1. Introduction
Cardiovascular disease (CVD) has emerged as a significant challenge for global livestock agriculture, severely impacting animal welfare, food security, and economic sustainability. Recent epidemiological studies indicate a troubling rise in bovine congestive heart failure (BCHF), with incidence rates doubling approximately every decade, affecting over 1.2 million cattle annually in the United States alone [
1]. Approximately 4.14% of feeder cattle evaluated at slaughter exhibit advanced cardiac pathology, underscoring the scale and urgency of this widespread issue [2-4]. Economically, BCHF imposes substantial costs on the livestock industry, generating annual losses exceeding
$250,000 per feedlot due to undiagnosed cardiac conditions—losses that surpass even respiratory diseases in their economic significance [
5]. Additionally, cattle suffering from undiagnosed cardiac disease endure prolonged distress and consume resources unnecessarily, thereby increasing greenhouse gas emissions and exacerbating agriculture’s environmental footprint.
Despite the severity and urgency of these issues, veterinary cardiology continues to depend heavily on traditional diagnostic methods such as electrocardiography (ECG), echocardiography, thoracic radiography, and conventional biochemical assays [
6,
7]. Although echocardiography can achieve diagnostic accuracy of up to 92% for cardiac dysfunction, its application is limited due to specialized equipment requirements and the scarcity of trained veterinary cardiologists, making it inaccessible to nearly 78% of cattle operations globally [
8,
9]. Thoracic radiographs offer restricted diagnostic resolution, while biochemical markers such as cardiac troponin I (cTnI) still lack standardized breed-specific reference values, limiting their practical clinical application [
10,
11]. Genetic approaches, including genomic tests like Igenity BCHF, can identify predisposition but fail to detect active cardiac disease, further contributing to a substantial gap in clinical diagnostics [
12]. Consequently, the majority of BCHF cases remain undiagnosed until postmortem examinations, highlighting critical limitations in both clinical practice and animal welfare standards.
These diagnostic challenges are further complicated by evolving epidemiological patterns. Historically confined primarily to cattle in high-altitude regions, BCHF is now increasingly detected at moderate elevations, a shift attributed to intensive genetic selection practices focused on traits like rapid growth and marbling, particularly in breeds such as Angus [
13]. Genomic research supports this observation, demonstrating considerable heritability (h² = 0.356) and significant genetic correlations between cardiac pathology and growth performance (r = 0.289–0.460), thus revealing unintended consequences associated with contemporary breeding strategies [
14]. These findings emphasize an urgent need for scalable, precise, and non-invasive diagnostic technologies, particularly as climate change further exacerbates metabolic stresses on livestock populations.
In parallel, human medicine has seen transformative advancements in early cardiovascular disease detection through artificial intelligence (AI)-based retinal imaging. Retinal microvascular changes, detectable through advanced AI algorithms, often precede clinical cardiovascular symptoms, enabling proactive interventions and improved patient outcomes [15-17]. Transformer-based neural networks, such as RetiCVD-Net, have demonstrated up to 89% accuracy in predicting left ventricular hypertrophy, while deep learning models can predict 10-year cardiovascular events with sensitivities near 74% [
18,
19]. Given the shared embryological origins between retinal and systemic vascular systems, the retina serves as an ideal non-invasive diagnostic window into systemic cardiovascular health, providing exciting translational possibilities for veterinary medicine [20-22].
However, direct application of human-developed retinal imaging models to cattle is challenged by significant anatomical and physiological distinctions between species. The bovine retina features a dominant central retinal artery and greater vascular complexity compared to human retinas, making direct transfer of AI models problematic [
23,
24]. Previous attempts to directly utilize advanced deep learning architectures, such as ResNet101, initially showed promise but ultimately revealed critical limitations. These models struggled to interpret bovine-specific retinal anatomy accurately, producing predictions lacking clinical relevance and interpretability. As a result, veterinarians have become increasingly cautious about relying on opaque AI systems that provide little insight into their decision-making processes. The demand for transparent, biologically meaningful explanations has highlighted the urgent need for explainable AI (XAI) approaches that effectively combine accuracy with clinical interpretability.
Hybrid AI models, integrating deep learning with clinically validated biomarkers, have already demonstrated significant efficacy in human cardiology. For example, the Singapore I Vessel Assessment-Deep Learning System (SIVA-DLS) has attained over 90% inter-rater agreement by combining quantitative microvascular analysis with clinician interpretation [
25,
26]. Such hybrid approaches are particularly valuable in veterinary medicine, where practical deployment requires robust, transparent systems capable of adaptation across diverse conditions. Recent advancements in explainable AI methods, including SHapley Additive exPlanations (SHAP) and Local Interpretable Model-agnostic Explanations (LIME), have significantly improved the clinical interpretability and practical utility of cardiovascular diagnostic models, facilitating their adoption [
27,
28].
Emerging veterinary research has validated specific retinal vascular biomarkers—especially vessel tortuosity and fractal dimension—as reliable clinical indicators strongly correlated with bovine cardiac dysfunction and pulmonary hypertension (r = 0.71, p < 0.001). These biomarkers closely parallel established human cardiovascular indicators, confirming their translational relevance and clinical value [
29,
30].
Implementing early, precise diagnostic methods for bovine cardiovascular disease offers substantial economic, ethical, and environmental advantages. Early detection could significantly mitigate financial losses by approximately 68% during the late feeding stages, amounting to annual savings of around
$1.2 billion across U.S. cattle operations [
5]. Diagnostic tools achieving sensitivities as high as 98.2% would considerably reduce undiagnosed cardiac conditions, directly improving animal welfare outcomes and aligning closely with ethical standards and the One Health approach. Moreover, timely intervention reduces unnecessary resource consumption and associated greenhouse gas emissions, advancing agricultural sustainability and climate resilience.
To address these urgent industry needs and critical diagnostic gaps, the present study introduces a fully interpretable artificial intelligence framework specifically designed for non-invasive, early detection of cardiovascular disease in cattle via retinal imaging. The framework incorporates three core components: first, a bovine-specific retinal vessel segmentation model utilizing a U-Net architecture, trained and optimized on a comprehensive dataset of annotated cattle retinal images; second, extraction and validation of five clinically relevant vascular biomarkers (vessel density, bifurcation angles, tortuosity, branching density, and fractal dimension) chosen based on their significance in human and veterinary cardiovascular research; and third, implementation of a hybrid classification system that integrates deep learning-derived image features from the ResNet18 model with these validated clinical biomarkers.
By employing this integrated, transparent AI methodology, this study seeks to bridge existing diagnostic limitations, offering a practical, scalable, and clinician-friendly diagnostic tool suitable for routine herd-level cardiovascular screening. The subsequent Materials and Methods section provides detailed descriptions of datasets, image acquisition processes, model architectures, feature extraction techniques, and evaluation criteria, ensuring clarity, reproducibility, and clinical applicability of the proposed diagnostic framework.
2. Materials and Methods
2.1. Study Overview
This study developed a novel framework for the early detection of cardiovascular disease (CVD) in cattle through retinal imaging. The approach combined a cattle-specific retinal vessel segmentation model with a hybrid classifier, integrating deep-learning-derived features and clinically validated vascular biomarkers. This methodology aimed to enhance diagnostic accuracy, transparency, and clinical interpretability, facilitating practical applications in veterinary diagnostics.
2.2. Datasets and Image Acquisition
2.2.1. Human Retinal Vessel Dataset
Initial model training employed the Retina Blood Vessel Dataset publicly available on Kaggle [
31]. The dataset comprises 100 human retinal fundus images (512 × 512 pixels, PNG format), divided into 80 training and 20 testing images. Each image has corresponding binary segmentation masks differentiating vessel pixels (label = 1) from background pixels (label = 0), providing a reliable foundation for initial segmentation model development.
2.2.2. Cattle Retinal Image Dataset
The veterinary component utilized the "CVD vs. Non-CVD Retinal Images of Cattle" dataset previously described by Ertas et al. [
23]. This dataset includes 1,118 retinal images collected from 100 cattle (591 diagnosed with CVD, 527 healthy). The images were captured using an Optomed Smartscope digital fundus camera with a resolution of 1536 × 1152 pixels (JPG format). Clinical diagnoses were confirmed by veterinary professionals through comprehensive biochemical, hematological, and clinical evaluations, ensuring robust categorization.
