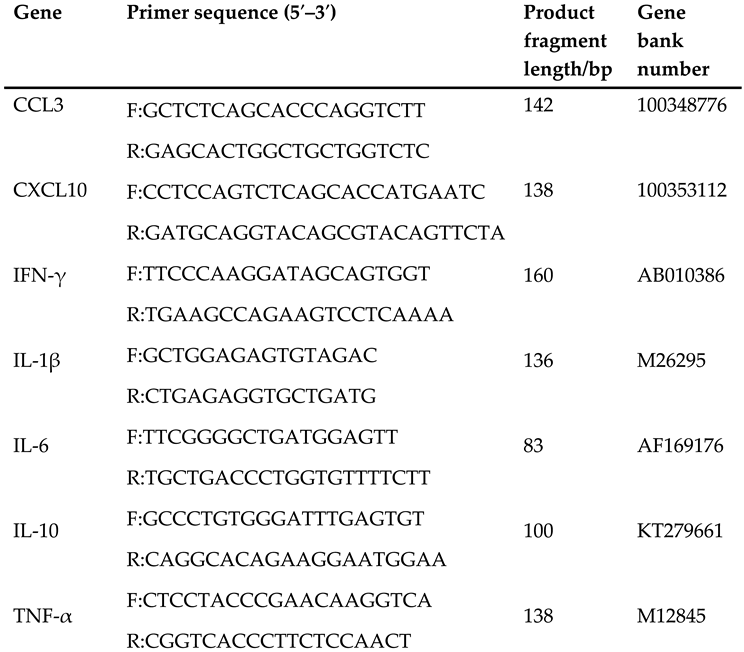
About 1g of jejunal and ileal mucosal tissues were taken and total RNA was extracted based on silica gel membrane purification technology and following the procedure mentioned in our previous study (Fang et al., 2020b) (The kit was purchased from Nanjing Vazyme Biotechnology Co., Ltd..). The sample was first lysed with buffer and then transferred to a gDNA filter column for centrifugation, and the filtrate was collected and mixed with absolute ethanol. The mixture was then transferred to Fast-Pure gDNA-Filter Column III for centrifugation, and the filtrate was discarded. Then buffer RW1 and buffer RW2 were added successively for centrifugation and discard the filtrate. Finally, RNA was eluted by centrifugation with enzyme-free sterile water. RNA concentration and purity were determined, and the obtained RNA was stored in a -80°C refrigerator for future use. Total RNA was reverse-transcribed to synthesize cDNA, following the instructions of reverse transcription (HiScriptP® III All-in-one RT Super-Mix Perfect for qPCR, Nanjing Vazyme Biotech Co., Ltd.), and the synthesized cDNA was stored in -20℃ refrigerator. The qPCR was demonstrated following the procedure of our previous study [
6]. The primer sequences of the detected genes shown in
Table 1 were created using Primer Premier 5.0 software, synthesized by China Shenzhen Huada Gene Co., Ltd. The detected genes were TNF-α, IL-1β, IL-6, IL-10, INF-γ, C-X-C Motif Chemokine Ligand 10 (CXCL10), and C-C Motif Chemokine Ligand 3 (CCL3). The relative expression of the targeted gene took Glyceraldehyde-3-phosphate dehydrogenase (
GAPDH) as the internal reference gene and the calculation of relative mRNA expression of T-bet and GATA-3mRNA was based on
.
2.6. Illumina MiSeq Double-Tailed Sequencing
First, the original offline data of high-throughput sequencing was preliminarily screened according to the sequence quality. Retesting and supplementary testing of problematic samples. The original sequence that had passed quality screening was divided into libraries and samples based on index and barcode information, and the barcode sequence was removed. Perform sequence denoising or OTU clustering according to QIIME2 dada2 analysis process or Vsearch software analysis process. Display the specific composition of each sample (group) at the taxonomy level of different species to understand the overall situation. Evaluate the alpha diversity level of each sample based on the distribution of ASV/OTU in different samples, and reflect the suitability of sequencing depth through sparse curves. At the level of ASV/OTU, calculate the distance matrix of each sample, and measure the difference and significance of beta diversity among different samples (groups) through a variety of unsupervised sorting and clustering methods, combined with corresponding statistical testing methods. At the level of species taxonomy composition, through various unsupervised and supervised sorting, clustering, and modeling methods, combined with the corresponding statistical test methods, further measures the differences in species abundance composition among different samples (groups), and tries to find marker species. Based on the composition and distribution of species in each sample, construct an association network, calculate the topological index, and attempt to identify key species. Based on the sequencing results of 16S rRNA, 18S rRNA, and ITS genes, it is also possible to predict the microbial metabolic function of the sample, identify differential pathways, and obtain the species composition of specific pathways.
