1. Introduction
Water-use efficiency (WUE) is a fundamental parameter in sustainable agriculture, particularly in water-limited environments where optimizing resource use is crucial for maintaining productivity and environmental resilience [
1,
2,
3]. Increasing WUE has been a primary goal in agronomic research, as it contributes to improved crop performance under fluctuating water availability while minimizing excessive water consumption [
1,
2]. While previous studies have demonstrated that certain cultivars exhibit enhanced WUE under specific conditions, most research has focused on instantaneous WUE measurements rather than long-term yield sustainability [
4]. Understanding the physiological and hydraulic mechanisms underlying WUE at different time scales remains a critical aspect of improving water management strategies in crop production [
1,
2,
5].
Water-use efficiency is often quantified through multiple physiological parameters, including intrinsic WUE (WUE
i), which accounts for the ratio of carbon assimilation to stomatal conductance, and instantaneous WUE (WUE
E), which reflects immediate responses to environmental conditions [
5,
6,
7]. These parameters provide insights into how plants regulate water loss and carbon gain under dynamic stress conditions [
6,
7,
8]. However, the extent to which short-term physiological adjustments correlate with long-term agronomic performance remains uncertain [
5,
6,
7,
9]. Recent studies suggest that WUE responses are highly cultivar-dependent, with trade-offs occurring between gas exchange efficiency, hydraulic traits, and yield stability under varying water conditions [
10].
Black pepper (
Piper nigrum) is a high-value crop cultivated in tropical and subtropical regions, where water availability significantly influences its productivity and quality [
10,
11,
12]. Given the increasing challenges posed by climate variability, optimizing WUE in
P. nigrum is essential for maintaining stable yields under diverse environmental conditions [
9,
10]. While studies have reported variability in WUE among black pepper cultivars, there is limited knowledge on how different cultivars balance physiological performance and yield potential under varying soil water tensions and over seasons. Investigating these responses at both physiological and agronomic levels is necessary to develop improved management strategies for sustainable pepper cultivation [
10,
11,
13].
Despite extensive research on WUE, most studies rely on instantaneous measurements without fully integrating the long-term consequences of water availability on productivity [
14,
15,
16]. On the other hand, there is a lack of understanding regarding the cultivar-specific hydraulic and physiological responses that contribute to WUE differences over time. Moreover, how and whether the hydraulic bases underpinning the performance of different
P. nigrum cultivars explain their responses to varying levels of water availability, and temporal dynamics remain largely unexplored. Therefore, identifying the physiological and structural adaptations that drive these differences can help refine irrigation strategies and breeding programs aimed at improving WUE without compromising yield.
In this sense, by analyzing cultivar-specific physiological parameters, including photosynthetic rates, stomatal regulation, and water potential, this study seeks to elucidate the fundamental drivers of WUE across different time frames and soil moisture conditions. We hypothesize that distinct P. nigrum cultivars exhibit divergent physiological and agronomic strategies in response to water stress, with one favoring high photosynthetic performance and gas exchange efficiency and the other prioritizing water conservation and yield stability. Specifically, we predict that Clonada will maintain higher photosynthetic rates and transpiration under moderate stress, leading to greater yields at lower soil water potentials, whereas Uthirankotta will exhibit superior WUE across all conditions due to its conservative water-use strategy.
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
The experiment was conducted at fields of the Tropical Products Company of Castanhal (Tropoc), municipality of Castanhal, PA, Brazil (01º 17' 50” S, 47º 55' 20” W, 40 m altitude). The trial was carried out in cooperation with the Study Group on Water and Soil Engineering in the Amazon (GEEASA) from the Universidade Federal Rural da Amazônia (UFRA). The field was composed of 2-year-old seedlings from two cultivars of Piper nigrum L., namely cv. Clonada and cv. Uthirankotta. They were arranged in an area of 1930 m2. The experiment was set up in randomized blocks design, in which each plot consisted of four plants per cultivar (Clonada and Uthirankotta), in a double-row, with a spacing of 4.0 m between rows and 2.20 x 2.20 m among plants.
The soil is classified as a dystrophic Yellow Argisol (medium texture), with predominating secondary vegetation [
17]. The soil chemical and acidity corrections were carried out by applying liming (3.7 t ha
-1) and both organic and mineral planting fertilizers as previously described by Oliveira and Nakayama (2007). Plants were cultivated under global radiation (GR) of 1303 W/m
2 (nearly 700 µmol de photons m
-2 s
-1), and daytime temperatures of 25.3 to 26.2 ± 2 °C (day/night), with relative air humidity between 60.5 and 86%. According to Köppen, the region's climate is classified as Af-type (wet equatorial climate), with annual precipitation averages of 2,432 mm and temperature of 26.5 °C. In particular, the rainfall regime in this Amazon region concentrates from December to May and presents the lowest volumes during the dry period, corresponding to June to November [
18].
The method of [
19] was applied to climatological data from the automatic station A202 of the National Institute of Meteorology – INMET from Castanhal during the period from 2003 to 2022, using the (Figure S1). Overall, the climatological water balance for Castanhal presented the greatest water oversupply between February and May, with a maximum in March (186 mm) and April (190 mm).
2.2. Irrigation System Setup and Management
Soil water availability was managed by a drip irrigation system, set up to a flow rate of 3.55 L h
-1, with emitters spaced 30 cm apart. The drip tubing was arranged superficially with a self-compensating drip-tech PC/AS flat emitter for irrigation, with DN 16 mm, set up to a working pressure of 1 bar at the end of the hose. The dripping tubes were positioned within the plot in a double line, to meet the plants' double spacing (7 drippers per plant). The automation consisted of a 24-volt time/sector programmer and a standard box with five solenoids. 1,250 m of 8 x 4 mm linear polyethene control and necessary connections were used. Five water tanks, 10,000 L each, a 1 hp electric pump (flow of 8.44 m 3 h
-1) and a disc filter were used to supply the irrigation system. The irrigation system was tested for efficiency by applying a Distribution Uniformity Coefficient (CUD). The uniformity analysis was carried out by 250 ml collection containers under eight emitters, which collected the drip water for 1 min. This procedure was performed with three replications. The system presented a rate of 97%, which is considered excellent according to the classification postulated by [
20].
To determine the critical soil tensions (or soil water potential), a set of three puncture tensiometers was installed: two installed at a depth of 20 cm, to indicate the time of irrigation; and one at a depth of 30 cm to check whether water percolation is occurring. The tensiometers were positioned in line with the plant set, about 15 cm from the drippers. Daily readings of tensions were carried around 8:30 am, which was followed by the irrigation system activation. Irrigations were performed when the tensiometers (at the same depth) presented the critical tension for each treatment, seeking to raise the soil to its respective soil moisture at field capacity (10, 25, 35, 45 and 55 kPa). For puncture tensiometer outputs, the value of matric potential (Ψ
m) was determined using the method described by [
21].