2.3. Retinal Vessel Segmentation Model Development
2.3.1. Initial U-Net Training on Human Dataset
The segmentation task utilized a U-Net convolutional neural network (CNN), recognized for its efficacy in medical image segmentation. Implemented using Keras, the model was trained from scratch on resized human retinal images (512 × 512 pixels) and corresponding masks. Training parameters included 300 epochs, the Adam optimizer with a learning rate of 0.001, a batch size of eight, and binary cross-entropy loss to classify pixels into vessel or background classes.
2.3.2. Initial Evaluation on Cattle Retinal Images
To test cross-species transferability, the human-trained U-Net model was evaluated on 16 cattle retinal images randomly selected from the veterinary dataset. Segmentation performance was initially inadequate (low Dice coefficient), attributed primarily to significant anatomical differences between human and bovine retinal structures, notably the cattle's distinct central retinal vessel.
2.3.3. Manual Annotation and Model Fine-Tuning
To address cross-species anatomical discrepancies, 20 cattle retinal images (10 healthy, 10 CVD-affected) were manually annotated using Roboflow Annotate’s Smart Polygon tool, powered by Meta’s Segment Anything Model (SAM). Annotated images underwent data augmentation through horizontal and vertical flips and rotations (90° clockwise, counterclockwise, and 180°), expanding the dataset to 60 images. This dataset was split into training (80%) and validation (20%) subsets.
The U-Net model was then fine-tuned for 100 epochs using the augmented cattle-specific dataset, maintaining the Adam optimizer but reducing the learning rate to 1e-4. To correct class imbalance between vessel and background pixels, the loss function was changed from binary cross-entropy to Dice loss, significantly improving segmentation accuracy on cattle images.
2.4. Clinical Biomarker Extraction from Segmented Images
Post-segmentation, five clinically relevant vascular biomarkers indicative of cardiovascular health were extracted from each retinal vessel mask:
Vessel Density: Calculated as the ratio of the area covered by vessel pixels to the total retinal image area.
Bifurcation Angle: Measured as the average angle formed at vessel branching points, providing insight into vascular branching patterns.
Tortuosity: Defined as the average ratio of each vessel’s curved path length to the straight-line distance between vessel endpoints, indicating abnormal vascular morphology.
Branching Density: Quantified as the number of vessel branch points per unit vessel length, indicating vascular complexity.
Fractal Dimension: Evaluated via the box-counting method, capturing the complexity and spatial pattern of the retinal vascular network.
These biomarkers were chosen based on their established clinical relevance to cardiovascular disease in previous veterinary and human studies.
2.5. Classification Framework Development
2.5.1. Initial Deep Learning Classification
As a preliminary step, segmented vessel masks were directly classified using five established CNN architectures: ResNet18, VGG16, DenseNet121, EfficientNetB0, and MobileNetV2. Vessel masks were resized to 224 × 224 pixels and normalized before training. Data were partitioned into training (80%) and validation (20%) subsets. Among these architectures, ResNet18 showed the highest diagnostic accuracy (82.14%), outperforming other models and justifying its selection for subsequent hybrid modeling.
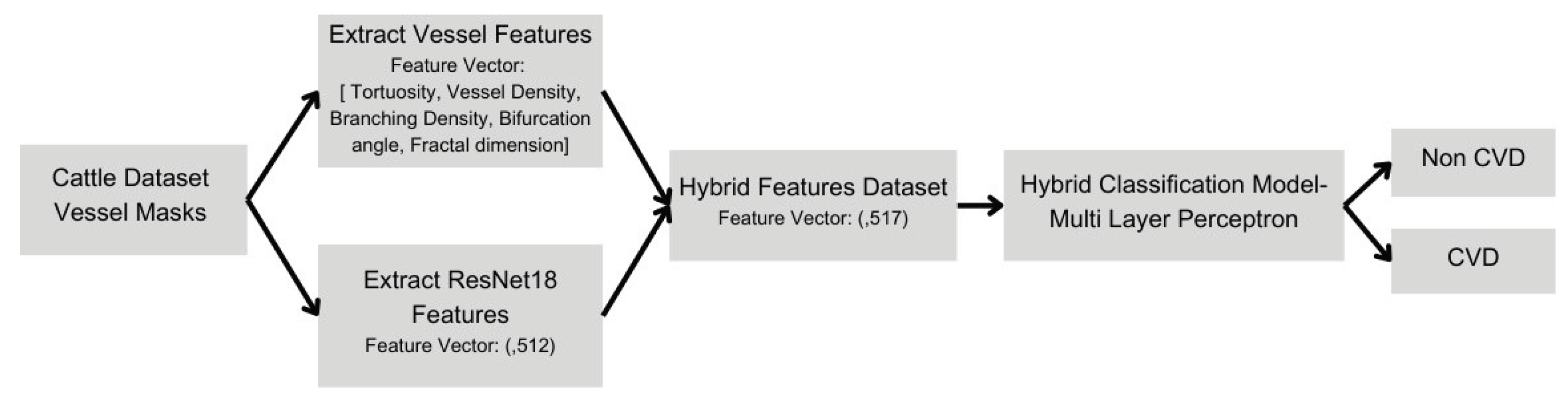
2.5.2. Hybrid Multilayer Perceptron (MLP) Classifier Development
To enhance model interpretability and accuracy, a hybrid classifier was constructed using both deep-learning-derived features (512 features extracted from the ResNet18 model) and the five extracted clinical biomarkers. This combined feature set was used to train a multilayer perceptron classifier with three hidden layers. Training occurred over 150 epochs, employing the Adam optimizer with a reduced learning rate of 1e-6. This hybrid classifier significantly improved accuracy (97.32%) and sensitivity (98.2%), indicating strong potential for clinical veterinary application.
2.6. Model Evaluation and Validation
To rigorously evaluate segmentation and classification performance, multiple quantitative metrics were employed. Segmentation performance was assessed using Dice coefficient and Jaccard index, standard metrics indicating overlap between predicted and ground-truth vessel masks. Classification models were evaluated using accuracy, precision, recall, F1-score, specificity, sensitivity, and area under the receiver operating characteristic curve (AUC-ROC). Consistent training-validation splits (80%-20%) were applied across all experimental setups, ensuring unbiased, reproducible, and robust evaluation of models. Confusion matrices and ROC curves provided additional visualization of model performance, aiding interpretability.
3. Results
3.1. Overview and Workflow Integration
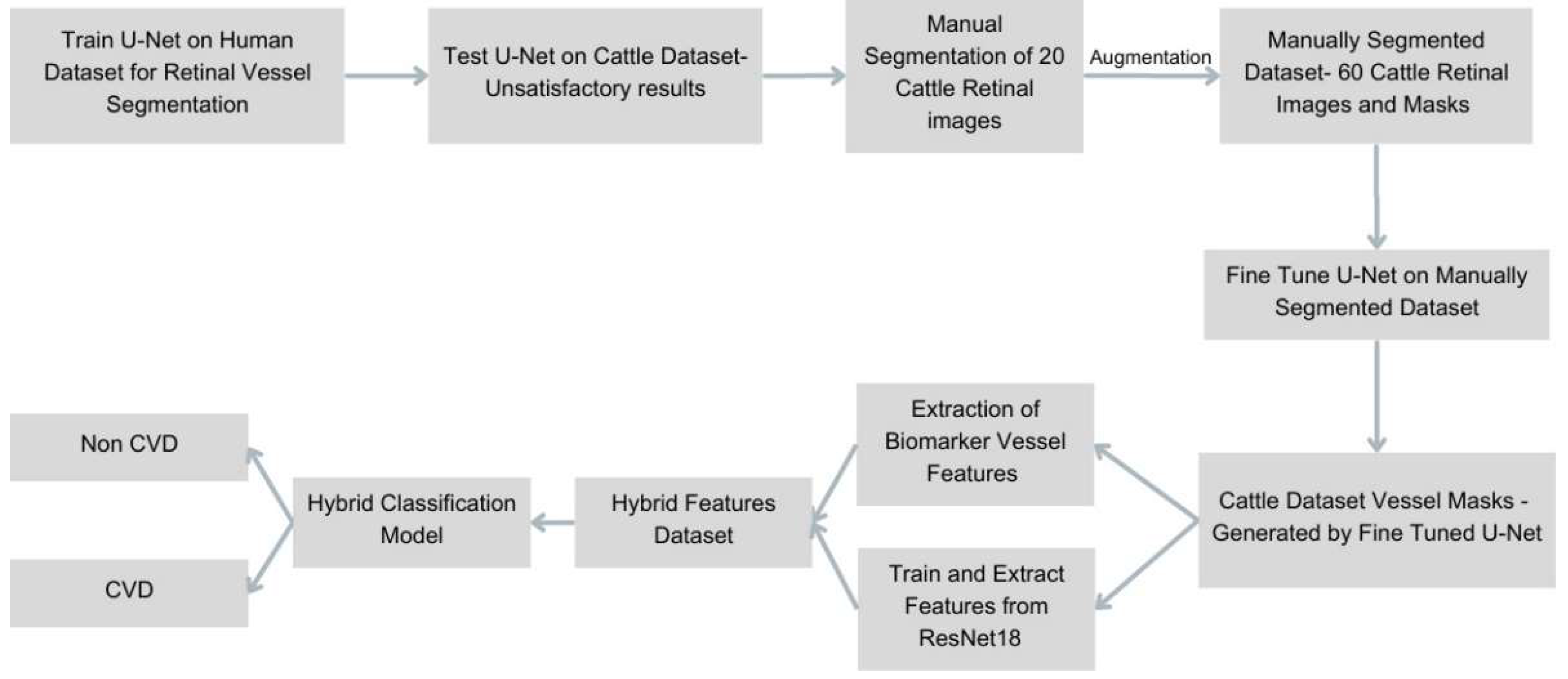
This study introduces an advanced and interpretable artificial intelligence (AI) pipeline for the early detection of cardiovascular disease (CVD) in cattle using retinal fundus images. The developed workflow (
Figure 1) systematically integrates sophisticated retinal vessel segmentation, quantitative extraction of clinically validated vascular biomarkers, and a hybrid classification framework that merges deep learning with interpretable clinical features. The primary goal of this integration is to address the critical limitations of traditional veterinary diagnostics, which are typically invasive, costly, and unsuitable for large-scale herd screenings. This pipeline strategically bridges precision medicine with practical livestock management, explicitly overcoming anatomical and physiological disparities between human and bovine retinas, historically restricting cross-species AI applicability.