2.7. Data Statistical Analysis
Experimental pictures were plotted using GraphPad Prism 8.0, and SPSS 23.0 software was used to conduct a two-factor analysis of variance to compare the Main effect (whether dandelion and Akebia extract are added to the diet) and its interaction. The results are expressed as mean ± standard deviation (SD). The difference is statistically significant with p < 0.05.
3. Results
3.1. Influence of Dandelion and Akebia Extract on Weaned Rabbits in Growth
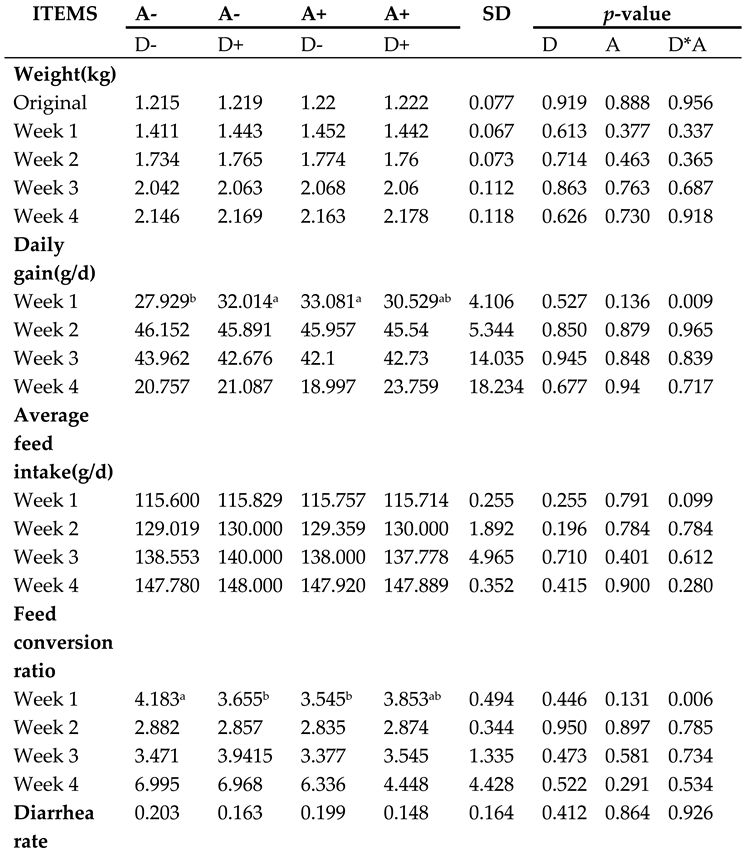
As shown in
Table 2, there was a significant interaction (
p < 0.05) between the addition of dandelion and akebia extract to the diet for rabbits on first week’s daily weight gain.
3.2. Influence of Dandelion and Akebia Extract Addition on IL-1β, IL-6, IgA, and IgG Concentration of Weaned Rabbits
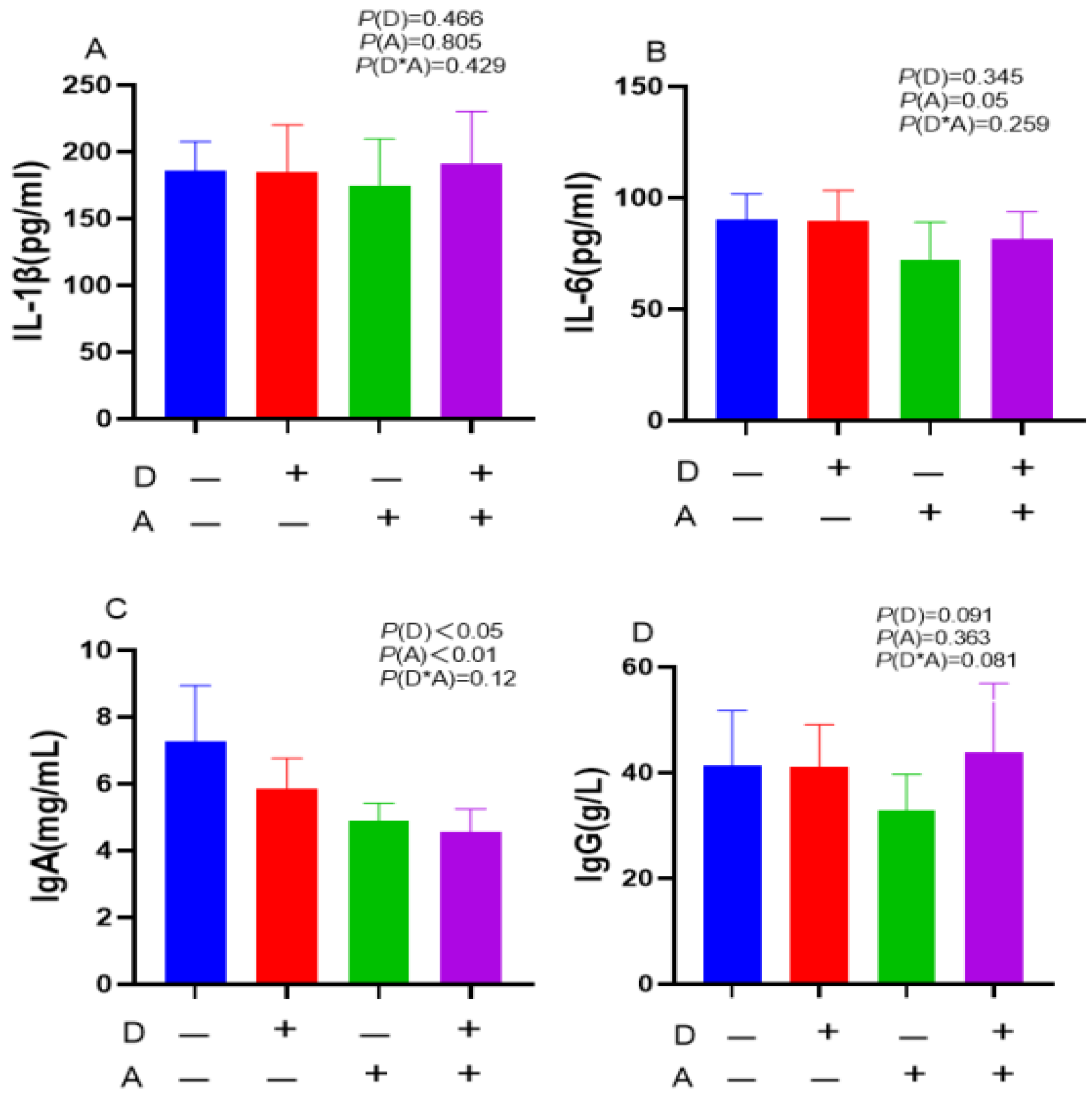
3.2.1. Influence of Dandelion and Akebia Extract Addition on Serum IL-1β, IL-6, IgA, and IgG Concentration of Weaned Rabbits
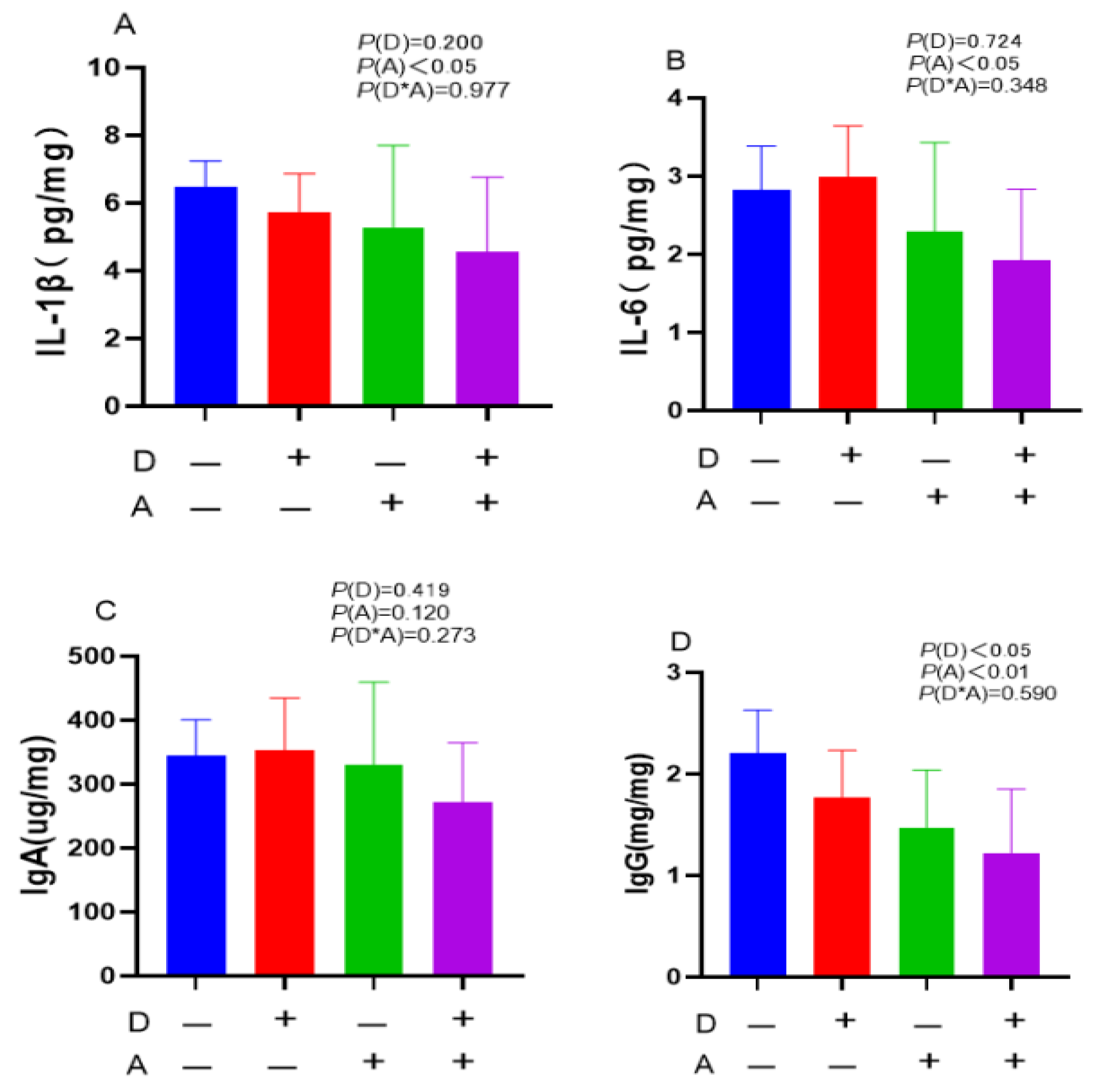
Figure 1 indicates a highly significant (
p < 0.01) decrease in serum IgA concentration with addition of akebia extract. In addition, there was a significant (
p < 0.05) decrease in IgA with the addition of dandelion. There was no detectable interaction between the akebia and dandelion extracts in terms of IgA content, IL-6, IgG, along with IL-1β.
3.2.2. Influence of Dandelion and Akebia Extract Addition on Liver IL-1β, IL-6,IgA and IgG Concentration of Weaned Rabbits
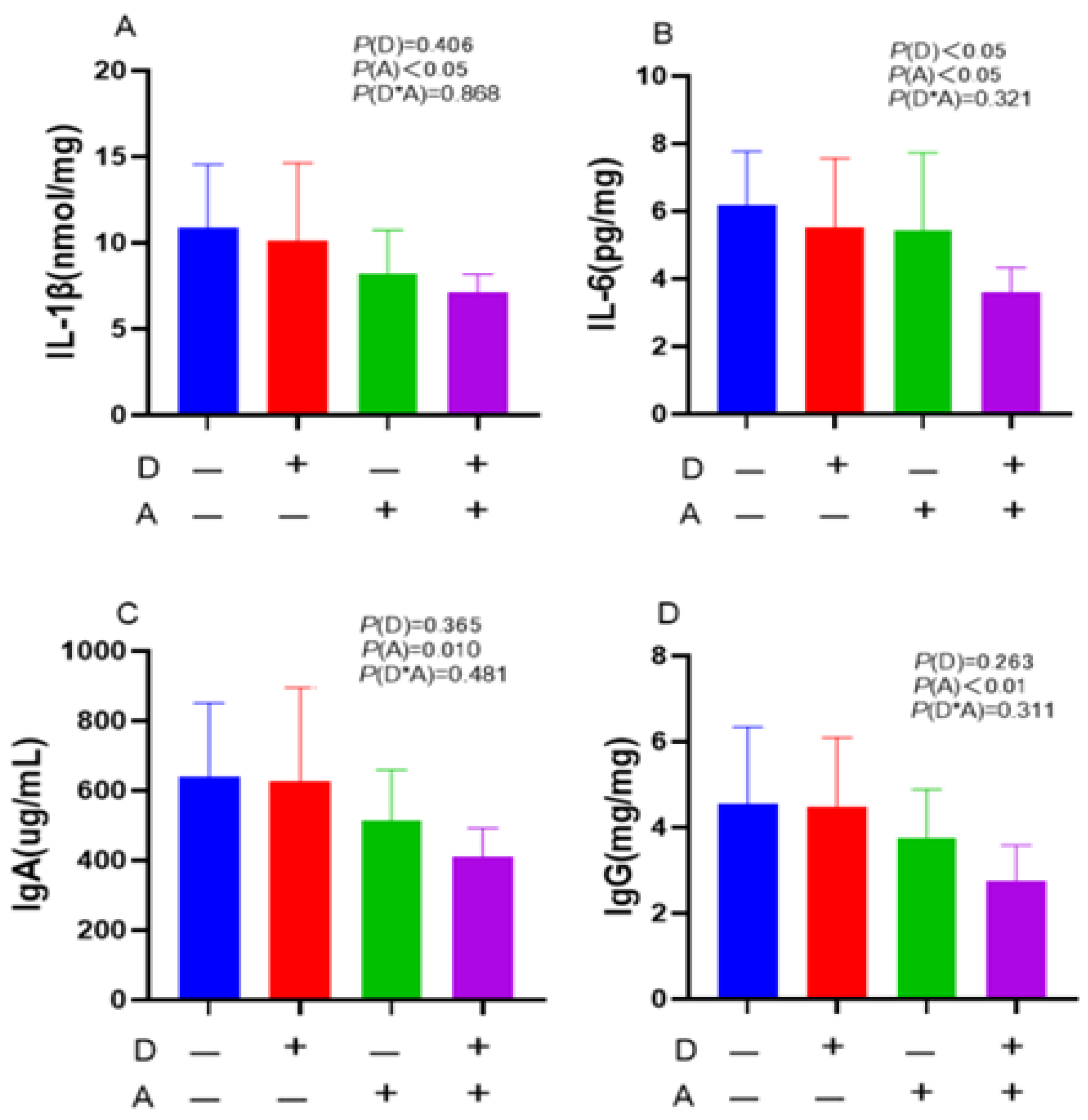
Addition of dandelion extract in the diet showed a significant (
p < 0.05) decrease in the content of Ig) in liver tissue (
Figure 2). Concentration of IgG, IL-6, and IL-1β in liver tissue was significantly (
p < 0.05) reduced with addition of akebia extract to the diet. Significant interaction between dandelion and akebia was not found in the amounts of IL-1β, IL-6, IgA, and IgG in liver tissue.
3.2.3. Influence of Dandelion and Akebia Extract Addition on Jejunal Mucosa IL-1β, IL-6,IgA and IgG Concentration of Weaned Rabbits
A significant (
p < 0.05) decrease in the levels of IL-6 in the jejunal mucosa was observed following administration of dandelion extract, as shown in
Figure 3. Additionally, after being exposed to akebia extract, the contents of IgA, IgG, IL-1β, and IL-6 in the jejunal mucosa were significantly (
p < 0.05) decreased. There was no significant interaction between dandelion and akebia on cytokine and antibody content of the jejunal mucosa.
3.2.4. Influence of Dandelion and Akebia Extract Addition on Ileal Mucosa IL-1β, IL-6, IgA and IgG Concentration of Weaned Rabbits
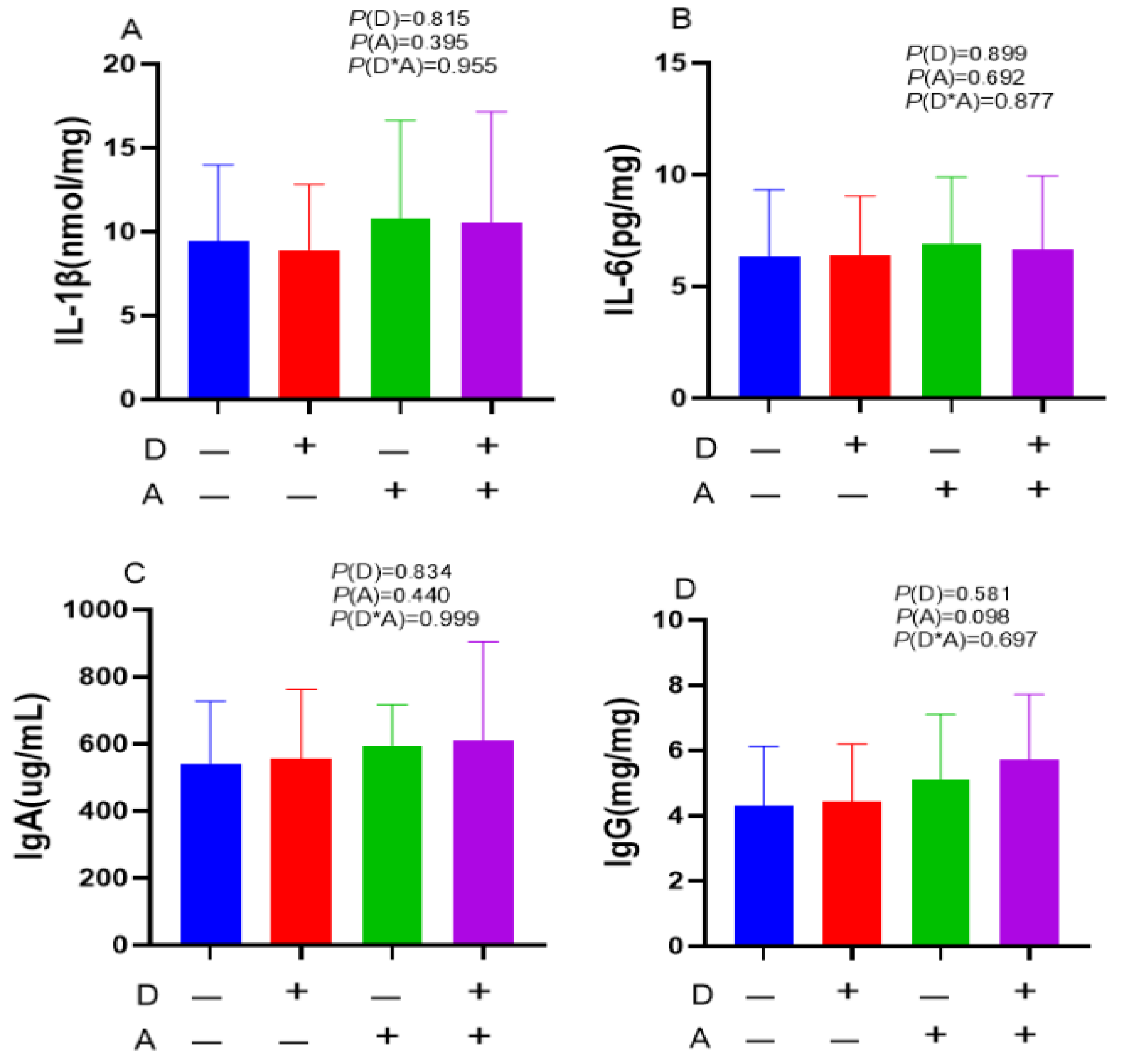
IgG concentration in the ileal mucosa was positively affected, although not significantly, as
Figure 4 demonstrates. Remarkably, about the quantities of IgA, IgG, IL-1β, and IL-6 in the ileal mucosa, no significant interaction between the dandelion and akebia extracts was observed.
