Submitted:
21 August 2024
Posted:
22 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
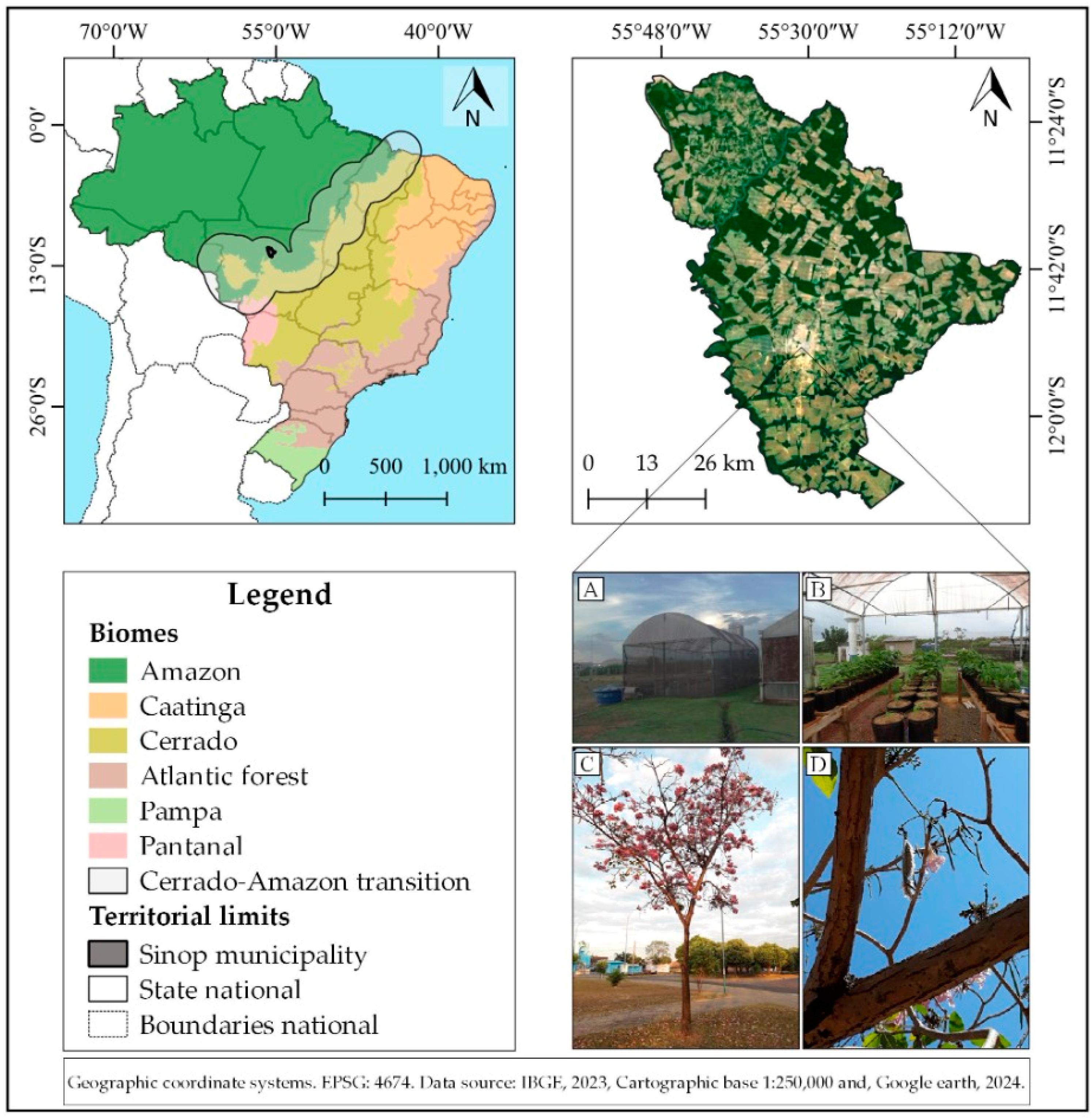
2.1. Obtaining and Germinating Seeds of Handroanthus impetiginosus
2.2. Transplanting and Substrates for Seedling Production
2.3. Substrate Moisture and Application of Treatments
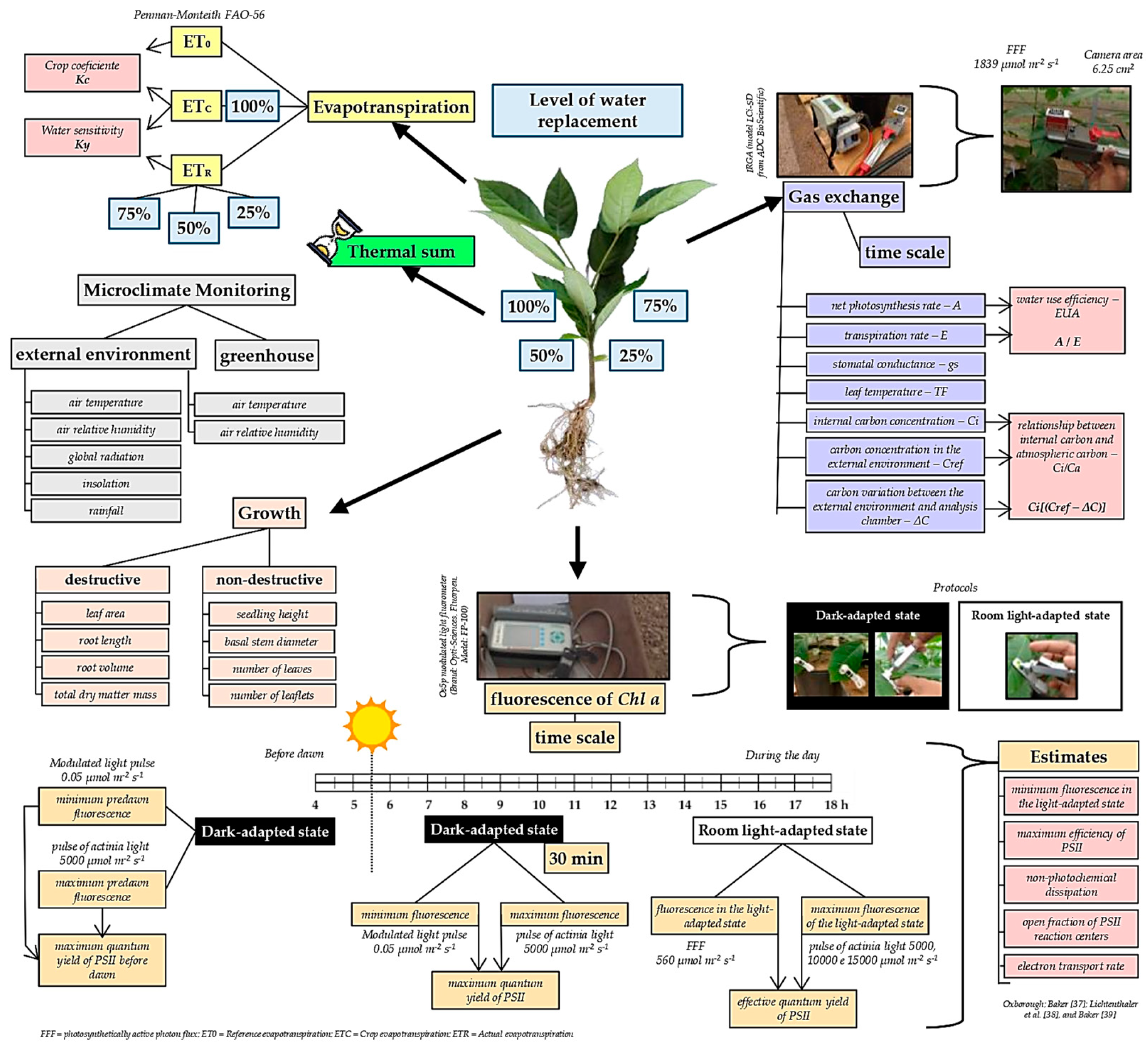
2.4. Micrometeorological Monitoring
2.4. Reference Evapotranspiration, Crop Coefficients and Water Sensitivity
2.5. Seedling Growth Analysis
2.6. Gas Exchange and Fluorescence
2.7. Basal Temperatures and Thermal Sum
2.8. Data Analysis
3. Results
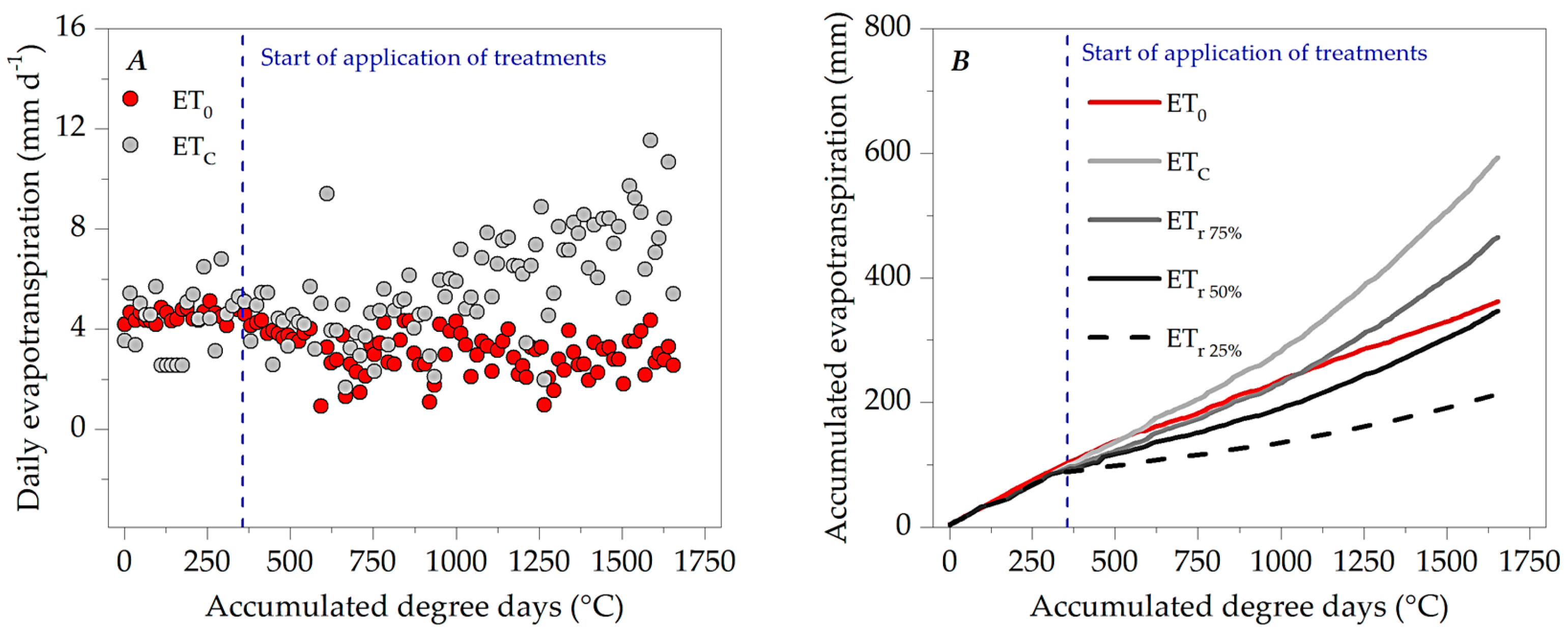
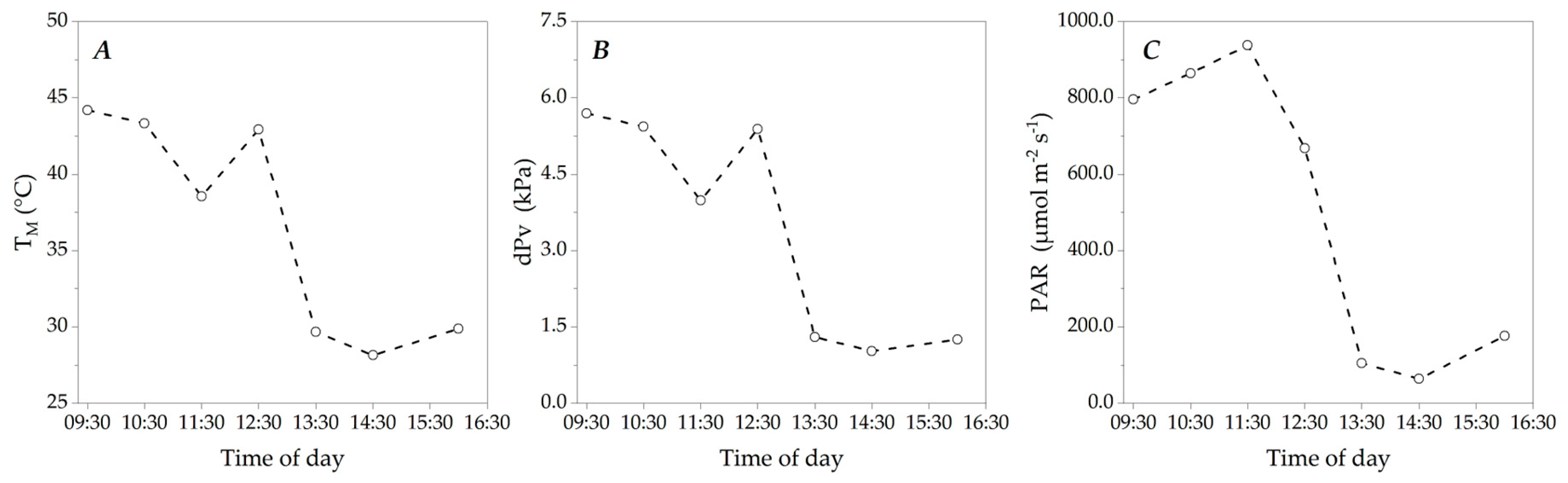
3.1. Microclimate Dynamics Throughout the Experimental Period
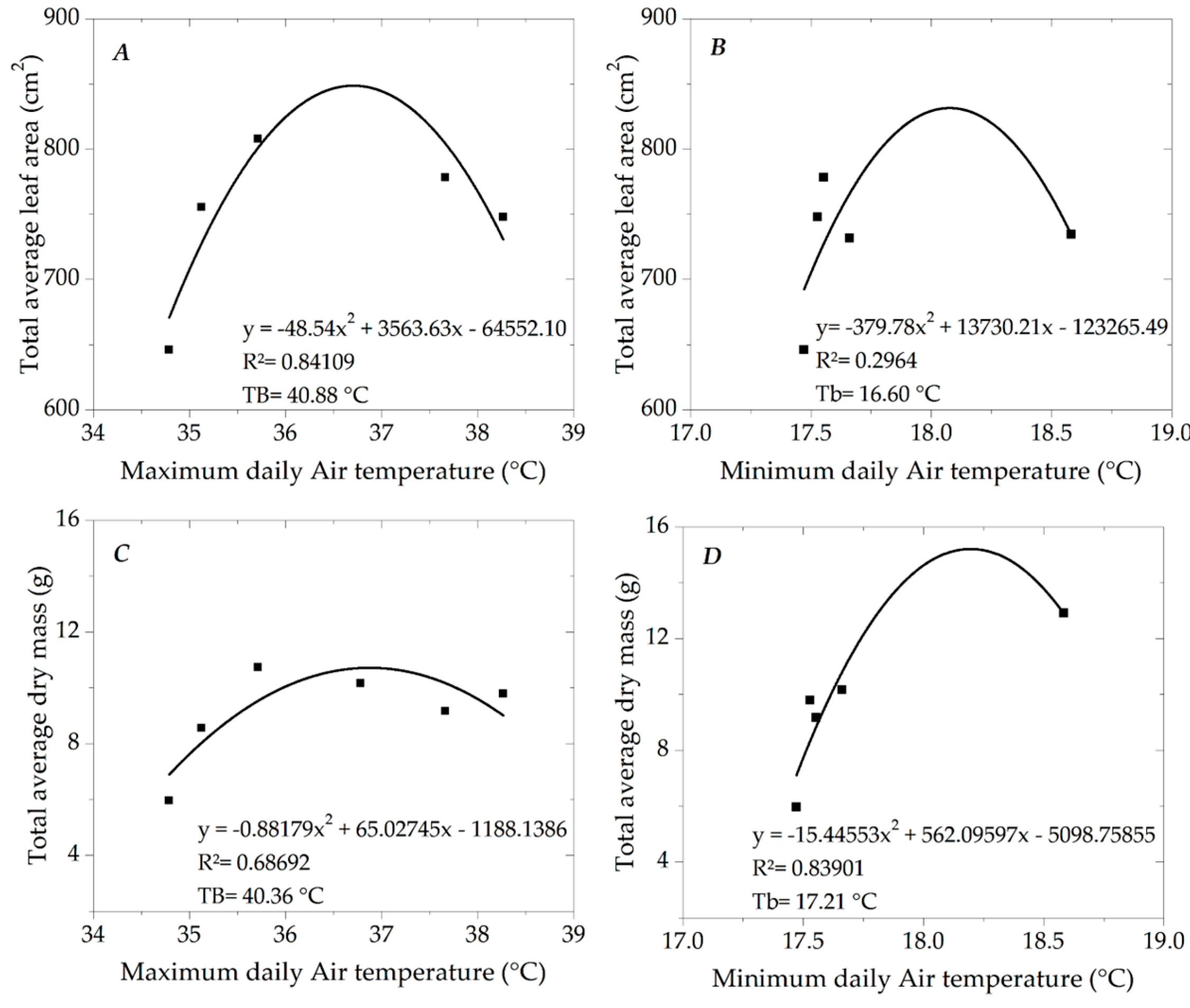
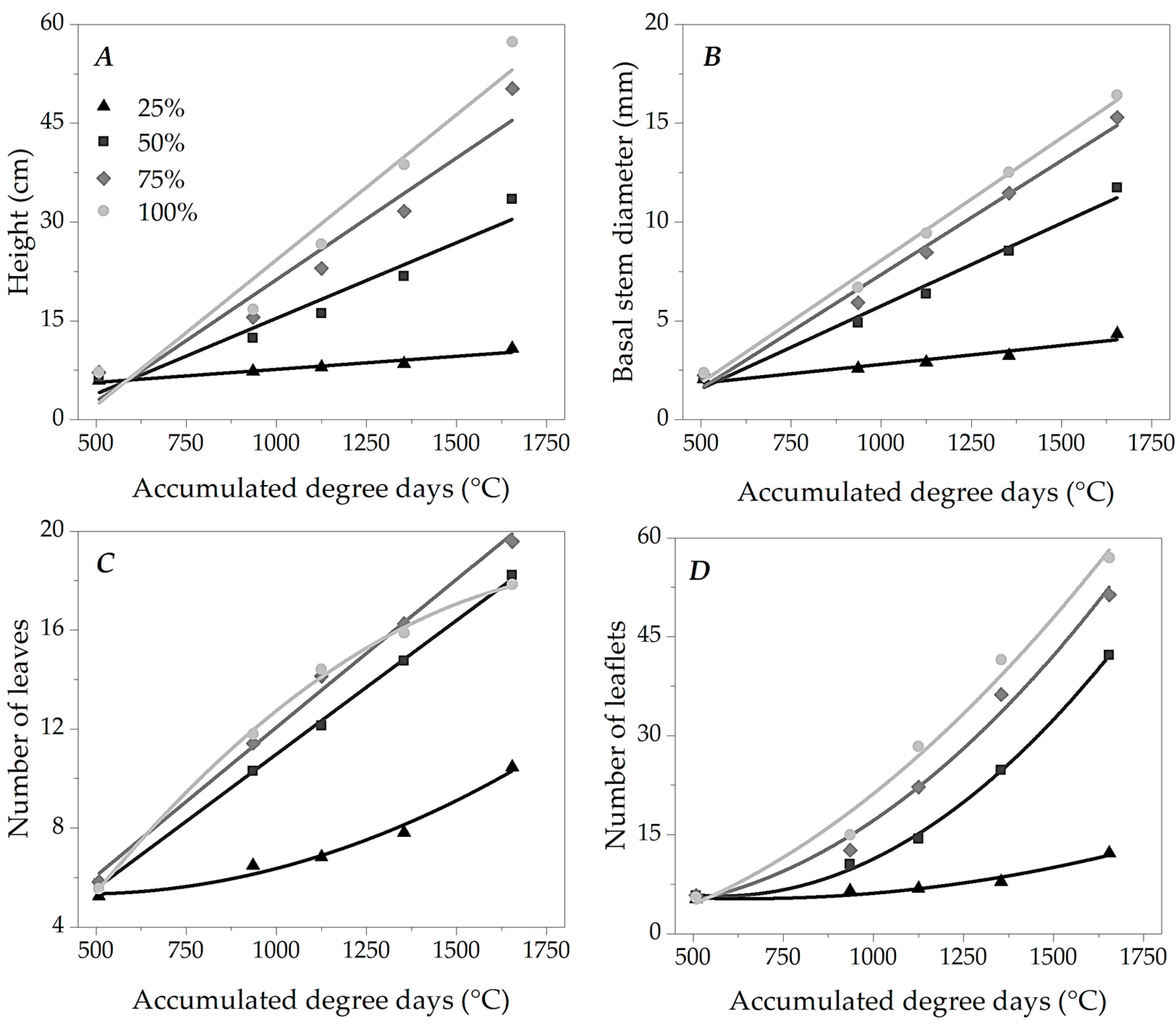
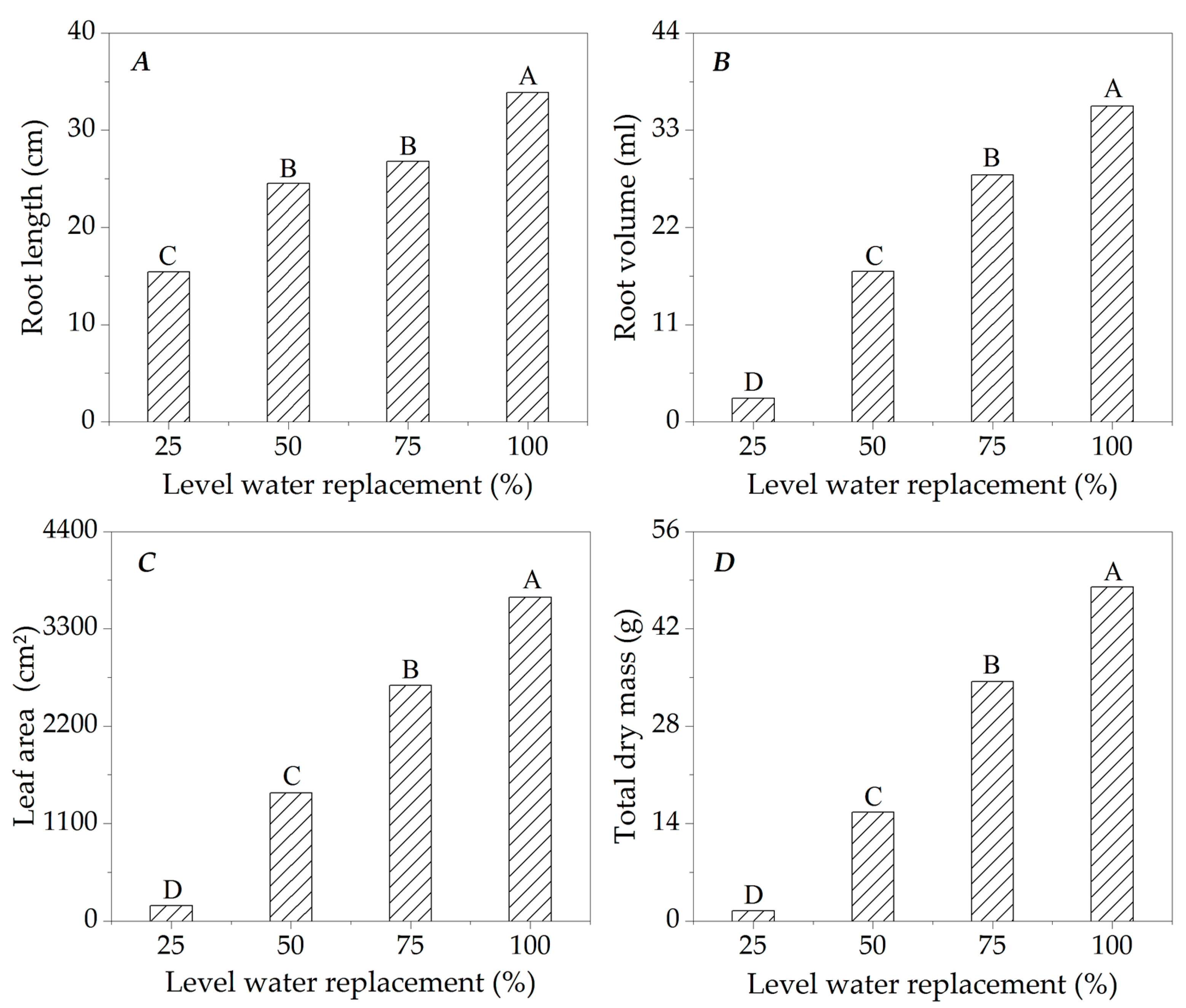
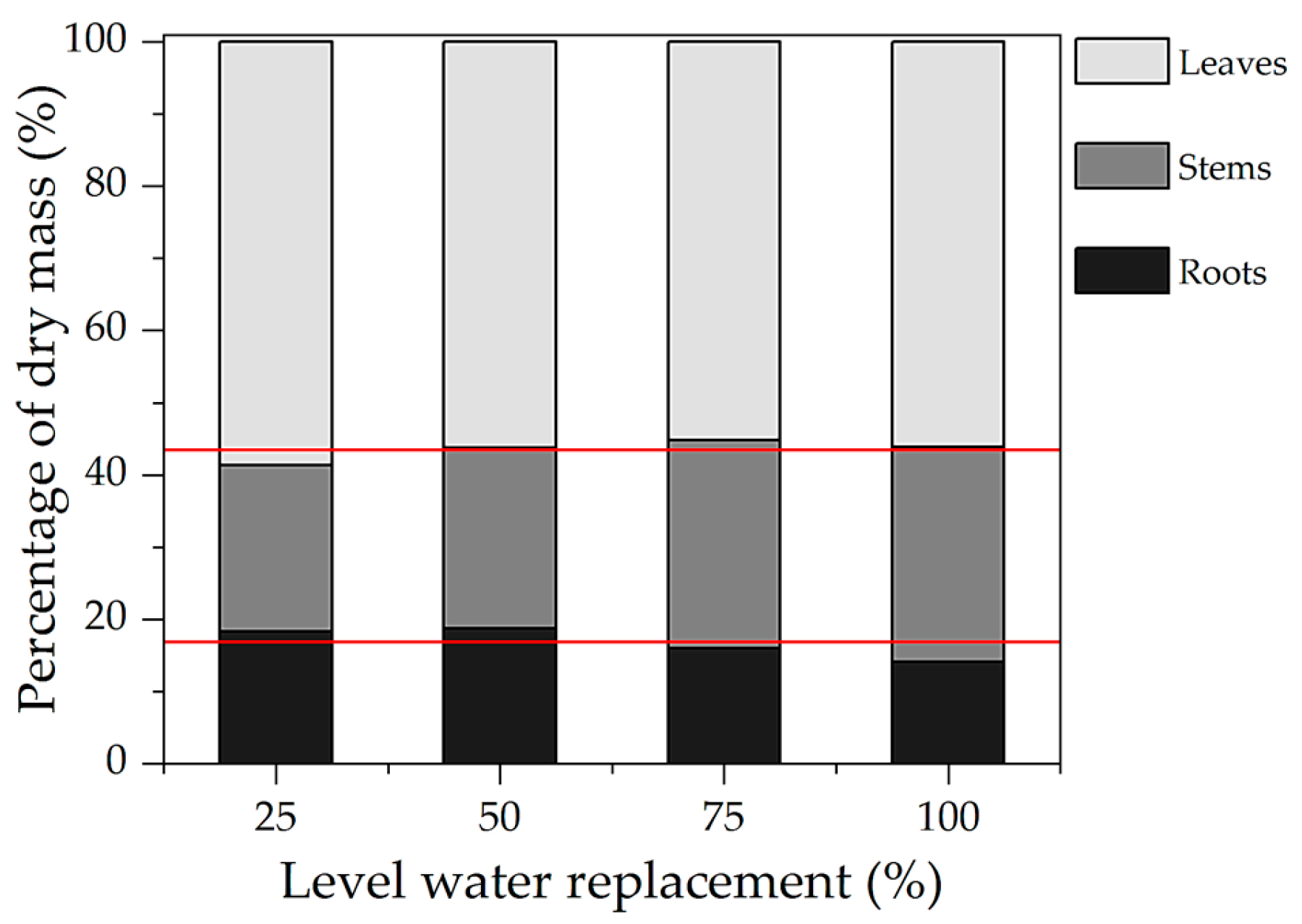
3.2. Growth of Ipê-Rosa Seedlings
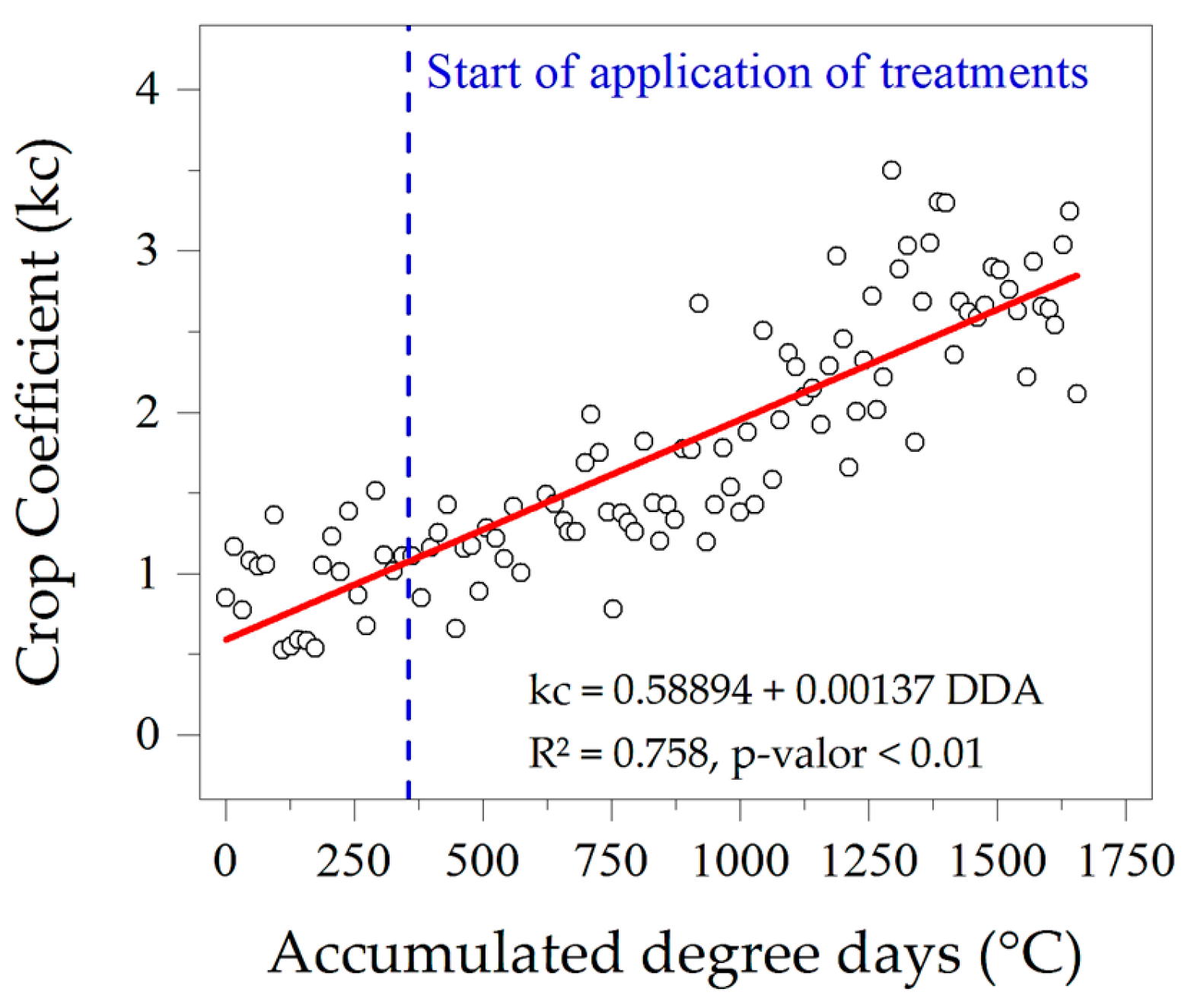
3.3. Evapotranspiration, Crop Coefficients and Water Sensitivity (Ky)
3.3. Diurnal Variations in Gas Exchange in Ipê-Rosa Seedlings
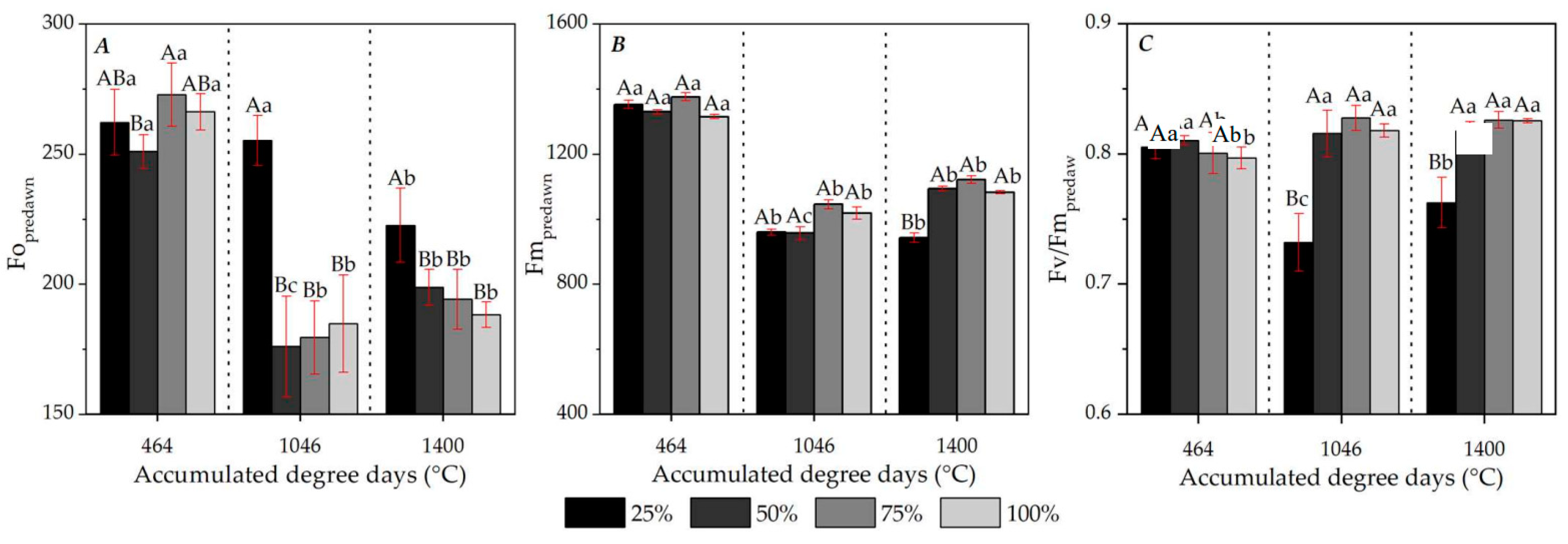
3.4. Chl a Fluorescence: Seasonality and Diurnal Variations
4. Discussion
4.1. Gas Exchange of Ipê-Rosa Seedlings
4.2. Chl a Fluorescence of Ipê-Rosa Seedlings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lorenzi, H. Brazilian trees: manual for identification and cultivation of tree plants in Brazil. 4nd ed.; Nova Odessa: Instituto Plantarum, 2002; v. 2; 368p.
- Pereira, M. S. Technical manual knowing and producing seeds and seedlings from the caatinga. Fortaleza: Association Caatinga, 2011; 60p.
- Mendonça, A.M.C.; Lira, J.M.S.; Vilela, A.L.O.; Vieira, D.A.; Melo, N.C.; Barbosa, J.P.R.A.D. High aluminum concentration and initial establishment of Handroanthus impetiginosus: clues about an Al non-resistant species in Brazilian Cerrado J. For. Res. 2020, 31, 2075–2082. [Google Scholar] [CrossRef]
- Benevides, D.S.; Carvalho, F.G. Survey of bee flora present in Caatinga areas in the municipality of Caraúbas- RN. Sociedade e Território 2009, 21, 44-54.
- Vieira, C.R.; Araujo, M.M. V.; Ferreira, A.F. Base saturation in the initial growth of Tabebuia impetiginosa seedlings. Revista de estudos ambientais 2020, 22 6-14. [CrossRef]
- Pimenta, J.M.A.; Souza, W.M.A.T.; Ferrari, C.S.; Vieira, F.A.; Fajardo, C.G.; Pacheco, M.V. Selection of Handroanthus impetiginosus mother trees to support seed collection areas. Revista Árvore 2023, 47, e4706. [CrossRef]
- Lisboa, M.A.N.; Silva, L.V.A.; Nascimento, A.S.; Silva, A.O.; Teixeira, M.R.A.; Ferreira, M.F.R.; Ferreira, S.C.; Silva, A.C.V.; Colares, A.V.; Calixto Júnior, J.T. Diversity, structure, and carbon sequestration potential of the woody flora of urban squares in the Brazilian semiarid region. Trees, Forests and People 2024, 16, 100561. [CrossRef]
- Morichetti, M.; Vangi, E.; Collalti, A. Predicted Future Changes in the Mean Seasonal Carbon Cycle Due to Climate Change. Forests 2024, 15, 1124. [Google Scholar] [CrossRef]
- Santos, M.E.C.d.; Melo, R.R.d.; Correia, D.; Sousa, J.A.d.; Santos, A.M.; Silva, A.K.V.d.; Paula, E.A.d.O.; Alves, A.R.; Scatolino, M.V.; Rusch, F.; et al. Variation in the Basic Density of Woods Produced in the Brazilian Semiarid Region Subjected to Different Irrigation Regimes. Forests 2023, 14, 2168. [Google Scholar] [CrossRef]
- Castellanos, J.R.G.; Prito, J.M.; Heinrich, M. Red Lapacho (Tabebuia impetiginosa) - a global ethnopharmacological commodity? Journal of Ethnopharmacology 2009, 121, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, J.A.; Pitangui, C.P.; Jordão, A.A.; Vannucchi, H.; Cecchi, A.O. Absence of mutagenicity and antimutagenicity of the extract obtained from the flowers of ipê-roxo Tabebuia impetiginosa (Mart. ex DC.) Standl. Revista Brasileira Plantas Medicinais 2010, 12, 414-420. [CrossRef]
- Zhang, J.; Hunto, S. T.; Yang, Y.; Lee, J.; Cho, J. Y. Tabebuia impetiginosa: A Comprehensive Review on Traditional Uses, Phytochemistry, and Immunopharmacological Properties. Molecules 2020, 25, 1–16. [Google Scholar] [CrossRef]
- Nahar, J.; Morshed, M.N.; Rupa, E.J.; Lee, J.H.; Kariyarath Valappil, A.; Awais, M.; Hun, K.J.; Sook, L.J.; Al-Amin, M.; Ahn, J.C.; et al. Roasting Extract of Handroanthus impetiginosus Enhances Its Anticancer Activity in A549 Lung Cancer Cells and Improves Its Antioxidant and Anti-Inflammatory Effects in Normal Cells. Appl. Sci. 2023, 13, 13171. [Google Scholar] [CrossRef]
- Santos, L.C.D.; Azevedo, L.S.; Siqueira, E.P.; Castro, A.H.F.; Lima, L.A.R.S. Chemical characterization, antioxidant activity, and cytotoxicity of fatty acids methyl esters from Handroanthus impetiginosus (Mart. Ex DC.) Mattos (Bignoniaceae) seeds. Natural Product Research 2024, 38, 619-623. [CrossRef]
- Oliveira, N.P.; Nascimento, J.W.S.; Madalena Jr, N.S.; Serafim, E.O.; Leandro, B.S.; Pereira, L.S.; Santos, M.C.C.; Nascimento, H.H.C. Ecophysiology of Handroanthus impetiginosus seedlings subjected to different irrigation cycles. Brazilian Journal of Development 2020, 6, 36563–36574. [Google Scholar] [CrossRef]
- Juárez, R.I.N.; Hodnett, M.G.; Fu, R.; Goulden, M.L.; Randow, C.V. Control of Dry Season Evapotranspiration over the Amazonian Forest as Inferred from Observations at a Southern Amazon Forest Site. Journal of Climate 2007, 20, 2827–2839. [Google Scholar] [CrossRef]
- Costa, A.C.; Rezende-Silva, S.L.; Megguer, C.A.; Moura, L.M.F.; Rosa, M.; Silva, A.A. The effect of irradiance and water restriction on photosynthesis in young jatobá-do-cerrado (Hymenea stigonocarpa) plants. Phoyosynthetica 2015, 53, 118–127. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Yin, C.Y.; Pang, X.Y.; Peuke, A.D.; Wang, X.; Chen, K.; Gong, R.G. Growth and photosynthetic responses in Jatropha curcas L. seedlings of different provenances to watering regimes. Phoyosynthetica 2016, 54, 367–373. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Tang, F.; Li, P.; Liu, J.; Fu, R.; Zheng, L.; Zhang, J.; Chao, N. MaMYBR30, a Novel 1R-MYB, Plays Important Roles in Plant Development and Abiotic Stress Resistance. Plants 2024, 13, 1794. [Google Scholar] [CrossRef] [PubMed]
- Shirke, P.A.; Pathre, U.V. Diurnal and Seasonal Changes in Photosynthesis and Photosystem 2 Photochemical Efficiency in Prosopis juliflora Leaves Subjected to Natural Environmental Stress. Phoyosynthetica 2003, 41, 83–89. [Google Scholar] [CrossRef]
- Bandurska, H. Drought Stress Responses: Coping Strategy and Resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef]
- Oliveira, B.; Marimon Junior, B.H.; Mews, H.A.; Valadão, M.B.X.; Marimon, B.S. Unraveling the ecosystem functions in the Amazonia–Cerrado transition: evidence of hyperdynamic nutrient cycling. Plant Ecology 2017, 218, 225–239. [Google Scholar] [CrossRef]
- Marques, E.Q.; Marimon-Junior, B.H.; Marimon, B.S.; Matricardi, E.A.; Mews, H.A.; Colli, G.R. Redefining the Cerrado–Amazonia transition: implications for conservation. Biodiversity and conservation 2019, 29, 1501–1517. [Google Scholar] [CrossRef]
- Santos, W.R.; Souza, L.S.B.; Jardim, A.M.R.F.; Morais, J.E.F.; Santos, M.M.P.; Souza, C.A.A.; Silva, T.G.F. How is the water footprint of the species Vachellia farnesiana, Amburana cearen-sis, and Handroanthus impetiginosus influenced by abiotic stresses as water deficit and salinity? International Journal of Phytoremediation 2024, 26, 784–792. [Google Scholar] [CrossRef]
- de Oliveira, I.P.; Schaaf, C.; de Setta, N. Drought Responses in Poaceae: Exploring the Core Components of the ABA Signaling Pathway in Setaria italica and Setaria viridis. Plants 2024, 13, 1451. [Google Scholar] [CrossRef]
- Scarpa, A.L.M.; Cruz, Y.C.; Duarte, V.P.; Castro, E.M.; Pasqual, M.; Oliveira, J.P.V.; Pereira, F.J. Growth Response, Gas Exchange, and Leaf Anatomy of Handroanthus spp. Seedlings in Mine Tailings Enriched with Nutrient Solution. Journal of Soil Science and Plant Nutrition 2022, 22, 3774–3787. [Google Scholar] [CrossRef]
- Monteiro, E.B.; Silva, A.C.; Souza, A.P.; Tanaka, A.A.; Ferneda, B.G.; Martim, C.C. Water requirements and crop coefficients of tropical forest seedlings in different shading conditions. Revista Brasileira de Engenharia Agrícola e Ambiental 2016, 20, 709-715. [CrossRef]
- Keffer, J.F.; Silva, C.C.; Souza, A.P.; Silva, A.C.; Bouvié, L.; Dias, T.K.R. Evapotranspiration and water sensitivity of Amazonian yellow ipe seedlings under different shading conditions. Revista Brasileira de Engenharia Agrícola e Ambiental 2019, 23, 733-740. [CrossRef]
- Borella, D.R.; Souza, A.P.; Silva, A.C.; Pizzatto, M.; Keffer, J.F.; Lima, D.C. Water requirements of Dipteryx alata Vogel Seedlings at different solar radiation levels in Cerrado-Amazon transition. Tropical and Subtropical Agroecosystems 2020, 23, 1–13. [Google Scholar] [CrossRef]
- . Sabino, M.; Ferneda, B.G.; Martim, C.C.; Bouvié, L.; Silva, C.C. Da; Souza, A.P.; Silva, A.C.; Felipe, R.T.A. Initial growth of amazonian and brazilian Cerrado yellow ipe cultivated under different shading intensities and spectral wavelength. Interciência 2020, 45, 183-191, 2020.
- Su, X.; Yang, Z.; Zhou, C.; Geng, S.; Chen, S.; Cai, N.; Tang, J.; Chen, L.; Xu, Y. The Response and Evaluation of Morphology, Physiology, and Biochemistry Traits in Triploid Passiflora edulis Sims ‘Mantianxing’ to Drought Stress. Plants 2024, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.P.; Mota, L.L.; Zamadei, T.; Martim, C.C.; Almeida, F.T.; Paulino, J. Climate classification and climatic water balance in Mato Grosso state, Brazil. Nativa 2013, 1, 34–43. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration: guidelines for computing crop water requirements. In: FAO Irrigation and Drainage Paper, 56nd ed. FAO, Rome, Italy, 1998; 300p.
- Martim, C.C.; Zamadei, T.; Souza, A.P.; Almeida, F.T.; Zolin, C.A. Angström-Prescott coefficients and reference evapotranspiration in the Cerrado-Amazon transition region of Mato Grosso. Revista Brasileira de Climatologia 2020, v. 26, 579- 594. [CrossRef]
- Elsheery, N.; Cao, K.F. Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiologiae Plantarum 2008, 30, 769–777. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components – calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynthesis Research 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Phoyosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annual Review of Plant Biology 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Monteiro, E.B. Growth and water requirements of forest seedlings under different shading conditions. Dissertation (Master’s in Agronomy) – Federal University of Mato Grosso, Sinop, 2015; 218p.
- Ometto, J. C. Plant bioclimatology. São Paulo: Agronômica Ceres, 1981. 425 p.
- Souza, A.P.; Leonel, S.; Silva, A.C. Basal temperature and thermal sum in phenological phases of nectarine and peach cultivars. Pesquisa Agropecuária Brasileira 2011, 46, 1588-1596. [CrossRef]
- Ferreira, D.F. Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia 2011, 35, 1039-1042. [CrossRef]
- Souza, A.P.; Zamadei, T.; Monteiro, E.B.; Casavecchia, B.H. Atmospheric Transmissivity of the Global Radiation in the Amazonic Region of Mato Grosso. Revista Brasileira de Meteorologia 2016, 31, 639–648. [Google Scholar] [CrossRef]
- Borella, D.R.; Souza, A.P.; Silva, K.N.C.; Santos, L.M.M.; Ximenes, E.S.O.C.; Anjos, A.M. Dynamics and estimates of air temperature and relative humidity in nurseries protected with different shading. Nativa 2021, 9, 62–75. [Google Scholar] [CrossRef]
- Monteiro, E.B.; Silva, C.C.; