1. Introduction
Persistent ductus arteriosus (PDA) is a common disorder in newborns and a significant predictor of morbidity and mortality. Several studies have been conducted to identify potential factors associated with delayed ductal closure in term newborns [
1], but reports of maternal factors associated with early PDA in newborns are limited [
2,
3].
The ductus arteriosus (DA) is a fetal structure that connects the main pulmonary artery and the aorta. Its main function is to shunt blood flow away from the pulmonary circulation, given that the vascular resistance in the pulmonary circulation is high, and toward the aorta, closer to the peripheral organs with lower vascular resistance. To provide flow-dependent vasodilation capability and the large variability in postnatal shunt size necessary for adapting to the systemic vascular resistance, the DA's structure is characterized by a uniformly developed middle layer of longitudinal smooth muscle cells, which acquire the capability of constriction after birth [
4]. In the human fetus, the previously described DA function results in left-to-right shunting of oxygenated blood from the aortic arch to the main pulmonary artery. After birth, the basic switch from a shunt to a reentry system and the consequent closure of the DA lead to right-to-left shunting of deoxygenated blood from the main pulmonary artery to the aorta, thereby increasing the amount of oxygenated blood sent toward the body [
5].
Traditionally, there is a tendency to treat neonatal PDA, and attention is urgently required. However, those treated pharmacologically alone are limited and there are more complicated outcomes [
6]. Compared to mothers with pathologic pregnancy, the risk of PDA is also higher for these newborns. However, there are few studies of PDA association with pathologic pregnancy such as non-infectious inflammatory diseases and autoimmune-related diseases [
7,
8]. Although the outcome is neonatal hypoxia, the mother's illness has not been relevant. Focusing on the fact that the fetus responds to maternal symptoms of hypoxia, the fetus of pathologic pregnancy, including maternal trauma, may exacerbate fetal damage by maternal hypoxia [
9].
Maternal hypertension impacts fetal circulatory development, potentially resulting in hypertrophy of left-heart structures and affecting the risk of patent ductus arteriosus due to modified blood flow and vascular resistance [
10,
11,
12]. Also, it is known that gestational diabetes and type 1 diabetes in pregnancy are linked to hyperglycemia, which may affect the cardiac development of neonates, raising the risk of PDA in offspring [
13,
14]. Maternal thyroid dysfunction, especially hypothyroidism, may impair fetal vascular physiology [
15], potentially postponing ductus arteriosus closure and elevating the risk of patent ductus arteriosus due to heightened prostaglandin levels and alterations in vascular resistance [
16,
17]. Other potential maternal contributing factors to PDA include infections, particularly urinary tract infections during pregnancy [
18,
19], but also viral infections during pregnacy, and maternal anemia, specifically iron deficiency anaemia [
20,
21,
22,
23].
Machine learning enables machines to learn from a training dataset and predict outcomes for new data. It uses statistical theory to build mathematical models, aiming to derive inferences from samples [
24]. Decision trees are classification techniques in data mining and machine learning, using a straightforward algorithm easily understood by researchers. As white-box models, they offer non-parametric flexibility, handle heterogeneous data, and classify sequential data without needing normalized features. They also require less execution time for classification than other methods [
25]. The Random Forest (RF) predictive model, based on decision trees, is a behavioral analysis tool that manages extensive data sets from modern supply chain operations, especially in healthcare [
26]. The RF model is highly precise among common data classification algorithms and can handle extensive datasets with specific parameters. It efficiently manages diverse variables, making it suitable for complex tasks like health supply chain management. If a class is less prevalent, datasets can be automatically balanced [
27]. The Extreme Gradient Boosting (XGBoost) model is prominent in machine learning for its generalizability, low overfitting risk, and high interpretability. It excels in predictive medicine tasks using tabular data like electronic health records and is used in critical care scenarios [
28].
The study seeks to identify critical predictive features and evaluate the relative efficacy of algorithms by analysing an extensive dataset of maternal and neonatal health indicators, focussing on accuracy, sensitivity, specificity, and additional diagnostic metrics. The primary objective is to develop a dependable predictive instrument to aid healthcare professionals in the early detection of neonates at elevated risk for PDA, thereby enabling prompt interventions and enhancing neonatal care outcomes.
2. Materials and Methods
A retrospective analysis was conducted based on a cohort of both preterm and term newborns who were hospitalized in the Neonatal Intensive Care Unit (NICU) in the Clinical Hospital of Obstetrics and Gynecology “Prof. Dr. Panait Sârbu” of Bucharest, Romania. We included all newborns admitted to NICU between 01.01.2021 to 31.12.2023, over a period of three years. Ethical approval was obtained from the Research Ethics Committee of the Clinical Hospital of Obstetrics and Gynecology “Prof. Dr. Panait Sârbu” of Bucharest, no. 16516/11.12.2024 The dataset included a total of 201 cases with detailed maternal and neonatal variables.
Inclusion criteria consisted of neonates born at the Clinical Hospital of Obstetrics and Gynecology “Prof. Dr. Panait Sârbu” and admitted to NICU within the study period along with documented maternal health data. Exclusion criteria were cases with missing critical data, such as gestational age, birth weight, APGAR score, maternal pathology or medication use during pregnancy.
2.1. Variables and Data Preprocessing
Data were preprocessed to convert categorical variables into a suitable format for analysis. Key predictor variables included maternal age, prenatal care level, specific maternal pathologies such as hypertension, diabetes, infections, and medications taken during pregnancy (antibiotics, antihypertensives, steroids). Neonatal outcomes included PDA diagnosis as the primary target variable (binary variable indicating presence or absence of PDA). For predictor variables we included maternal and medication variables, as follows:
Maternal Pathologies: Conditions such as hypertension, diabetes, anemia, infections (e.g., GBS, SARS-CoV-2), and pregnancy complications (e.g., preeclampsia, prolonged rupture of membranes).
Medication During Pregnancy: Administration of medications including aspirin, nifedipine, antibiotics, and insulin.
Missing values were handled using the imputation method, and outliers were reviewed to ensure data consistency.
2.2. Statistical Analysis
We initially performed univariate analyses employing logistic regression and chi-square tests to investigate the correlation between maternal factors and the risk of neonatal PDA.
2.3. Machine Learning Models
A Random Forest model was initially created to assess the significance of maternal and medication variables in predicting PDA. The model was trained using 500 trees, employing a subset of features at each split. Feature importance scores were extracted from the RF model to determine the most significant predictors. Subsequent to the Random Forest model, XGBoost was employed to augment predictive precision and assess the impact of each predictor on PDA risk. The parameters of XGBoost, such as learning rate, maximum depth, and subsampling rate, were optimised through cross-validation to mitigate overfitting. The employed objective function was logistic regression for binary classification, utilising the area under the ROC curve (AUC) as the principal evaluation metric.
