Submitted:
14 April 2025
Posted:
14 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Strain Isolation
2.2. Morphology and Maximum Growth Temperature
2.3. DNA extraction, PCR Amplification, and Sequencing
2.4. Phylogenetic Analyses
3. Results
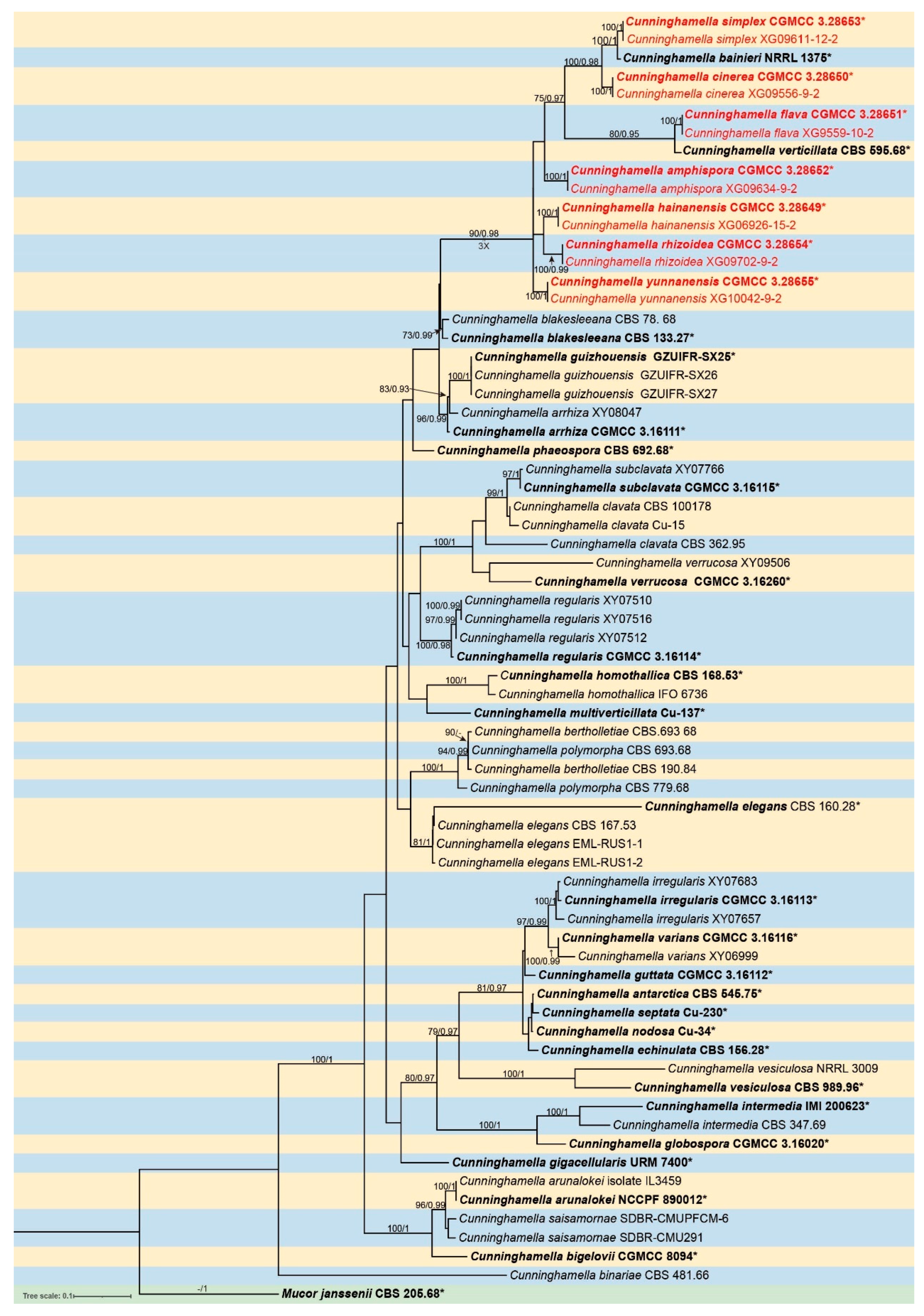
3.1. Phylogeny
3.2. Taxonomy
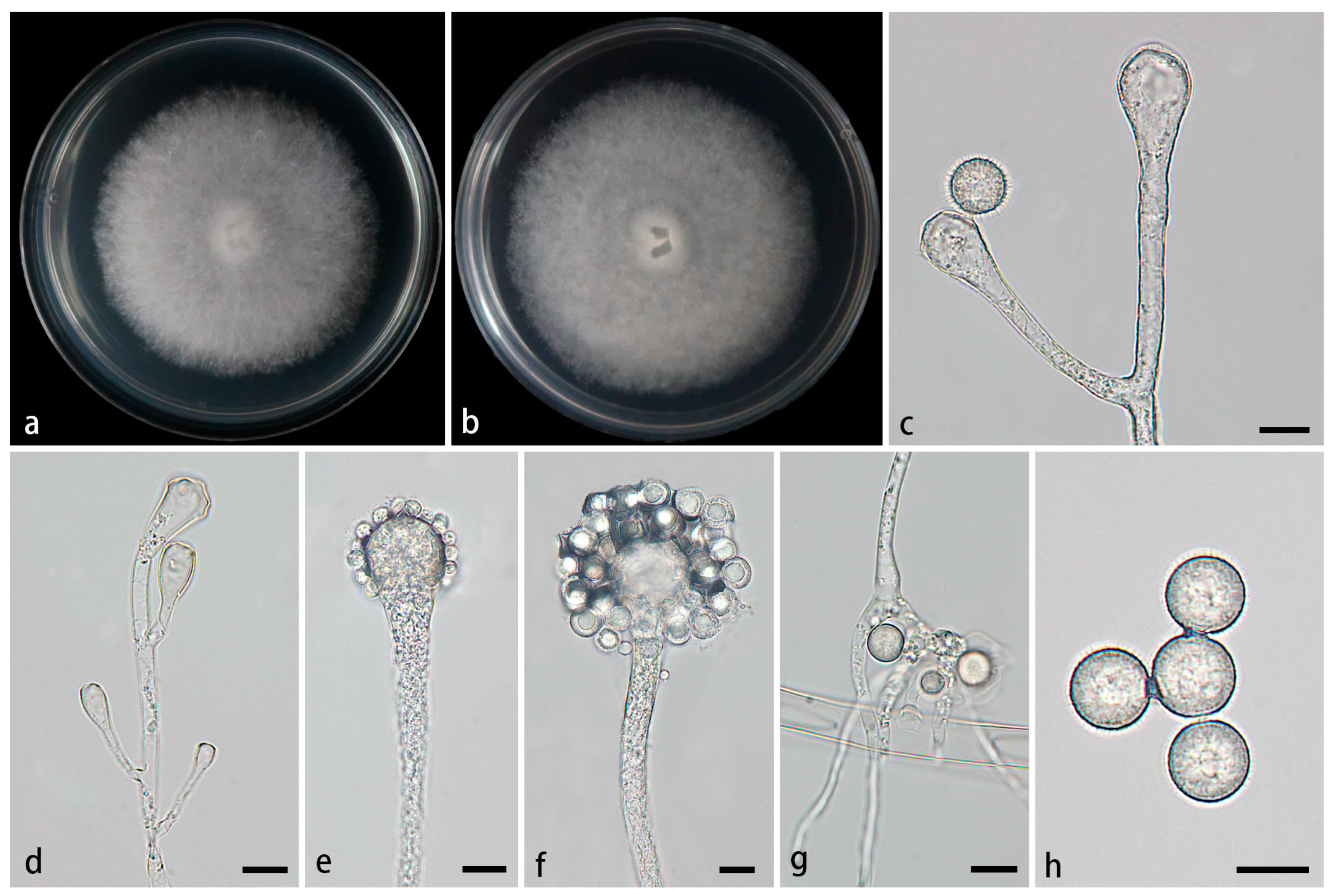
3.2.1. Cunninghamella amphispora Z.Y. Ding & X.Y. Liu, sp. nov., Figure 2.
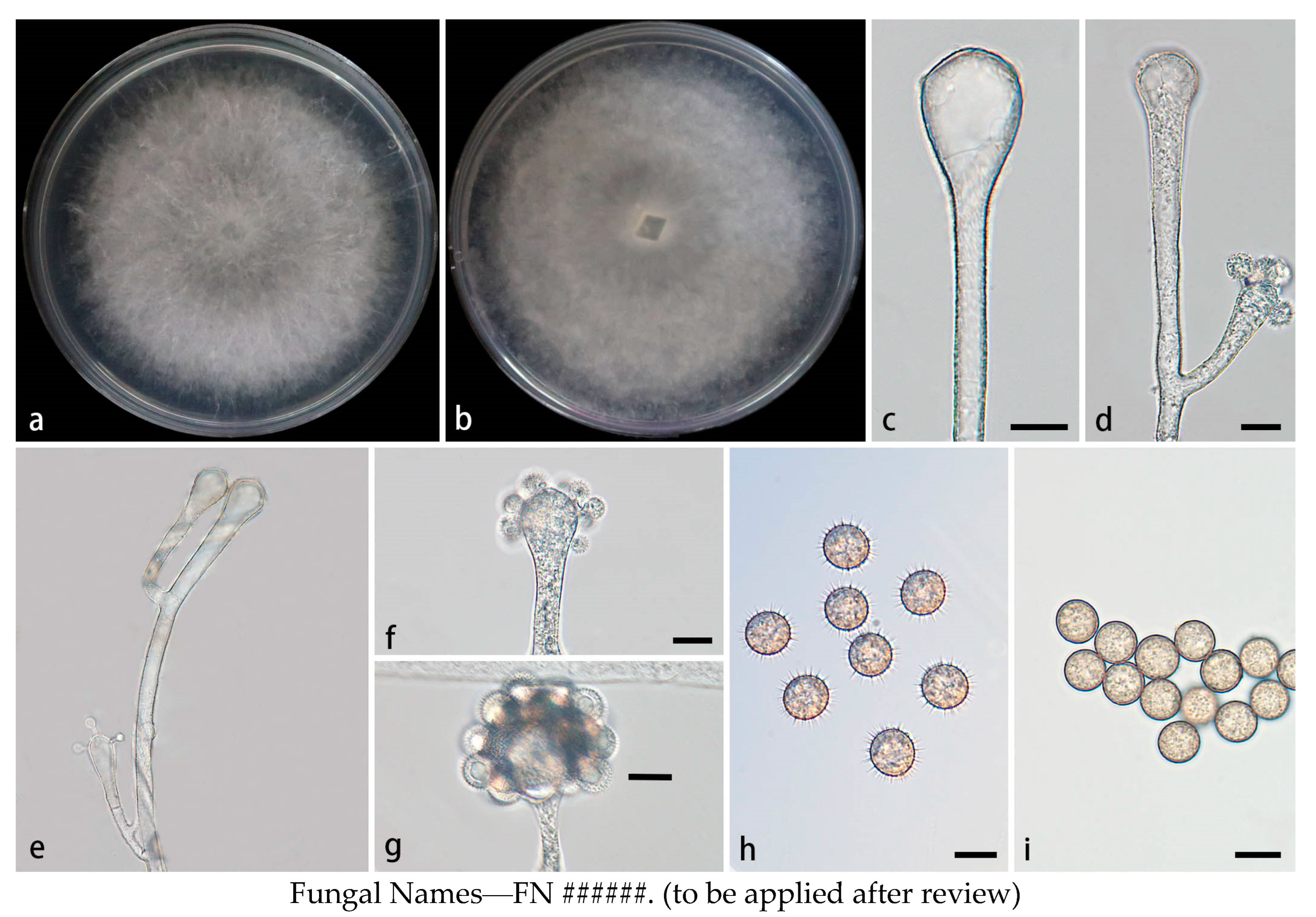
3.2.2. Cunninghamella cinerea Z.Y. Ding & X.Y. Liu, sp. nov., Figure 3.
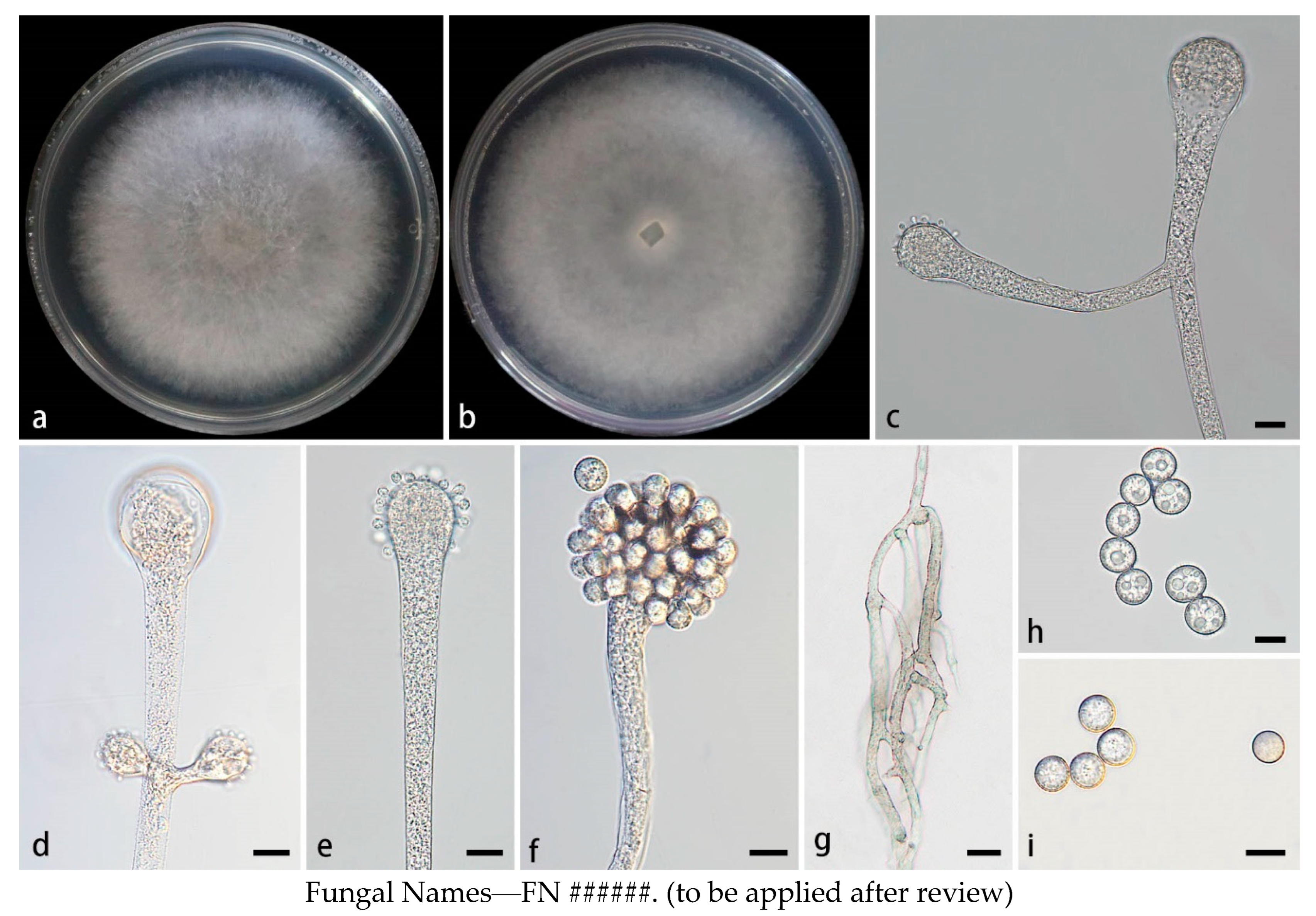
3.2.3. Cunninghamella flava Z.Y. Ding & X.Y. Liu, sp. nov., Figure 4.
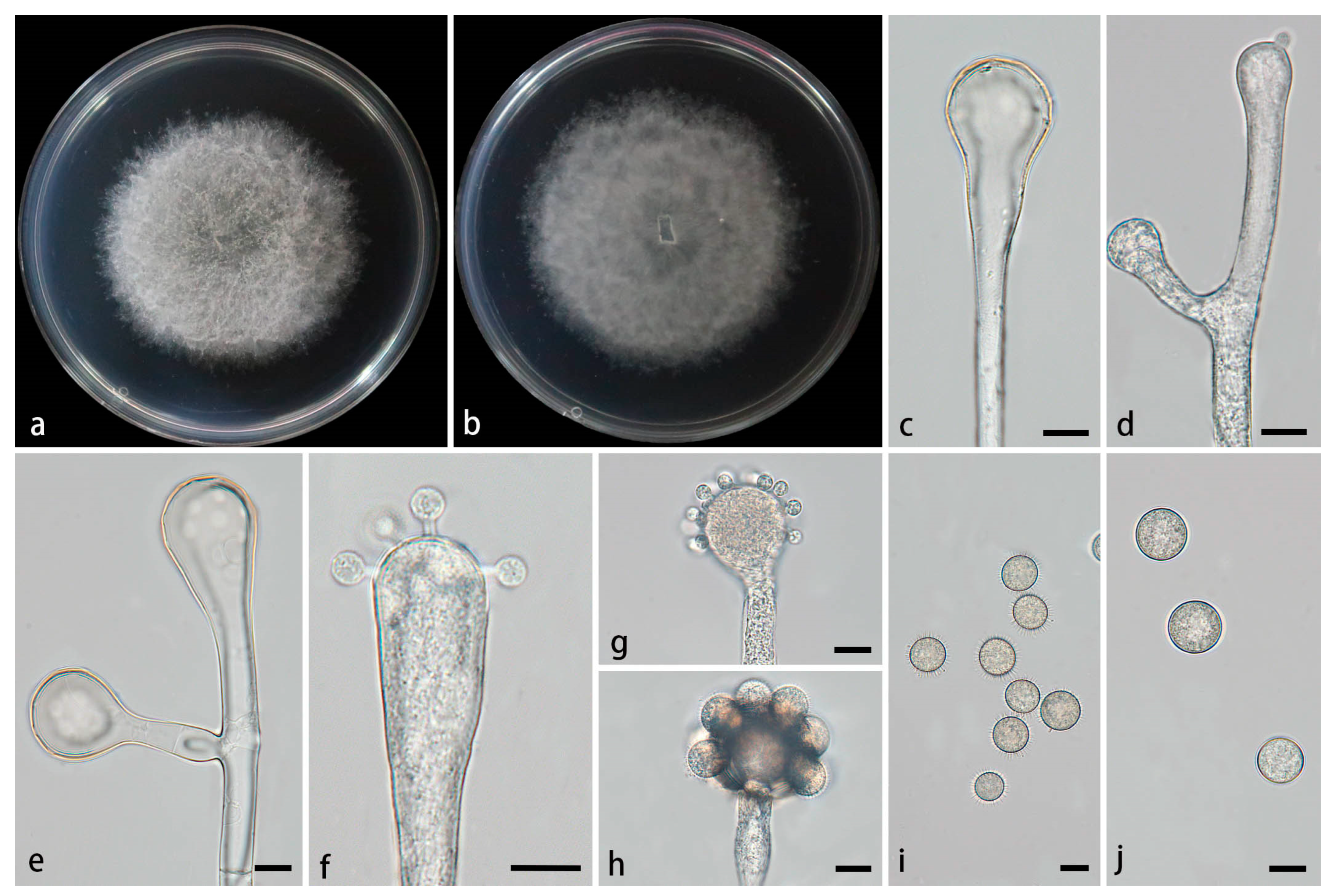
3.2.4. Cunninghamella hainanensis Z.Y. Ding & X.Y. Liu, sp. nov., Figure 5.
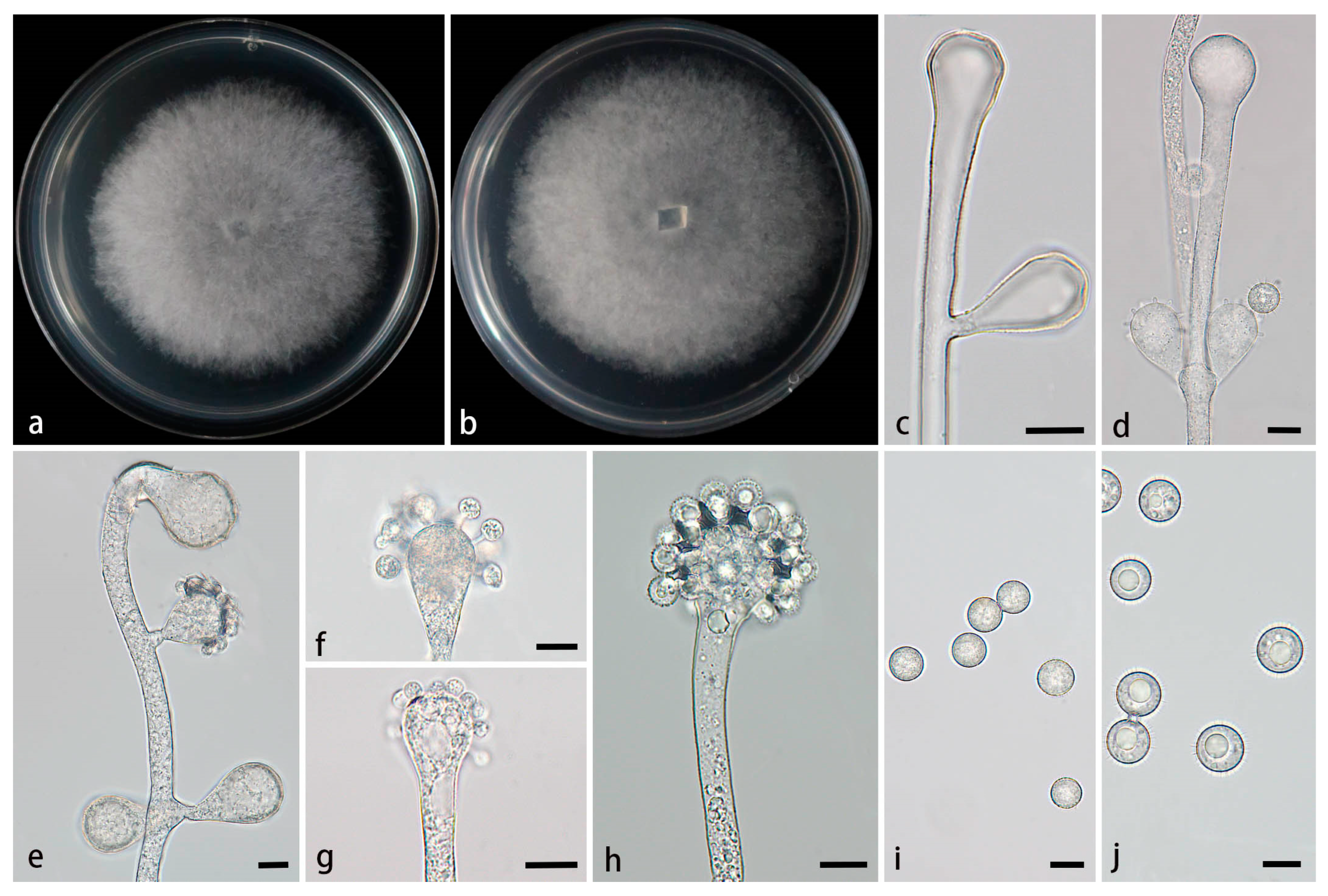
3.2.5. Cunninghamella rhizoidea Z.Y. Ding & X.Y. Liu, sp. nov., Figure 6.
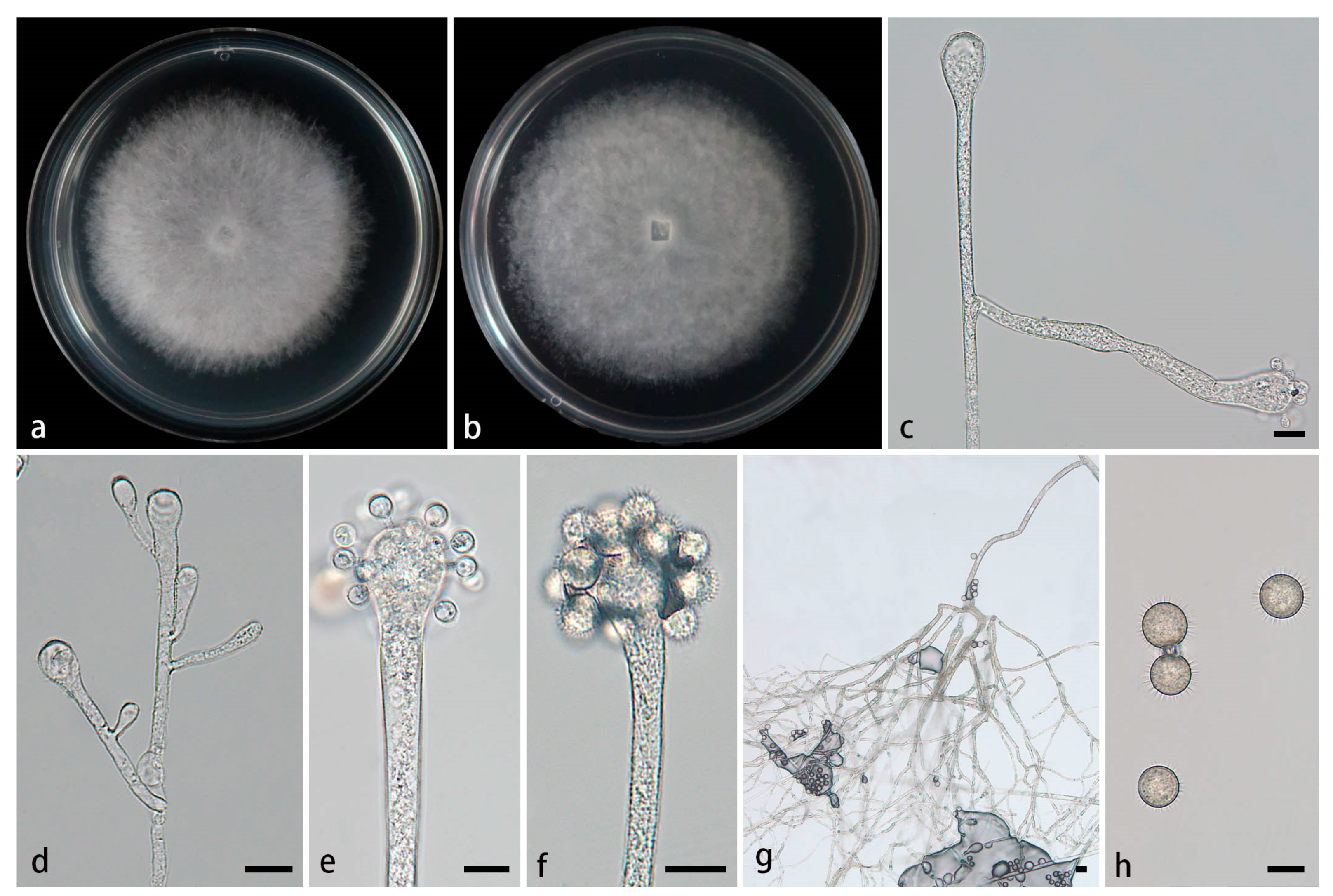
3.2.6. Cunninghamella simplex Z.Y. Ding & X.Y. Liu, sp. nov., Figure 7.
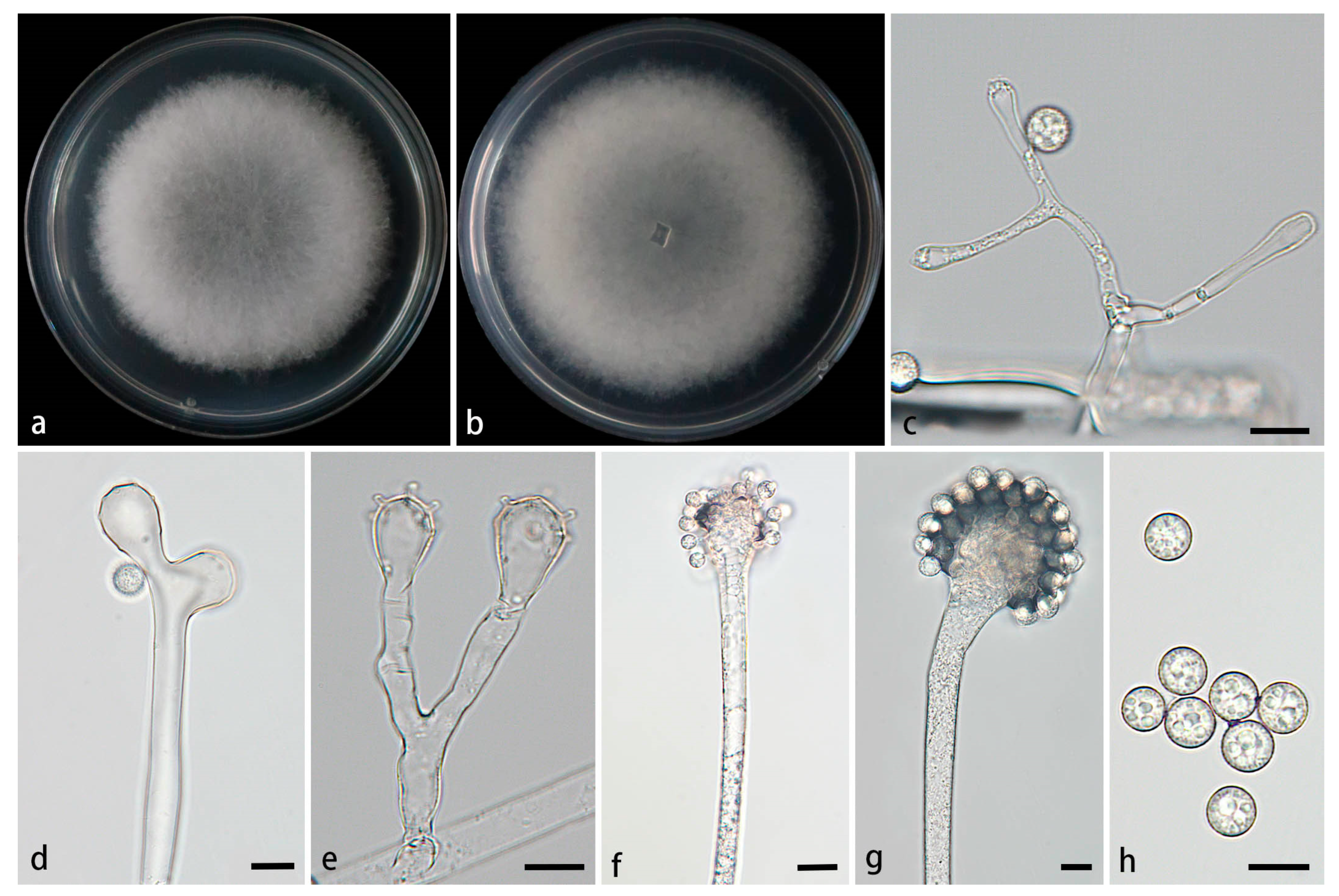
3.2.7. Cunninghamella yunnanensis Z.Y. Ding & X.Y. Liu, sp. nov., Figure 8.
4. Discussion