Finally, the irrigation regime and management were based on the soil water characteristic curve obtained in the 0 – 20 cm deep layer. The outputs of soil water retention were achieved using the Richards pressure chamber at potentials of 0, 6, 10, 30, 100, 500 and 1500 kPa [
22]. The data obtained were fitted to the model proposed by Van Genuchten (1980) with the R software, and the parameters of the adjustment equation for the soil water retention curve were determined (Figure S2). Both water levels and the operating time of the irrigation system were calculated according to [
21], considering the effective depth of the root system equal to 20 cm, as previously described by [
23]. The summary of irrigation regime and management, such as irrigation levels, the total amount of water applied, number of irrigations, irrigation frequency and daily water demand are available in Table S1.
2.3. Green and Black Pepper Yield Coupled To Water-Use Efficiency
Fruits were harvested at the corresponding harvest months for each cultivar. For cv. Clonada, between July and October, while between August and November for cv. Uthirankotta. Using data of fresh pepper fruits weight, green pepper yield (GPY) was calculated as GPY=P/A, where Y, as yield (kg ha
-1); P, as production (kg); and A, as area (ha). The water balance was monitored throughout the experiment and expressed in mm. Based on this, the water-use efficiency (WUE) between cultivars was obtained through the relationship between black pepper fruit productivity (kg ha
-1) and water consumption (mm) [
24], as WUE=Y/w, where: WUE, as water-use efficiency, kg ha
-1 mm
-1; Y, as total yield, kg ha
-1; w, as the volume of water applied, mm. The black pepper yield (BPY) was calculated using the percentage of yield related to the production of black pepper with the production of harvested green pepper, calculated by the equation BPY=(BPP/GPP)*100%, where: BPY, black pepper yield, %; BPP, black pepper production, kg plant
-1; GPP, green pepper production, kg plant
-1. The yield was determined at the end of the harvest season. The entire experiment period comprised 36 months.
2.4. Gas Exchange
Gas exchange parameters were determined using the infrared gas analyser (LCpro-SD, ADC BioScientific Ltd, United Kingdom). Analyses were performed on the third to fourth fully expanded leaf of the twelfth branch from the base to the apex. Net carbon assimilation rate (A), stomatal conductance (gs), transpiration (E) and intercellular CO2 concentration (Ci), were measured at a diurnal period under photosynthetically active radiation (PAR) of 1000 µmol (photons) m–2 s–1 and 400 ppm CO2 at leaf level (Yin et al. 2009). Instantaneous (WUEleaf) and intrinsic water-use efficiency (WUEi) were estimated based on the ratio between A/E and A/gs, respectively. Such procedures were repeatedly performed 40, 80, 120 and 160 days after the experiment.
2.5. Water Status
Leaf water potential measurements were carried out at both predawn (Ψ
pd) and midday (Ψ
md) using the Scholander pressure chamber [
25], model M 1505D (Pressure Chamber Instruments, PMS), on completely healthy expanded leaves from the 12th plagiotropic branch of two plants from each cultivar and replication. The predawn water potential (Ψ
pd) was performed between 2:30 am and 5:30 am, while the midday water potential (Ψ
md) was performed between 12:00 pm and 3:00 pm. The predawn water potential (Ψ
pd) was assessed at 40, 80, 120 and 160 days after the experiment was initiated; and the midday water potential (Ψ
md) was at only both the 80
th and 120
th days.
2.6. Data Analysis
The data were obtained from the experiments using a randomized blocks design, in a factorial scheme in split plots of 5 x 2, using 10 treatments and three blocks. The treatments consisted of five soil water tensions (15, 25, 35, 45 and 55 kPa) indicating the time to irrigate – critical tension, and two black pepper cultivars (Clonada and Uthirankotta. The homoscedasticity and normality of the data were verified to meet assumptions surrounding ANOVA. If ANOVA showed significant effects, a Tukey test (
P< 0.05) was used to determine differences between cultivars either over time or among water availability levels (see Table S2, S3). Furthermore, a principal component analysis (PCA) was conducted considering both cultivars and variables. Statistical procedures were performed using R software [
26].
3. Results
3.1. Green and Black Pepper Yield And Water-Use Efficiency Under Tensions
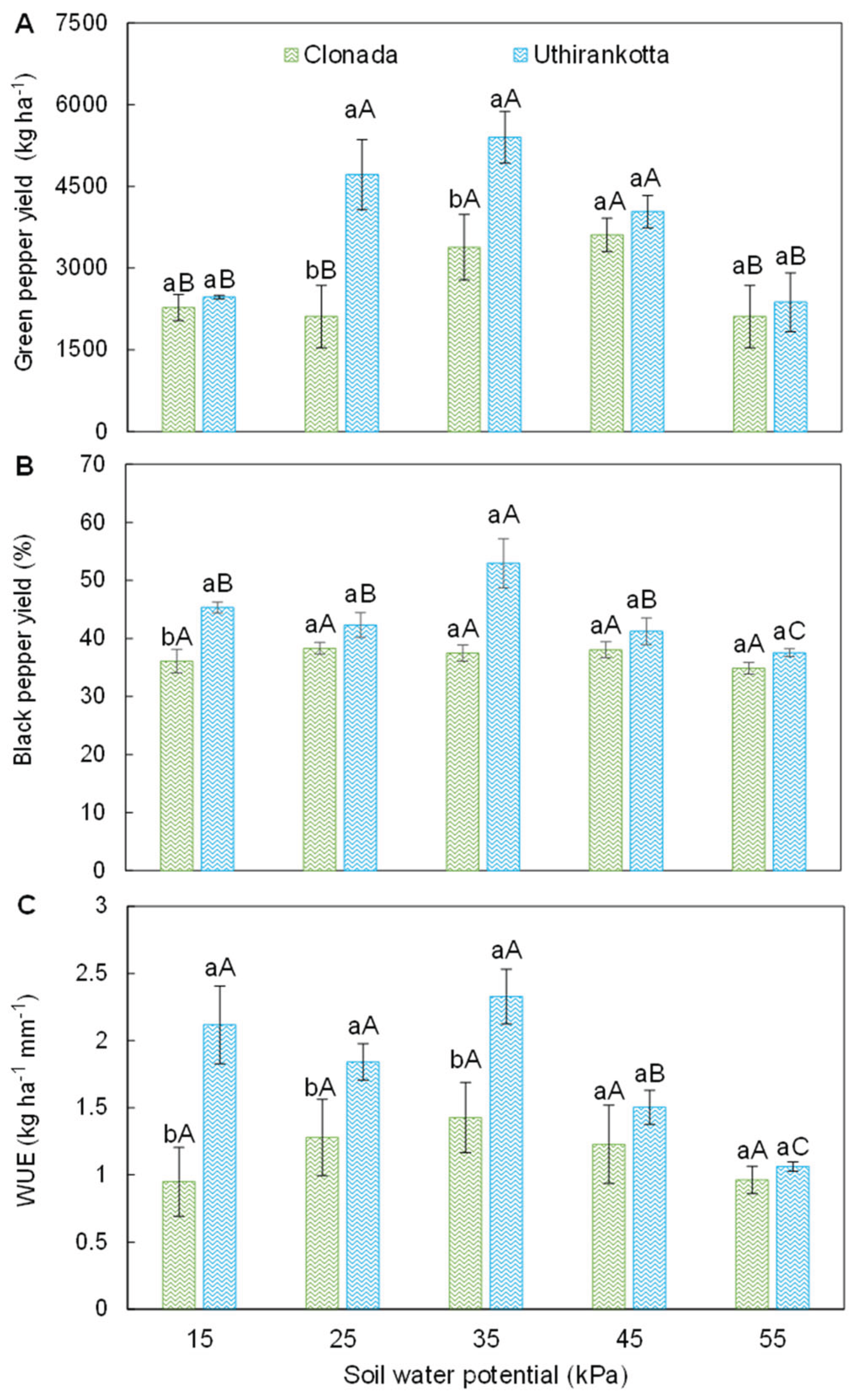
Green pepper yield (GPY), black pepper yield (BPY) and water use efficiency (WUE) were significantly (
P<0.05) influenced by soil water tension. [GPY] (
Figure 1A-C). The green pepper yield (
Figure 1A) demonstrated significant variations across different soil water potentials (SWP) for both Clonada and Uthirankotta (
Figure 1A). In Clonada, the yield increased from 15 kPa (3500 kg ha⁻¹) to its maximum at 35 kPa (4500 kg ha⁻¹). This peak was statistically higher than at 15, 25, and 45 kPa. Beyond 35 kPa, the yield declined, reaching its lowest value at 55 kPa (3000 kg ha⁻¹,), significantly lower than all other tensions. Similar to Clonada, the yield increased with decreasing soil water availability, peaking at 35 kPa (4500 kg ha⁻¹) in Uthirankotta. Yields were lower at both 15 kPa (3000 kg ha⁻¹) and 55 kPa (3000 kg ha⁻¹). There was no significant difference between the 15, 45, and 55 kPa yields. Finally, at 15 kPa, Clonada produced significantly higher yields than Uthirankotta. At 35 kPa, both varieties performed equally, achieving their maximum yields. At 55 kPa, Clonada showed statistically similar yields to Uthirankotta.