3.3. Influence of Dandelion and Akebia Extract on mRNA Expression Related to Genes of Inflammatory Response
3.3.1. Influence of Dandelion and Akebia Extract on Liver mRNA Expression Related to Genes of Inflammatory Response
As shown in
Figure 5, in comparison to groups who received dandelion extract, it was shown that groups that were not treated with dandelion extract had significantly lower (
p < 0.05) gene mRNA expression levels of IFN-γ and IL-10 in their liver tissue. Moreover, dandelion extract had a tendency to decrease the mRNA expression levels of TNF-αand CCL3 in liver tissue. The treatment of akebia extract in liver tissue led to a significant (
p < 0.05) drop in the mRNA expression levels of IFN-γ, CCL3, CXCL10, and IL-1β. Furthermore, there was a decrease in IL-6 mRNA expression, although not to a statistically significant level. The mRNA expression level of IFN-γ was shown to decrease in response to both dandelion and akebia extracts, however, this reduction did not show statistical significance (
p = 0.053).
3.3.2. Influence of Dandelion and Akebia Extract on Jejunum Mucosa mRNA Expression Related to Genes of Inflammatory Response
The mRNA expression levels of IL-1β and TNF-α in the jejunum were significantly (
p < 0.05) decreased following dandelion extract administration, as shown in
Figure 6. The mRNA expression of CCL3 showed a declining trend (P = 0.074). In the jejunum, akebia extract significantly reduced IL-1β mRNA expression (
p < 0.05). Significant interactions were seen in the mRNA expression levels of IL-10 and CXCL-10 between dandelion and akebia. Higher levels of jejunal CXCL10 and IL-10 gene expression were observed in the diets without the addition of dandelion and akebia extracts, and the addition of dandelion and akebia extracts significantly reduced the levels of CXCL10 and IL-10 gene expression.
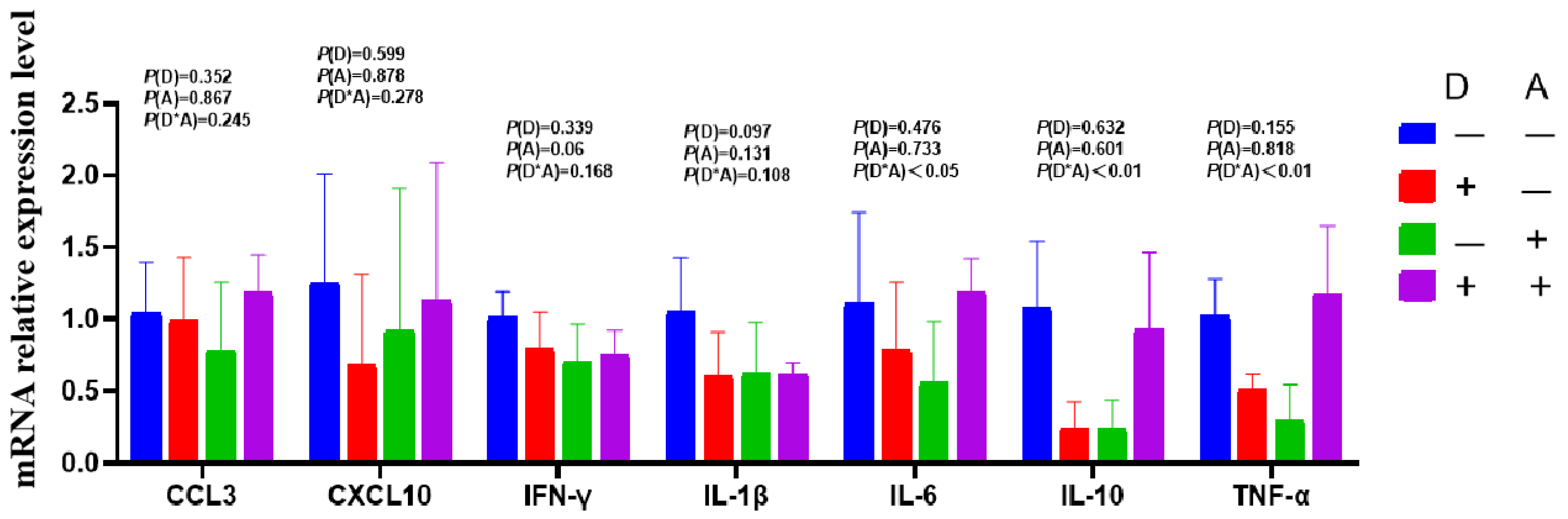
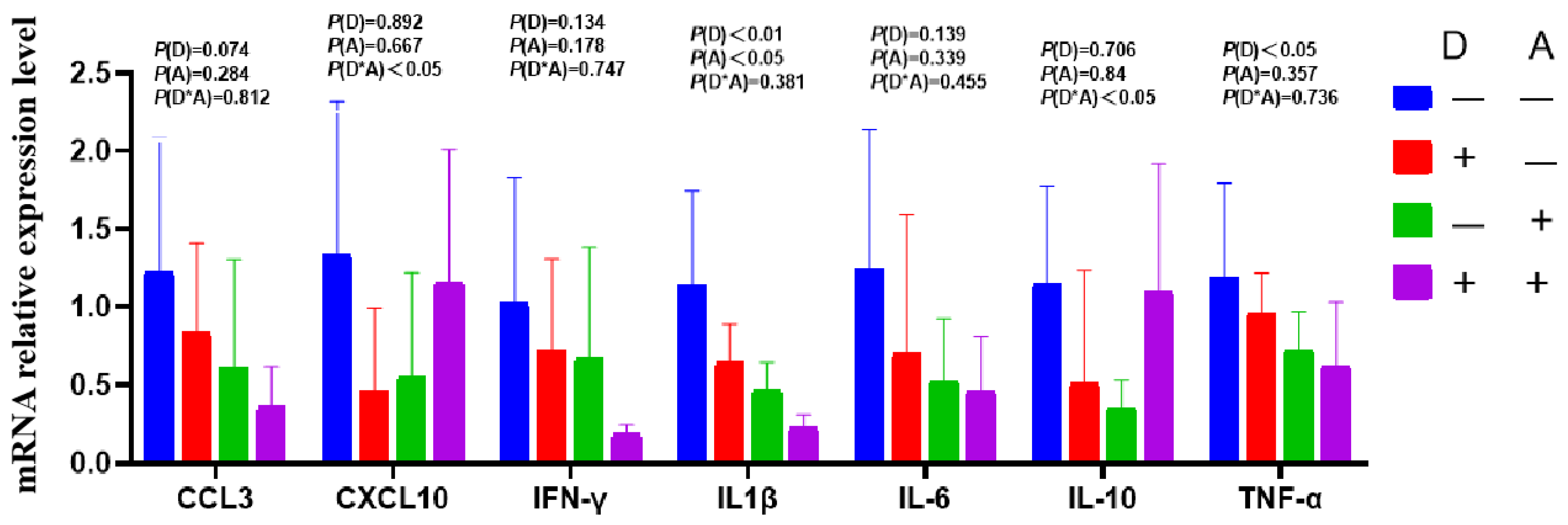
3.3.3. Influence of Dandelion and Akebia Extract on Ileum Mucosa mRNA Expression Related to Genes of Inflammatory Response
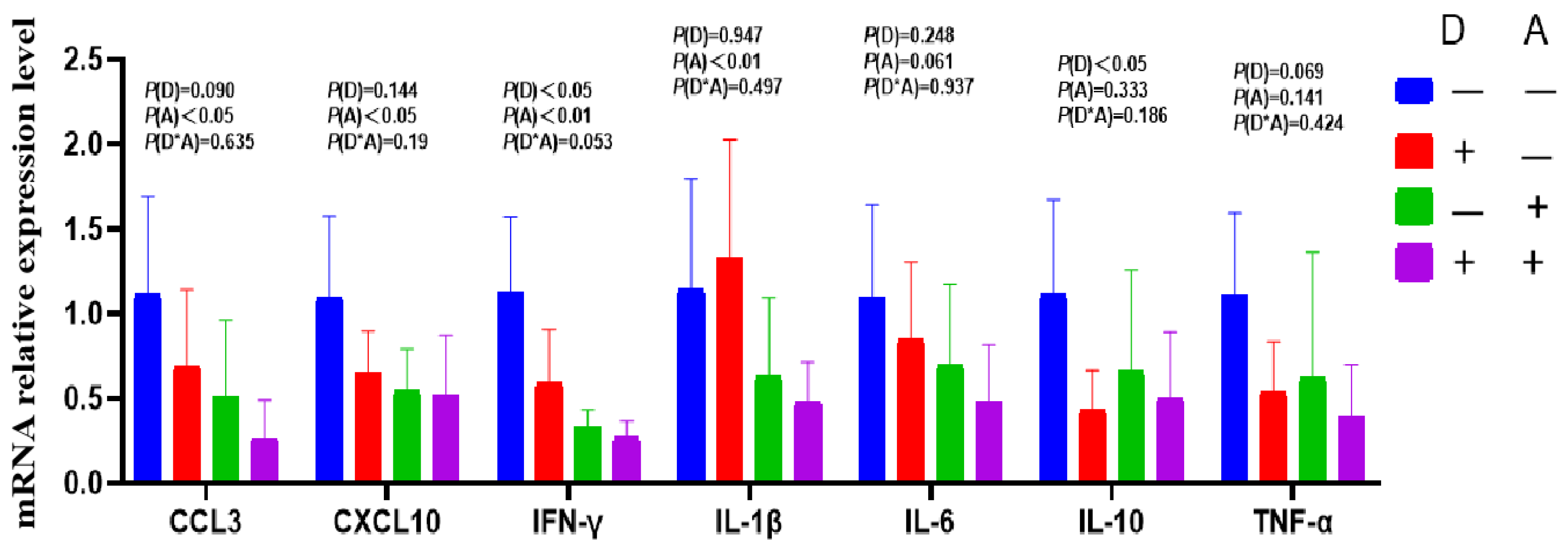
Dandelion extract was found to decrease the expression of IL-1β mRNA in the ileum (
Figure 7), although the change was not statistically significant. Similarly, IFN-γ mRNA expression in the ileum decreased in response to akebia extract but not significantly. The mRNA expression levels of IL-10, IL-6, and TNF-α were found to exhibit a significant interaction (p < 0.05) between the extracts of dandelion and akebia. The addition of dandelion and akebia extracts resulted in a significant increase (p < 0.05) in IL-10 mRNA expression compared to a significant decrease in mRNA expression of TNF-α and IL-6 without the addition of these extracts.
Figure 7.
Influence of dandelion and akebia extract on ileum mucosa mRNA expression related to genes of inflammatory response. Akebia extract (A); dandelion extract (D); interleukin-1 beta (IL-1β); interleukin-6 (IL-6); interleukin-10 (IL-10); tumor necrosis factor-alpha (TNF-α); interferon-gamma (IFN-γ); chemokine ligand 3(CCL3); chemokine (C-X-C motif) ligand 10 (CXCL10).
Figure 7.