| Species | Colonies | Sporangiophores | Vesicles | Pedicels | Sporangiola | Reference |
| C. hainanensis | PDA: 26°C 4 d, 85 mm, 21.25 mm/d, initially white, gradually becoming light gray, floccose | 2.9-10.6 µm wide, mostly erect, a few slightly bent, occasionally verticillate, unbranced or 1–3 branched, opposite, in pairs | spherical, hemispherical, elliptic, terminal vesicles 9.4–27 × 7.9–26.5µm,lateral vesicles 19.6–28.4 × 12.2–22.9 µm | 1.2–5.8 µm long | mostly spherical, 7.3–14 × 7.8–13.4µm, with or without spines, 0.7–1.9 µm long | This study |
| C.cinerea | PDA: 26°C 4 d, 85 mm, 21.25 mm/d, initially white, gradually turning to smoky gray with age, floccose | 3.1–11.7 µm wide, erect or slightly bent, mainly unbranched or simply branched, mainly single or recumbent, never verticillate | spherical to elliptic, terminal vesicles 9.6–32.3 × 6.9–28.3 µm,lateral vesicles 11.9–20.3 ×9.9–17.3 µm | 1.2–2.2 µm long | globose to ovoid, 5.5–16.7 c × 4.9–15.5 µm, with or without spines, 0.8–1.7 µm long | This study |