3.2. Retinal Vessel Segmentation Performance
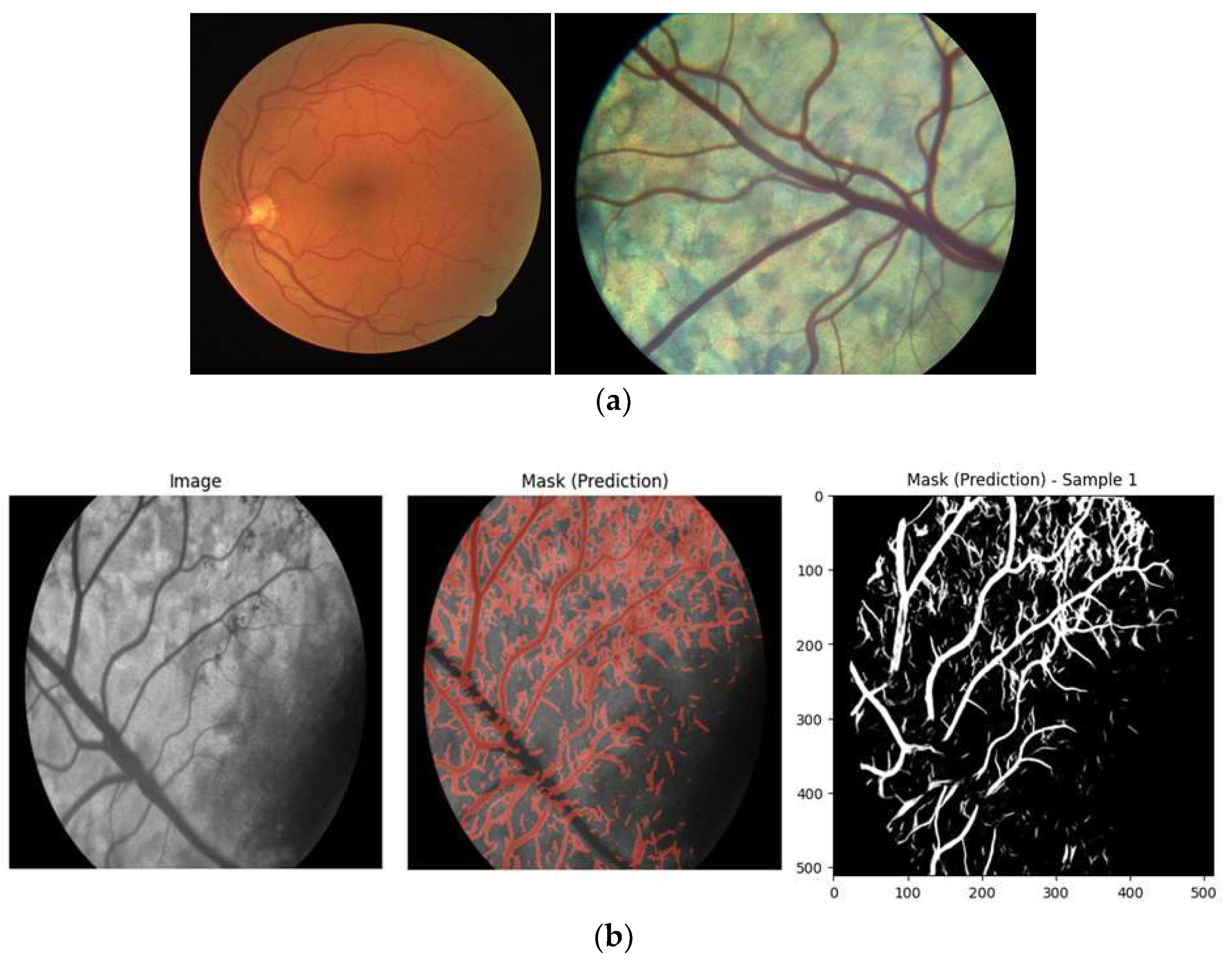
Initial attempts at segmentation using a U-Net model trained exclusively on human retinal images resulted in insufficient segmentation performance when directly applied to cattle retinal images (
Figure 2). Major inaccuracies included frequent misclassification of the distinctive central retinal vessel in bovines as background and erroneous identification of spurious vessels in darker regions. These issues stem from fundamental anatomical differences; bovine retinas predominantly feature a single dominant vessel with asymmetric branching compared to multiple major vessels emanating from the human optic disc.
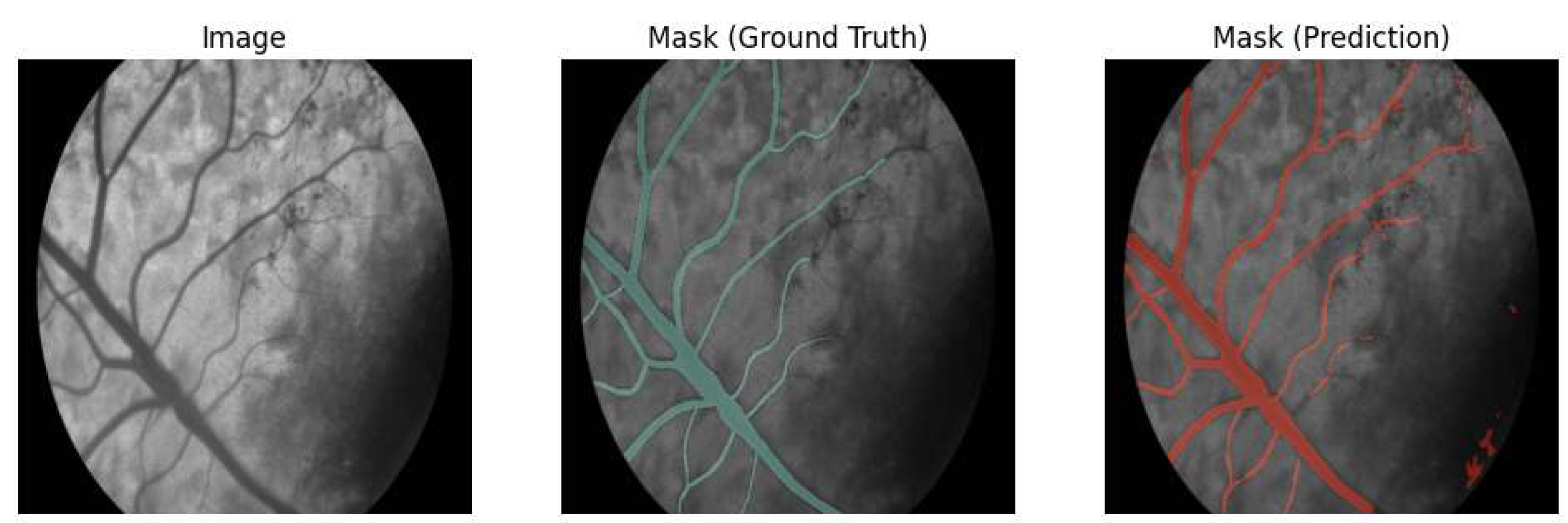
To rectify these anatomical discrepancies, 20 cattle retinal images underwent meticulous manual segmentation to create accurate ground truth masks, which were then augmented extensively through geometric transformations such as flips and rotations. Fine-tuning the U-Net model on this augmented bovine-specific dataset, utilizing dice loss to address the class imbalance, significantly improved segmentation quality.