Influence of dandelion and akebia extract on ileum mucosa mRNA expression related to genes of inflammatory response. Akebia extract (A); dandelion extract (D); interleukin-1 beta (IL-1β); interleukin-6 (IL-6); interleukin-10 (IL-10); tumor necrosis factor-alpha (TNF-α); interferon-gamma (IFN-γ); chemokine ligand 3(CCL3); chemokine (C-X-C motif) ligand 10 (CXCL10).
Figure 8.
Influence of dandelion and akebia extract on Alpha diversity of weaned rabbits. C: control group; D: dandelion group, with 0.5% dandelion extract addition in the diet; A: akebia group, with 0.5% akebia extract addition in the diet; DA: dandelion and akebia group, with 0.5% dandelion and akebia in the diet.
Figure 8.
Influence of dandelion and akebia extract on Alpha diversity of weaned rabbits. C: control group; D: dandelion group, with 0.5% dandelion extract addition in the diet; A: akebia group, with 0.5% akebia extract addition in the diet; DA: dandelion and akebia group, with 0.5% dandelion and akebia in the diet.
3.4. Influence of Dandelion or Akebia Extract Addition on Weaned Rabbits' Intestinal Microbial Flora
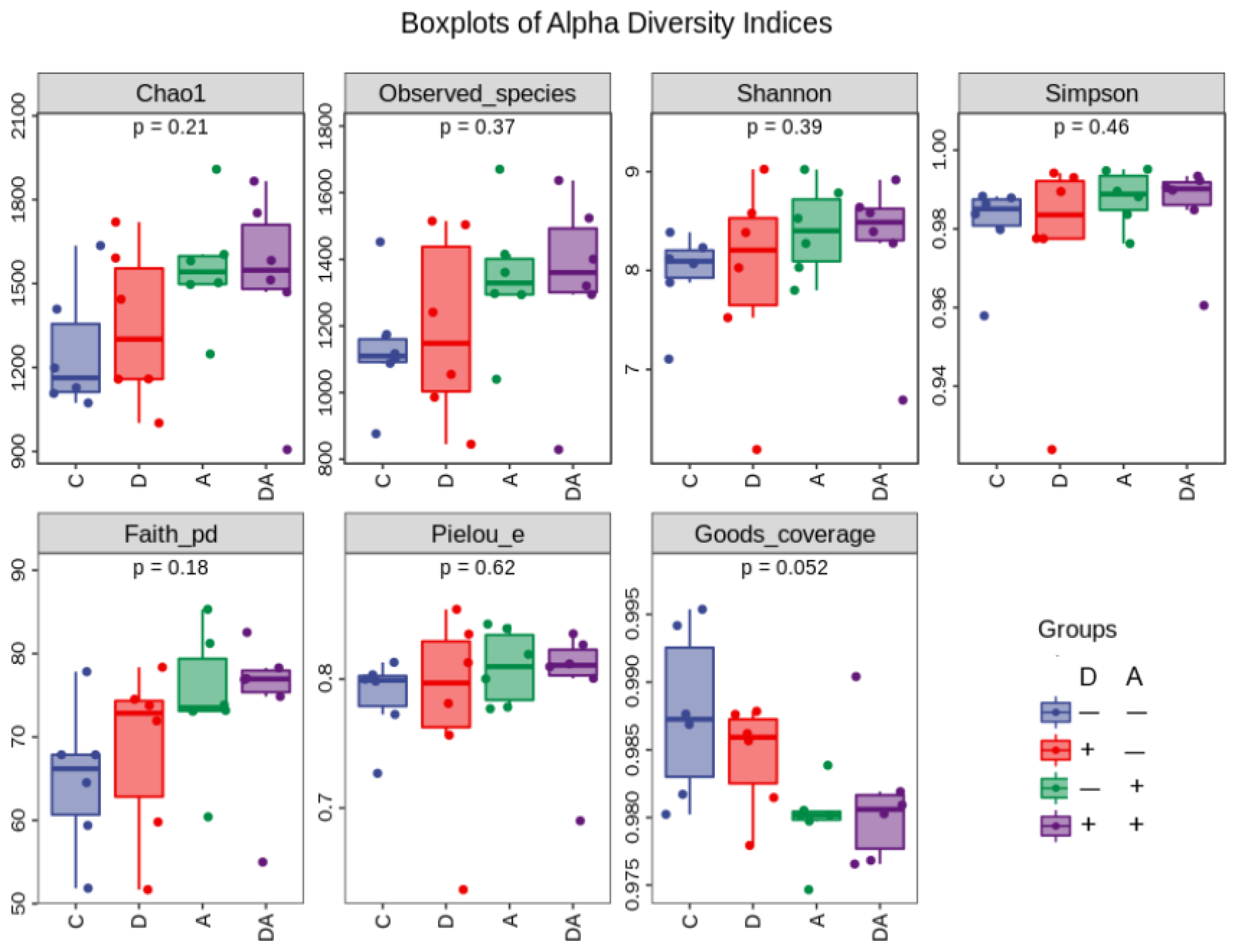
Alpha Diversity of Microbial Flora
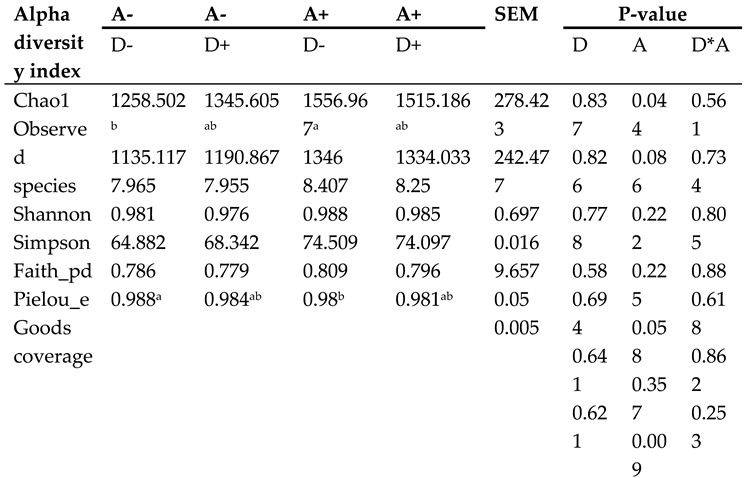
As shown in
Figure 9, the Chao1 index, which ranged from 906 to 1908, suggested a high abundance of microbial species in the samples. With a coverage score over 0.9, thought to show appropriate sequencing coverage and hence effectively represent the actual intestinal flora situation, a greater coverage value was indicative of a lower risk of unreported samples. The average coverage index in this experiment was found to be 0.98. While addition of akebia in the diet significantly reduced the coverage index (
p < 0.05).
The groups that with addition of akebia extract in the diet showed a significant rise (
p < 0.05) in the Chao1 index (
Table 3). Furthermore, although not achieving statistical significance, both the observed index (
p = 0.086) and faith index (
p = 0.058) showed a rising tendency. There was no significant interaction between dandelion and akebia extracts on weaned rabbits' cecum flora's alpha diversity index.
3.5. Microbial Flora Composition of Samples
3.5.1. Microbiota Composition of Weaned Rabbits in the Level of Phylum
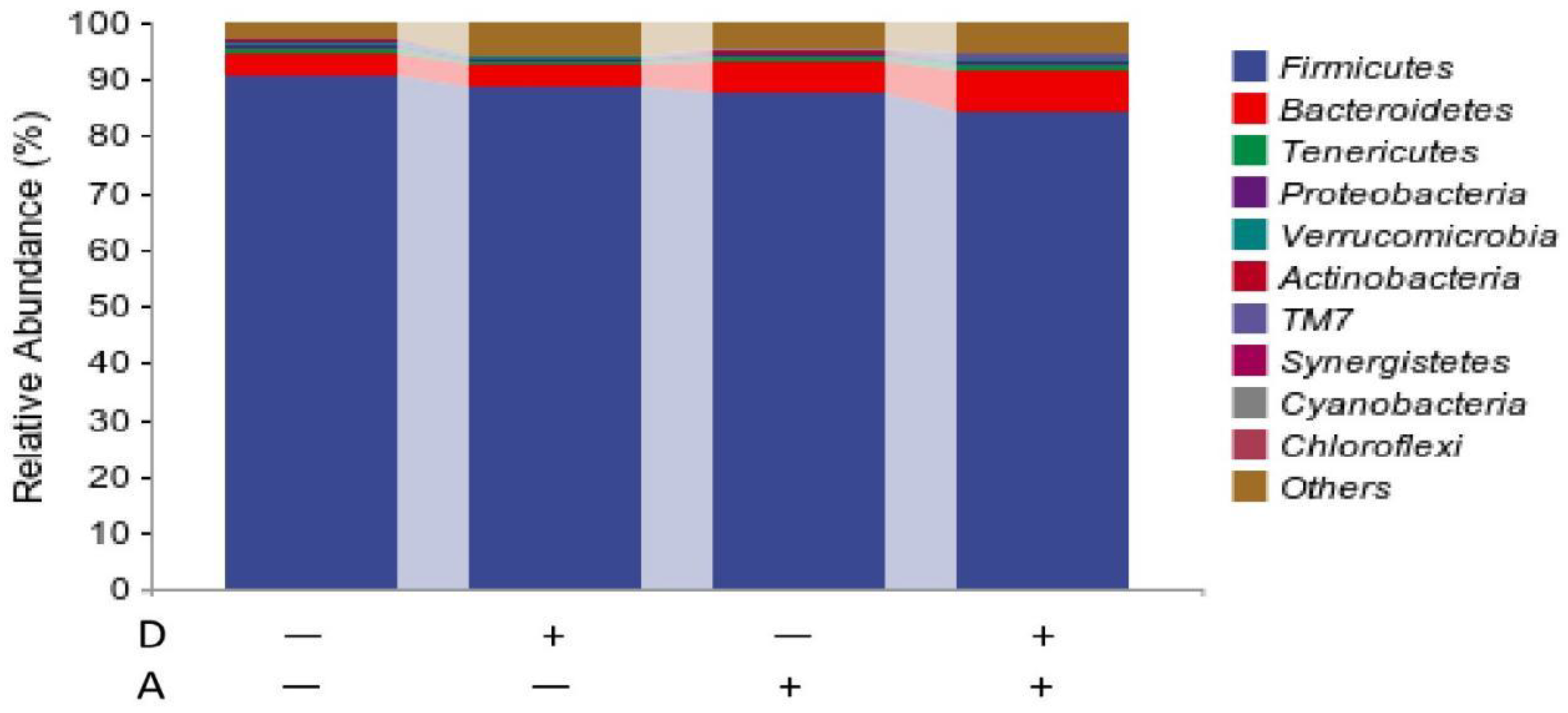
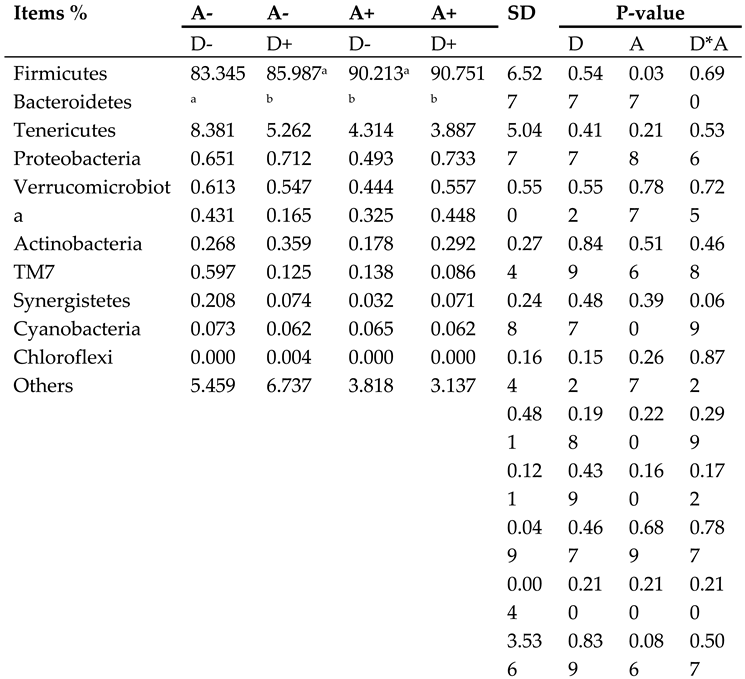
The result at phylum level is shown in
Figure 10. The 10 species with the highest abundance at phylum level were presented, and the rest species were classified into others with their relative abundance merged. The dominant phylum categories were Firmicutes, Bacteroidetes, Tenericutes, Verrucomicrobiota, Proteobacteria, and Actinobacteria. As can be seen from the stacked histogram of the percentage of each phylum, relative abundance greater than 1% is considered to be the dominant phylum. The dominant flora in the samples of the four groups was Firmicutes, with Bacteroidetes. The relative abundance of Tenericutes and Proteobacteria was less than 1% on average for each group.