The black pepper yield (
Figure 1B) followed a trend similar to the green pepper yield, with variations depending on the variety and soil water potential. In Clonada, Black peper yield increased from 15 kPa (1750 kg ha⁻¹) to its maximum at 35 kPa (2250 kg ha⁻¹). Beyond this point, the yield decreased, with the lowest value recorded at 55 kPa (1500 kg ha⁻¹). The difference between 15, 25, and 45 kPa was not statistically significant. On the other hand, in Uthirankotta, yield peaked at 35 kPa (2250 kg ha⁻¹), with significantly lower yields at 15 kPa (1500 kg ha⁻¹) and 55 kPa (1500 kg ha⁻¹). Finally, there were no significant differences among 15, 25, and 45 kPa. At 15 kPa, Clonada produced significantly higher yields than Uthirankotta. At 35 kPa, both varieties achieved their maximum yields without statistical differences. At 55 kPa, Clonada outperformed Uthirankotta, with a statistically significant difference.
Water use efficiency (
Figure 1C) displayed distinct trends across soil water potentials and between the two varieties. In Clonada, WUE increased from 15 kPa (1.5 kg ha⁻¹ mm⁻¹) to its maximum at 35 kPa (2.5 kg ha⁻¹ mm⁻¹). At 45 kPa (2.0 kg ha⁻¹ mm⁻¹), WUE decreased significantly compared to 35 kPa. The lowest WUE was observed at 55 kPa (1.0 kg ha⁻¹ mm⁻¹), which was significantly lower than all other tensions. In Uthirankotta, WUE was consistently higher than Clonada at all soil tensions. It increased from 15 kPa (2.0 kg ha⁻¹ mm⁻¹) to its maximum at 35 kPa (2.6 kg ha⁻¹ mm⁻¹). Beyond 35 kPa, WUE declined, reaching its lowest value at 55 kPa (1.2 kg ha⁻¹ mm⁻¹). At all soil tensions, Uthirankotta exhibited statistically higher WUE compared to Clonada. For example, at 15 kPa, Uthirankotta recorded 2.0 kg ha⁻¹ mm⁻¹ compared to Clonada's 1.5 kg ha⁻¹ mm⁻¹. Both varieties reached their maximum WUE at 35 kPa, with Uthirankotta significantly outperforming Clonada.
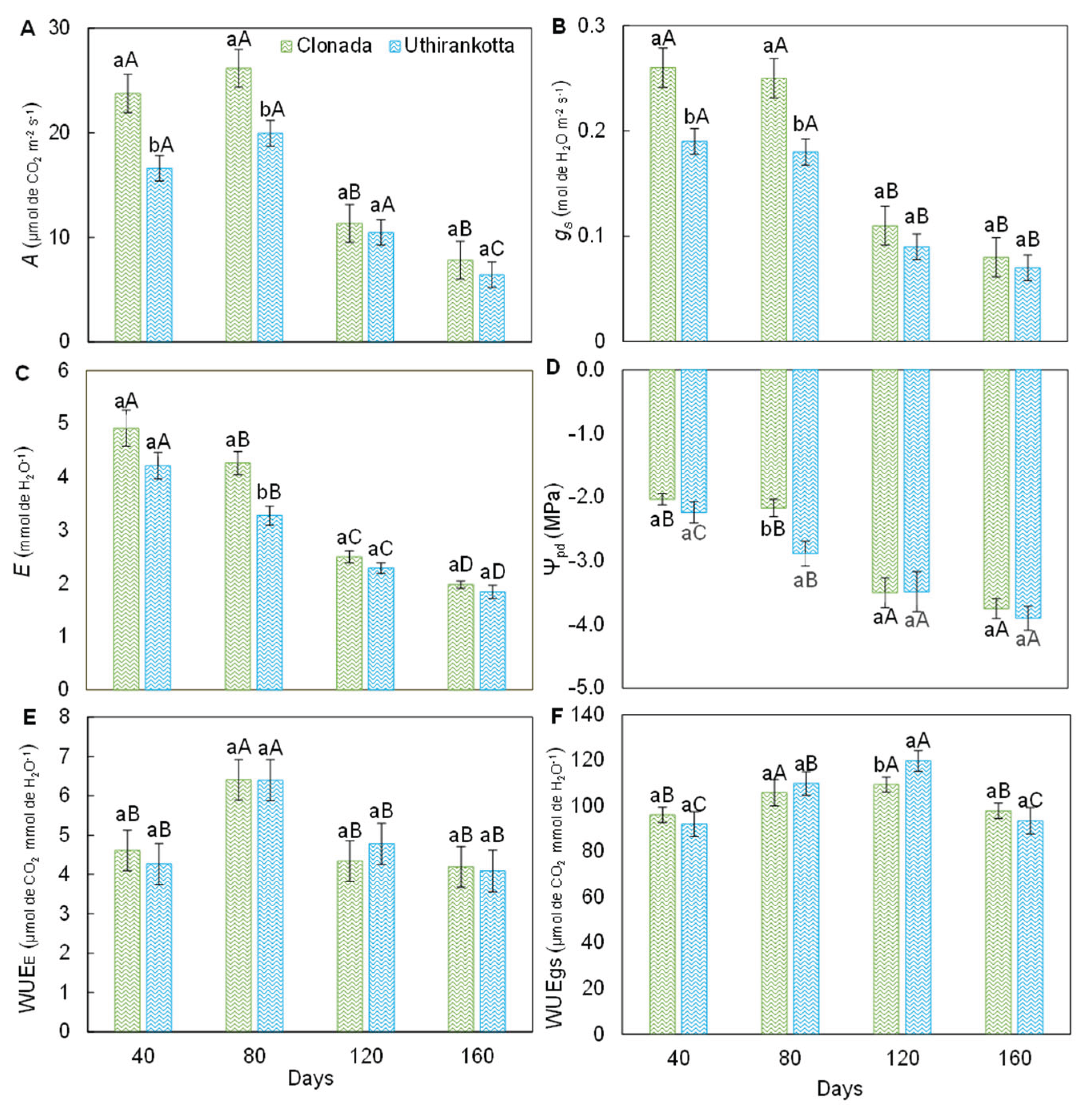
3.2. Physiological Parameters Under The Influence of Time Of Evaluation
The net photosynthetic rate (
Figure 2A) varied significantly between measurement days (40, 80, 120, and 160 days) and between the two varieties, Clonada and Uthirankotta. In Clonada, the highest photosynthetic rate was observed at 40 days (approximately 25 µmol CO₂ m⁻² s⁻¹) and 80 days (25 µmol CO₂ m⁻² s⁻¹), with no statistical difference between these two time points. The rate declined significantly after 80 days, reaching its lowest value at 160 days (10 µmol CO₂ m⁻² s⁻¹. In Uthirankotta, a similar trend was observed, with maximum rates at 40 and 80 days (20 µmol CO₂ m⁻² s⁻¹) and a significant decline by 160 days (8 µmol CO₂ m⁻² s⁻¹). The photosynthetic rate for Uthirankotta was consistently lower than Clonada at all time points, with statistical differences at 40, 80, and 160 days.