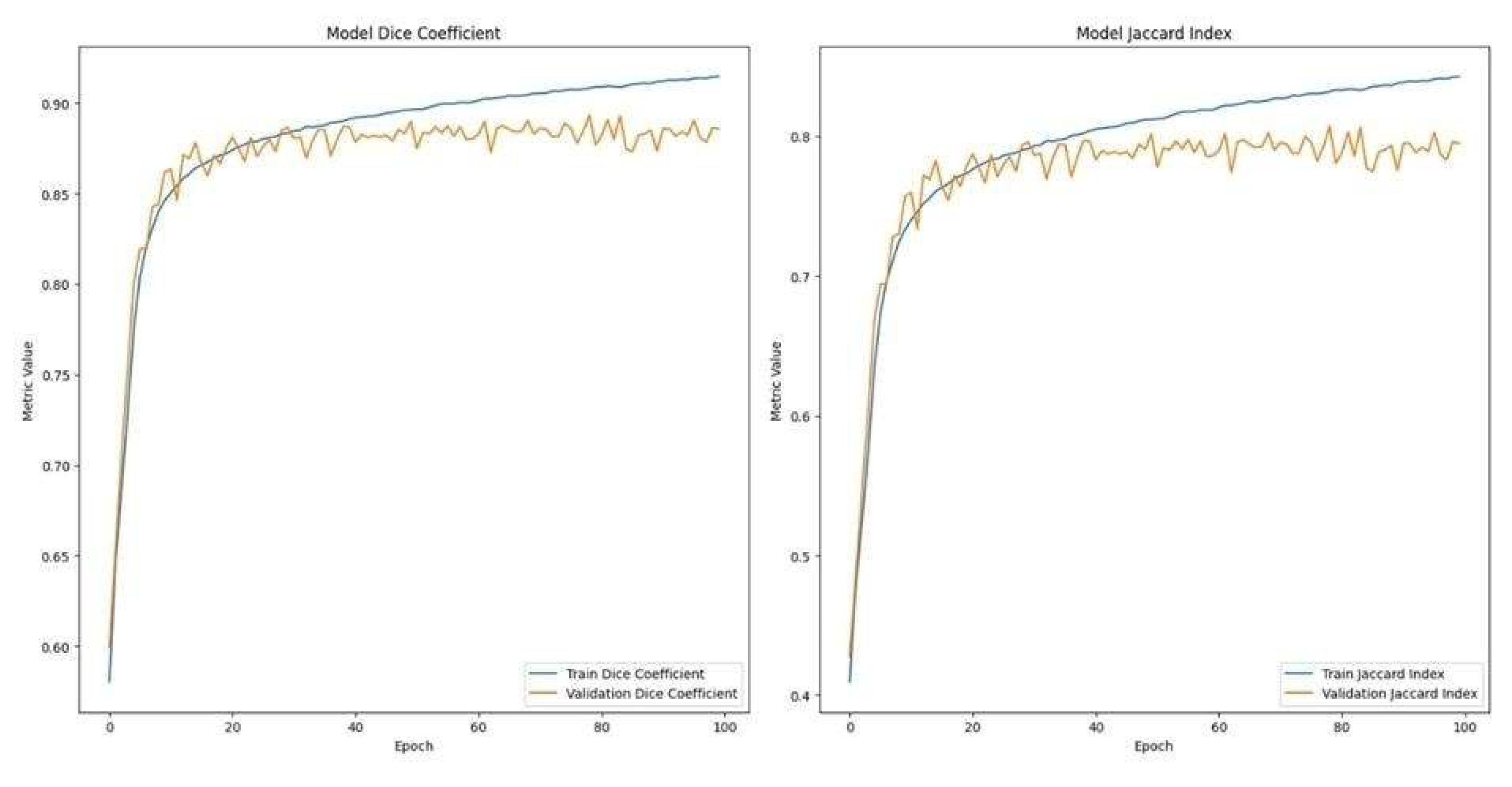
The refined U-Net model demonstrated substantial performance improvements post-fine-tuning, achieving a Dice coefficient and Jaccard index of 89.34% and 80.73%, respectively (
Figure 3). Training and validation performance metrics, including Dice coefficient and Jaccard index over epochs, are shown in
Figure 4, confirming the model’s learning stability and convergence. Visual evaluation confirmed accurate delineation of both primary and finer vessels, effectively resolving previous segmentation inaccuracies. This confirms the importance of species-specific AI model adjustments in veterinary medical imaging.
3.3. Clinical Biomarker Extraction and Analysis
Following successful segmentation, five clinically validated biomarkers indicative of cardiovascular health—vessel density, bifurcation angle, tortuosity, branching density, and fractal dimension—were quantitatively extracted (
Figure 5). These biomarkers have robust clinical validation in human cardiovascular research and growing recognition in veterinary studies.
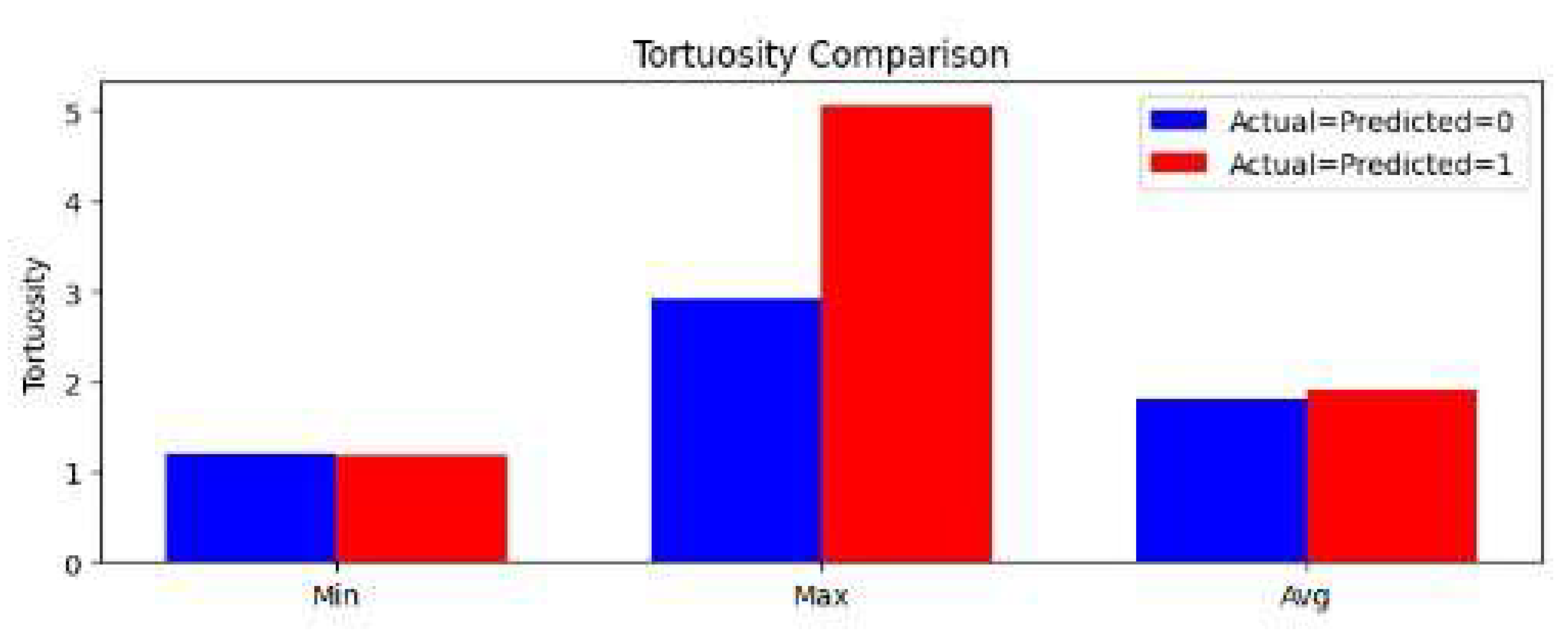
Statistical analyses revealed pronounced differences between CVD-affected and healthy cattle, particularly highlighting retinal vessel tortuosity (
Figure 6). CVD-affected cattle consistently presented higher maximum tortuosity, reinforcing its potential as a significant biomarker for cardiovascular pathology. This finding aligns with human cardiovascular studies that link increased vessel tortuosity to systemic vascular disorders, emphasizing its clinical relevance.