As shown in
Table 4, addition of akebia extract to diet significantly decreased (
p < 0.05) the relative abundance of Firmicutes in the cecum of weaned rabbits. There was a trend of synergistic reduction in the relative abundance of Verrucomicrobiota by the extracts of akebia and dandelion. No significant interactions were found on the relative abundance of other phyla.
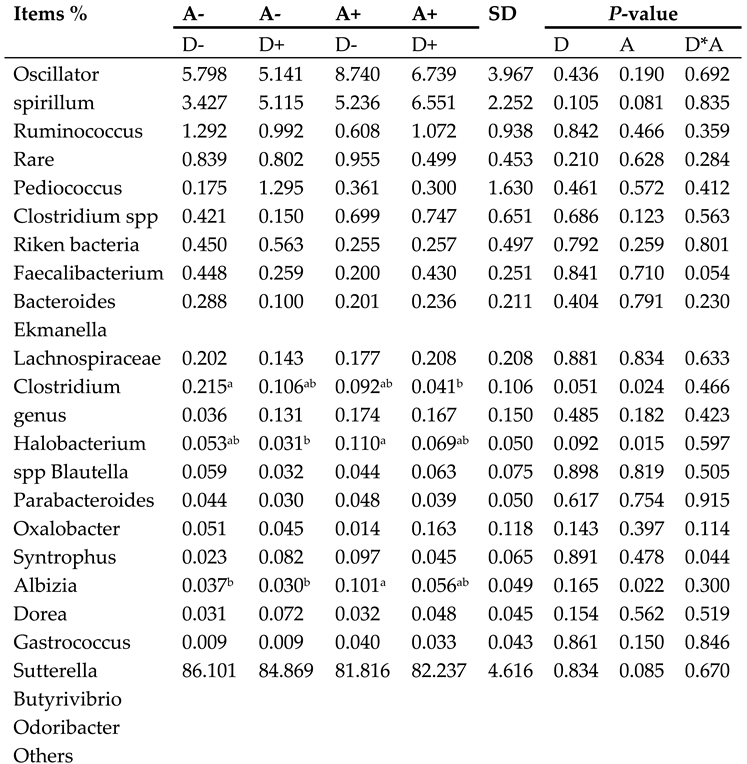
3.5.2. Microbiota Composition of Weaned Rabbits in the Level of Genus
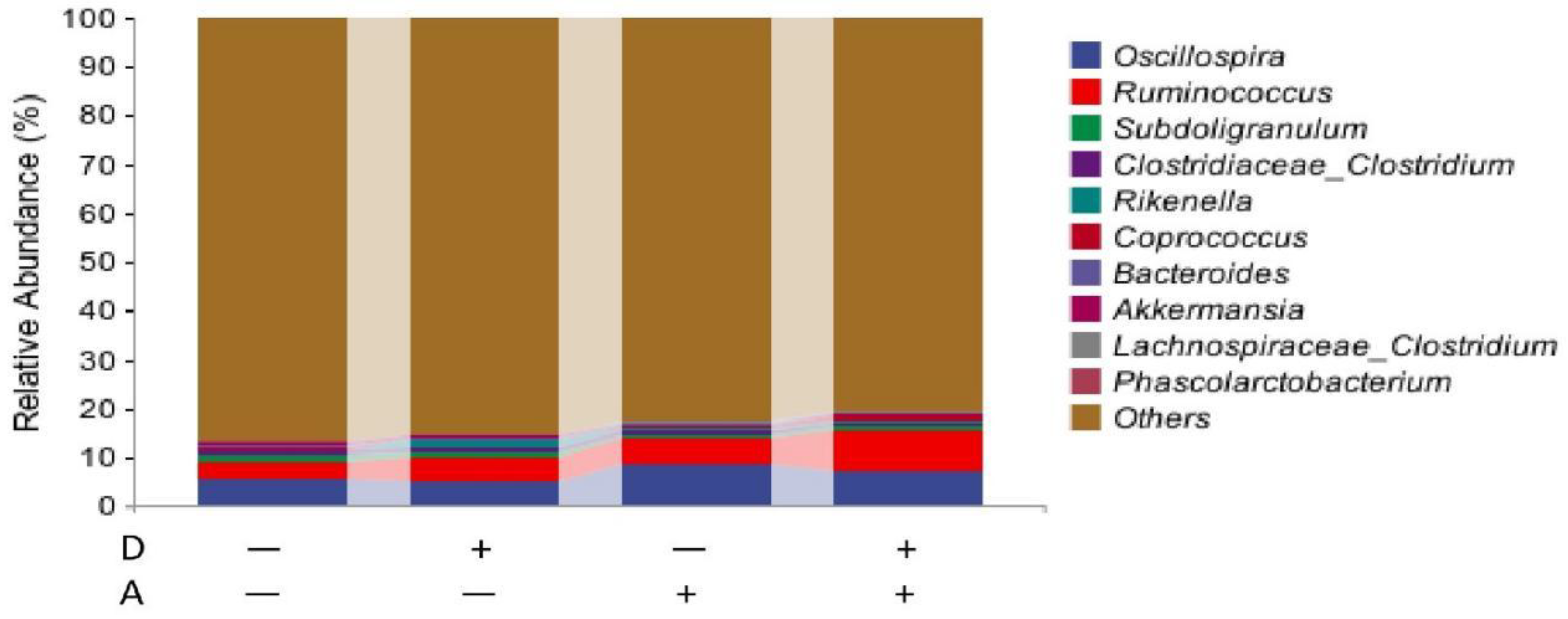
The results in the genus are presented in
Figure 11. The top 20 genera were selected and the rest were classified into others with merged relative abundance. The species with a relative abundance of more than 1% were regarded as dominant species. The dominant species in each group were Oscillospira and Ruminococcus, and the rare Subdoligranulum in comparing groups was also classified as a dominating species.
Dietary addition of dandelion extract had an effect on increasing the relative abundance of Ruminococcus and Butyrivibrio in the cecum of weaned rabbits (
p = 0.105,
p = 0.154), and on decreasing the relative abundance of Sutterella, but there was no significant difference. A significantly increased (
p < 0.05) relative abundance of cecum Oxalobacter and Sutterella, as well as a significantly decreased (
p < 0.05) relative abundance of Blautella, was observed with akebia extract addition. The relative abundance of Ruminococcus was affected by akebia extract to a certain degree, but not significantly (
p = 0.081). Dandelion and akebia extracts had a significant interaction effect on elevating the relative abundance of Gastrococcus (
p < 0.05), and the interaction had an effect on decreasing Eckermannia spp. but no significant difference was observed (
p = 0.054).
Table 5.
Microbiota composition of weaned rabbits in the level of genus.
Table 5.
Microbiota composition of weaned rabbits in the level of genus.
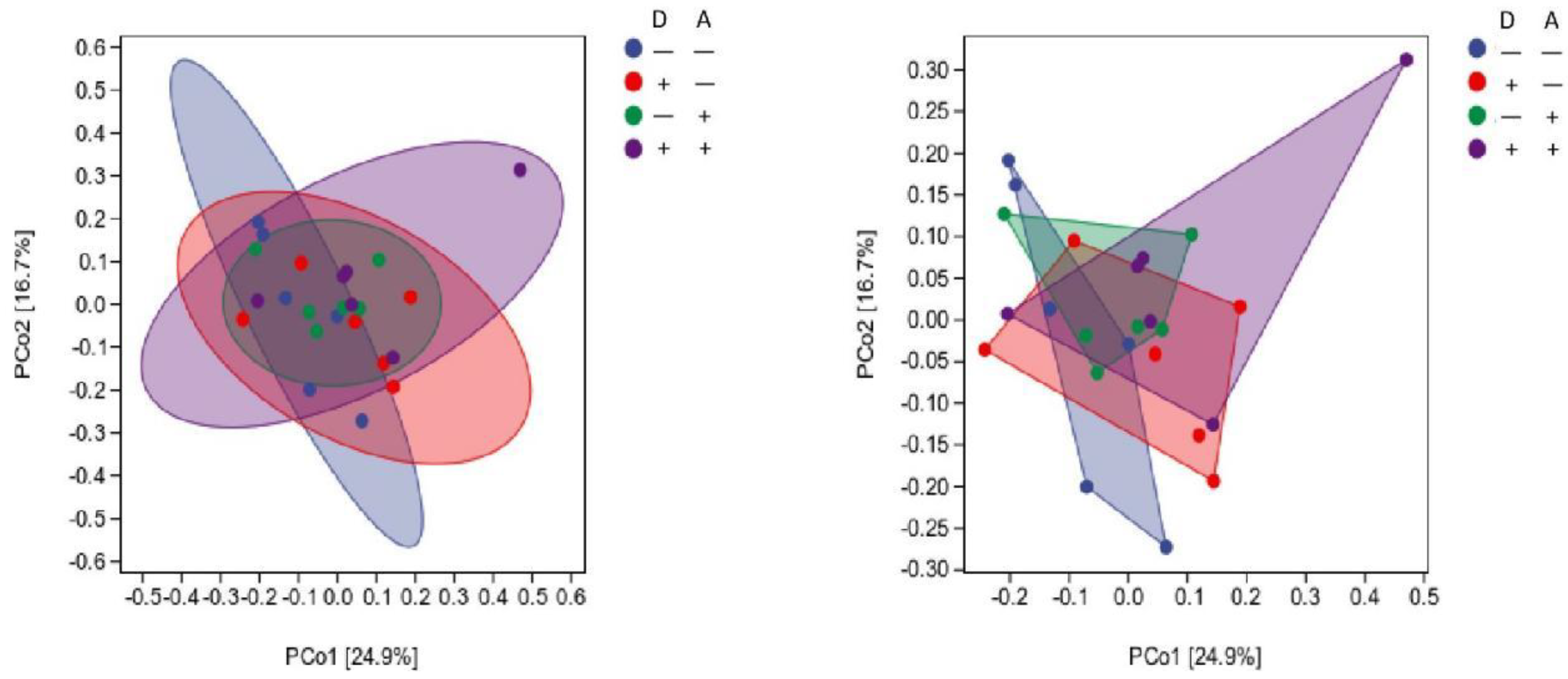
3.6. Beta Diversity
Beta diversity stands for the difference of species composition among the flora varying with the environmental gradient or the changing rate of species under the influence of environmental gradient, and thus being referred to as eco-environmental diversity.
Figure 11 indicates that the groups' points appeared in the same area of the chart and the clouds are close to each other, suggesting that the 24 rabbits in the 4 groups displayed no difference in gut microbial composition.