Stomatal conductance (
Figure 2B) followed a pattern similar to net photosynthesis, with significant differences between days and varieties. In Clonada, the highest stomatal conductance was recorded at 40 and 80 days (both approximately 0.25 mol H₂O m⁻² s⁻¹), followed by a decline at 120 days (0.15 mol H₂O m⁻² s⁻¹) and 160 days (0.10 mol H₂O m⁻² s⁻¹). In Uthirankotta, the stomatal conductance was slightly lower than Clonada at all time points, with maximum values at 40 and 80 days (approximately 0.20 mol H₂O m⁻² s⁻¹) and the lowest at 160 days (0.08 mol H₂O m⁻² s⁻¹). Statistical differences were observed between varieties at 40, 80, and 160 days.
The transpiration rate (
Figure 2C) displayed significant variation over time and between varieties. In Clonada, transpiration was highest at 40 days (6 mmol H₂O m⁻² s⁻¹) and decreased significantly over time, reaching its lowest value at 160 days (2 mmol H₂O m⁻² s⁻¹). Intermediate values were observed at 80 and 120 days, with statistical differences between these time points. In Uthirankotta, a similar declining trend was observed, with the highest value at 40 days (4 mmol H₂O m⁻² s⁻¹) and the lowest at 160 days (1.5 mmol H₂O m⁻² s⁻¹). Uthirankotta consistently exhibited lower transpiration rates than Clonada, with significant differences at 40 and 80 days.
Leaf water potential (
Figure 2D) became progressively more negative with time for both varieties. In Clonada, the least negative water potential was recorded at 40 days (-1.5 MPa), which became increasingly negative, reaching its lowest value at 160 days (-3.5 MPa). In Uthirankotta, a similar trend was observed, with water potential ranging from -2.0 MPa at 40 days to -4.0 MPa at 160 days. Uthirankotta consistently displayed more negative values compared to Clonada, with significant differences at 40, 80, and 120 days.
Instantaneous water use efficiency (
Figure 2E) demonstrated variations over time and between varieties. In Clonada, WUEₑ was highest at 80 days (4.5 µmol CO₂ mmol⁻¹ H₂O) and remained stable between 40 and 80 days. However, a significant decline was observed at 120 and 160 days, reaching 3.0 µmol CO₂ mmol⁻¹ H₂O. In Uthirankotta, WUEₑ followed a similar trend, peaking at 80 days (4.0 µmol CO₂ mmol⁻¹ H₂O) and declining thereafter to 2.8 µmol CO₂ mmol⁻¹ H₂O at 160 days. Statistical differences between varieties were observed at 40 and 80 days, with Uthirankotta showing lower WUEₑ.
Water use efficiency based on stomatal conductance (
Figure 2F) increased over time for both varieties. In Clonada, WUEₛ₉ was highest at 160 days (100 µmol CO₂ mmol⁻¹ H₂O) and lowest at 40 days (80 µmol CO₂ mmol⁻¹ H₂O). A significant increase was observed over time. In Uthirankotta, WUEₛ₉ followed a similar pattern, increasing from 40 days (70 µmol CO₂ mmol⁻¹ H₂O) to 160 days (90 µmol CO₂ mmol⁻¹ H₂O). Uthirankotta showed consistently lower WUE
gs compared to Clonada, with statistical differences at all time points except 160 days.