Other biomarkers, including fractal dimension and bifurcation angles, also displayed significant differences, underscoring reduced vascular complexity and narrower angles in diseased animals, respectively. These findings provide comprehensive support for using retinal vascular biomarkers as non-invasive diagnostic tools in veterinary cardiovascular assessments.
3.4. Direct Deep Learning Classification Performance
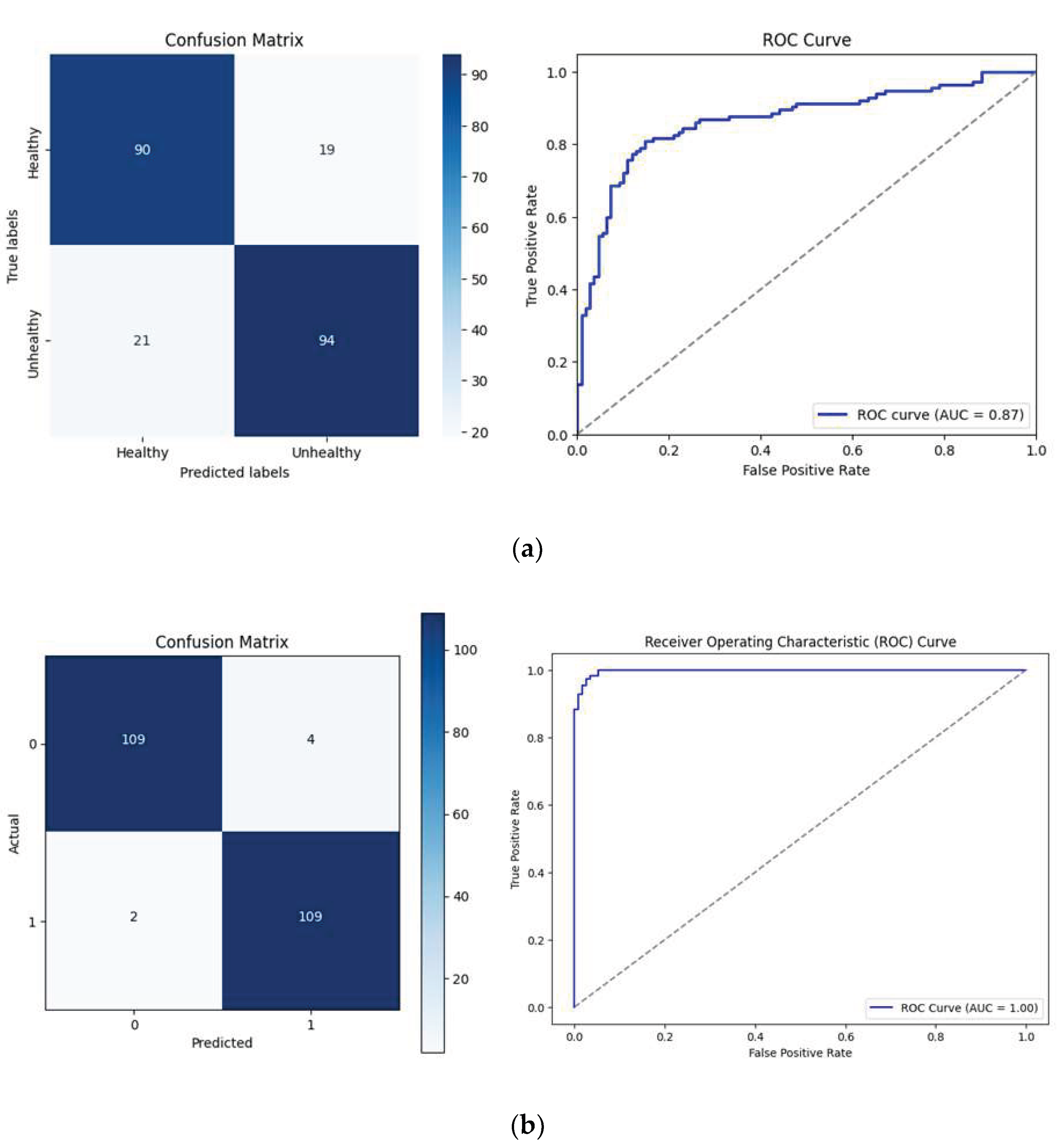
The feasibility of direct deep learning-based classification was evaluated using several prominent CNN architectures - ResNet18, VGG16, DenseNet121, EfficientNetB0, and MobileNetV2. Among these, ResNet18 performed best, achieving 82.14% accuracy (
Figure 6). Nevertheless, the diagnostic performance was limited by notable false positives and negatives, highlighting inherent limitations of standalone deep learning models. The "black-box" nature of purely deep learning-based approaches further limits clinical interpretability, presenting a significant barrier to veterinary adoption due to a lack of transparency and explanatory power in model predictions.
3.5. Enhanced Diagnostic Accuracy with Hybrid Classification
To overcome the limitations of direct CNN approaches, a hybrid multilayer perceptron (MLP) classifier was developed, integrating deep learning features from ResNet18 and the five clinical biomarkers (
Figure 7). This integration significantly elevated diagnostic performance, achieving an overall accuracy of 97.32% and sensitivity of 98.2%, as demonstrated by confusion matrix and ROC analyses (
Figure 7). The hybrid approach substantially reduced false negatives and enhanced diagnostic reliability. Beyond improved accuracy, the hybrid classifier provided critical interpretability advantages by incorporating biologically meaningful biomarkers, thus offering veterinary clinicians transparent, actionable insights for clinical decision-making.
Figure 8 illustrates the architecture of the hybrid multilayer perceptron model, showing the integration of 512 deep learning features from ResNet18 with five vascular biomarkers
3.6. Clinical and Practical Veterinary Implications
The hybrid AI framework's ability to non-invasively and accurately detect bovine cardiovascular diseases has significant implications for veterinary practice, potentially revolutionizing cattle health management. Early diagnosis facilitates prompt interventions, dramatically improving herd health, productivity, animal welfare, and economic sustainability. Practically, the model’s non-invasive nature and scalability render it ideal for extensive herd screenings, circumventing the constraints and high costs of traditional diagnostic methods. Its rapid, on-site diagnostic capability democratizes access to advanced diagnostics, especially in resource-limited agricultural settings. Economically, early diagnosis and intervention significantly reduce late-cycle feedlot losses and associated resource wastage, aligning closely with sustainability objectives and responsible agricultural practices.
4. Discussions
4.1. Limitations and Future Research Directions
Despite the encouraging outcomes, several limitations require attention. Foremost, the cattle retinal image dataset, although carefully annotated and augmented, remains relatively limited in scale and diversity. This constrains the model’s ability to generalize across varied breeds, geographical environments, and diverse disease phenotypes. Future research should aim to substantially expand the dataset, incorporating a broader representation of cattle breeds, multiple geographic and climatic conditions, and a variety of cardiovascular disease phenotypes. This approach will facilitate the development of more universally applicable and robust diagnostic models. Additionally, further enrichment of the diagnostic framework can be achieved by integrating additional biomarkers such as artery-to-vein ratios, retinal surface abnormalities, and potential indicators of inflammation or edema. These supplementary biomarkers may provide complementary insights, enhancing the diagnostic precision of retinal imaging. Moreover, incorporating genomic analyses, physiological monitoring through wearable biosensors, and biochemical assays could yield a multifaceted and comprehensive approach to cardiovascular risk stratification, significantly enhancing the precision and personalization of veterinary cardiovascular diagnostics.