3.7. Species Differences and Marker Species Analysis
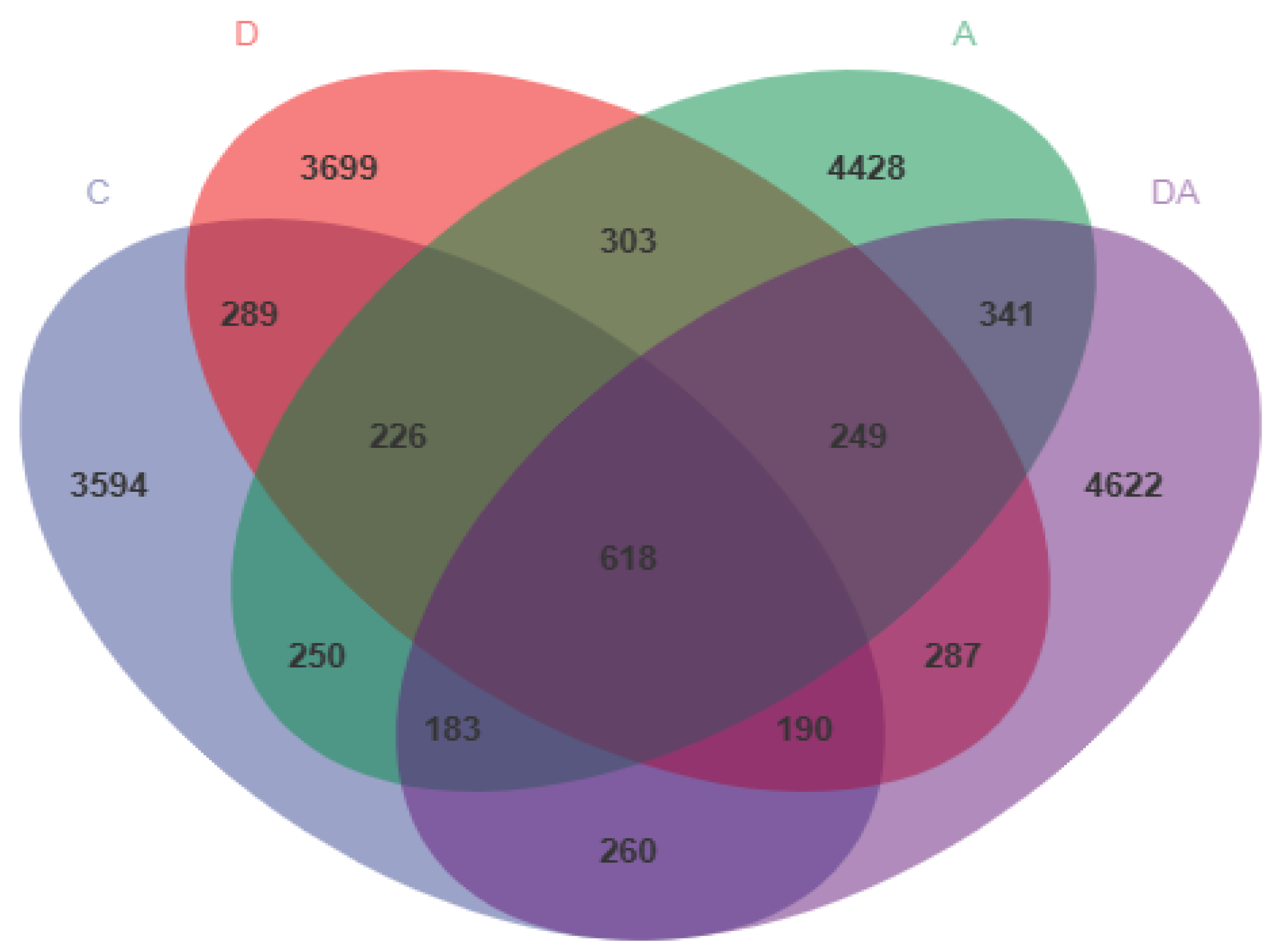
There were 19,539 OTUs in these 4 groups, of which 618 OTUs were found in both 4 groups, 848 OTUs in 3 groups, and 1730 OTUs in just 2 groups. The total OUT among the four groups was 618, and the unique OUT in the comparison group was 3594. Dandelion-only group had 3699 unique OUTs, and the akebia-only group had 4428 unique OUTs, while 4622 unique OUTs were observed in both the dandelion-and-akebia groups. In comparison to the other three groups, the DA group had the largest number of unique OTUs (
Figure 12).
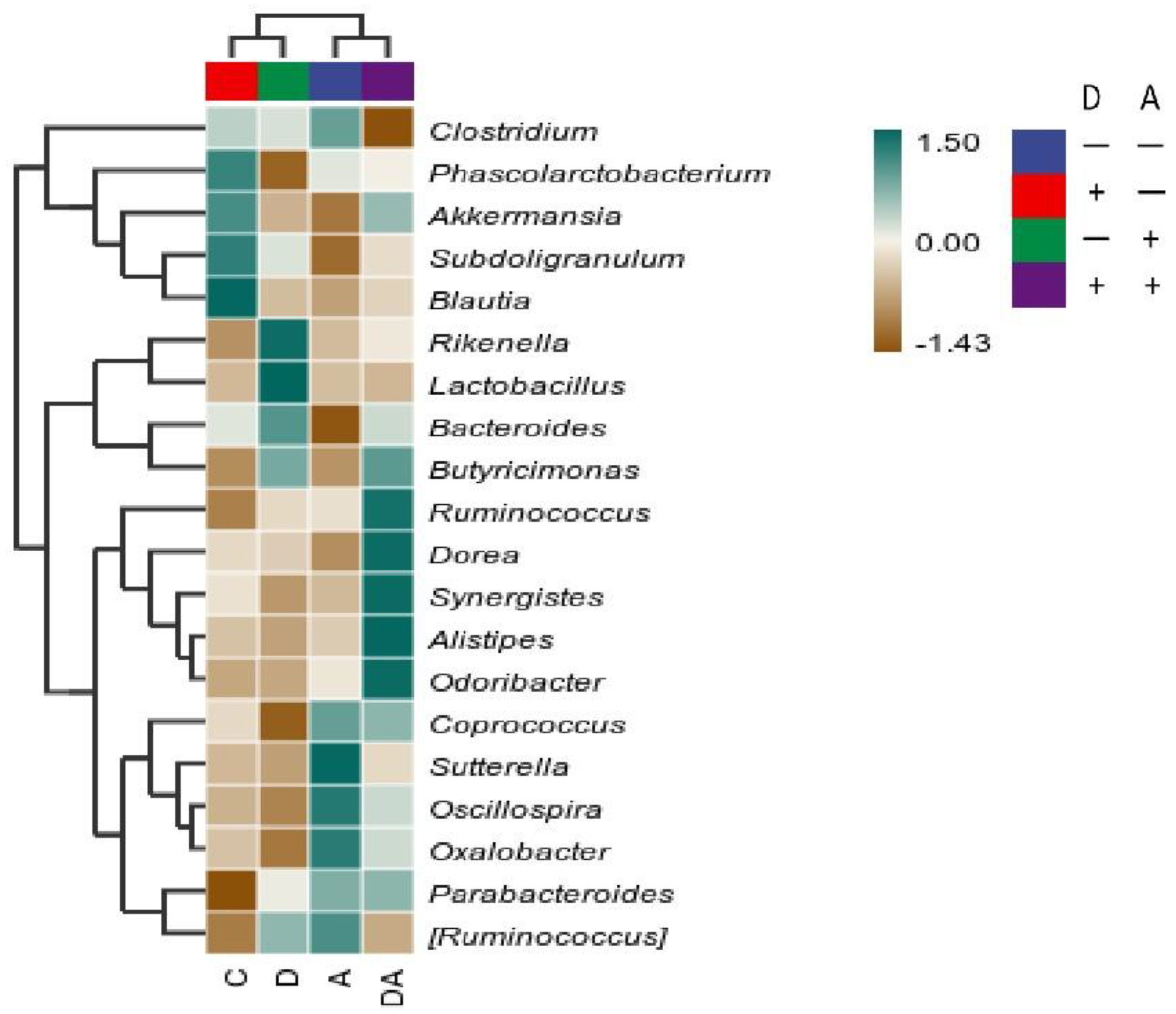
As shown in
Figure 13, at the generic level, the relative abundances of Phascolarctobacterium and Coprococcus were found to be lower after dandelion extract was administered. Meanwhile, the abundance of Rikenella and Lactobacillus was found to be higher compared to other groups. In the same way, consumption of akebia extract was associated with higher relative abundances of Sutterella, Oscillospira, and Oxalobacter in the cecum as well as lower Bacteroides abundances. Furthermore, the relative abundances of Ruminococcus, Dorea, Synergistes, Alistipes, and Odoribacter increased in combination with the interactions between dandelion and akebia extract, whereas Clostridium's abundance decreased.
4. Discussion
Dandelion extract is rich in polysaccharide, which plays a vitally important role in the growth and development process of animals. The addition of dandelion and akebia extract significantly increased the daily gain of weaned rabbits corporately in the first week and significantly decreased the average feed efficiency of weaned rabbits in the first week. The results were similar to the research of Mao et al. (2023), that addition of fermented dandelion can increase the ADG of broilers in the first 21 days and the carcass traits were improved with a high concentration of the extract. Dandelion extract promotes weight reduction and sustained normal body weight in high-fat diet rats (Davaatseren et al., 2013), indicating the important role of dandelion in the growth of animals. According to Park et al. (2018), after being treated with 200mg/kg akebia, the mean body weight of mice was significantly increased, and the treatment group also showed better physical performance. The positive effect of Dandelion and Akebia towards animal body condition is undeniable, and the results of this study further investigate that the combination effect of these two herbs plays an important role in improving the production performance and feed efficiency of livestock.
The level of serum immunoglobulin is an important indicator of animal humoral immunity (Khan et al., 2021). IgA is the dominant antibody in the exocrine fluid and plays its role in the local mucosal immunity, such as the respiratory tract and the intestinal tract. IgG is the dominant antibody produced by the organism after active immunity and is the main force of the anti-infection of the organism, playing immune activity of antibacterial, antiviral and antitoxin activities, especially is important in humoral immunity (Sun et al., 2023). The trend of increased IgG level was observed with dandelion extract addition, indicating that dandelion and akebia extract enhanced the immunity of weaned rabbits, which was similar to the research results of Wang et al. (2020).
Serum and intestinal tissue inflammatory cytokine levels reflect changes in intestinal inflammation (Bamias & Cominelli, 2016). The over-secretion of inflammatory mediators, including IL-1β, IL-6, and TNF-α, exhibits a potential pro-inflammatory effect (Sang et al., 2019). Inhibiting the excessive release of these inflammatory mediators is crucial, as these specific cytokines play a pivotal role in the prevention or inhibition of various inflammatory diseases (Zhou et al., 2020). This experiment exhibited similar results to Choi et al. (2012) and Wang et al. (2013) that akebia extracts addition significantly decreased serum IL-6 concentration and also significantly decreased IL-6 and IL-1β concentration in liver tissue and jejunum. The findings of this study is similar with those reported by Wang et al. (2020), who demonstrated that the total flavonoid concentration in trifoliate akebia inhibits the expression of nuclear factor-kappa B (NF-κB) by reducing the levels of inflammatory factors such as TNF-α, IL-1β, and IL-6 in the liver. Similarly, our results suggest that akebia extract mitigates inflammation and protects the liver and intestines by decreasing the secretion of inflammatory factors, including IL-6 and IL-1β, in weaned rabbits. This protective effect against inflammation is consistent with the broader understanding of the role of anti-inflammatory agents in liver health.
The significant reduction in jejunal expression of interleukin (IL)-1β and tumor necrosis factor (TNF)-α following the addition of dandelion extract is consistent with the findings of Che et al. (2019), who reported that dandelion significantly decreased the expression levels of TNF-α and IL-6 in mice with ulcerative colitis. Furthermore, our results align with those of Yao et al. (2013), who demonstrated that taraxasterol inhibits inflammatory factors, including TNF-α, IL-10, and IL-6, by modulating the NF-κB signaling pathway, thereby attenuating inflammation. These findings collectively underscore the anti-inflammatory potential of dandelion extract and its active components in mitigating inflammatory responses in the gastrointestinal tract. Sang et al. (2019) found that taraxasterol significantly inhibited the secretion of TNF-α, IL-6, IL-1β, IFN-γ and IL-4 through regulating TLRs/NF-kB and Bax/Bc11-2 signaling pathway to prevent acute liver damage of mice. This experiment also indicated that dandelion extract addition significantly decreased the relative expression of liver IFN-γ, indicating that dandelion extract possibly prevents inflammation by decreasing the secretion of liver IFN-γ and jejunum TNF-α, IL-6 and IL-β of weaned rabbits.
