Submitted:
23 May 2025
Posted:
26 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Method
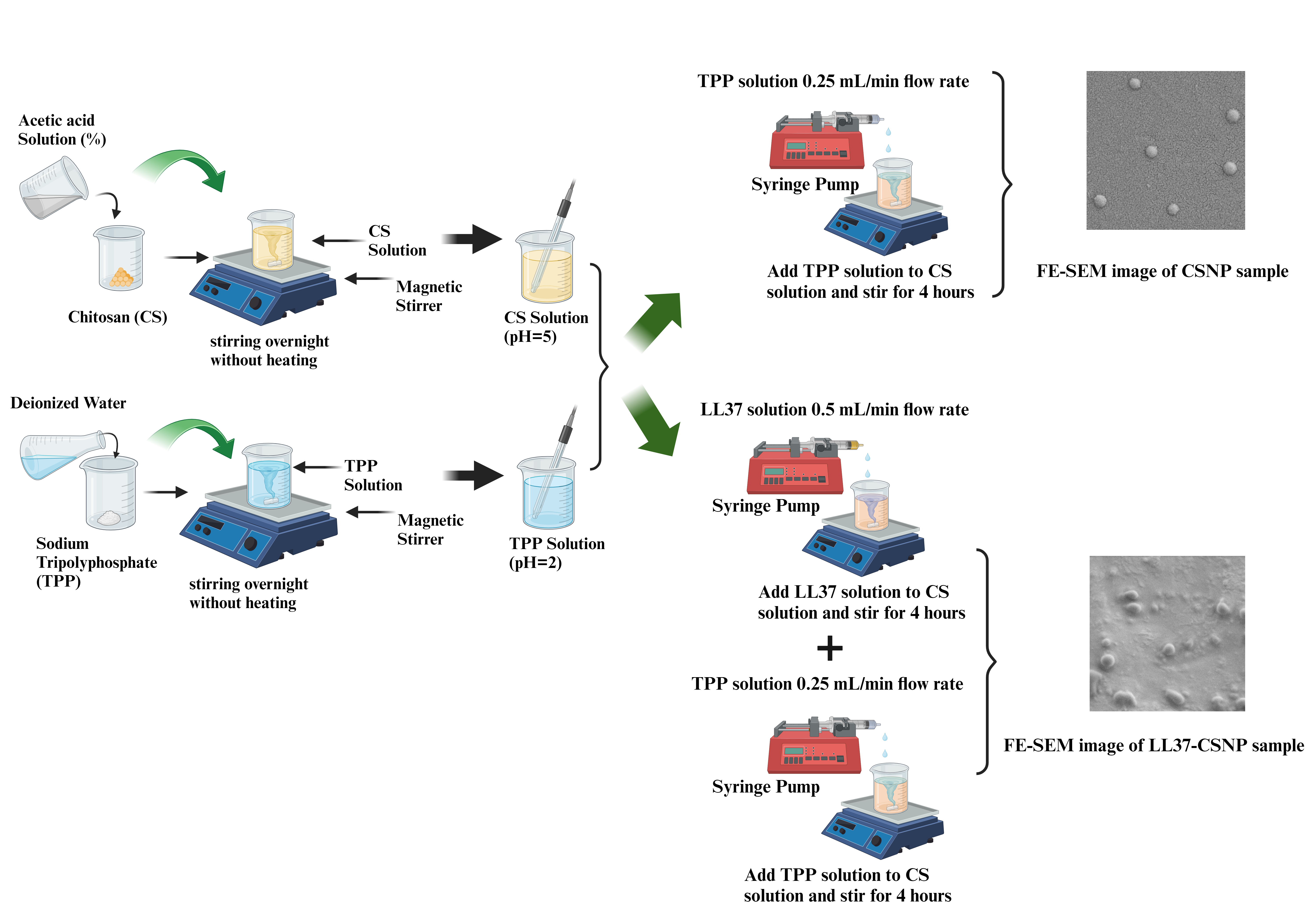
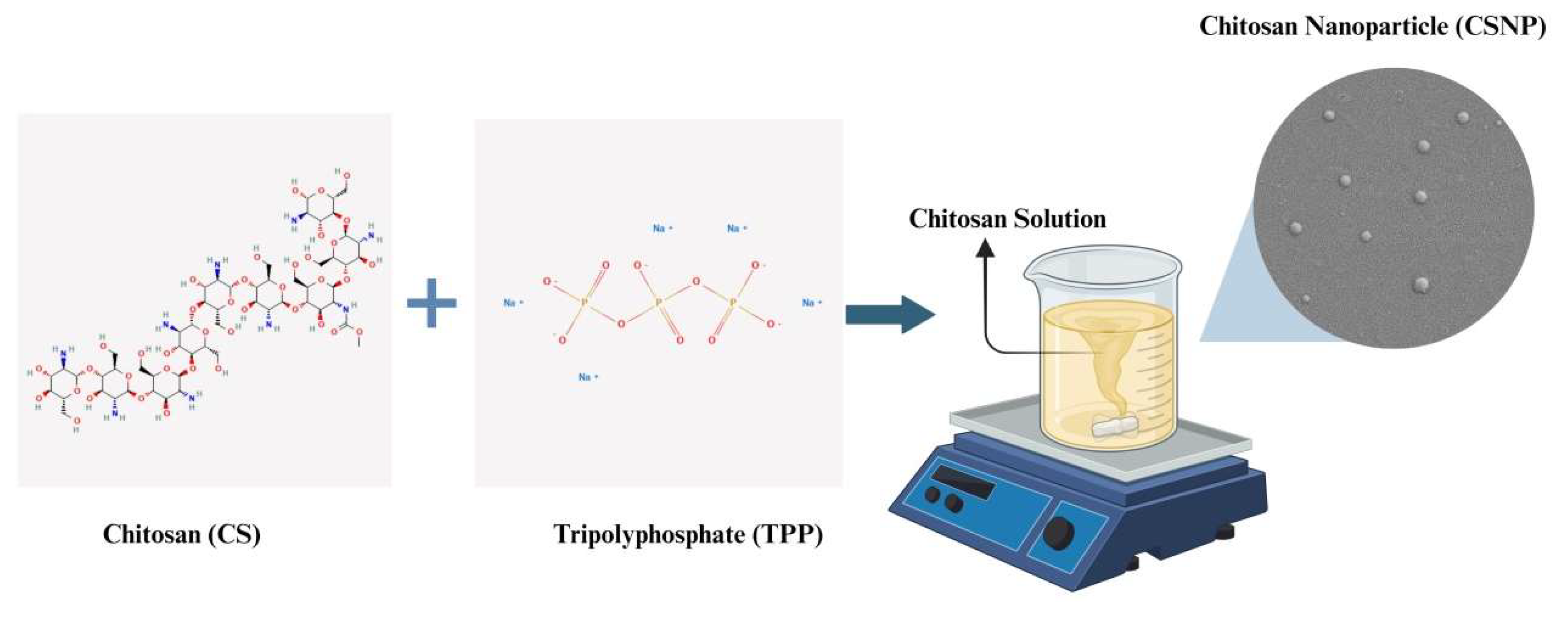
2.2.1. Development of CSNPs
Optimization of Development of Blank CSNPs
Development of LL37-Loaded CSNPs
2.2.2. Physicochemical Characterization of CSNPs
2.2.3. Encapsulation Efficiency of LL-37 and In-Vitro Release Kinetics
2.2.4. Evaluation of In-Vitro Biocompatibility
2.2.5. Evaluation of Antibacterial Activity
3. Results and Discussion
3.1. Physicochemical Characteristics of CSNPs and LL37-CSNPs
3.2. Encapsulation Efficiency
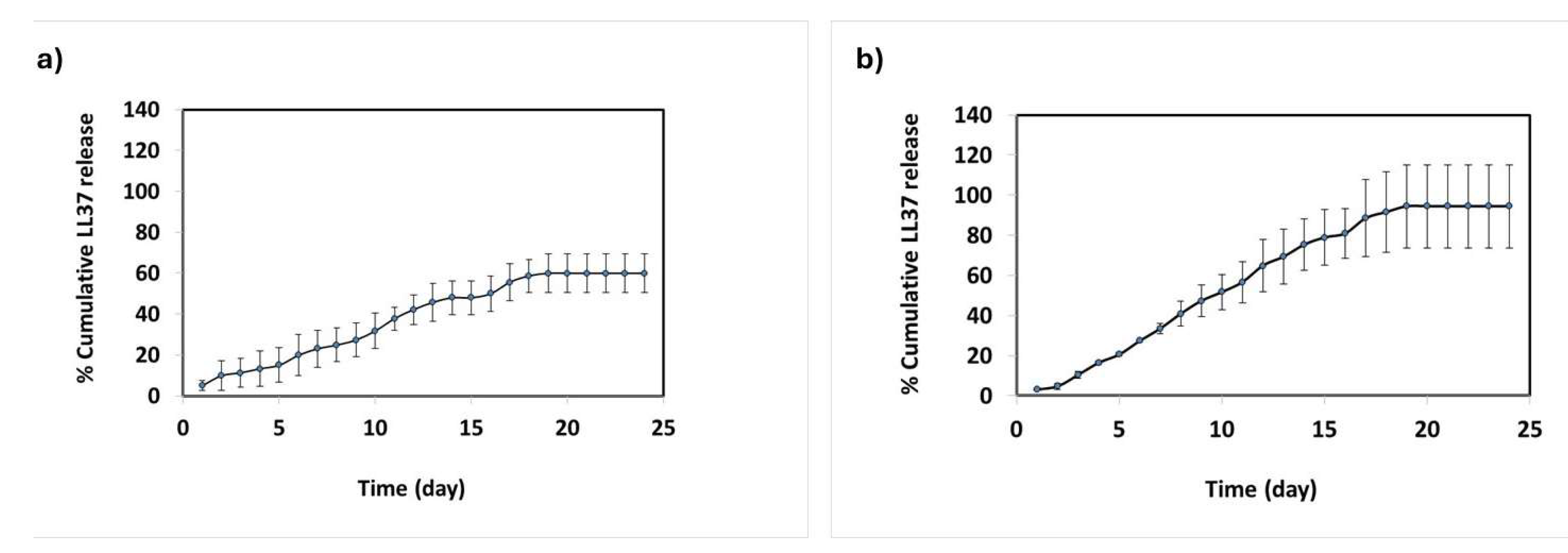
3.3. In Vitro LL37 Release from CSNPs
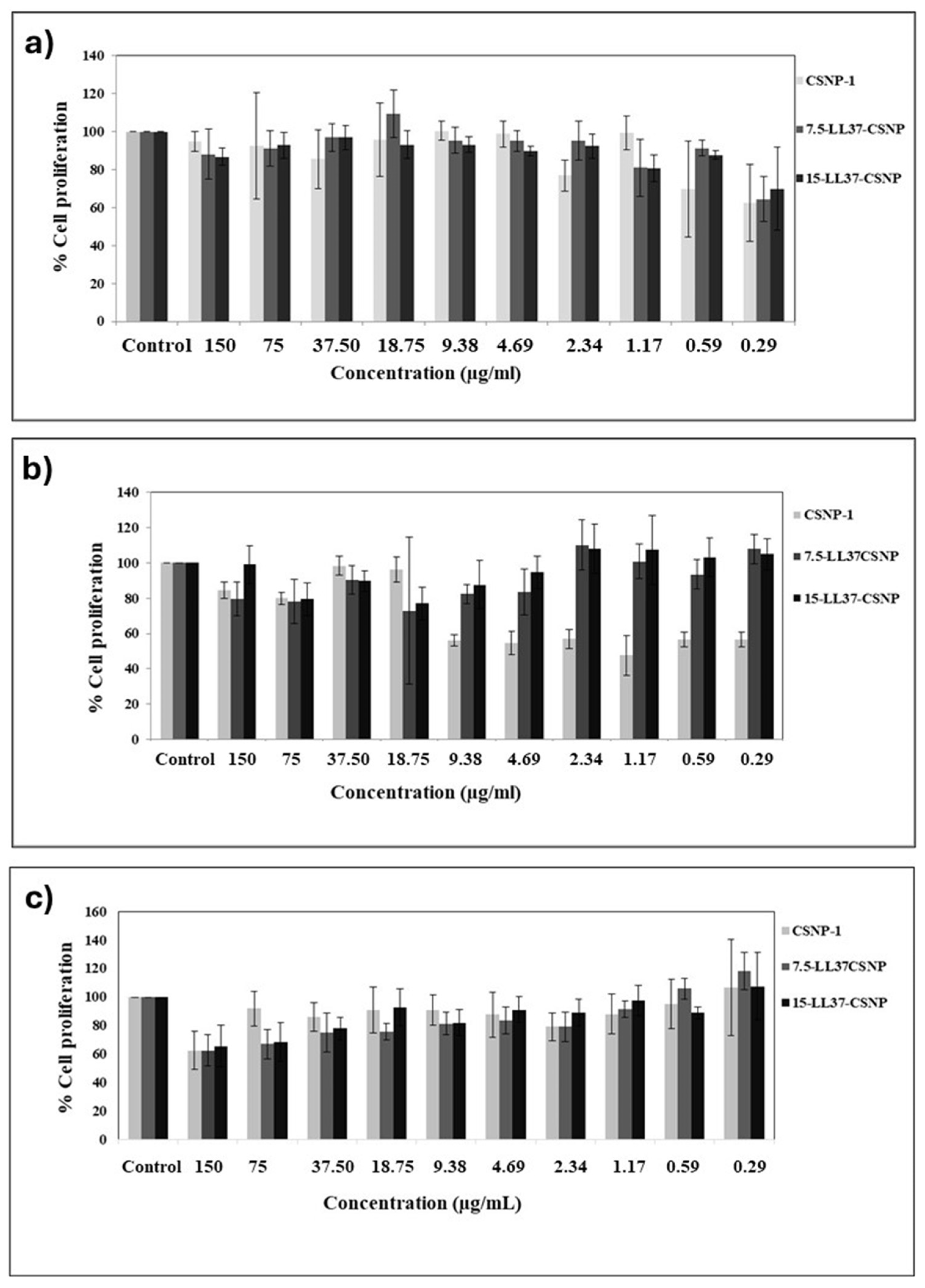
3.5. Biocompatibility of CSNPs
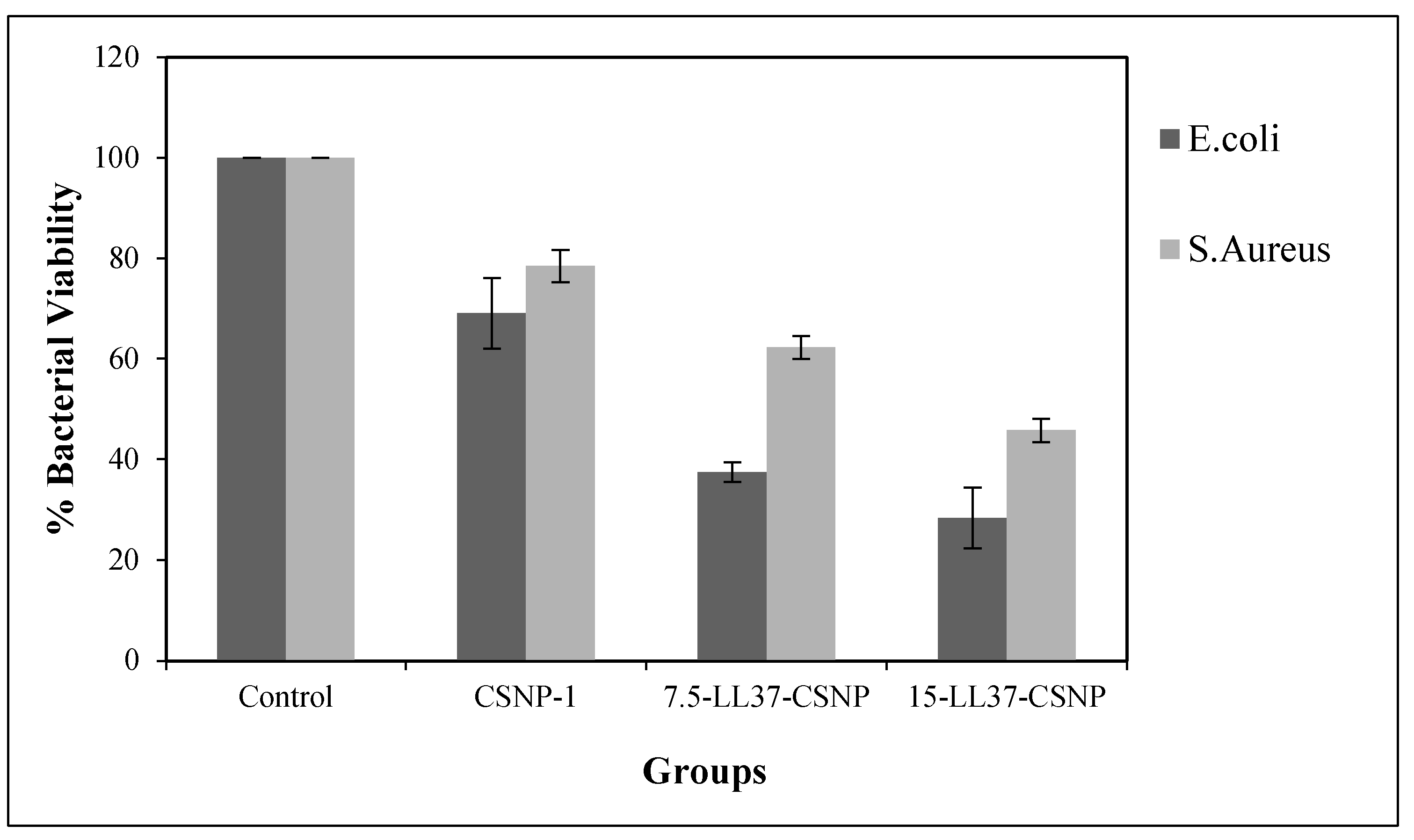