4.2. Comparative Analysis of Veterinary Diagnostics
The AI-driven retinal imaging method introduced in this study presents significant advantages over traditional veterinary diagnostic tools such as echocardiography, thoracic radiography, and biochemical assays. Conventional methods are often invasive, resource-intensive, costly, and dependent on specialized equipment and expert personnel. In contrast, the retinal imaging-based AI method described here offers rapid, non-invasive, scalable herd-level screening, eliminating the dependency on expensive equipment and highly specialized veterinary professionals. Real-time diagnostic capabilities are particularly transformative, enabling immediate, actionable clinical insights necessary for timely interventions and proactive herd management. The integration of clinically relevant biomarkers significantly enhances the interpretability of the diagnostic outcomes, addressing common veterinary concerns surrounding opaque, "black-box" AI approaches. Thus, the presented method not only improves diagnostic accuracy but also fosters clinical adoption by bridging the gap between technological innovation and practical veterinary application, empowering veterinarians with reliable and transparent diagnostic tools.
4.3. Broader Veterinary AI Applications
This study underscores the broader potential and applicability of AI-driven diagnostic solutions in veterinary medicine. By successfully adapting retinal imaging techniques from human medicine to veterinary use, the research sets an important precedent for cross-species translational diagnostics. This methodological innovation provides a valuable blueprint for future veterinary diagnostic tool developments, promoting interdisciplinary collaboration between veterinary practitioners, biomedical researchers, and computer scientists. Additionally, public release and sharing of the annotated cattle retinal datasets could catalyze broader comparative evolutionary studies and stimulate collaborative research initiatives across different disciplines. This openness can accelerate the discovery of novel insights into cardiovascular diseases and other systemic conditions across mammalian species, thus enhancing our understanding of disease mechanisms and supporting the development of universally applicable diagnostic technologies.
4.4. Critical Insights and Future Prospects
The research presented significantly advances veterinary diagnostic technologies and highlights the transformative role of AI integration in veterinary practice. By establishing retinal vascular biomarkers as reliable, non-invasive systemic health indicators, the study challenges conventional diagnostic paradigms and emphasizes the importance of translational and interdisciplinary research. The hybrid AI framework developed in this study exemplifies the powerful potential of technology as an enhancer, rather than a replacer, of veterinary expertise. By providing transparent, interpretable diagnostic outcomes, this framework empowers veterinary practitioners with meaningful insights, thus maintaining and reinforcing the centrality of professional veterinary judgment in clinical decision-making. Future prospects include integrating AI with wearable health-monitoring sensors, telemedicine solutions, and mobile diagnostic platforms, which promise significant advancements in real-time precision livestock health management. These technological advancements could enable veterinarians to remotely monitor animal health status continuously, detect early signs of disease, and implement timely interventions, thereby improving animal welfare, productivity, and overall sustainability of livestock operations. This vision represents a dynamic evolution in veterinary practice, one driven by technology, yet deeply grounded in clinical expertise and ethical responsibility.
5. Conclusions
This study presents an innovative approach to diagnosing bovine cardiovascular disease (CVD), integrating retinal imaging, advanced deep learning, and clinically meaningful biomarkers, effectively addressing current diagnostic limitations in veterinary medicine. The research pioneers the first cattle-specific hybrid artificial intelligence (AI) framework, offering a robust, non-invasive, and scalable diagnostic tool with significant implications for animal welfare, sustainability in agriculture, and economic viability.
Our tailored U-Net model addresses the unique anatomical features of bovine retinas. Initial trials using human-trained retinal imaging models demonstrated substantial interspecies anatomical differences, particularly in central vessel morphology and branching patterns. Recognizing the necessity for species-specific adaptation, meticulous manual annotation and fine-tuning on cattle-specific datasets significantly improved segmentation accuracy, achieving a high Dice coefficient (89.34%) and Jaccard index (80.73%), highlighting the critical importance of customized AI applications in veterinary diagnostics.
The study further extracted and analyzed five key vascular biomarkers—vessel density, bifurcation angle, tortuosity, branching density, and fractal dimension—from segmented retinal images. Vessel tortuosity emerged as notably discriminative, aligning closely with human cardiovascular research, where increased retinal tortuosity correlates strongly with systemic vascular disease. This cross-species consistency underlines the universal relevance of retinal biomarkers and advances veterinary medicine within the broader scope of translational research.
The hybrid classification model, integrating deep learning features from ResNet18 and validated clinical biomarkers, significantly improved diagnostic accuracy to 97.32% and sensitivity to 98.2%. This combined approach outperformed traditional deep learning classifiers and effectively addressed the interpretability challenge prevalent in medical AI, offering transparent and actionable insights for veterinarians, thereby enhancing trust, clinical adoption, and practical deployment.
Clinically and economically, the benefits of this diagnostic framework are substantial. Early and precise detection of bovine CVD enables timely interventions, significantly reducing herd mortality, animal distress, and economic losses. By identifying cardiovascular risks at earlier stages, our approach potentially reduces economic losses by up to 68% during late cattle feeding cycles, equating to annual savings of approximately $1.2 billion across U.S. cattle operations. Moreover, proactive interventions decrease unnecessary resource consumption, aligning closely with sustainability goals by reducing greenhouse gas emissions from agriculture.
Despite promising results, this study acknowledges certain limitations, including the need for expanded and diverse datasets to enhance model generalizability across different breeds and geographic conditions. Future research should incorporate additional biomarkers such as artery-to-vein ratios and retinal surface abnormalities, and explore multimodal diagnostic approaches integrating genomic, biochemical, and physiological data. Such comprehensive, precision-based strategies would further strengthen veterinary cardiovascular diagnostics.
In conclusion, this research not only delivers a practical, interpretable AI-driven diagnostic tool but significantly advances AI integration into veterinary practice. By validating retinal vascular biomarkers as reliable indicators of systemic cardiovascular health in cattle, the study bridges precision medicine and livestock management, setting new benchmarks in veterinary cardiology. Amidst growing pressures from climate change, economic demands, and ethical animal welfare concerns, our framework presents a scalable, effective, and ethically responsible solution for proactive livestock health management, exemplifying the future of veterinary diagnostics where technology, interpretability, and clinical expertise converge.
Author Contributions
Conceptualization, S.N.; methodology, C.S.; N.R.; S.C.; G.V.; validation, C.S., N.R. and S.C.; formal analysis, C.S.; investigation, S.C.; resources, S.N.; data curation, X.X.; writing—original draft preparation, C.S.; writing—review and editing, S.C.; S.N.; visualization, S.C.; supervision, G.V.; project administration, S.N.; funding acquisition, S.N. All authors have read and agreed to the published version of the manuscript.
Funding
The authors sincerely thank the Natural Sciences and Engineering Research Council of Canada for funding this study.
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CVD |
Cardiovascular disease |
| AI |
Artificial intelligence |
| BCHF |
Bovine congestive heart failure |
| CNN |
Convolutional neural network |
| MLP |
Multilayer perceptron |
| AUC-ROC |
Area under the receiver operating characteristic curve |
References
- Buchanan, J.W.; Flagel, L.E.; MacNeil, M.D.; Nilles, A.R.; Hoff, J.L.; Pickrell, J.K.; Raymond, R.C. Variance component estimates, phenotypic characterization, and genetic evaluation of bovine congestive heart failure in commercial feeder cattle. Frontiers in Genetics 2023, 14, 1148301. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.T.; Amrine, D.E.; Larson, R.L.; Weaber, R.L.; White, B.J. Retrospective analysis of cohort risk factors and feeding phase timing associated with noninfectious heart disease deaths in US feedlot cattle. Translational Animal Science 2021, 5, txab220. [Google Scholar] [CrossRef] [PubMed]
- Moxley, R.A.; Smith, D.R.; Grotelueschen, D.M.; Edwards, T.; Steffen, D.J. Investigation of congestive heart failure in beef cattle in a feedyard at a moderate altitude in western Nebraska. Journal of Veterinary Diagnostic Investigation 2019, 31, 509–522. [Google Scholar] [CrossRef]
- Kukor, I. (2022). Feedlot heart disease: Understanding heart score and its relationships to economically relevant traits. Colorado State University.
- US Meat Animal Research Center. (n.d.). BCHF - Bovine Congestive Heart Failure. U.S. Department of Agriculture. https://www.ars.usda.gov/plains-area/clay-center-ne/marc/bchf/bchf-main/.