A study on liver ischemia reperfusion by Zhai et al. (2011) indicated that IL-10 was an inflammatory factor that was produced from macrophages and could inhibit the secretion of inflammatory mediators such as TNF-α, IL-β and IL-6 to prevent inflammation. Under normal physical conditions, pro-inflammatory and anti-inflammatory factors were dynamically balanced, while the over-inflammation triggered by numerous over-secreted pro-inflammatory factors increased the concentration of anti-inflammatory factor IL-10 (Cicchese et al., 2018). The concentration of liver IL-10 was significantly decreased by dandelion extract addition, indicating that TNF-α and CCL3 had demonstrated a declining trend in weaned rabbits under a normal physical condition to maintain the dynamic balance of pro-inflammatory factors and anti-inflammatory, leading to the decreased concentration of IL-10. Macrophage M1 secretes both anti and pro-inflammatory factors (Liu et al., 2014). Pro-inflammatory factors, which could be divided into CC family (such as CCL3) and CXC family (such as CXCL10), could contribute to the migration and infiltration of inflammatory cells towards inflammatory spots and the secretion of pro-inflammatory factors such as IFN-γ, IL-6 and IL-8 (Elemam et al., 2022). Studies have found that chemokines such as CCL2, CCL3, and CXCL10 exhibited increased expression during the process of liver damage or liver inflammation (Saiman & Friedman, 2012; Song et al., 2021), to which it was mentioned by Brass & Brenndörfer (2014) that liver damage could be released by focusing on the recruitment and infiltration of neutrophils decreased by chemokine receptors. Karthika et al. (2022) and Zhou et al. (2016) studied rectal cancer treatment through the compatibility of curcuma zedoary and 5-fluorouracil, finding that curcuma zedoary decreased the expression of mRNA and protein of liver and colon CCL3 and CXCL10. Wang et al. (2019) found that pre-treatment with oleanolic acid in a mouse model of liver ischemia-reperfusion injury significantly reduced neutrophil recruitment and inflammation. The results of this experiment indicated that akebia with extract rich in oleanolic acid significantly decreased mRNA expression of liver CCL3 and CXCL10, implying that akebia plays its anti-inflammatory function through inhibiting chemokine CCL3 and CXCL10 secretion.
This experiment studied whether dandelion or akebia extract would affect the gut diversity of weaned rabbits by Illumina MiSeq technology, which made the detection data more comprehensively covered and the microbiota composition and variation verily and comprehensively reflected. Intestinal flora closely related to the physical functions of the organism (Hildebrand et al., 2013), and rabbits have particularly developed cecum, making the whole digestive tract susceptible to various pathological reactions and causing digestion and absorption problems when the normal cecum flora got destructed, which would even decrease organism immunity and lead to systemic pathological reaction (De Blas, 2013). The results of this experiment exhibited a significantly increased Chao1 index and an increased Shannon index, although not significantly, thus it can be implied that akebia extract increased species abundance and diversity in the cecum of weaned rabbits. The results of this experiment showed that Firmicutes and Bacteroidetes were the most abundant at the phylum level, and these two phyla accounted for over 90%, which is consistent with the study of Eckburg et al. (2005). Firmicutes were an important source of butyrate, which was the dominant cellular energy source. The decrease of Firmicutes might trigger or worsen the local inflammatory reaction (Kho & Lal, 2018). Bacteroidetes is the flora involved in the metabolism of sugar, bile acid, and steroids, such as decomposing peptone or glucose and producing metabolites such as acetic acid, formic acid, lactic acid, and propionic acid, with its main function of absorbing and decomposing polysaccharides (Zafar & Saier, 2021). The decreased ratio of Firmicutes and Bacteroidetes directly affects the digestive tract flora for the metabolism of dietary fiber, leading to a decreased short-chain fatty acid (SCFA) concentration. SCFAs are an important product from the dietary fiber fermented by gut probiotics, mainly involving acetic acid, propionic acid, butyric acid, etc. SCFAs prevent inflammation through significantly declining pro-inflammatory chemotactic factors such as CCL2, CCL3, CXCL10, and CXCL11, so as to increase the anti-inflammatory factors such as IL-10. Akebia extract addition significantly decreased the abundance of Firmicutes and increased the abundance of Bacteroidetes, although the difference did not reach a significant level. The synergistic effect of Mucuna pruriens and Taraxacum officinale was more effective in regulating the structure of intestinal flora and increasing the abundance of beneficial flora than the effects of Mucuna pruriens and Taraxacum officinale alone.
Oscillospira and Ruminococcus both ferment fibers to produce SCFAs, which can prevent inflammation. The decreased Oscillospira abundance was observed in inflammatory diseases and was positively related to human health (Konikoff & Gophna, 2016). Through Beta diversity, it was found that the extracts of dandelion and akebia can significantly improve the diversity of the intestinal flora and increase the abundance of butyric acid-producing bacteria, such as Blautella. The results of this experiment implied addition of dandelion extract or akebia extract potentially exhibited the trend of increased Oscillospira and Ruminococcus abundance, indicating that dandelion or akebia extract prevents inflammation through increasing the abundance of Oscillospira and Ruminococcus.
5. Conclusions
Dandelion extract addition increased serum immunoglobulin levels and decreased the expression of inflammatory factors, and akebia extract addition decreased the mRNA expression of inflammatory factors and chemokines, contributing to the inhibition of inflammatory diseases. Moreover, akebia extract plays its anti-inflammatory function by creating an increased hind-gut flora diversity and increased abundance of certain microbiota species.
References
- Bamias, G., & Cominelli, F. (2016). Cytokines and intestinal inflammation. Current Opinion in Gastroenterology, 32(6), 437–442. [CrossRef]
- Brass, A., & Brenndörfer, E. (2014). The Role of Chemokines in Hepatitis C Virus-Mediated Liver Disease. International Journal of Molecular Sciences, 15(3), 4747–4779. [CrossRef]
- Chagas, M. D. S. S., Behrens, M. D., Moragas-Tellis, C. J., Penedo, G. X. M., Silva, A. R., & Gonçalves-de-Albuquerque, C. F. (2022). Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Medicine and Cellular Longevity, 2022, 1–21. [CrossRef]
- Che, L., Li, Y., Song, R., Qin, C., Hao, W., Wang, B., Yang, L., Peng, P., & Xu, F. (2019). Anti-inflammatory and anti-apoptosis activity of taraxasterol in ulcerative colitis in vitro and in vivo. Experimental and Therapeutic Medicine. [CrossRef]
- Choi, J. N., Choi, Y.-H., Lee, J.-M., Noh, I. C., Park, J. W., Choi, W. S., & Choi, J. H. (2012). Anti-inflammatory effects of β-sitosterol-β- D -glucoside from Trachelospermum jasminoides (Apocynaceae) in lipopolysaccharide-stimulated RAW 264.7 murine macrophages. Natural Product Research, 26(24), 2340–2343. [CrossRef]
- Cicchese, J. M., Evans, S., Hult, C., Joslyn, L. R., Wessler, T., Millar, J. A., Marino, S., Cilfone, N. A., Mattila, J. T., Linderman, J. J., & Kirschner, D. E. (2018). Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunological Reviews, 285(1), 147–167. [CrossRef]
- Davaatseren, M., Hur, H. J., Yang, H. J., Hwang, J.-T., Park, J. H., Kim, H.-J., Kim, M. J., Kwon, D. Y., & Sung, M. J. (2013). Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food and Chemical Toxicology, 58, 30–36. [CrossRef]
- De Blas, J. C. (2013). Nutritional impact on health and performance in intensively reared rabbits. Animal, 7, 102–111. [CrossRef]
- Dendrou, C. A., Fugger, L., & Friese, M. A. (2015). Immunopathology of multiple sclerosis. Nature Reviews Immunology, 15(9), 545–558. [CrossRef]
- Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., Gill, S. R., Nelson, K. E., & Relman, D. A. (2005). Diversity of the Human Intestinal Microbial Flora. Science, 308(5728), 1635–1638. [CrossRef]
- Elemam, N., Talaat, I., & Maghazachi, A. (2022). CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses, 14(11), 2445. [CrossRef]
- Fang, S., Chen, X., Pan, J., Chen, Q., Zhou, L., Wang, C., Xiao, T., & Gan, Q. F. (2020a). Dynamic distribution of gut microbiota in meat rabbits at different growth stages and relationship with average daily gain (ADG). BMC Microbiology, 20(1), 116. [CrossRef]
- Fang, S., Chen, X., Pan, J., Chen, Q., Zhou, L., Wang, C., Xiao, T., & Gan, Q. F. (2020b). Dynamic distribution of gut microbiota in meat rabbits at different growth stages and relationship with average daily gain (ADG). BMC Microbiology, 20(1), 116. [CrossRef]
- Grigoriadis, N., & Van Pesch, V. (2015). A basic overview of multiple sclerosis immunopathology. European Journal of Neurology, 22(S2), 3–13. [CrossRef]
- Harrison, T. M., & Churgin, S. M. (2022). Acupuncture and Traditional Chinese Veterinary Medicine in Zoological and Exotic Animal Medicine: A Review and Introduction of Methods. Veterinary Sciences, 9(2), 74. [CrossRef]
- Hildebrand, F., Nguyen, T. L. A., Brinkman, B., Yunta, R. G., Cauwe, B., Vandenabeele, P., & Raes, J. (2013). Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biology, 14, 1–15.
- Karthika, C., Rahman, Md. H., Sureshkumar, R., Akter, R., Khan, A. A., Alanazi, A. M., Azad, A. K., Barai, P., & Barai, H. R. (2022). 5-Fluorouracil and Curcumin Combination Coated with Pectin and Its Strategy towards Titanium Dioxide, Dimethylhydrazine Colorectal Cancer Model with the Evaluation of the Blood Parameters. Polymers, 14(14), 2868. [CrossRef]
- Khan, S. R., Van Der Burgh, A. C., Peeters, R. P., Van Hagen, P. M., Dalm, V. A. S. H., & Chaker, L. (2021). Determinants of Serum Immunoglobulin Levels: A Systematic Review and Meta-Analysis. Frontiers in Immunology, 12, 664526. [CrossRef]
- Kho, Z. Y., & Lal, S. K. (2018). The Human Gut Microbiome – A Potential Controller of Wellness and Disease. Frontiers in Microbiology, 9, 1835. [CrossRef]
- Khoshnam, S. E., Sarkaki, A., Rashno, M., & Farbood, Y. (2018). Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sciences, 211, 126–132. [CrossRef]
- Kim, J. Y., Choi, M. J., Ha, S., Hwang, J., Koyanagi, A., Dragioti, E., Radua, J., Smith, L., Jacob, L., Salazar De Pablo, G., Lee, S. W., Yon, D. K., Thompson, T., Cortese, S., Lollo, G., Liang, C., Chu, C., Fusar-Poli, P., Cheon, K., … Solmi, M. (2022). Association between autism spectrum disorder and inflammatory bowel disease: A systematic review and meta-analysis. Autism Research, 15(2), 340–352. [CrossRef]
- Konikoff, T., & Gophna, U. (2016). Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends in Microbiology, 24(7), 523–524. [CrossRef]
- Li, M., Zhang, H., Hu, X., Liu, Y., Liu, Y., Song, M., Wu, R., & Wu, J. (2022). Isolation of a New Polysaccharide from Dandelion Leaves and Evaluation of Its Antioxidant, Antibacterial, and Anticancer Activities. Molecules, 27(21), 7641. [CrossRef]
- Liu, Y. C., Wang, H. M., & Zeng, X. H. (2018). Research progress of active compounds and pharmacological effects in Akebia trifoliata (Thunb) koidz stems. IOP Conference Series: Earth and Environmental Science, 185, 012034. [CrossRef]
- Liu, Y.-C., Zou, X.-B., Chai, Y.-F., & Yao, Y.-M. (2014). Macrophage Polarization in Inflammatory Diseases. International Journal of Biological Sciences, 10(5), 520–529. [CrossRef]
- Mao, J., Wang, Y., Duan, T., Yin, N., Dong, C., Ren, X., Liu, N., An, X., & Qi, J. (2023). Effect of fermented dandelion on productive performance, meat quality, immune function, and intestinal microbiota of broiler chickens. BMC Veterinary Research, 19(1), 178. [CrossRef]
- Oglesbee, B. L., & Lord, B. (2020). Gastrointestinal Diseases of Rabbits. In Ferrets, Rabbits, and Rodents (pp. 174–187). Elsevier. [CrossRef]
- Park, S. H., Jang, S., Lee, S. W., Park, S. D., Sung, Y.-Y., & Kim, H. K. (2018). Akebia quinata Decaisne aqueous extract acts as a novel anti-fatigue agent in mice exposed to chronic restraint stress. Journal of Ethnopharmacology, 222, 270–279. [CrossRef]
- Qi, H.-P., Gao, X.-C., Zhang, L.-Q., Wei, S.-Q., Bi, S., Yang, Z.-C., & Cui, H. (2013). In vitro evaluation of enhancing effect of borneol on transcorneal permeation of compounds with different hydrophilicities and molecular sizes. European Journal of Pharmacology, 705(1–3), 20–25. [CrossRef]
- Saiman, Y., & Friedman, S. L. (2012). The Role of Chemokines in Acute Liver Injury. Frontiers in Physiology, 3. [CrossRef]
- Sang, R., Yu, Y., Ge, B., Xu, L., Wang, Z., & Zhang, X. (2019). Taraxasterol from Taraxacum prevents concanavalin A-induced acute hepatic injury in mice via modulating TLRs/NF-κB and Bax/Bc1-2 signalling pathways. Artificial Cells, Nanomedicine, and Biotechnology, 47(1), 3929–3937. [CrossRef]
- Song, X., Gao, X., Wang, Y., Raja, R., Zhang, Y., Yang, S., Li, M., Yao, Z., & Wei, L. (2021). HCV Core Protein Induces Chemokine CCL2 and CXCL10 Expression Through NF-κB Signaling Pathway in Macrophages. Frontiers in Immunology, 12, 654998. [CrossRef]
- Sun, H., Ni, X., Song, X., Wen, B., Zhou, Y., Zou, F., & Wang, P. (2016). Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Applied Microbiology and Biotechnology, 100, 8105–8120.