| C. flava | PDA: 26°C 6 d, 72 mm, 12 mm/d, initially white, gradually turning to dry yellow with age, floccose | 3.8–25.6 µm wide, erect or slightly bent, unbranched or 1–7 branched, recumbent, opposite, in pairs, 1–3 verticillate | globose, pear-shaped, elliptic, terminal vesicles 12.8–43.1 ×11.3–44.5 µm, ateral vesicles 9.3–28.1 × 9.4–25.3 µm | 1.6–2.2 µm long | globose to ovoid, 8.8–18.6 × 8.6–18.7 µm wide, with or without spines, 1.4–2.8 µm long | This study |
| Species | Colonies | Sporangiophores | Vesicles | Pedicels | Sporangiola | Reference |
| C.amphispora | PDA: 26°C 4 d, 85 mm, 21.25 mm/d, initially white, gradually becoming light gray, floccose | 2.3–19.1 µm wide, erect or few slightly bent, unbranced or simply branched, hyaline, single, no verticillate | hemispherical, spherical, oval, pillar-shaped, terminal vesicles 6.4–56.6 × 3.2–46.7 µm, lateral vesicles 8–18.9 × 5.1–14.8 µm | 1.4–3.4 µm long | Globose, 6.3–16.7 × 6.3–15.8 µm, with or without spines, 0.5–2.6 µm long | This study |
| C. simplex | PDA: 26°C 4 d, 85 mm, 21.25 mm/d, nitially white, gradually turning to dusky gray, floccose | 3.1–12.2 µm wide, erect or slightly bent, hyaline, unbranced or simply branched, in pairs, never verticillate | pherical, oval, cylindrical, terminal vesicles 5.0–28.4 ×4.1–25.8 µm, lateral vesicles 6.7–17.0 ×3.8–10.8 µm | 1.1–2.9 µm long | spherical to oval, 5.8–10.2 × 5.5–9.7 µm | This study |