2.4. Model Training and Evaluation
The dataset was divided into training (70%) and testing (30%) subsets applying stratified sampling to preserve the ratio of PDA cases. Cross-validation was conducted within the training set to optimise model parameters and assess model stability. The model's performance was assessed on the test set utilising accuracy, sensitivity, specificity, and AUC. Confusion matrices were created to illustrate model performance regarding accurately and inaccurately classified instances. Hyperparameters for both RF and XGBoost were optimised through a grid search employing 5-fold cross-validation to enhance AUC while balancing sensitivity and specificity.
2.5. Software and Tools
The study was conducted using R Studio (version 2023.09.1+494 for Mac) with packages caret, xgboost, and randomForest for machine learning modeling. Data preprocessing and visualization were performed using dplyr and ggplot2. The stats library was utilised for logistic regression (glm function) to compute odds ratios and p-values, whereas the MASS library was employed for determining confidence intervals for odds ratios. Furthermore, chi-square tests were conducted utilising the chisq.test function within the stats library to assess categorical associations between maternal variables and the presence of PDA. The dplyr and tidyverse libraries enabled data cleaning and preparation, ensuring precise and efficient data manipulation during the study.
3. Results
The study population consisted of 201 neonates admitted to the NICU during the specified period. Maternal conditions included urinary tract infections, chorioamnionitis, positive endocervical cultures, diabetes, pregnancy induced hypertension, anemia, prolonged rupture of membranes (>18 hours), thyroid disorders, viral infections, and other conditions. The principal pathologies are detailed in
Table 1, along with their statistical significance. 89.1% of mothers exhibited at least one pathology during pregnancy, with 95.52% originating from supervised pregnancies (44.28% receiving basic care and 51.24% receiving comprehensive care), while a mere 4.48% were unmonitored pregnancies.
Maternal pathologies, notably prolonged rupture of membranes (PROM), show a strong correlation with increased PDA risk (Odds Ratio: 13.03, p<0.001). This suggests PROM may affect fetal circulatory dynamics, raising PDA risk. Hypertension and diabetes showed moderate, non-significant correlations with PDA; thyroid conditions had no impact. Anemia and IVF showed important odds but had non-significant associations. Medications like enoxaparin and amoxicillin with clavulanic acid slightly increased PDA odds without statistical significance. Methyldopa and cefuroxime didn't significantly affect PDA odds. Prenatal care didn't influence PDA risk significantly, although high-risk cases might increase its likelihood.
Our analysis confirmed that gestational age and low birthweight significantly predict patent ductus arteriosus (PDA) risk in neonates (
Table 2). Each additional week of gestation reduced PDA probability, highlighting preterm infants' susceptibility due to underdeveloped circulatory systems. Lower birthweight was linked to higher PDA risk. The maternal ages in our cohort ranged from 19 to 42 years, while gestational ages ranged from 28 to 41 weeks. Birth weights varied from 1,200 to 4,200 grams.
3.1. Machine Learning Analysis for PDA Prediction
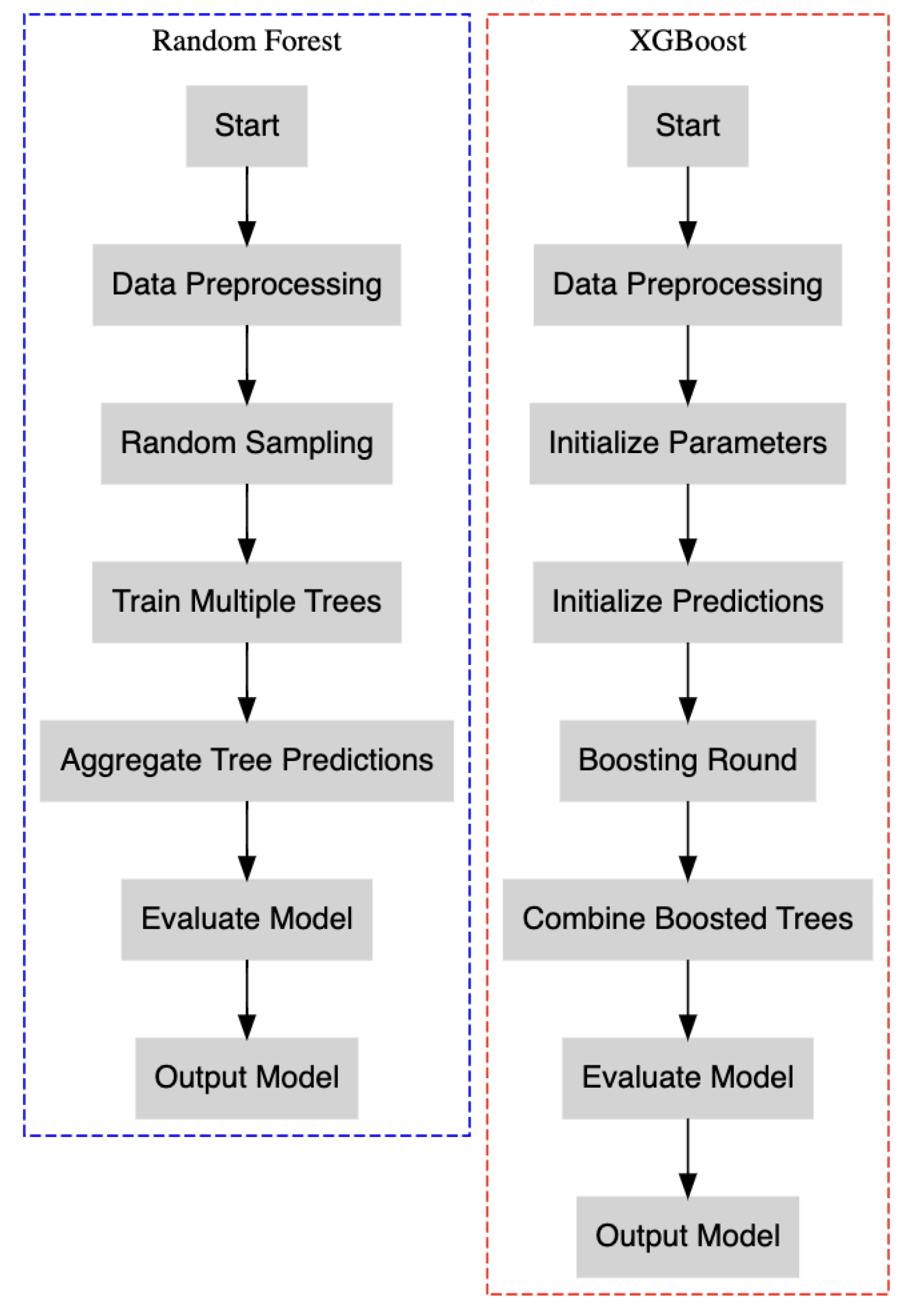
To improve our predictive modelling of PDA risk, we expanded our analysis utilising machine learning algorithms: Random Forest and XGBoost (
Figure 1). These models were selected for their robustness in managing complex, nonlinear interactions and their effectiveness in identifying critical features associated with PDA risk.
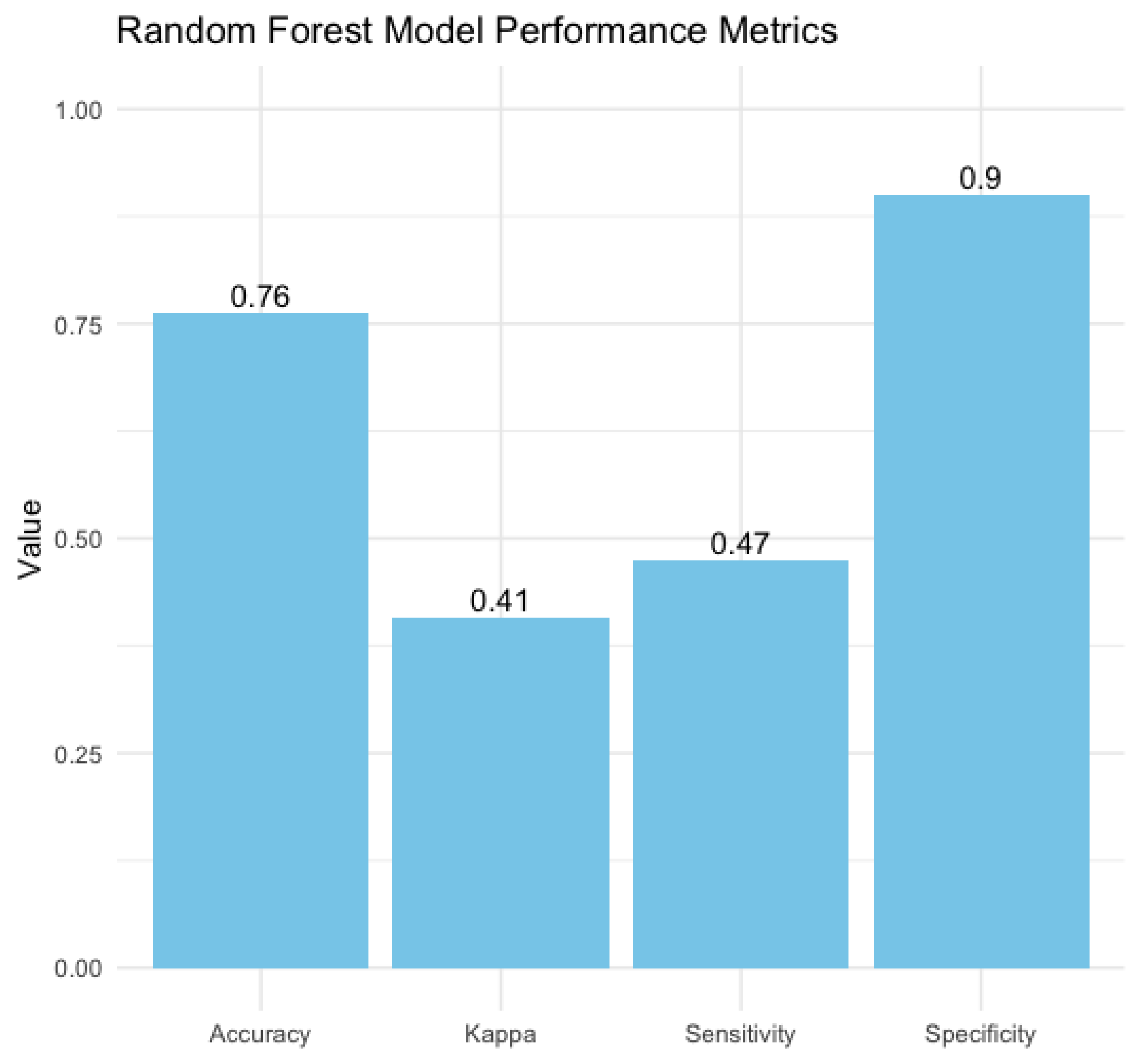
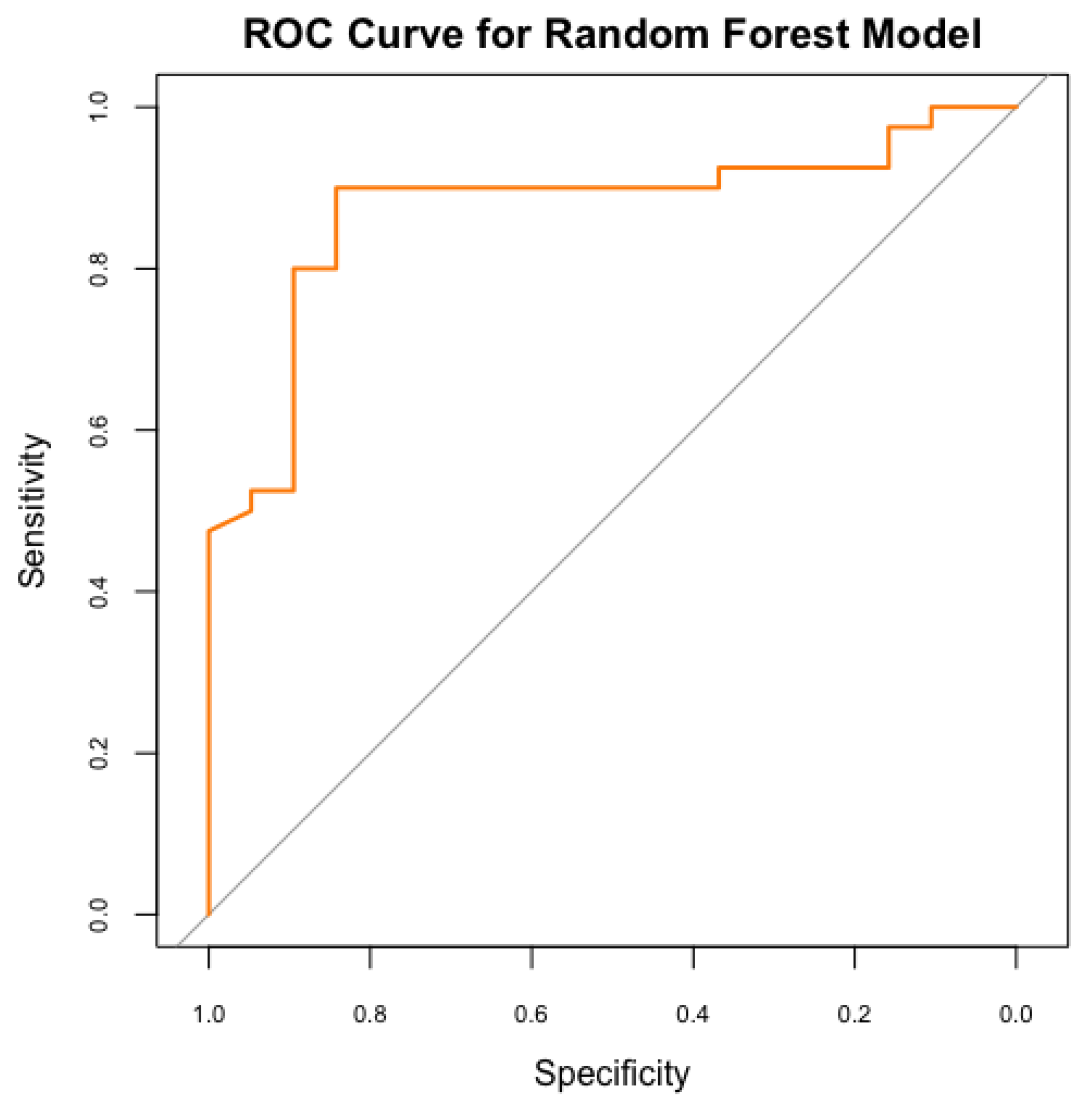
A Random Forest model was employed to forecast the risk of PDA in neonates, utilising maternal and neonatal variables. Our model achieved an accuracy of 76.3%, demonstrating moderate efficacy in distinguishing between neonates at high and low risk of PDA. However, the sensitivity was 47.4%, reflecting the model's ability to detected less than fifty percent of actual PDA cases, indicating limitations in recognizing all at-risk neonates, possibly due to the multifactorial nature of PDA and the variability of risk factors. Moreover, our model’s specificity of 90%, reliably identifies neonates without PDA. This indicates that the model effectively minimizes false-positive results, making it valuable for excluding PDA in low-risk scenarios. The Kappa value of 0.41 indicates moderate agreement between predicted and actual classifications, accounting for random chance (
Figure 2 and
Figure 3).
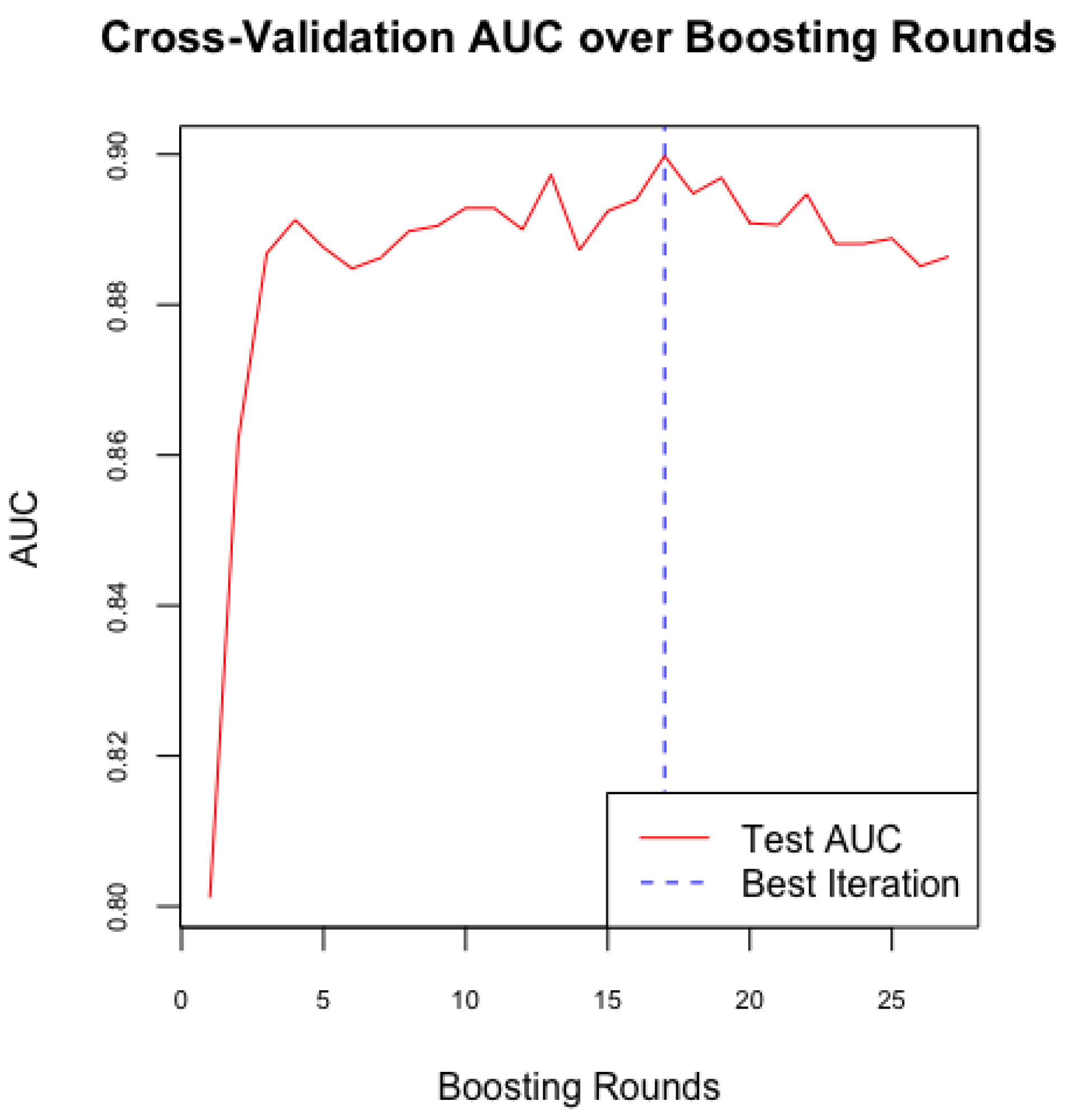
Following the establishment of a baseline model with Random Forest, we implemented the XGBoost algorithm to enhance predictive performance and address the imbalance in sensitivity and specificity observed in the Random Forest model. In our study, we configured XGBoost with optimized hyperparameters, including the learning rate, maximum tree depth, and number of boosting rounds, to improve the model's accuracy in predicting the likelihood of PDA in neonates. We used cross-validation (
Figure 4) to determine the optimal number of boosting rounds and mitigate overfitting, ensuring the model's effective generalization to new data.
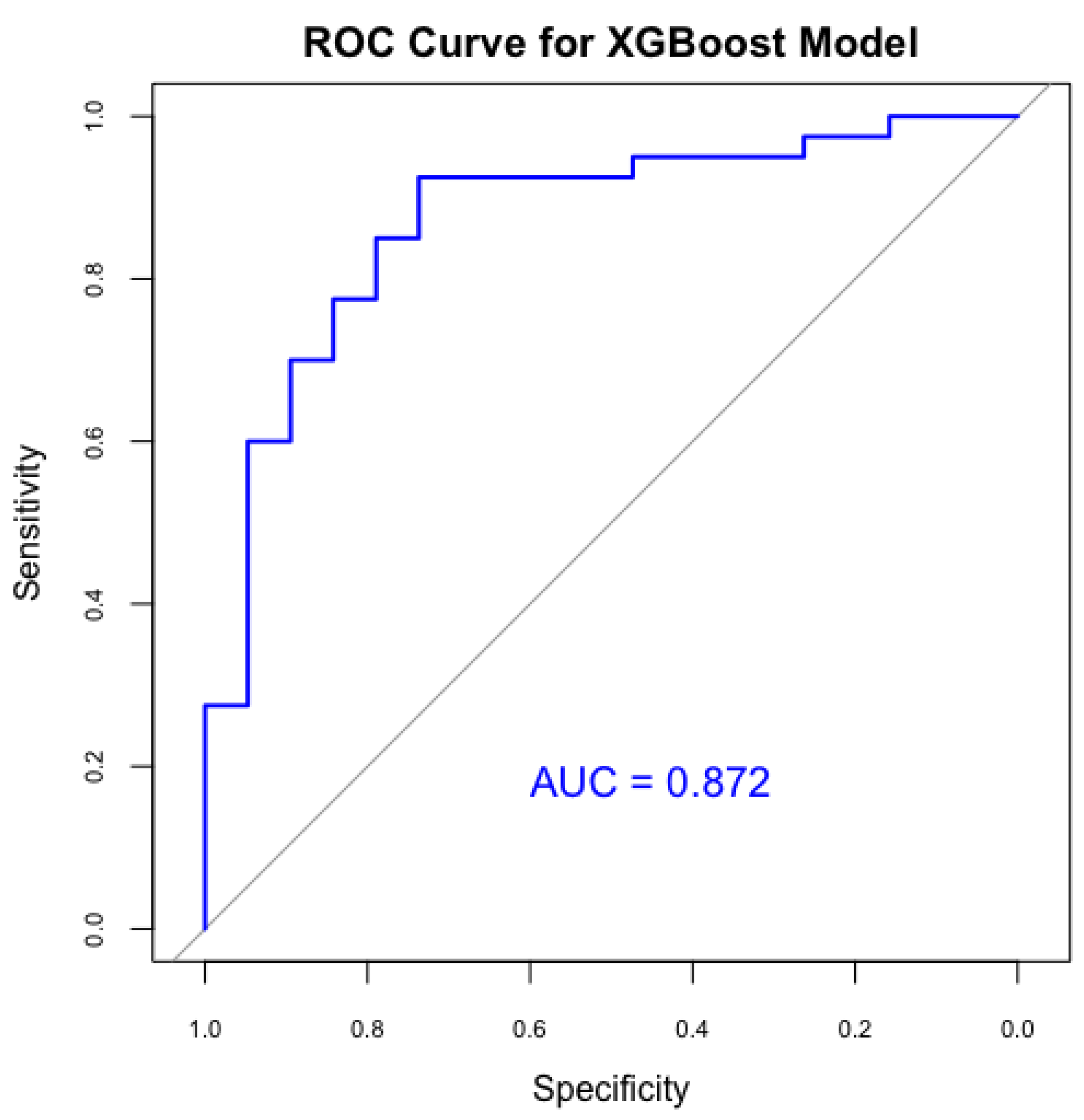
The XGBoost model was used to improve predictive efficacy. Therefore, it achieved an accuracy of 81.4% and an AUC of 0.872 (
Figure 5), indicating a strong ability to differentiate between PDA and non-PDA cases. Furthermore, the XGBoost showed a sensitivity of 92.5%, significantly enhancing the detection of true PDA cases, but with a specificity of 57.9%, indicating moderate precision in recognizing non-PDA cases, but a better result compared to the previous model. The Kappa statistic for XGBoost was 0.54, showing improved concordance between predictions and actual results. Our XGBoost model demonstrated superior sensitivity and overall accuracy compared to the Random Forest model, making it more effective for identifying at-risk neonates for PDA.
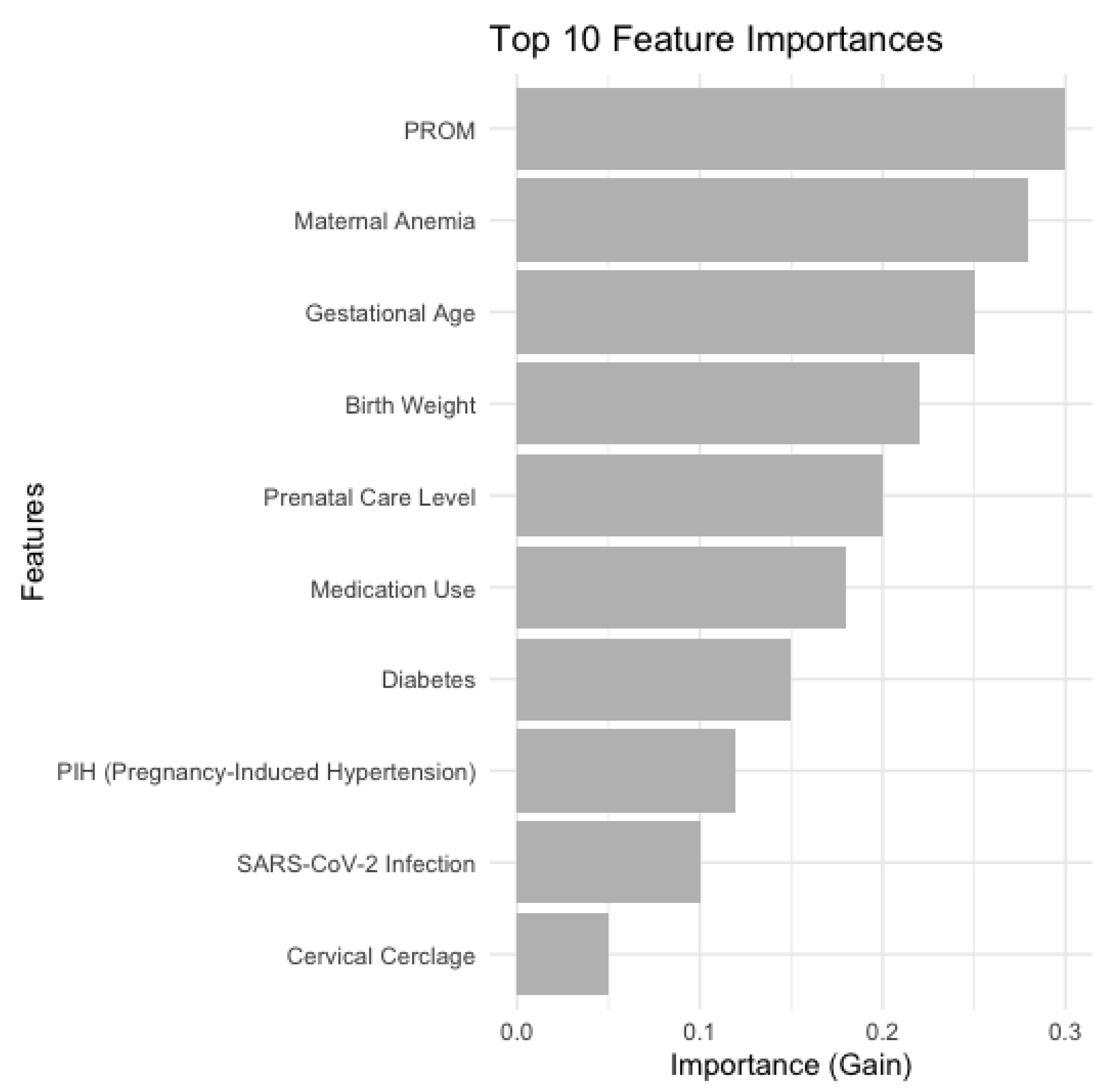
Figure 6 provides a bar chart which illustrates the ten most significant features correlated with the probability of Patent Ductus Arteriosus (PDA) in neonates, as determined by the XGBoost model. The primary predictors provide a comprehensive view of the factors affecting PDA risk, emphasizing maternal health conditions, fetal development metrics, and prenatal care as essential components in PDA forecasting.
4. Discussion
Our study identified multiple significant maternal and neonatal factors linked to an elevated risk of patent ductus arteriosus in neonates, including prolonged rupture of membranes (>18 hours), maternal anemia, gestational age, and birth weight. Lee et al. describes lower gestational age, female gender, and pregnancy induced hypertension a significant risk factor for symptomatic PDA [
29]. PROM surfaced as the paramount predictor, exhibiting a substantial odds ratio and feature significance in both Random Forest and XGBoost models. This association may be attributed to the potential of PROM to precipitate infection or other complications that impact neonatal cardiovascular development. Kusuma et al. concluded that rupture of membranes >12 hours and thrombocytopenia in the first 24 hours were proven to increase the risk of PDA in preterm infants [
30].
Another observation identified gestational age and birth weight as significant predictors, aligning with the existing literature. Low gestational age and low birth weight frequently indicate immature circulatory and pulmonary systems, thereby elevating the risk of PDA [
31,
32]. Bernati et al. noted that newborns <2000 grams at birth tended to have a 1.9 times higher risk for PDA [
33].
Maternal anaemia demonstrated considerable feature importance, suggesting that inadequate maternal nutrition or oxygenation during gestation may impede foetal development, resulting in neonatal anaemia and potentially causing delayed ductal closure, demanding postnatal anaemia treatment, as suggested by literature [
34,
35]. Pregnancy-induced hypertension and diabetes were correlated with elevated odds of patent ductus arteriosus, albeit not consistently with statistical significance. These conditions may indirectly influence fetal cardiovascular development by modifying maternal blood flow and nutrient delivery, thereby affecting the neonate's capacity to regulate ductal closure [
36,
37]. Maternal infections, including SARS-CoV-2 and GBS, exhibited a potential association with PDA, although this was not statistically significant. However, infection-induced inflammation may elevate the risk of circulatory complications in neonates, indicating that infection control and maternal prevention could be significant in mitigating PDA risk [
38,
39].
Extensive prenatal care did not exhibit a significant correlation with the reduction of PDA risk, indicating that standard prenatal care alone may be insufficient to alleviate particular risks associated with PDA, with specialised prenatal interventions aimed at high-risk pregnancies yielding a more significant effect [
40]. Medications, specifically enoxaparin and methyldopa, exhibited modest correlations with the risk of PDA. Hoeltzenbein et al describes a higher rate of major birth defects in methyldopa-exposed pregnancies (3.7%) compared to cohort (2.5%), but the difference was statistically not significant [
41]. Similarly, Bar et al. observed 3 out of 46 neonates born from mothers with Enoxaparin treatment during pregnancy exhibited persistent ductus arteriosus [
42].
Timely recognition of PDA risk factors, including PROM, reduced gestational age, and maternal anaemia, may facilitate targeted interventions, thereby enhancing neonatal outcomes. Healthcare providers may prioritise these factors in prenatal and postnatal care to mitigate the incidence of PDA. For preterm infants with recognised PDA risk factors, enhanced monitoring and management protocols may be instituted to reduce complications related to PDA. These findings may inform clinical decisions regarding antenatal and postnatal care, especially in NICUs that allocate resources to the management of high-risk neonates.
Our study was constrained by sample size and the omission or incompleteness of certain variables. Subsequent research should target larger cohorts to validate these associations and enhance the predictive accuracy of the models. Further research is required to elucidate the mechanisms connecting maternal health conditions and particular medications to PDA, in order to clarify potential causal relationships. Additional model optimisation and evaluation using alternative machine learning techniques, such as neural networks or ensemble methods, could improve predictive accuracy and provide greater understanding of the interactions among maternal, neonatal, and PDA risk factors. The incorporation of machine learning models such as Random Forest and XGBoost in clinical environments may enhance personalised medicine by enabling clinicians to more precisely stratify PDA risk according to specific maternal and neonatal variables.
5. Conclusions
This study provides a comprehensive examination of maternal and neonatal factors influencing the risk of patent ductus arteriosus in neonates, combining conventional statistical methods with advanced machine learning techniques. Logistic regression and chi-square tests highlighted prolonged rupture of membranes (>18 hours) as a key predictor, while XGBoost identified a broader range of influential variables, including maternal anemia, gestational age, birth weight, and prenatal care level. The results underscore the crucial impact of maternal health and pregnancy-related factors on neonatal outcomes. Future research should confirm these findings in larger, diverse populations and explore using advanced predictive models in clinical decisions to improve neonatal care and outcomes.
Author Contributions
Conceptualization, A.M.C.J., D.E.P. and I.R.; methodology, A.M.C.J.; software, A.M.C.J..; validation, D.E.P., I.R. and O.M.P.; formal analysis, C.P.; investigation, M.B.; resources, I.R.; data curation, I.R.; writing—original draft preparation, A.M.C.J.; writing—review and editing, C.P., I.R., and A.D.; visualization, A.T.C.; supervision, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Clinical Hospital of Obstetrics and Gynecology “Prof. Dr. Panait Sârbu” of Bucharest, no. 16516/11.12.2024 . The data was fully anonymized, with no identifiable personal information, ensuring compliance with privacy and confidentiality standards.
Informed Consent Statement
Patient consent was waived because it utilized retrospective data collected from existing medical records without directly involving human participants or interventions.
Data Availability Statement
All data is available in this paper.
a: We would like to acknowledge University of Medicine and Pharmacy “Victor Babeș” Timișoara for their support in covering the costs of publication for this research paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, N.; Malhotra, N.; Mahey, R.; Patel, G.; Saini, M. In Vitro Fertilization as an Independent Risk Factor for Perinatal Complications: Single-Center 10 Years Cohort Study. JBRA Assist Reprod 2023, 27, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Bossung, V.; Fortmann, M.I.; Fusch, C.; Rausch, T.; Herting, E.; Swoboda, I.; Rody, A.; Härtel, C.; Göpel, W.; Humberg, A. Neonatal Outcome After Preeclampsia and HELLP Syndrome: A Population-Based Cohort Study in Germany. Frontiers in Pediatrics 2020, 8, 579293. [Google Scholar] [CrossRef] [PubMed]
- Wender-Ozegowska, E.; Gutaj, P.; Mantaj, U.; Kornacki, J.; Ozegowski, S.; Zawiejska, A. Pregnancy Outcomes in Women with Long-Duration Type 1 Diabetes-25 Years of Experience. J Clin Med 2020, 9, 3223. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.C.G.; Peterson, J.C.; Wisse, L.J.; Roest, A.A.W.; Poelmann, R.E.; Bökenkamp, R.; Elzenga, N.J.; Hazekamp, M.; Bartelings, M.M.; Jongbloed, M.R.M.; et al. Pulmonary Ductal Coarctation and Left Pulmonary Artery Interruption; Pathology and Role of Neural Crest and Second Heart Field during Development. PLoS ONE 2020, 15, e0228478. [Google Scholar] [CrossRef]
- Chen, L.; Xu, H.; Zhou, L.; Liu, C.; Xi, J.; Wu, Y.; Yang, L.; Guo, Y. Prenatal Diagnosis of Ductal Origin of Distal Pulmonary Artery: Presentation of Three Cases and Literature Review. Ultrasound Obstet Gynecol 2022, 60, 284–290. [Google Scholar] [CrossRef]
- Bussmann, N.; Smith, A.; Breatnach, C.R.; McCallion, N.; Cleary, B.; Franklin, O.; McNamara, P.J.; El-Khuffash, A. Patent Ductus Arteriosus Shunt Elimination Results in a Reduction in Adverse Outcomes: A Post Hoc Analysis of the PDA RCT Cohort. J Perinatol 2021, 41, 1134–1141. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int J Mol Sci 2021, 22, 2965. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Hoong, M.FW.; Li, C.-S.; Li, W.-F.; You, S.-H.; Lee, Y.-C.; Peng, H.-H.; Chueh, H.-Y.; Chao, A.-S.; Cheng, P.-J.; et al. Association between Intrauterine Growth Restriction and Patent Ductus Arteriosus: Use of a Dichorionic Twin Pregnancy Model. Taiwanese Journal of Obstetrics and Gynecology 2021, 60, 517–522. [Google Scholar] [CrossRef]
- Gillam-Krakauer, M.; Mahajan, K. Patent Ductus Arteriosus. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Maged, A.M.; Elsherief, A.; Hassan, H.; Salaheldin, D.; Omran, K.A.; Almohamady, M.; Dahab, S.; Fahmy, R.; AbdelHak, A.; Shoab, A.Y.; et al. Maternal, Fetal, and Neonatal Outcomes among Different Types of Hypertensive Disorders Associating Pregnancy Needing Intensive Care Management. J Matern Fetal Neonatal Med 2020, 33, 314–321. [Google Scholar] [CrossRef]
- Cífková, R. Hypertension in Pregnancy: A Diagnostic and Therapeutic Overview. High Blood Press Cardiovasc Prev 2023, 30, 289–303. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, K.; Magee, L.A.; von Dadelszen, P.; Ekeroma, A.; Li, X.; Zhang, E.; Bhutta, Z.A. A Global View of Hypertensive Disorders and Diabetes Mellitus during Pregnancy. Nat Rev Endocrinol 2022, 18, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Gojnic, M.; Todorovic, J.; Stanisavljevic, D.; Jotic, A.; Lukic, L.; Milicic, T.; Lalic, N.; Lalic, K.; Stoiljkovic, M.; Stanisavljevic, T.; et al. Maternal and Fetal Outcomes among Pregnant Women with Diabetes. Int J Environ Res Public Health 2022, 19, 3684. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Peng, Y.; Wang, L.; Song, L.; Sun, B.; Wei, J.; Wang, T.; Mi, Y.; Cui, W. Risk Factors Screening for Gestational Diabetes Mellitus Heterogeneity in Chinese Pregnant Women: A Case-Control Study. Diabetes Metab Syndr Obes 2021, 14, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Năstase, L.; Cristea, O.; Diaconu, A.; Stoicescu, S.-M.; Mohora, R.; Pascu, B.M.; Tala, S.T.; Roșca, I. Two Cases of Congenital Hypothyroidism Revealing Thyroid Agenesis. Medicina 2023, 59, 1887. [Google Scholar] [CrossRef]
- Li, Y.; Johnson, J.P.; Yang, Y.; Yu, D.; Kubo, H.; Berretta, R.M.; Wang, T.; Zhang, X.; Foster, M.; Yu, J.; et al. Effects of Maternal Hypothyroidism on Postnatal Cardiomyocyte Proliferation and Cardiac Disease Responses of the Progeny. Am J Physiol Heart Circ Physiol 2023, 325, H702–H719. [Google Scholar] [CrossRef]
- Ovalı, F. Molecular and Mechanical Mechanisms Regulating Ductus Arteriosus Closure in Preterm Infants. Front Pediatr 2020, 8, 516. [Google Scholar] [CrossRef]
- Lungu, N.; Popescu, D.-E.; Jura, A.M.C.; Zaharie, M.; Jura, M.-A.; Roșca, I.; Boia, M. Enhancing Early Detection of Sepsis in Neonates through Multimodal Biosignal Integration: A Study of Pulse Oximetry, Near-Infrared Spectroscopy (NIRS), and Skin Temperature Monitoring. Bioengineering 2024, 11, 681. [Google Scholar] [CrossRef]
- Popescu, D.E.; Jura, A.M.C.; Știube, D.; Ciulpan, A.; Stoica, F.; Șipoș, S.I.; Cîtu, C.; Gorun, F.; Boia, M. How Much Does SARS-CoV-2 Infection during Pregnancy Affect the Neonatal Brain, Heart, and Kidney? A Parallel between COVID-19, Vaccination, and Normal Pregnancy. Life 2024, 14, 224. [Google Scholar] [CrossRef]