- Stern, J. (Ed.). (2023). Advancements in Companion Animal Cardiology, An Issue of Veterinary Clinics of North America: Small Animal Practice (Vol. 53, No. 6). Elsevier Health Sciences. Stern, J. (Ed.).
- Varshney, J.P. (2020). Electrocardiography in veterinary medicine. Springer Singapore.
- Kaffas, A.E.; Vo-Phamhi, J.M.; Griffin, J.F.I.V.; Hoyt, K. Critical advances for democratizing ultrasound diagnostics in human and veterinary medicine. Annual Review of Biomedical Engineering 2024, 26. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Kumar, A.; Singh, N. An overview on the diagnostic and therapeutic aspects of cardiac diseases in bovine. Journal of Entomology and Zoology Studies 2019, 7, 855–863. [Google Scholar]
- Feitoza, L.F.; White, B.J.; Larson, R.L. Thoracic ultrasound in cattle: Methods, diagnostics, and prognostics. Veterinary Sciences 2025, 12, 16. [Google Scholar] [CrossRef]
- Hennessey, E.; DiFazio, M.; Hennessey, R.; Cassel, N. Artificial intelligence in veterinary diagnostic imaging: A literature review. Veterinary Radiology & Ultrasound 2022, 63, 851–870. [Google Scholar]
- Heaton, M.P.; Harhay, G.P.; Bassett, A.S.; Clark, H.J.; Carlson, J.M.; Jobman, E.E.; Sadd, H.R.; Pelster, M.C.; Workman, A.M.; Kuehn, L.A.; Kalbfleisch, T.S. Association of ARRDC3 and NFIA variants with bovine congestive heart failure in feedlot cattle. F1000Research 2024, 11, 385. [Google Scholar] [CrossRef]
- Grandin, T. Problems with congestive heart failure and lameness that have increased in grain-fed steers and heifers. Animals 2024, 14, 2824. [Google Scholar] [CrossRef]
- Freebern, E. (2022). The genetic architecture of complex traits and diseases in dairy cattle (Doctoral dissertation, University of Maryland, College Park).
- Ghenciu, L.A.; Dima, M.; Stoicescu, E.R.; Iacob, R.; Boru, C.; Hațegan, O.A. Retinal imaging-based oculomics: Artificial intelligence as a tool in the diagnosis of cardiovascular and metabolic diseases. Biomedicines 2024, 12, 2150. [Google Scholar] [CrossRef]
- Arnould, L.; Meriaudeau, F.; Guenancia, C.; Germanese, C.; Delcourt, C.; Kawasaki, R.; Cheung, C.Y.; Creuzot-Garcher, C.; Grzybowski, A. Using artificial intelligence to analyse the retinal vascular network: The future of cardiovascular risk assessment based on oculomics? A narrative review. Ophthalmology and Therapy 2023, 12, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cheung, C.Y.; Li, D.; Tham, Y.C.; Sheng, B.; Cheng, C.Y.; Wang, Y.X.; Wong, T.Y. AI-integrated ocular imaging for predicting cardiovascular disease: Advancements and future outlook. Eye 2024, 38, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Bagardi, M.; Locatelli, C.; Zanaboni, A.; Galizzi, A.; Malchiodi, D.; Brambilla, P.G. Multiple retrospective analysis of survival and evaluation of cardiac death predictors in a population of dogs affected by degenerative mitral valve disease in ACVIM class C treated with different therapeutic protocols. Polish Journal of Veterinary Sciences 2021. [Google Scholar] [CrossRef] [PubMed]
- Ranchod, T.M. Systemic retinal biomarkers. Current Opinion in Ophthalmology 2021, 32, 439–444. [Google Scholar] [CrossRef]
- Cihan, P.; Saygılı, A.; Ermutlu, C.Ş.; Aydın, U.; Aksoy, Ö. AI-aided cardiovascular disease diagnosis in cattle from retinal images: Machine learning vs. deep learning models. Computers and Electronics in Agriculture 2024, 226, 109391. [Google Scholar] [CrossRef]
- Cihan, P.; Saygılı, A.; Akyüzlü, M.; Özmen, N.E.; Ermutlu, C.Ş.; Aydın, U.; Yılmaz, A.; Aksoy, Ö. Performance of machine learning methods for cattle identification and recognition from retinal images. Applied Intelligence 2025, 55, 1–20. [Google Scholar] [CrossRef]
- Li, N.; Kondo, N.; Ogawa, Y.; Shiraga, K.; Shibasaki, M.; Pinna, D.; Fukushima, M.; Nagaoka, S.; Fujiura, T.; De, X.; Suzuki, T. Fundus camera-based precision monitoring of blood vitamin A level for Wagyu cattle using deep learning. Scientific Reports 2025, 15, 4125. [Google Scholar] [CrossRef]
- Ertaş, T.D.; Kahvecioğlu, K.O.; Erdoğan, S. Morphological aspects and microscopic analyses of fibrous tunic and uveal components in bovine eye. Microscopy and Microanalysis 2022, 28, 1794–1807. [Google Scholar] [CrossRef]
- Dubielzig, R.R.; Ketring, K.L.; McLellan, G.J.; Albert, D.M. (2010). Veterinary ocular pathology: A comparative review.
- Wong, D.Y.; Lam, M.C.; Ran, A.; Cheung, C.Y. Artificial intelligence in retinal imaging for cardiovascular disease prediction: Current trends and future directions. Current Opinion in Ophthalmology 2022, 33, 440–446. [Google Scholar] [CrossRef]
- Tseng, R.M.W.W.; Rim, T.H.; Cheung, C.Y.; Wong, T.Y. (2021). Artificial intelligence using the eye as a biomarker of systemic risk. In Artificial Intelligence in Ophthalmology (pp. 243–255).
- Rezk, N.G.; Alshathri, S.; Sayed, A.; El-Din Hemdan, E.; El-Behery, H. XAI-augmented voting ensemble models for heart disease prediction: A SHAP and LIME-based approach. Bioengineering 2024, 11, 1016. [Google Scholar] [CrossRef]
- Samaras, A.D.; Moustakidis, S.; Apostolopoulos, I.D.; Papageorgiou, E.; Papandrianos, N. Uncovering the black box of coronary artery disease diagnosis: The significance of explainability in predictive models. Applied Sciences 2023, 13, 8120. [Google Scholar] [CrossRef]
- Murray, O.N.; Liu, T.; Zhou, Y.; Williamson, D.; Struyven, R.; Wagner, S.; Balaskas, K.; Borja, M.C.; Petzold, A.; Rahi, J.; Denniston, A.K. Associations between COPD and retinal vessel density, fractal dimension, and tortuosity density. Investigative Ophthalmology & Visual Science 2023, 64, 249–249. [Google Scholar]
- Chew, E.Y.; Burns, S.A.; Abraham, A.G.; Bakhoum, M.F.; Beckman, J.A.; Chui, T.Y.; Finger, R.P.; Frangi, A.F.; Gottesman, R.F.; Grant, M.B.; Hanssen, H. Standardization and clinical applications of retinal imaging biomarkers for cardiovascular disease: A roadmap from an NHLBI workshop. Nature Reviews Cardiology 2025, 22, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.W. (2022). Retina blood vessel dataset [Data set]. Kaggle. https://www.kaggle.com/datasets/abdallahwagih/retina-blood-vessel.
Figure 1.
Workflow for AI-Based Cardiovascular Disease Detection in Cattle. Schematic overview illustrating the comprehensive artificial intelligence pipeline, encompassing retinal image acquisition, vessel segmentation, vascular biomarker extraction, and hybrid deep learning classification to facilitate early cardiovascular disease diagnosis in cattle.
Figure 1.
Workflow for AI-Based Cardiovascular Disease Detection in Cattle. Schematic overview illustrating the comprehensive artificial intelligence pipeline, encompassing retinal image acquisition, vessel segmentation, vascular biomarker extraction, and hybrid deep learning classification to facilitate early cardiovascular disease diagnosis in cattle.
Figure 2.
(a) Sample images from the Human dataset (left) and the Cattle dataset (right); (b) Initial Retinal Vessel Segmentation Performance on Cattle Retinal Images. Comparison of cattle retinal fundus images with corresponding segmentation masks generated by the U-Net model initially trained solely on human retinal data, demonstrating inadequate segmentation performance due to anatomical differences between human and bovine retinas.