3.6. Antibacterial Activities of Blank and LL37-CSNPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
References
- Agarwal, M., Agarwal, M. K., Shrivastav, N., Pandey, S., Das, R., & Gaur, P. (2018). Preparation of chitosan nanoparticles and their in-vitro characterization. International Journal of Life-Sciences Scientific Research, 4(2), 1713-1720. [CrossRef]
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. J. Adv. Drug. Delivery Revi., 2001; 47:65–81. [CrossRef]
- Vasile, C., Pamfil, D., Stoleru, E., & Baican, M. (2020). New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules, 25(7), 1539.
- Thapa, R. K., Diep, D. B., & Tønnesen, H. H. (2020). Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta biomaterialia, 103, 52-67.
- Kahya, N. (2019). Water soluble chitosan derivatives and their biological activities: a review. Polym. Sci, 5(1), 1-11. [CrossRef]
- Algharib, S. A., Dawood, A., Zhou, K., Chen, D., Li, C., Meng, K., ... & Xie, S. (2020). Designing, structural determination and biological effects of rifaximin loaded chitosan-carboxymethyl chitosan nanogel. Carbohydrate polymers, 248, 116782. [CrossRef]
- Calvo, P., Remuñan-López, C., Vila-Jato, J. L., & Alonso, M. J. (1997). Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharmaceutical research, 14, 1431-1436. [CrossRef]
- Algharib, S. A., Dawood, A., & Xie, S. (2020-1). Nanoparticles for treatment of bovine Staphylococcus aureus mastitis. Drug delivery, 27(1), 292-308. [CrossRef]
- Algharib, S. A., Dawood, A., Zhou, K., Chen, D., Li, C., Meng, K., ... & Xie, S. (2022). Preparation of chitosan nanoparticles by ionotropic gelation technique: Effects of formulation parameters and in vitro characterization. Journal of Molecular Structure, 1252, 132129. [CrossRef]