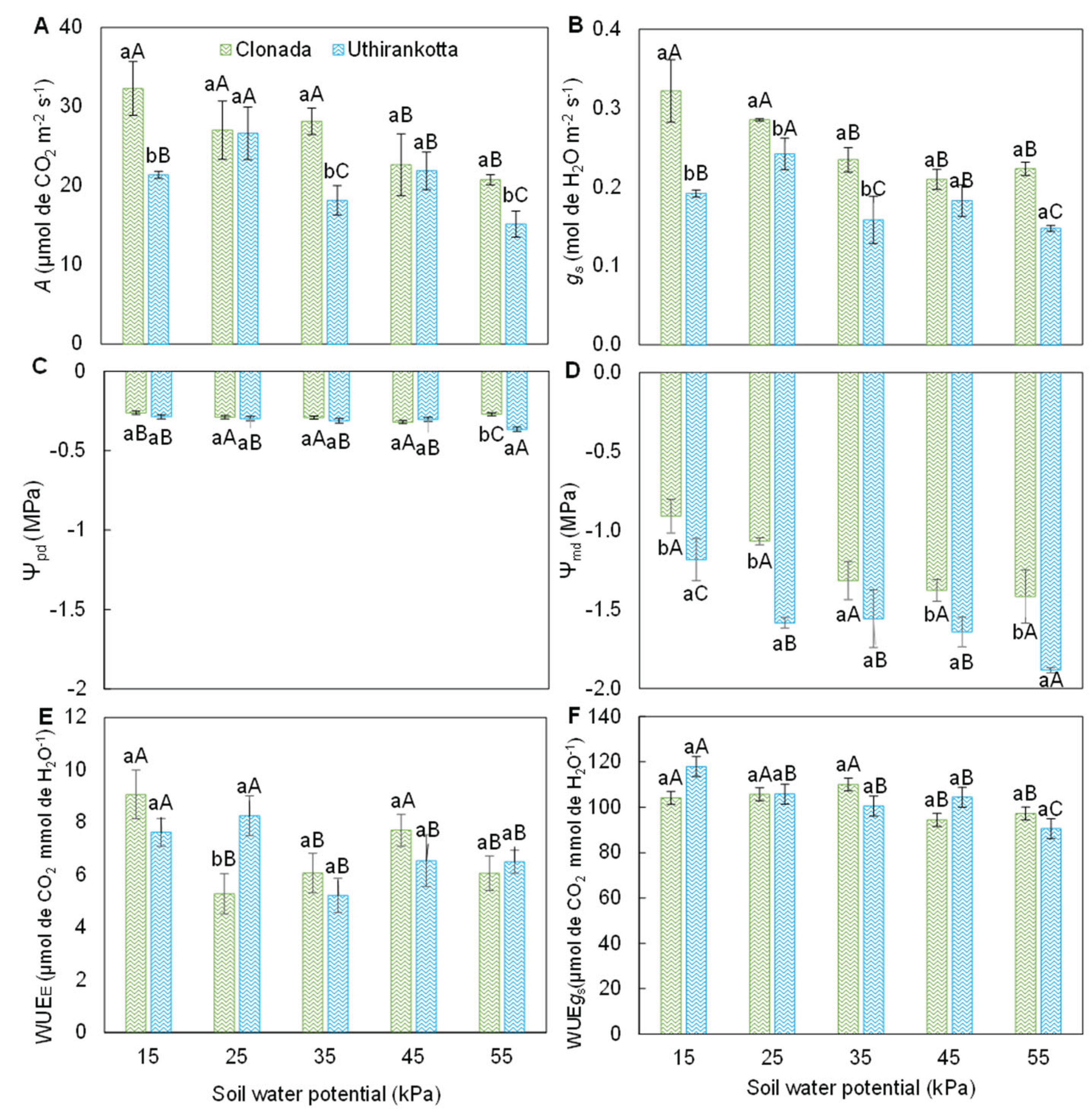
3.3. Physiological Parameters Under the Influence of Soil Water Tension in Both Cultivars
The net photosynthetic rate (
Figure 3A) showed significant variations with soil water potential (15, 25, 35, 45, and 55 kPa) and between the two varieties, Clonada and Uthirankotta. In Clonada, the highest rate was observed at 15 kPa (approximately 30 µmol CO₂ m⁻² s⁻¹) and remained high at 25 and 35 kPa (27–28 µmol CO₂ m⁻² s⁻¹). A significant decline occurred at 45 and 55 kPa, reaching a minimum at 55 kPa (approximately 12 µmol CO₂ m⁻² s⁻¹). In Uthirankotta, the highest rate was also at 15 kPa (approximately 25 µmol CO₂ m⁻² s⁻¹) and decreased progressively with increasing soil water potential, reaching a minimum at 55 kPa (10 µmol CO₂ m⁻² s⁻¹). At all soil water potentials, Uthirankotta showed statistically lower photosynthetic rates compared to Clonada, except at 55 kPa, where differences were not significant.
Stomatal conductance (
Figure 3B) was significantly affected by soil water potential and varied between varieties. In Clonada, the highest stomatal conductance was at 15 and 25 kPa (approximately 0.3 mol H₂O m⁻² s⁻¹). It declined significantly with increasing soil water potential, reaching its lowest value at 55 kPa (0.1 mol H₂O m⁻² s⁻¹). In Uthirankotta, A similar trend was observed, with maximum stomatal conductance at 15 kPa (0.25 mol H₂O m⁻² s⁻¹) and minimum at 55 kPa (0.08 mol H₂O m⁻² s⁻¹). Uthirankotta consistently showed lower stomatal conductance than Clonada at all soil water potentials.
The predawn leaf water potential (Ψ
pd,
Figure 3C) became more negative with increasing soil water potential for both varieties. In Clonada, Ψ
pd ranged from approximately -0.5 MPa at 15 kPa to -1.5 MPa at 55 kPa. There were no significant differences between 25, 35, and 45 kPa, but a significant drop occurred at 55 kPa. In Uthirankotta, Ψ
pd followed a similar trend, ranging from -0.6 MPa at 15 kPa to -1.8 MPa at 55 kPa. At all soil water potentials, Uthirankotta showed more negative Ψ
pd compared to Clonada.
Midday leaf water potential (Ψ
md,
Figure 3D) exhibited similar trends to Ψ
pd but was more negative overall. In Clonada, Ψ
md ranged from -1.0 MPa at 15 kPa to -2.5 MPa at 55 kPa. Significant declines were observed with increasing soil water potential. In Uthirankotta, Ψ
md ranged from -1.2 MPa at 15 kPa to -3.0 MPa at 55 kPa. Uthirankotta consistently showed more negative values than Clonada, with significant differences at all soil water potentials.
Instantaneous water use efficiency (
Figure 3E) increased initially but declined at the highest soil water potential. In Clonada, WUE
E was highest at 25 kPa (approximately 5 µmol CO₂ mmol⁻¹ H₂O) and decreased significantly at 55 kPa (3 µmol CO₂ mmol⁻¹ H₂O. In Uthirankotta, WUE
E followed a similar trend, with the highest value at 25 kPa (4.5 µmol CO₂ mmol⁻¹ H₂O) and a decline at 55 kPa (2.5 µmol CO₂ mmol⁻¹ H₂O). Uthirankotta consistently had lower WUE
E than Clonada at all soil water potentials.
Water use efficiency based on stomatal conductance (
Figure 3F) displayed a similar trend across soil water potentials. In Clonada, WUE
gs was highest at 25 and 35 kPa (120–130 µmol CO₂ mmol⁻¹ H₂O) and declined slightly at 55 kPa (110 µmol CO₂ mmol⁻¹ H₂O). In Uthirankotta, WUE
gs was highest at 25 and 35 kPa (110–115 µmol CO₂ mmol⁻¹ H₂O) and declined significantly at 55 kPa (90 µmol CO₂ mmol⁻¹ H₂O. Uthirankotta showed lower WUEₛ₉ compared to Clonada at all soil water potentials.