Figure 2.
(a) Sample images from the Human dataset (left) and the Cattle dataset (right); (b) Initial Retinal Vessel Segmentation Performance on Cattle Retinal Images. Comparison of cattle retinal fundus images with corresponding segmentation masks generated by the U-Net model initially trained solely on human retinal data, demonstrating inadequate segmentation performance due to anatomical differences between human and bovine retinas.
Figure 3.
Improved Retinal Vessel Segmentation Performance after Model Fine-Tuning. Visual comparison highlighting significant improvement in segmentation accuracy following fine-tuning of the U-Net model on manually segmented, cattle-specific retinal datasets, emphasizing enhanced delineation of the central retinal vessel and finer vascular structures.
Figure 3.
Improved Retinal Vessel Segmentation Performance after Model Fine-Tuning. Visual comparison highlighting significant improvement in segmentation accuracy following fine-tuning of the U-Net model on manually segmented, cattle-specific retinal datasets, emphasizing enhanced delineation of the central retinal vessel and finer vascular structures.
Figure 4.
Training and Validation Metrics for the Fine-Tuned U-Net Segmentation Model on Cattle Data Graphs depicting the progression of Dice coefficient and Jaccard index values over training epochs during fine-tuning of the U-Net segmentation model on the cattle-specific retinal dataset, confirming substantial improvements and robustness of the final segmentation performance.
Figure 4.
Training and Validation Metrics for the Fine-Tuned U-Net Segmentation Model on Cattle Data Graphs depicting the progression of Dice coefficient and Jaccard index values over training epochs during fine-tuning of the U-Net segmentation model on the cattle-specific retinal dataset, confirming substantial improvements and robustness of the final segmentation performance.
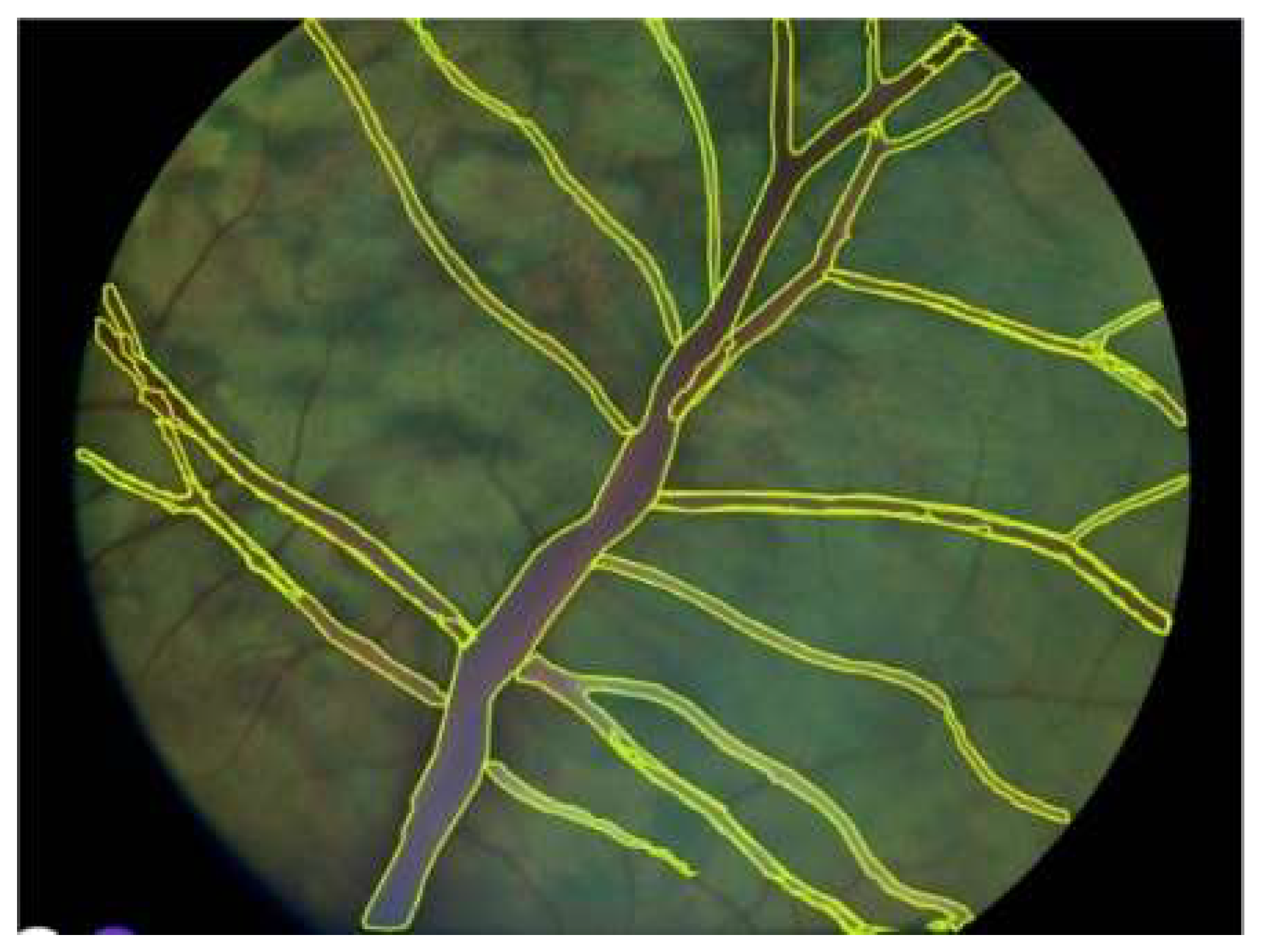
Figure 5.
Quantitative Vascular Biomarker Extraction from a Segmented Cattle Retinal Image. An illustrative example showing automated extraction of clinically relevant vascular biomarkers, including vessel density, bifurcation angles, tortuosity, branching density, and fractal dimension. Segmented vessels are overlaid in yellow on the original cattle retinal image.
Figure 5.
Quantitative Vascular Biomarker Extraction from a Segmented Cattle Retinal Image. An illustrative example showing automated extraction of clinically relevant vascular biomarkers, including vessel density, bifurcation angles, tortuosity, branching density, and fractal dimension. Segmented vessels are overlaid in yellow on the original cattle retinal image.
Figure 6.
Comparative Analysis of Retinal Vessel Tortuosity in Healthy versus CVD-Affected Cattle. Box plot representation comparing the minimum, maximum, and average vessel tortuosity values between healthy and cardiovascular disease-affected cattle, highlighting tortuosity as a significant and discriminative biomarker for CVD.
Figure 6.
Comparative Analysis of Retinal Vessel Tortuosity in Healthy versus CVD-Affected Cattle. Box plot representation comparing the minimum, maximum, and average vessel tortuosity values between healthy and cardiovascular disease-affected cattle, highlighting tortuosity as a significant and discriminative biomarker for CVD.
Figure 7.
Diagnostic Performance of Direct Deep Learning (ResNet18) (a) versus Hybrid Classification Models. Confusion matrices and receiver operating characteristic (ROC) curves contrasting the diagnostic accuracy of the standalone ResNet18 model with the hybrid multilayer perceptron classifier; (b) integrating deep learning features and clinically validated biomarkers, demonstrating superior accuracy and interpretability of the hybrid approach.
Figure 7.
Diagnostic Performance of Direct Deep Learning (ResNet18) (a) versus Hybrid Classification Models. Confusion matrices and receiver operating characteristic (ROC) curves contrasting the diagnostic accuracy of the standalone ResNet18 model with the hybrid multilayer perceptron classifier; (b) integrating deep learning features and clinically validated biomarkers, demonstrating superior accuracy and interpretability of the hybrid approach.
Figure 8.
Hybrid Classification Model Architecture with Integrated Biomarkers and Deep Learning Features Detailed schematic illustrating the hybrid multilayer perceptron classification model, emphasizing the integration of 512-dimensional deep learning features from ResNet18 with five quantitative vascular biomarkers, facilitating enhanced diagnostic accuracy and clinical interpretability.
Figure 8.
Hybrid Classification Model Architecture with Integrated Biomarkers and Deep Learning Features Detailed schematic illustrating the hybrid multilayer perceptron classification model, emphasizing the integration of 512-dimensional deep learning features from ResNet18 with five quantitative vascular biomarkers, facilitating enhanced diagnostic accuracy and clinical interpretability.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).