- Villamor, E.; Borges-Luján, M.; González-Luis, G. Association of Patent Ductus Arteriosus with Fetal Factors and Endotypes of Prematurity. Semin Perinatol 2023, 47, 151717. [Google Scholar] [CrossRef]
- Rafi, M.A.; Miah, M.M.Z.; Wadood, M.A.; Hossain, M.G. Risk Factors and Etiology of Neonatal Sepsis after Hospital Delivery: A Case-Control Study in a Tertiary Care Hospital of Rajshahi, Bangladesh. PLoS One 2020, 15, e0242275. [Google Scholar] [CrossRef]
- Petrolini, C.; Chiara, L.; Chiara, B.; Mario, S.; Buonocore, G.; Perrone, S. The Anemic Newborn at Birth: From Diagnosis to Treatment. Curr Pediatr Rev 2023, 19, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.; Pereira, S.; Antunes-Sarmento, J.; Flôr-de-Lima, F.; Soares, H.; Guimarães, H. Early Anemia and Neonatal Morbidity in Extremely Low Birth-Weight Preterm Infants. J Matern Fetal Neonatal Med 2021, 34, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.Z.T.; Fong, Q.W.; Huang, W.; Tan, C.H. Machine Learning in Medicine: What Clinicians Should Know. Singapore Medical Journal 2021, 64, 91. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, S.; Rabiei, N.; Soltanian, A.R.; Mamani, M. Application of Machine Learning Models Based on Decision Trees in Classifying the Factors Affecting Mortality of COVID-19 Patients in Hamadan, Iran. BMC Medical Informatics and Decision Making 2022, 22, 192. [Google Scholar] [CrossRef]
- Belle, A.; Thiagarajan, R.; Soroushmehr, S.M.R.; Navidi, F.; Beard, D.A.; Najarian, K. Big Data Analytics in Healthcare. Biomed Res Int 2015, 2015, 370194. [Google Scholar] [CrossRef]
- Mbonyinshuti, F.; Nkurunziza, J.; Niyobuhungiro, J.; Kayitare, E. Application of Random Forest Model to Predict the Demand of Essential Medicines for Non-Communicable Diseases Management in Public Health Facilities. The Pan African Medical Journal 2022, 42, 89. [Google Scholar] [CrossRef]
- Moore, A.; Bell, M. XGBoost, A Novel Explainable AI Technique, in the Prediction of Myocardial Infarction: A UK Biobank Cohort Study. Clin Med Insights Cardiol 2022, 16, 11795468221133611. [Google Scholar] [CrossRef]
- Lee, J.A.; Sohn, J.A.; Oh, S.; Choi, B.M. Perinatal Risk Factors of Symptomatic Preterm Patent Ductus Arteriosus and Secondary Ligation. Pediatrics & Neonatology 2020, 61, 439–446. [Google Scholar] [CrossRef]
- Kusuma, A.; Gunawijaya, E.; Putra, I.; Yantie, N.; Kardana, I.; Lingga, D.; Gustawan, I.W. Risk Factors of Patent Ductus Arteriosus in Preterm. American Journal of Pediatrics 2020, 6, 168. [Google Scholar] [CrossRef]
- Pourarian, S.; Farahbakhsh, N.; Sharma, D.; Cheriki, S.; Bijanzadeh, F. Prevalence and Risk Factors Associated with the Patency of Ductus Arteriosus in Premature Neonates: A Prospective Observational Study from Iran. J Matern Fetal Neonatal Med 2017, 30, 1460–1464. [Google Scholar] [CrossRef]
- Na, J.Y.; Kim, D.; Kwon, A.M.; Jeon, J.Y.; Kim, H.; Kim, C.-R.; Lee, H.J.; Lee, J.; Park, H.-K. Artificial Intelligence Model Comparison for Risk Factor Analysis of Patent Ductus Arteriosus in Nationwide Very Low Birth Weight Infants Cohort. Sci Rep 2021, 11, 22353. [Google Scholar] [CrossRef] [PubMed]
- Bernati, N.; Nova, R.; Tasli, J.M.; Theodorus, T. Risk Factors for Patent Ductus Arteriosus in Preterm Neonates. Paediatrica Indonesiana 2014, 54, 132–136. [Google Scholar] [CrossRef]
- Chen, J.-Y. Patent Ductus Arteriosus in Preterm Infants. Pediatrics & Neonatology 2012, 53, 275. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, M.; Wu, T.; Li, J.; Shi, H.; Wei, Y. The Association between Maternal Anemia and Neonatal Anemia: A Systematic Review and Meta-Analysis. BMC Pregnancy and Childbirth 2024, 24, 677. [Google Scholar] [CrossRef]
- Capobianco, G.; Gulotta, A.; Tupponi, G.; Dessole, F.; Pola, M.; Virdis, G.; Petrillo, M.; Mais, V.; Olzai, G.; Antonucci, R.; et al. Materno-Fetal and Neonatal Complications of Diabetes in Pregnancy: A Retrospective Study. Journal of Clinical Medicine 2020, 9, 2707. [Google Scholar] [CrossRef]
- Roodpeyma, S.; Rafieyian, S.; Khosravi, N.; Hashemi, A. Cardiovascular Complications in Infants of Diabetic Mothers: An Observational Study in a Pediatric Cardiology Clinic in Tehran. J Compr Ped 2013, 4, 119–123. [Google Scholar] [CrossRef]
- Roşca, I.; Oriță, V.; Popescu, R.; Șerban, M.; Smadeanu, R.; Mitran, M. The Risk of Materno-Fetal Infection. Importance of Common Laboratory Tests. BMC Infectious Diseases 2013, 13, P112. [Google Scholar] [CrossRef]
- Popescu, D.-E.; Cîtu, C.; Jura, A.M.C.; Lungu, N.; Navolan, D.; Craina, M.; Semenescu, A.; Gorun, F.; Jura, M.-A.; Belengeanu, V.; et al. The Benefits of Vaccination against SARS-CoV-2 during Pregnancy in Favor of the Mother/Newborn Dyad. Vaccines 2022, 10, 848. [Google Scholar] [CrossRef]
- Zhang, Z.; Wengrofsky, A.; Wolfe, D.S.; Sutton, N.; Gupta, M.; Hsu, D.T.; Taub, C.C. Patent Ductus Arteriosus in Pregnancy: Cardio-Obstetrics Management in a Late Presentation. CASE : Cardiovascular Imaging Case Reports 2021, 5, 119. [Google Scholar] [CrossRef]
- Hoeltzenbein, M.; Beck, E.; Fietz, A.-K.; Wernicke, J.; Zinke, S.; Kayser, A.; Padberg, S.; Weber-Schoendorfer, C.; Meister, R.; Schaefer, C. Pregnancy Outcome After First Trimester Use of Methyldopa. Hypertension 2017, 70, 201–208. [Google Scholar] [CrossRef]
- Bar, J.; Cohen-Sacher, B.; Hod, M.; Blickstein, D.; Lahav, J.; Merlob, P. Low-Molecular-Weight Heparin for Thrombophilia in Pregnant Women. Int J Gynaecol Obstet 2000, 69, 209–213. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).