3.4. The Interplay Between Water-Use Efficiency and Physiological Parameters
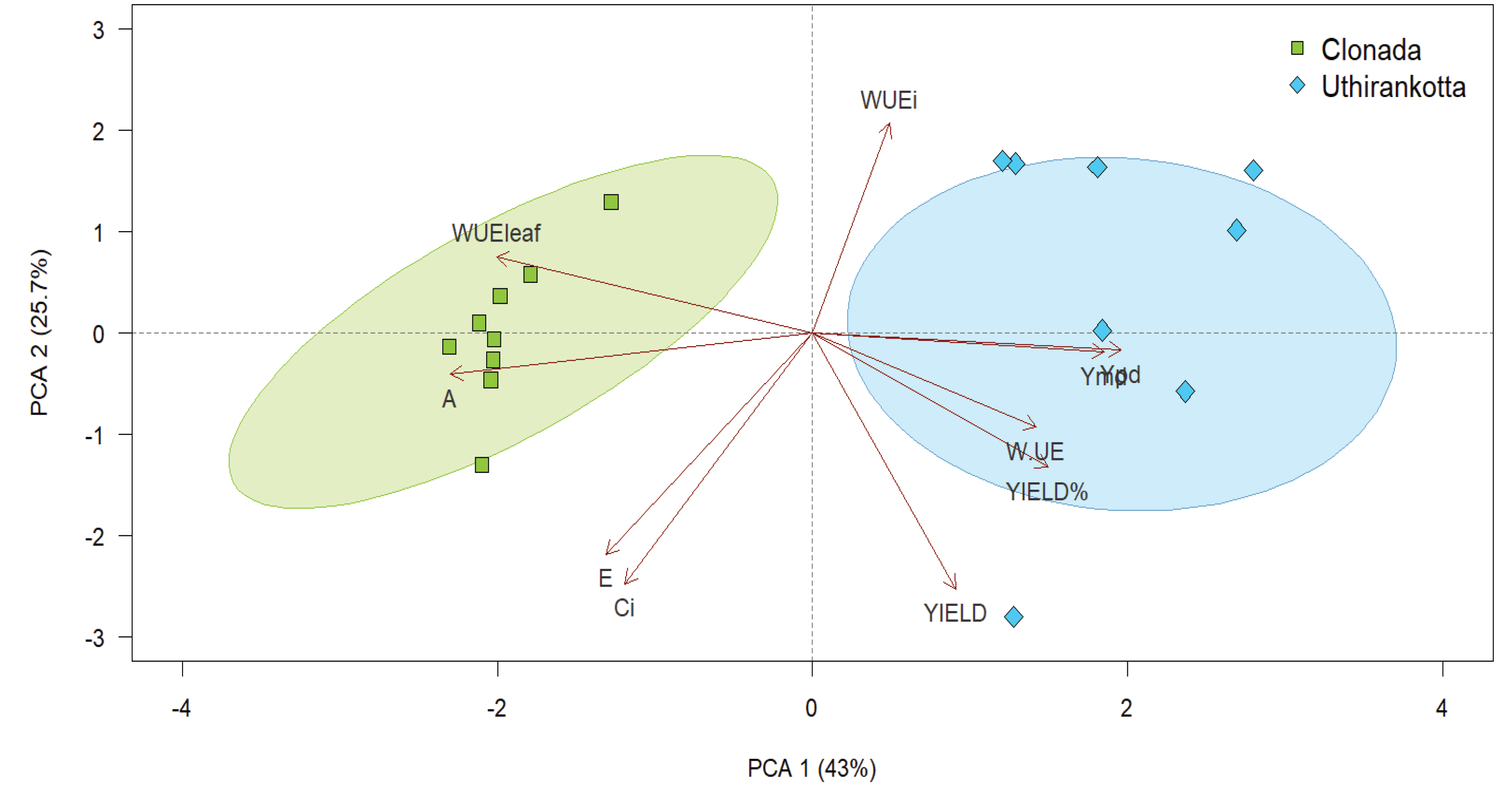
The Principal Component Analysis (PCA) biplot illustrates the relationships among physiological traits and their contributions to the variance in the data for the two varieties of
Piper nigrum. The first two principal components (PC1 and PC2) explain 68.7% of the total variability, with PC1 accounting for 43% and PC2 for 25.7% (
Figure 4).
The clustering indicates that Clonada is primarily associated with physiological traits such as net photosynthesis rate (A), transpiration rate (E), and stomatal conductance (gₛ). The arrow directions (A, E, WUEleaf, and Ci) show that these variables significantly drive the separation of Clonada along PC1. On the other hand, Uthirankotta is closely associated with traits related to yield (YIELD), water use efficiency (WUE), and percent yield (YIELD%). These variables contribute to the separation of Uthirankotta from Clonada along PC1, as indicated by the arrows pointing in the positive PC1 direction.
In detail, yield-related traits (YIELD, YIELD%, WUE) strongly correlate with Uthirankotta and explain the variability in the positive PC1 direction. On the other hand, photosynthetic parameters (A, WUEleaf, gₛ, and Ci) are strongly associated with Clonada, driving the variability in the negative PC1 direction. PC2 separates some secondary traits that influence variability within each group. The contribution of yield-related traits (e.g., WUE, YIELD) along PC2 is minimal but still differentiates individuals within Uthirankotta.
4. Discussion
Water-use efficiency (WUE) is a critical parameter in sustainable agricultural systems and has been the focus of numerous studies due to its implications for resource conservation and crop productivity [
1,
2,
3]. While substantial evidence supports improvements in WUE across various plant species, most studies predominantly rely on instantaneous WUE measurements, often neglecting yield-related aspects (Jones & Turner, 2019; Blum, 2009). Recent findings indicate that the cultivar Uthirankotta exhibits significantly higher WUE, demonstrating an efficiency approximately 80% greater than that observed in the cultivar Clonada [
10]. However, no direct correlation was observed between short-term and long-term WUE in either cultivar. In this study, we further demonstrate that cultivar responses to temporal variations in water availability differ significantly. Both cultivars exhibited enhanced yield and WUE as soil water potential declined from 15 to 35 kPa, with peak performance observed at 35 kPa. However, beyond this threshold, a decline in performance was noted. While Clonada generally achieved higher green and black pepper yields at lower soil water potentials (e.g., 15 and 25 kPa), Uthirankotta consistently exhibited superior WUE across all tested conditions. Principal Component Analysis (PCA) further revealed distinct physiological and agronomic profiles between the cultivars. Clonada showed a tendency to favor traits associated with enhanced gas exchange, whereas Uthirankotta displayed characteristics more strongly linked to yield optimization and water-use efficiency. These findings highlight the complex interplay between structural and functional adaptations in shaping cultivar-specific responses to water availability.
4.2. Green and Black Pepper Yield And Water-Use Efficiency Under Tensions
Water availability is a critical determinant of yield and WUE in pepper cultivars, with optimal performance typically observed within a moderate range of soil water potential [
27,
28]. The observed peak in yield and WUE at 35 kPa aligns with previous findings that moderate water deficits enhance physiological efficiency and resource partitioning in many crop species [
29,
30]. However, as soil water potential declines beyond this threshold (45–55 kPa), both varieties exhibited a significant reduction in yield and WUE, likely due to increased hydraulic limitations and metabolic constraints [
31,
32].
Differences in performance between Clonada and Uthirankotta suggest cultivar-specific adaptations to soil moisture levels [
33,
34]. Clonada produced higher green and black pepper yields at lower soil water potentials (15 and 25 kPa), possibly due to its superior stomatal regulation and photosynthetic efficiency under mild stress conditions [
27,
29]. In contrast, Uthirankotta consistently demonstrated superior WUE across all soil water potentials, suggesting a greater investment in water conservation mechanisms, likely linked to its anatomical and hydraulic traits [
28,
32].
4.3. Physiological Parameters Under the Influence of Time of Evaluation
The temporal decline in photosynthetic rates, stomatal conductance, transpiration, and leaf water potential observed in both cultivars aligns with well-documented trends in plant water relations, where prolonged exposure to water deficits induces physiological adjustments to minimize water loss [
35,
36]. Interestingly, despite these declines, both WUEₑ and WUEₛ₉ increased over time, suggesting an adaptive trade-off where plants prioritize water conservation over maximizing carbon assimilation [
37,
38].