| C. rhizoidea | PDA: 26°C 4 d, 85 mm, 21.25 mm/d, initially white, gradually becoming gray with age, floccose | .8–11.4 µm wide, erect or slightly bent, simple branches or ccasionally multiple branches, recumbent, opposite, occasionally verticillate | globose, club-shaped, terminal vesicles 7.9–35.3 × 4.9–32.0 µm; lateral vesicles 2.7–26.6 ×2.4–27.1 µm | 1.5–5.4 µm long | globose to ovoid,6.2–13.4 × 5.6–12.9 µm, with short spines, 1.3–2.0 µm long | This study |
| C. yunnanensis | PDA: 26°C 4 d, 85 mm, 21.25 mm/d, initially white, gradually becoming light gray with age, floccose | 1.8–14.6 µm wide, rect or few slightly bent, mainly unbranched, or occasionally 1–7 branched, straight or recumbent, few verticillate | emispherical, spherical, oval, elliptic, terminal vesicles 7.1–33.8 × 4.4–33.5 µm; lateral vesicles 5.5–13.9 × 3.2–12.4 µm | 1.1–4.1 µm long | mainly globose, sometimes oval, 3.3–12.4 × 3.3–12.0 µm wide, with short spines, 0.7–2.0 µm long | This study |
| C. bainieri | SMA: 27°C 4 d, 90mm, at first white, soon becoming light grey, grey, to ‘Light Mouse Grey’, reverse cream, floccose | rect, bent, or recumbent, main axes of sporangiophores (8–) 11–21µm wide; primary branches (1–) 4–10 (–18) µm wide, monopodial, pseudoverticillate or verticillate in 1–2(–3) whorls of 3–8, typically in pairs | globose, subglobose to ovoid, sometimes irregular, axial vesicles 18.5–32(–40) μm; lateral ones (8–)13.5–30 μm | 2.5–3.5 (–6) um long | ovoid to ellipsoid and 7–14.5 (–20) × 6.5-11 (–14.5) µm globose and 5.5–12.5µm, lacrymoid and 9–20 (–32.5) × 7–14.5 (–20) µm | [2] |
| Species | Colonies | Sporangiophores | Vesicles | Pedicels | Sporangiola | Reference |
| C. verticillata | SMA: 28°C 5–6 d, 90mm, at first white, from the sixth day near ‘Avellaneous’ to near ‘Colonial Buff’, revcrse yellowish cream, floccose | erect, straight or recumbent, main axes of sporangiophores 7.5–17.5 (–25) µm; branches (0–) 4-15 (–25), verticillate to pseudoverticillate, rarely singly or in pairs, mostly simple, very rarely re-branched | axial ones slightly depressed-globose, subglobose to globose, 