- Sun, W., Chen, Z., Huang, Z., Wan, A., Zhou, M., & Gao, J. (2023). Effects of dietary traditional Chinese medicine residues on growth performance, intestinal health and gut microbiota compositions in weaned piglets. Frontiers in Cellular and Infection Microbiology, 13, 1283789. [CrossRef]
- Van Der Sluis, M., Van Zeeland, Y. R. A., & De Greef, K. H. (2024). Digestive problems in rabbit production: Moving in the wrong direction? Frontiers in Veterinary Science, 11, 1354651. [CrossRef]
- Wang, W., Wu, L., Li, J., Ji, J., Chen, K., Yu, Q., Li, S., Feng, J., Liu, T., Zhang, J., Chen, J., Zhou, Y., Mao, Y., Wang, F., Dai, W., Fan, X., Guo, C., & Wu, J. (2019). Alleviation of Hepatic Ischemia Reperfusion Injury by Oleanolic Acid Pretreating via Reducing HMGB1 Release and Inhibiting Apoptosis and Autophagy. Mediators of Inflammation, 2019, 1–10. [CrossRef]
- Wang, X., Liu, R., Zhang, W., Zhang, X., Liao, N., Wang, Z., Li, W., Qin, X., & Hai, C. (2013). Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Molecular and Cellular Endocrinology, 376(1–2), 70–80. [CrossRef]
- Wang, X., Yu, N., Wang, Z., Qiu, T., Jiang, L., Zhu, X., Sun, Y., & Xiong, H. (2020). Akebia trifoliata pericarp extract ameliorates inflammation through NF-κB/MAPK signaling pathways and modifies gut microbiota. Food & Function, 11(5), 4682–4696. [CrossRef]
- Wen, Y., Wang, Y., Zhao, C., Zhao, B., & Wang, J. (2023). The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. International Journal of Molecular Sciences, 24(11), 9317. [CrossRef]
- Yao, X., Li, G., Bai, Q., Xu, H., & Lü, C. (2013). Taraxerol inhibits LPS-induced inflammatory responses through suppression of TAK1 and Akt activation. International Immunopharmacology, 15(2), 316–324. [CrossRef]
- Zafar, H., & Saier, M. H. (2021). Gut Bacteroides species in health and disease. Gut Microbes, 13(1), 1848158. [CrossRef]
- Zhai, Y., Busuttil, R. W., & Kupiec-Weglinski, J. W. (2011). Liver Ischemia and Reperfusion Injury: New Insights into Mechanisms of Innate—Adaptive Immune-Mediated Tissue Inflammation. American Journal of Transplantation, 11(8), 1563–1569. [CrossRef]
- Zhao, J., Zhang, G., Zhou, X., Dong, W., Wang, Q., Xiao, C., & Zhang, S. (2019). Effect of Dandelion root extract on growth performance, immune function and bacterial community in weaned pigs. Food and Agricultural Immunology, 30(1), 95–111. [CrossRef]
- Zhou, H., Cao, H., Zheng, Y., Lu, Z., Chen, Y., Liu, D., Yang, H., Quan, J., Huo, C., Liu, J., & Yu, L. (2020). Liang-Ge-San, a classic traditional Chinese medicine formula, attenuates acute inflammation in zebrafish and RAW 264.7 cells. Journal of Ethnopharmacology, 249, 112427. [CrossRef]
- Zhou, X., Wang, W., Li, P., Zheng, Z., Tu, Y., Zhang, Y., & You, T. (2016). Curcumin Enhances the Effects of 5-Fluorouracil and Oxaliplatin in Inducing Gastric Cancer Cell Apoptosis Both In Vitro and In Vivo. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics, 23(1), 29–34. [CrossRef]
Figure 1.
Influence of dandelion and akebia extract addition on serum IL-1β (A), IL-6 (B), IgA (C), and IgG (D) concentration of weaned rabbits. interleukin-1 beta (IL-1β); interleukin-6 (IL-6); immunoglobulin A (IgA); immunoglobulin G (IgG). Akebia extract (A), Dandelion extract (D).
Figure 1.
Influence of dandelion and akebia extract addition on serum IL-1β (A), IL-6 (B), IgA (C), and IgG (D) concentration of weaned rabbits. interleukin-1 beta (IL-1β); interleukin-6 (IL-6); immunoglobulin A (IgA); immunoglobulin G (IgG). Akebia extract (A), Dandelion extract (D).
Figure 2.
Influence of dandelion and akebia extract addition on liver IL-1β (A), IL-6 (B), IgA (C) and IgG (D) concentration of weaned rabbits. interleukin-1 beta (IL-1β); interleukin-6 (IL-6); immunoglobulin A (IgA); immunoglobulin G (IgG). A: akebia extract ; D: dandelion extract.
Figure 2.
Influence of dandelion and akebia extract addition on liver IL-1β (A), IL-6 (B), IgA (C) and IgG (D) concentration of weaned rabbits. interleukin-1 beta (IL-1β); interleukin-6 (IL-6); immunoglobulin A (IgA); immunoglobulin G (IgG). A: akebia extract ; D: dandelion extract.
Figure 3.
Influence of dandelion and akebia extract addition on jejunal mucosa IL-1β (A), IL-6 (B), IgA (C) and IgG (D) concentration of weaned rabbits. interleukin-1 beta (IL-1β); interleukin-6 (IL-6); immunoglobulin A (IgA); immunoglobulin G (IgG). A: akebia extract ; D: dandelion extract.
Figure 3.
Influence of dandelion and akebia extract addition on jejunal mucosa IL-1β (A), IL-6 (B), IgA (C) and IgG (D) concentration of weaned rabbits. interleukin-1 beta (IL-1β); interleukin-6 (IL-6); immunoglobulin A (IgA); immunoglobulin G (IgG). A: akebia extract ; D: dandelion extract.
Figure 4.
Influence of dandelion and akebia extract addition on ileal mucosa IL-1β (A), IL-6 (B) IgA (C) and IgG (D) concentration of weaned rabbits. interleukin-1 beta (IL-1β); interleukin-6 (IL-6); immunoglobulin A (IgA); immunoglobulin G (IgG). A: akebia extract; D: dandelion extract.
Figure 4.
Influence of dandelion and akebia extract addition on ileal mucosa IL-1β (A), IL-6 (B) IgA (C) and IgG (D) concentration of weaned rabbits. interleukin-1 beta (IL-1β); interleukin-6 (IL-6); immunoglobulin A (IgA); immunoglobulin G (IgG). A: akebia extract; D: dandelion extract.
Figure 5.
Influence of dandelion and akebia extract in liver mRNA expression related to genes of inflammatory response. Akebia extract (A); dandelion extract (D); interleukin-1 beta (IL-1β); interleukin-6 (IL-6); interleukin-10 (IL-10); tumor necrosis factor-alpha (TNF-α); interferon-gamma (IFN-γ); chemokine ligand 3(CCL3); chemokine (C-X-C motif) ligand 10 (CXCL10).
Figure 5.
Influence of dandelion and akebia extract in liver mRNA expression related to genes of inflammatory response. Akebia extract (A); dandelion extract (D); interleukin-1 beta (IL-1β); interleukin-6 (IL-6); interleukin-10 (IL-10); tumor necrosis factor-alpha (TNF-α); interferon-gamma (IFN-γ); chemokine ligand 3(CCL3); chemokine (C-X-C motif) ligand 10 (CXCL10).
Figure 6.
Influence of dandelion and akebia extract in jejunum mucosa mRNA expression related to genes of inflammatory response. Akebia extract (A); dandelion extract (D); interleukin-1 beta (IL-1β); interleukin-6 (IL-6); interleukin-10 (IL-10); tumor necrosis factor-alpha (TNF-α); interferon-gamma (IFN-γ); chemokine ligand 3(CCL3); chemokine (C-X-C motif) ligand 10 (CXCL10).
Figure 6.
Influence of dandelion and akebia extract in jejunum mucosa mRNA expression related to genes of inflammatory response. Akebia extract (A); dandelion extract (D); interleukin-1 beta (IL-1β); interleukin-6 (IL-6); interleukin-10 (IL-10); tumor necrosis factor-alpha (TNF-α); interferon-gamma (IFN-γ); chemokine ligand 3(CCL3); chemokine (C-X-C motif) ligand 10 (CXCL10).
Figure 9.
Microbiota composition of weaned rabbits at phylum level.
Figure 9.
Microbiota composition of weaned rabbits at phylum level.
Figure 10.
Microbiota composition of weaned rabbits in the level of genus.
Figure 10.
Microbiota composition of weaned rabbits in the level of genus.
Figure 11.
Influence of dandelion and akebia extract on Beta diversity of weaned rabbits.
Figure 11.
Influence of dandelion and akebia extract on Beta diversity of weaned rabbits.
Figure 12.
Effects of dandelion and akebia on cecum microbial Venn diagram of OTUs of weaned rabbits.
Figure 12.
Effects of dandelion and akebia on cecum microbial Venn diagram of OTUs of weaned rabbits.
Figure 13.
Differences in species abundance at the genus level of the top 20 genera in average abundance.
Figure 13.
Differences in species abundance at the genus level of the top 20 genera in average abundance.
Table 1.
Primer sequence of fluorescent quantitative PCR.
Table 1.
Primer sequence of fluorescent quantitative PCR.
Table 2.
Influence of dandelion and akebia extract on weaned rabbits in growth.
Table 2.
Influence of dandelion and akebia extract on weaned rabbits in growth.
Table 3.
Influence of dandelion and akebia extract on Alpha diversity of weaned rabbits.
Table 3.
Influence of dandelion and akebia extract on Alpha diversity of weaned rabbits.
Table 4.
Microbiota composition of weaned rabbits at phylum level.
Table 4.
Microbiota composition of weaned rabbits at phylum level.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).