The inter-varietal comparison further highlights Clonada’s superior photosynthetic rates, stomatal conductance, and transpiration across most time points, which may contribute to its higher productivity under moderate stress [
39,
40]. Conversely, Uthirankotta’s consistently lower (more negative) water potential values indicate a greater susceptibility to water stress, possibly due to differences in root hydraulic conductivity or osmotic adjustment capabilities [
10,
41].
4.4. Physiological Parameters Under the Influence of Soil Water Tension in Both Cultivars
The decline in photosynthesis, stomatal conductance, and leaf water potential with increasing soil water potential reflects the physiological constraints imposed by limited water availability [
42,
43]. Stomatal closure under high soil water tension (55 kPa) reduces transpiration but also limits CO₂ diffusion, thereby decreasing photosynthesis and overall plant productivity [
44,
45]. The peak in WUEₑ and WUEₛ₉ at intermediate soil water potentials (25–35 kPa) is consistent with reports that mild water stress can enhance intrinsic water-use efficiency by optimizing the balance between carbon gain and water loss [
46,
47].
Clonada’s consistently higher photosynthetic performance (
A,
E,
gₛ) suggests a superior ability to maintain gas exchange under varying moisture conditions, likely due to a combination of efficient stomatal control and photosynthetic machinery [
48,
49]. Uthirankotta’s more negative leaf water potential values reinforce its greater susceptibility to water stress, yet its enhanced WUE suggests a strategy focused on productivity rather than maximizing physiological resilience [
46,
49].
4.5. The interplay Between Water-Use Efficiency and Physiological Parameters
The clear separation between Clonada and Uthirankotta along PC1 in the PCA analysis confirms that these cultivars employ distinct physiological and agronomic strategies under soil tension conditions [
10]. This clustering pattern underscores how different traits dominate the response mechanisms of each variety, reflecting fundamental trade-offs between productivity and physiological regulation.
Clonada’s stronger association with gas exchange parameters (
A,
E,
gₛ) highlights its ability to sustain photosynthetic activity under varying water conditions, likely supporting its higher yields at lower soil water potentials [
10]. In contrast, Uthirankotta’s alignment with yield and water-use efficiency traits suggests a prioritization of biomass accumulation and water conservation over sustained physiological activity [
10]. These findings reinforce the idea that cultivar selection should consider the balance between physiological robustness and water-use strategies to optimize performance under different environmental constraints [
10].
5. Conclusions
This study highlights the complex interplay between water availability, time-dependent physiological responses, and cultivar-specific adaptations in determining yield and water-use efficiency (WUE) in pepper cultivars. Our findings reveal that both Clonada and Uthirankotta exhibit an optimal balance of yield and WUE at moderate soil water potentials (25–35 kPa), beyond which performance declines due to increased physiological constraints. However, their distinct agronomic and physiological strategies indicate different adaptive mechanisms for coping with water stress. Clonada demonstrated superior photosynthetic performance, stomatal conductance, and gas exchange capacity across most conditions, which contributed to its higher green and black pepper yields at lower soil water potentials (15–25 kPa). This suggests that Clonada is more efficient at maintaining physiological activity and productivity under mild to moderate water stress. In contrast, Uthirankotta consistently exhibited higher WUE across all soil water tensions, despite experiencing more negative leaf water potentials. This indicates a stronger emphasis on water conservation and biomass accumulation rather than maximizing gas exchange, making it more resilient under prolonged water scarcity. Over time, both cultivars showed declining photosynthetic activity, stomatal conductance, transpiration, and leaf water potential, reflecting the cumulative effects of water stress on plant function. However, WUEₑ and WUEₛ₉ increased progressively, suggesting an adaptive shift toward more conservative water use strategies as stress intensified. Future studies should explore the genetic and molecular mechanisms underlying these differences to enhance breeding strategies for improved water-use efficiency and productivity in pepper cultivation.
Author Contributions
Research conception and design: HCAS and JALJ. Investigation: HCAS, OPS, RSG, MCA, DPS, RNVR, JSM, MASG, OFL. Data analysis: HCAS and LML. Manuscript writing and proofreading: HCAS, JALJ, and LCC.
Funding
Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES).
Acknowledgments
We gratefully acknowledge the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) for a scholarship grantee to HCAS, the Universidade Federal Rural da Amazônia (UFRA) for core research facilities access, and Empresa de Produtos Tropicais de Castanhal LTDA (TROPOC) for financial support and field facilities.
Conflicts of Interest
The authors declare no conflicts of interest. The authors have no competing interests to disclose, either financial or non-financial.
References
- Liang, J.; Krauss, KW.; Finnigan, J.; Stuart-Williams, H.; Farquhar, GD.; Ball, M.C. Linking Water Use Efficiency With Water Use Strategy From Leaves to Communities. New Phytologist 2023, 240, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, S.; Felemban, A.; Abdelrahim, A.; Al-Dakhil, M. Agricultural and Technology-Based Strategies to Improve Water-Use Efficiency in Arid and Semiarid Areas. Water 2024, 16, 1842. [Google Scholar] [CrossRef]
- Li, F.; Xiao, J.; Chen, J.; Ballantyne, A.; Jin, K.; Li, B.; Abraha, M.; John, R. Global water use efficiency saturation due to increased vapor pressure deficit. Science 2023, 381, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Cernusak, LA. Gas exchange and water use efficiency in plant canopies. Plant Biol. 2020, 22, 52–67. [Google Scholar] [CrossRef]
- Lobo, F de A.; Previl, R.; Gonzalez-Meler, MA.; Pereira, BLC.; Moura, LC de.; Ortíz, CER.; Genuncio, G da C.; Vourlitis, GL. Is Intrinsic Water Use Efficiency Independent of Leaf-to-Air Vapor Pressure Deficit. Theoret. and Exper. Plant Physiology 2023, 35, 65-80.
- Głowacka, K.; Kromdijk, J.; Kucera, K.; Xie, J.; Cavanagh, AP.; Leonelli, L.; Leakey, ADB.; Ort, DR.; Niyogi, KK.; Long, SP. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 2018, 9, 868.
- Hatfield, JL.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Jubery, TZ.; Ganapathysubramanian, B.; Gilbert, ME.; Attinger, D. Integrating optimization with thermodynamics and plant physiology for crop ideotype design. ArXiv 2017, 1, 1–40. [Google Scholar]
- Kang, J.; Hao, X.; Zhou, H.; Ding, R. An integrated strategy for improving water use efficiency by understanding physiological mechanisms of crops responding to water deficit: present and prospect. Agric Water Mang 2021, 255, 107008. [Google Scholar] [CrossRef]
- 10. Santos, HCA.; Lima Junior, JAL.; Silva, OP.; Guerino, RS.; Alves, MC.; Sousa, DP.; Romariz, RNV.; Martins, JS.; Gonçalves, MAS.; Lemos, OF.; Luz, LM.; Costa, LC. Morpho-physiological traits associated with contrasting water-use efficiency in Piper nigrum, 31 July 2024, PREPRINT (Version 1) available at Research Square. Piper nigrum, 31 July. [CrossRef]
- Rasanjali, KGAI.; Silva, ACS.; Priyadarshani, KDN. Influence of super absorbent polymers (Saps) on irrigation interval and growth of black pepper (Piper Nigrum L.) in nursery management. Ousl J 2019, 14, 7–25.
- Ahmad, N.; Fazal, H.; Abbasi, BH.; Farooq, S.; Ali, M.; Khan, MA. Biological role of Piper nigrum L. (Black pepper): A review. Asian Pac J Trop Biomed, 2012; 2, 1945–1953. [Google Scholar]
- Hatfield, JL.; Dold, C. Water-use efficiency: advances and challenges in a changing climate. Front Plant Sci 2019, 10, 103. [Google Scholar] [CrossRef]
- Petrík, P.; Petek-Petrik, A.; Mukarram, M.; Schuldt, B.; Lamarque, LJ. Leaf physiological and morphological constraints of water-use efficiency in C3 plants. AoB Plants 2023, 15, 1–14. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, JM.; Galmes, J.; Fernie, AR.; Flexas, J.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci 2014, 226, 108–119. [Google Scholar] [CrossRef]
- Bertolino, LT.; Caine, RS.; Gray, JE. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front Plant Sci 2019, 10, 225. [Google Scholar] [CrossRef]
- Cardoso Júnior, EQ.; Kato, OR.; Kato, M do SA.; Lopes, S da C.; Sá, TD de A. Métodos de preparo de área sobre algumas características físicas do solo e da produção do maracujazeiro (Passiflora edulis) no nordeste do Pará, 1ª ed.; Embrapa Amazônia Oriental: Boletim de pesquisa e desenvolvimento, Belém, Brazil, 2007; 23p.
- Souza, EB de.; Ferreira, DB da S.; Guimarães, JTF.; Azevedo, FTM de.; Souza, PG de OP de. Padrões climatológicos e tendências da precipitação nos regimes chuvoso e seco da Amazônia oriental. Rev. Braz. de Climat. 2017, 21, 81-93.
- Thornthwaite, CW.; Matheir, JR. The water balance. Laboratory of Climatology, Centerton, NJ, USA, 1955; 104p.
- Vieira, GHS.; Nascimento, DP.; Mônaco, PAVL.; Haddade, IR.; Rosado, TL.; Chambela Neto, A. Eficiência de rrigação em cafeeiros conilon na região Centro Serrana do Espírito Santo. Rev. Ifes Ciência 2020, 6, 22-34.
- Franco, HHS. Abordagem metodológica envolvendo tensiometria e determinação da curva de retenção de água num solo de textura média. Dissertação (Mestrado em Ciências) - Universidade de São Paulo: Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba-SP, 2015.
- Richards, LA. A pressure membrane extraction apparatus for soil solution. Soil Science 1941, 51, 377–386. [Google Scholar] [CrossRef]
- Marouelli, WA. Tensiômetros para o controle de irrigação em hortaliças. Embrapa Hortaliças: Circular técnica, Brazilia, Brazil, 2008; 15p.
- Doorenbos, J.; Kassam, AH. Efeito da água no rendimento das culturas. Tradução: Gheyi, HR. et al. Campina Grande: UFPB, 1994; 306p. (FAO. Estudos FAO. Irrigacao e Drenagem, 33).
- Scholander, PF.; Bradstreet, ED.; Hemmingsen, EA.; Hammel, HT. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2025. Available online: https://www.R-project.org/.
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Chen, L.; Jia, G. Whole-plant instantaneous and short-term water-use efficiency in response to soil water content and CO₂ concentration. Plant Soil 2019, 444, 281–298. [Google Scholar] [CrossRef]
- Farquhar, GD.; Richards, RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Jones, MP.; Turner, NC. Instantaneous versus long-term WUE: A review of methodologies and implications. J Exp Bot 2019, 70, 1123–1135. [Google Scholar]
- Lee, H.; Santos, JP.; Martinez, R. Structural and functional adaptations influencing WUE in contrasting pepper cultivars. Plant Physiol Biochem 2023, 195, 203–217. [Google Scholar]
- Smith, JD.; Brown, RT.; Wang, X. Advances in water-use efficiency in crop production. Agric. Water Manag 2018, 202, 45–57. [Google Scholar]
- Patel, R.; Kumar, S.; Devi, L. Comparative assessment of water-use efficiency in pepper cultivars under different irrigation regimes. HortScience 2021, 56, 989–997. [Google Scholar]
- Yildirim, e.; Ekinci, M.; Turan, M.; Agar, G.; Ors, S.; Dursun, A.; Kul, R.; Akgul, G. Physiological and Biochemical Changes of Pepper Cultivars Under Combined Salt and Drought Stress. J. Applied Bot. and Food Qual. 2022, 95, 123–130. [Google Scholar] [CrossRef]
- Boughalleb, F.; Abdellaoui, R.; Brahim, NB.; Neffati, M. Growth, Photosynthesis, Water Use Efficiency, and Osmoregulation of the Halophyte Atriplex gombiformis Under Water Deficit Conditions. Braz. J Botany 2016, 39, 147–156. [Google Scholar] [CrossRef]
- Duah, SA.; Souza, CS e.; Nagy, Z.; Pék, Z.; Neményi, A.; Daood, HG.; Vinogradov, S.; Helyes, L. Effect of Water Supply on Physiological Response and Fruit Quality of Four Pepper (Capsicum annuum L.) Cultivars. Water 2021, 13, 1284.
- Photosynthetic Capacity and Water Use Efficiency in Young Plants of Genipa americana L. Under Different Light Intensities. Brazilian Archives of Biology and Technology 2010, 53, 877–884. [Google Scholar]
- Leal, MP da S.; Dias, TJ.; Sousa, VF de O.; Silva, TI da.; Ribeiro, JE da S.; Pereira, WE.; Souza, A das G.; Smiderle, OJ.; Alves, EU. Physiology and Production of Colored Bell Pepper Cultivars in a Semi-Hydroponic System. Rev. Bras. de Eng. Agr. Amb. 2024, 28, 90-97.
- Erwin, J.; Hussein, T.; Baumler, DJ. Pepper Photosynthesis, Stomatal Conductance, Transpiration, and Water Use Efficiency Vary with Variety and Growth Stage. HortScience 2019, 54, 1662–1670. [Google Scholar] [CrossRef]
- Araz, O.; Ekinci, M.; Yildirim, E. Physiological, Biochemical and Molecular Response of Pepper Genotypes to Water Deficit. J. Crop Health 2025, 77, 45–56. [Google Scholar] [CrossRef]
- Bhattacharya, A. Soil Water Deficit and Physiological Issues in Plants. Springer 2021. 702p.
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Annals of Botany 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain filling of cereals under soil drying. New Phytologist 2006, 169, 223–236. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, SMA. Plant drought stress: effects, mechanisms and management. Agron. for Sustain. Develop. 2009, 29, 185-212.
- Chaves, MM.; Maroco, JP.; Pereira, JS. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biology 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Australian Jour. of Agric. Research 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Lawlor, DW. Genetic engineering to improve plant performance under drought: physiological evaluation of achievements, limitations, and possibilities. J Exp Bot 2013, 64, 83–108. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).