225–50 (–70) μm; lateral ones usually globose to subglobose, sometimes broadly ovoid, 8 5–27.5 (–32.5) μm | 2.5–4 (–6.5) um long | Two kinds: globose, broadly ellipsoid ovoid and bluntly pointed at one end, 6–17.5 (–20) × 5.5–13.5 (–17.5) µm; dark giant sp orangiola, globose, 11.5-17.5 (-25) µm | [2] |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; L azarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; James, T.Y.; OʹDonnell, K.; Roberson, R.W.; Taylor, T.N.; Uehling, J.; Vilgalys, R.; White, M.M.; Stajich, J.E. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia. 2016, 108, 1028–1046. [CrossRef]
- Zheng, R.Y.; Chen, G.Q. A monograph of Cunninghamella. Mycotaxon. 2001, 80, 1–75.
- Walther, G.; Pawłowska, J.; Alastruey-Izquierdo, A.; Wrzosek, M.; Rodriguez-Tudela, J.L.; Dolatabadi, S.; Chakrabarti, A.; de Hoog, G.S. DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia. 2013, 30, 11–47. [CrossRef]
- Matruchot, L. Une Mucorinée purement conidienne, Cunninghamella africana. Annales Mycologici. 1903, 1, 45–60.
- Baijai, U.; Mehrotra, B.S. The genus Cunninghamella - a reassessment. Sydowia. 1980, 33, 1–13.