- Al-Zahrani, S. S., Bora, R. S., & Al-Garni, S. M. (2021). Antimicrobial activity of chitosan nanoparticles. Biotechnology & Biotechnological Equipment, 35(1), 1874–1880. [CrossRef]
- Batoni, G., Maisetta, G., Lisa Brancatisano, F., Esin, S., & Campa, M. (2011). Use of antimicrobial peptides against microbial biofilms: advantages and limits. Current medicinal chemistry, 18(2), 256-279. [CrossRef]
- Lei, J., Sun, L., Huang, S., Zhu, C., Li, P., He, J., ... & He, Q. (2019). The antimicrobial peptides and their potential clinical applications. American journal of translational research, 11(7), 391. [PubMed]
- Neshani, A., Zare, H., Eidgahi, M. R. A., Kakhki, R. K., Safdari, H., Khaledi, A., & Ghazvini, K. (2019). LL-37: Review of antimicrobial profile against sensitive and antibiotic-resistant human bacterial pathogens. Gene Reports, 100519. [CrossRef]
- Najmi, Z., Kumar, A., Scalia, A. C., Cochis, A., Obradovic, B., Grassi, F. A., ... & Rimondini, L. (2020). Evaluation of nisin and LL-37 antimicrobial peptides as tool to preserve articular cartilage healing in a septic environment. Frontiers in Bioengineering and Biotechnology, 8, 561. [CrossRef]
- Brogden, N. K., & Brogden, K. A. (2011). Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals?. International journal of antimicrobial agents, 38(3), 217-225. [CrossRef]
- Ridyard, K. E., & Overhage, J. (2021). The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics, 10(6), 650. [CrossRef]
- Debnath, S., Kumar, R. S., & Babu, M. N. (2011). Ionotropic gelation–a novel method to prepare chitosan nanoparticles. Research Journal of Pharmacy and Technology, 4(4), 492-495.
- Elbehairi, S. E. I., Ismail, L. A., Alfaifi, M. Y., Elshaarawy, R. F., & Hafez, H. S. (2020). Chitosan nano-vehicles as biocompatible delivering tools for a new Ag (I) curcuminoid-Gboxin analog complex in cancer and inflammation therapy. International Journal of Biological Macromolecules, 165, 2750-2764. [CrossRef]
- Nair, R. S., Morris, A., Billa, N., & Leong, C. O. (2019). An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. Aaps Pharmscitech, 20(2), 69. [CrossRef]
- Costa, P., & Lobo, J. M. S. (2001). Modeling and comparison of dissolution profiles. European journal of pharmaceutical sciences, 13(2), 123-133.
- Sreekumar, S., Goycoolea, F. M., Moerschbacher, B. M., & Rivera-Rodriguez, G. R. (2018). Parameters influencing the size of chitosan-TPP nano-and microparticles. Scientific reports, 8(1), 4695. [CrossRef]
- Antoniou, J., Liu, F., Majeed, H., Qi, J., Yokoyama, W., & Zhong, F. (2015). Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 465, 137-146. [CrossRef]
- Huang, Y., & Lapitsky, Y. (2011). Monovalent salt enhances colloidal stability during the formation of chitosan/tripolyphosphate microgels. Langmuir, 27(17), 10392-10399. [CrossRef]
- Fan, W., Yan, W., Xu, Z., & Ni, H. (2012). Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids and surfaces B: Biointerfaces, 90, 21-27. [CrossRef]
- Bangun, H., Tandiono, S., & Arianto, A. (2018). Preparation and evaluation of chitosan-tripolyphosphate nanoparticles suspension as an antibacterial agent. Journal of Applied Pharmaceutical Science, 8(12), 147-156. [CrossRef]
- Ozturk, K., Arslan, F. B., Tavukcuoglu, E., Esendagli, G., & Calis, S. (2020). Aggregation of chitosan nanoparticles in cell culture: Reasons and resolutions. International Journal of Pharmaceutics, 578, 119119. [CrossRef]
- Gan, Q., Wang, T., Cochrane, C., & McCarron, P. (2005). Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids and Surfaces B: Biointerfaces, 44(2-3), 65-73. [CrossRef]
- Zhao, J., & Wu, J. (2006). Preparation and characterization of the fluorescent chitosan nanoparticle probe. Chinese Journal of Analytical Chemistry, 34(11), 1555-1559. [CrossRef]
- Csaba, N., Köping-Höggård, M., & Alonso, M. J. (2009). Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. International journal of pharmaceutics, 382(1-2), 205-214. [CrossRef]
- Majedi, F. S., Hasani-Sadrabadi, M. M., VanDersarl, J. J., Mokarram, N., Hojjati-Emami, S., Dashtimoghadam, E., ... & Renaud, P. (2014). On-chip fabrication of paclitaxel-loaded chitosan nanoparticles for cancer therapeutics. Advanced Functional Materials, 24(4), 432-441. [CrossRef]
- Pilipenko, I., Korzhikov-Vlakh, V., Sharoyko, V., Zhang, N., Schäfer-Korting, M., Rühl, E., ... & Tennikova, T. (2019). pH-sensitive chitosan–heparin nanoparticles for effective delivery of genetic drugs into epithelial cells. Pharmaceutics, 11(7), 317. [CrossRef]
- Hussain, Z., and S. Sahudin. “Preparation, Characterisation and Colloidal Stability of Chitosan-Tripolyphosphate Nanoparticles: Optimisation of Formulation and Process Parameters”. International Journal of Pharmacy and Pharmaceutical Sciences, vol. 8, no. 3, Mar.2016, pp. 297-08.
- Jonassen, H., Kjoniksen, A. L., & Hiorth, M. (2012). Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules, 13(11), 3747-3756. [CrossRef]
- Hassan, A., Sahudin, S., Hussain, Z., Hussain, M., & Hussain, M. (2018). Self-assembled chitosan nanoparticles for percutaneous delivery of caffeine: Preparation, characterization and in vitro release studies. Int J App Pharm, 10(4), 172-185.
- Nallamuthu, I., Devi, A., & Khanum, F. (2015). Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian journal of pharmaceutical sciences, 10(3), 203-211. [CrossRef]
- Lazaridou, M., Christodoulou, E., Nerantzaki, M., Kostoglou, M., Lambropoulou, D. A., Katsarou, A., ... & Bikiaris, D. N. (2020). Formulation and in-vitro characterization of chitosan-nanoparticles loaded with the iron chelator deferoxamine mesylate (DFO). Pharmaceutics, 12(3), 238. [CrossRef]
- Rashki, S., Safardoust-Hojaghan, H., Mirzaei, H., Abdulsahib, W. K., Mahdi, M. A., Salavati-Niasari, M., ... & Mousavi, S. G. A. (2022). Delivery LL37 by chitosan nanoparticles for enhanced antibacterial and antibiofilm efficacy. Carbohydrate Polymers, 291, 119634. [CrossRef]
- Piras, A. M., Maisetta, G., Sandreschi, S., Gazzarri, M., Bartoli, C., Grassi, L., ... & Batoni, G. (2015). Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Frontiers in microbiology, 6, 372. [CrossRef]
- Herdiana, Y., Wathoni, N., Shamsuddin, S., & Muchtaridi, M. (2022). Drug release study of the chitosan-based nanoparticles. Heliyon, 8(1). [CrossRef]
- Iacob, A. T., Lupascu, F. G., Apotrosoaei, M., Vasincu, I. M., Tauser, R. G., Lupascu, D., ... & Profire, L. (2021). Recent biomedical approaches for chitosan based materials as drug delivery nanocarriers. Pharmaceutics, 13(4), 587. [CrossRef]
- Tığlı Aydın, R. S., & Pulat, M. (2012). 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. Journal of Nanomaterials, 2012(1), 313961. [CrossRef]
- Lin, X., Wang, R., & Mai, S. (2020). Advances in delivery systems for the therapeutic application of LL37. Journal of Drug Delivery Science and Technology, 60, 102016.
- Ramos, R., Silva, J. P., Rodrigues, A. C., Costa, R., Guardão, L., Schmitt, F., ... & Gama, M. (2011). Wound healing activity of the human antimicrobial peptide LL37. Peptides, 32(7), 1469-1476. [CrossRef]
- Fahimirad, S., Ghaznavi-Rad, E., Abtahi, H., & Sarlak, N. (2021). Antimicrobial activity, stability and wound healing performances of chitosan nanoparticles loaded recombinant LL37 antimicrobial peptide. International journal of peptide research and therapeutics, 27(4), 2505-2515. [CrossRef]
- Sun, L., Chen, Y., Zhou, Y., Guo, D., Fan, Y., Guo, F., ... & Chen, W. (2017). Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian journal of pharmaceutical sciences, 12(5), 418-423.
- Nasri, R., Hamdi, M., Touir, S., Li, S., Karra-Chaâbouni, M., & Nasri, M. (2021). Development of delivery system based on marine chitosan: Encapsulationand release kinetic study of antioxidant peptides from chitosan microparticle. International Journal of Biological Macromolecules, 167, 1445-1451.
- Costa, E. M., Silva, S., & Pintado, M. (2023). Chitosan nanoparticles production: optimization of physical parameters, biochemical characterization, and stability upon storage. Applied Sciences, 13(3), 1900. [CrossRef]
- Shukla, S. K., Mishra, A. K., Arotiba, O. A., & Mamba, B. B. (2013). Chitosan-based nanomaterials: A state-of-the-art review. International journal of biological macromolecules, 59, 46-58. [CrossRef]
- Ing, L. Y., Zin, N. M., Sarwar, A., & Katas, H. (2012). Antifungal activity of chitosan nanoparticles and correlation with their physical properties. International journal of Biomaterials, 2012(1), 632698. [CrossRef]
- Patrulea, V., Ostafe, V., Borchard, G., & Jordan, O. (2015). Chitosan as a starting material for wound healing applications. European Journal of Pharmaceutics and Biopharmaceutics, 97, 417-426.
- Keong, L. C., & Halim, A. S. (2009). In vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management. International journal of molecular sciences, 10(3), 1300-1313.
- Fahimirad, S., Ajalloueian, F., & Ghorbanpour, M. (2019). Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicology and environmental safety, 168, 260-278.
- Yu, H., Ma, Z., Meng, S., Qiao, S., Zeng, X., Tong, Z., & Jeong, K. C. (2021). A novel nanohybrid antimicrobial based on chitosan nanoparticles and antimicrobial peptide microcin J25 with low toxicity. Carbohydrate Polymers, 253, 117309.
- Sun, T., Zhan, B., Zhang, W., Qin, D., Xia, G., Zhang, H., ... & Lee, W. H. (2018). Carboxymethyl chitosan nanoparticles loaded with bioactive peptide OH-CATH30 benefit nonscar wound healing. International journal of nanomedicine, 5771-5786.
- Noore, J., Noore, A., & Li, B. (2013). Cationic antimicrobial peptide LL-37 is effective against both extra-and intracellular Staphylococcus aureus. Antimicrobial agents and chemotherapy, 57(3), 1283-1290. [CrossRef]
- Almaaytah, A., Mohammed, G. K., Abualhaijaa, A., & Al-Balas, Q. (2017). Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug design, development and therapy, 3159-3170. [CrossRef]
- Pan, C., Qian, J., Fan, J., Guo, H., Gou, L., Yang, H., & Liang, C. (2019). Preparation nanoparticle by ionic cross-linked emulsified chitosan and its antibacterial activity. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 568, 362-370. [CrossRef]
- Li, F., Jin, H., Xiao, J., Yin, X., Liu, X., Li, D., & Huang, Q. (2018). The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food research international, 111, 351-360. [CrossRef]


| Groups | CS (mg/mL, pH 5) | TPP (mg/mL, pH 2) | CS/TPP (v/v) | NaCI (mM) | Tween80 |
| CSNP-1 | 0.7 | 0.3 | 2:1 | - | + |
| CSNP-2 | 0.7 | 0.3 | 2:1 | 100 | - |
| CSNP-3 | 0.3 | 0.1 | 2:1 | - | + |
| CSNP-4 | 0.3 | 0.1 | 2:1 | 100 | - |
| Groups | Mean Particle Size (nm) | PDI | ZP (mV) |
| CSNP-1 | 180.40±2.16 | 0.289±0.00 | 40.57±1.82 |
| CSNP-2 | 198.00±4.48 | 0.259±0.03 | 43.68±1.40 |
| CSNP-3 | 291.90±6.65 | 0.364±0.01 | 46.26±0.72 |
| CSNP-4 | 496.40±9.80 | 0.321±0.00 | 35.75±0.97 |
| 7.5-LL37-CSNP | 195.60±3.50 | 0.257±0.02 | 19.26±1.39 |
| 15-LL37-CSNP | 210.90±2.59 | 0.306±0.02 | 51.21±0.93 |
| LL37 (µg/mL) | EE (%) |
| 30 µg/mL | 55.57±11.90 |
| 15 µg/mL | 80.32±.,94 |
| 7.5 µg/mL | 97.81±2.72 |
| 7.5-LL37-CSNP | 15-LL37-CSNP | |||||||||
| Release kinetics model | R2 | RMSE | RSS | AIC | BIC | R2 | RMSE | RSS | AIC | BIC |
| First Order Kinetics | 0.9771 | 2.8708 | 197.7961 | 54.6204 | 56.9765 | 0.9724 | 5.3215 | 679.6389 | 84.2442 | 86.6003 |
| Korsmeyer Peppas | 0.9680 | 3.3915 | 276.0563 | 62.6212 | 64.9773 | 0.9616 | 6.2766 | 945.5007 | 92.1679 | 94.5240 |
| Zero Order Kinetics | 0.9569 | 3.9350 | 371.6292 | 69.7562 | 72.1123 | 0.9518 | 7.0313 | 1186.5542 | 97.6181 | 99.9742 |
| Hixson Crowell | 0.8958 | 6.1189 | 898.5776 | 90.9462 | 93.3023 | 0.8744 | 11.3493 | 3091.3721 | 120.5996 | 122.9557 |
| Higuchi | 0.8760 | 6.6747 | 1069.2336 | 93.1194 | 94.2975 | 0.8454 | 12.5909 | 3804.7675 | 123.5830 | 124.7610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