- Reed, M.D.A.; Body, P.B.; Austin, M.D.M.B.; Jr Frierson, M.D.H. Cunninghamella bertholletiae and Pneumocystis carinii pneumonia as a fatal complication of chronic lymphocytic leukemia. Human Pathology. 1988, 19, 1470–1472. [CrossRef]
- Nguyen, T.T.T.; Choi, Y.J.; Lee, H.B. Zygomycete fungi in Korea: Cunninghamella bertholletiae, Cunninghamella echinulata, and Cunninghamella elegans. Mycobiology 2017, 45, 318–326. [CrossRef]
- Gomes, M.Z.; Lewis, R.E.; Kontoyiannis, D.P. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia. Clin. Microbiol. Rev. 2011, 24, 411–45.
- Kwon-Chung, K.J. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin. Infect. Dis. 2012, 54 Suppl 1, S8–S15. [CrossRef]
- Yu, J.; Walther, G.; Van Diepeningen, A.D.; Gerrits Van Den Ende, A.H.G.; Li, R.Y.; Moussa, T.A.A.; Almaghrabi, O.A.; De Hoog, G.S. DNA barcoding of clinically relevant Cunninghamella species. Med. Mycol. 2015, 53, 99–106. [CrossRef]
- Fakas, S.; Papanikolaou, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis. G. Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J. Appl. Microbiol. 2008, 105, 1062–1070. [CrossRef]
- Alakhras, R.; Bellou, S.; Fotaki, G.; Stephanou, G.; Demopoulos, N. A.; Papanikolaou, S.; Aggelis, G. Fatty acid lithium salts from Cunninghamella echinulata have cytotoxic and genotoxic effects on HL-60 human leukemia cells. Eng. Life. Sci. 2015, 15, 243–253. [CrossRef]
- Zhao H, Lv ML, Liu Z, et al. High-yield oleaginous fungi and high-value microbial lipid resources from Mucoromycota. Bioenerg. Res. 2020, 14, 1196–1206. [CrossRef]
- Liu, C.W.; Liou, G.Y.; Chien, C.Y. New records of the genus Cunninghamella (Mucorales) in Taiwan. Fungal Science. 2005, 20, 1–9.
- Guo, J.; Wang, H.; Liu, D.; Zhang, J.N.; Zhao, Y.H.; Liu, T.X.; Xin, Z.H. Isolation of Cunninghamella bigelovii sp. nov. CGMCC 8094 as a new endophytic oleaginous fungus from Salicornia bigelovii. Mycol. Prog. 2015, 14, 1–8. 11. [CrossRef]
- Alves, A.L.; de Souza, C.A.; de Oliveira, R.J.; Cordeiro, T.R.; de Santiago, A.L. 2017 Cunninghamella clavata from Brazil: a new record for the western hemisphere. Mycotaxon. 2017, 132, 381–389.
- Bragulat, M.R.; Castella, G.; Isidoro-Ayza, M.; Domingo, M.; Cabanes, F.J. Characterization and phylogenetic analysis of a Cunninghamella bertholletiae isolate from a bottlenose dolphin (Tursiops truncatus). Revista Iberoamericana de Micología 2017, 34, 215–219. [CrossRef]
- Weitzman, I.; Crist, M.Y. Studies with clinical isolates of Cunninghamella II. Physiological and morphological studies. Mycologia 1980, 72, 661–9.
- Liu, X.Y.; Huang, H.; Zheng, R.Y. Relationships within Cunninghamella based on sequence analysis of ITS rDNA. Mycotaxon 2001, 80, 77–95.
- Hyde, K.D.; Hongsanan, S.; Jeewon, R.; Bhat, D.J.; McKenzie, E.H.C.; Jones, E.B.G.; Phookamsak, R.; Ariyawansa, H.A.; Boonmee, S.; Zhao, Q.; Abdel-Aziz, F.A.; Abdel-Wahab, M.; Banmai, S.; Chomnunti, P.; Cui, B.K.; Daranagama, D.A.; Das, K.; Dayarathne, M.C.; De Silva, N.I.; Dissanayake, A.J.; Doilom, M.; Ekanayaka, A.H.; Gibertoni, T.B.; Góes-Neto, A.; Huang, S.K.; Jayasiri, S.C.; Jayawardena, R.S.; Konta, S.; Lee, H.B.; Li, W.J.; Lin, C.G.; Liu, J.K.; Lu, Y.Z.; Luo, Z.L.; Manawasinghe, I.S.; Manimohan, P.; Mapook, A.; Niskanen, T.; Norphanphoun, C.; Papizadeh, M.; Perera, R.H.; Phukhamsakda, C.; Richter, C.; De Santiago, A.L.C.M.; Drechsler-Santos, E.R.; Senanayake, I.C.; Tanaka, K. Fungal diversity notes 367-490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 2016, 80, 1–270. [CrossRef]
- Suwannarach, N.; Kumla, J.; Supo, C.; Honda, Y.; Nakazawa, T.; Khanongnuch, C.; Wongputtisin, P. Cunninghamella saisamornae (Cunninghamellaceae, Mucorales), a new soil fungus from northern Thailand. Phytotaxa 2021,509, 291–300. [CrossRef]
- Zhang, Z.Y.; Zhao, Y.X.; Shen, X.; Chen, W.H.; Han, Y.F.; Huang, J.Z.; Liang, Z.Q. Molecular phylogeny and morphology of Cunninghamella guizhouensis sp. nov. (Cunninghamellaceae, Mucorales), from soil in Guizhou, China. Phytotaxa 2020, 455, 31–39. [CrossRef]
- Wang, Y.J.; Zhao, T.; Wu, W.Y.; Wang, M.; Liu, X.Y. Cunninghamella verrucosa sp. nov. (Mucorales, Mucoromycota) from Guangdong province in China. Phytotaxa 2022, 560: 274–284.
- Zhao, H.; Zhu, J.; Zong, T.K.; Liu, X.L.; Ren, L.Y.; Lin, Q.; Qiao, M.; Nie, Y.; Zhang, Z.D.; Liu, X.Y. Two new species in the family Cunninghamellaceae from China. Mycobiology 2021, 49, 142–150. [CrossRef]
- Zhao, H.; Nie, Y.; Zong, T.; Wang, K.; Lv, M.; Cui, Y.; Tohtirjap, A.; Chen, J.; Zhao, C.; Wu, F.; Cui, B.; Yuan, Y.; Dai, Y.; Liu, X.Y. Species diversity, updated classification and divergence times of the phylum Mucoromycota. Fungal Diversity 2023, 123, 49–157. [CrossRef]
- Zhao, H.; Nie, Y.; Huang, B.; Liu, X.Y. Unveiling species diversity within early-diverging fungi from China I: three new species of Backusella (Backusellaceae, Mucoromycota). MycoKeys 2024, 109, 285–304. [CrossRef]
- Tao, M.F.; Ding, Z.Y.; Wang, Y.X.; Zhang, Z.X.; Zhao, H.; Meng, Z.; Liu, X.Y. Unveiling species diversity within early-diverging fungi from China II: Three new species of Absidia (Cunninghamellaceae, Mucoromycota) from Hainan Province. MycoKeys 2024, 110, 255–272. [CrossRef]
- Wang, Y.X.; Zhao, H.; Jiang, Y.; Liu, X.Y.; Tao, M.F.; Liu, X.Y. Unveiling species diversity within early-diverging fungi from China III: Six new species and a new record of Gongronella. (Cunninghamellaceae, Mucoromycota). MycoKeys 2024, 110, 287–317. [CrossRef]
- Ding, Z.Y.; Ji, X.Y.; Tao, M.F.; Jiang, Y.; Liu, W.X.; Wang, Y.X.; Meng, Z.; Liu, X.Y. Unveiling species diversity within early-diverging fungi from China IV: Four new species of Absidia (Cunninghamellaceae, Mucoromycota). MycoKeys 2025. (under review).
- Ji, X.Y.; Ding, Z.Y.; Nie, Y.; Zhao, H.; Wang, S.; Huang, B.; Liu, X.Y. Unveiling species diversity: within early-diverging fungi. From China V: Five new species of Absidia (Cunninghamellaceae, Mucoromycota) MycoKeys 2025. (under review).
- Wang, Y.X.; Ding, Z.Y.; Ji, X.Y.; Meng, Z.; Liu, X.Y. Unveiling species diversity within early-diverging fungi from China VI: Four Absidia sp. nov. (Mucorales) in Guizhou and Hainan. Microorganisms 2025. (under review).
- Li, W. X.; Wei, Y. H.; Zou, Y.; Liu, P.; Li, Z.; Gontcharov, A. A.; Stephenson, S. L.; Wang, Q.; Zhang, S. H.; Li, Y. Dictyostelid cellular slime molds from the Russian Far East. Protist 2020, 171, 125756. [CrossRef]
- Zou, Y.; Hou, J. G.; Guo, S. N.; Li, C. T.; Li, Z.; Stephenson, S. L.; Pavlov, I. N.; Liu, P.; Li, Y. Diversity of dictyostelid cellular slime molds, including two species new to science, in forest soils of Changbai Mountain, China. Microbiol. Spectr. 2022, 10, e0240222. [CrossRef]
- Zhao, H.; Nie, Y.; Zong, T.; Dai, Y.; Liu, X.Y. Three new species of Absidia (Mucoromycota) from China based on phylogeny, morphology and physiology. Diversity 2022, 14, 132. [CrossRef]
- Zhao, H.; Nie, Y.; Zong, T.; Wang, Y.; Wang, M.; Dai, Y.; Liu, X.Y. Species diversity and ecological habitat of Absidia (Cunninghamellaceae, Mucorales) with emphasis on five new species from forest and grassland soil in China. Journal of Fungi 2022, 8, 471. [CrossRef]
- Corry, J.E.L. Rose bengal chloramphenicol (RBC) agar. In: Progress in Industrial Microbiology; Elsevier: Amsterdam, The Netherlands, 1995, pp. 431–433.
- Zheng, R.Y.; Chen, G.Q.; Huang, H.; Lt g t S, Liu, X.Y. A monograph of Rhizopus. Sydowia 2007, 59, 273–372.
- Zheng, R.Y.; Liu, X.Y.; Li, R.Y. More Rhizomucor causing human mucormycosis from China: R. chlamydosporus sp. nov. Sydowia 2009, 61, 135–147.
- Zheng, R.Y.; Liu, X.Y. Taxa of Pilaira (Mucorales, Zygomycota) from China. Nova Hedwigia 2009, 88, 255–267. [CrossRef]
- Zong, T.K.; Zha, H.; Liu, X.L.; Ren, L.Y.; Zhao, C.L.; Liu, X.Y. Taxonomy and phylogeny of four new species in Absidia (Cunninghamellaceae, Mucorales) from China. Frontiers in Microbiology 2021, 12, 677836. [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [CrossRef]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000, 147, 617–630. http://doi.org/10.1046/j.1469-8137.2000.00716.x.
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; pp. 315–322.
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [CrossRef]
- O’Donnell, K.; Lutzoni, F.M.; Ward, T.J.; Benny, G.L. Evolutionary relationships among mucoralean fungi Zygomycota: Evidence for family polyphyly on a large scale. Mycologia 2001, 93, 286–297. [CrossRef]
- Zhang, Z.; Liu, R.; Liu, S.; Mu, T.; Zhang, X.; Xia, J. Morphological and phylogenetic analyses reveal two new species of Sporocadaceae from Hainan, China. MycoKeys 2022, 88, 171–192. [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [CrossRef]
- Nylander, J. MrModeltest V2: Program distributed by the author. Bioinformatics 2004, 24, 581–583. [CrossRef]
- Bauer, R.; Garnica, S.; Oberwinkler, F.; Riess, K.; Weiß, M.; Begerow, D. Entorrhizomycota: a new fungal phylum reveals new perspectives on the evolution of fungi. PLoS One 2015, 10, e0128183. [CrossRef]
- Jeewon, R.; Hyde, K.D. Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 2016, 7, 1669–1677. [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Jones, E.B.G.; McKenzie, E.H.C.; Bhat, J.D.; Dayarathne, M.C.; Huang, S.K.; Norphanphoun, C.; Senanayake, I.C.; Perera, R.H.; Shang, Q.J.; Xiao, Y.P.; D’souza, M.J.; Hongsanan, S.; Jayawardena, R.S.; Daranagama, D.A.; Konta, S.; Goonasekara, I.D.; Zhuang, W.Y.; Jeewon, R.; Phillips, A.J.L.; Abdel-Wahab, M.A.; Al-Sadi, A.M.; Bahkali, A.H.; Boonmee, S.; Boonyuen, N.; Cheewangkoon, R.; Dissanayake, A.J.; Kang, J.C.; Li, Q.R.; Liu, J.K.; Liu, X.Z.; Liu, Z.Y.; Luangsaard, J.J.; Pang, K.L.; Phookamsak, R.; Promputtha, I.; Suetrong, S.; Stadler, M.; Wen, T.C.; Wijayawardene, N.N. Families of Sordariomycetes. Fungal Diversity 2016, 79, 1–317. [CrossRef]
- Hu, D.M.; Wang, M.; Cai, L. Phylogenetic assessment and taxonomic revision of Mariannaea. Mycological Progress 2016, 16, 271–283. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





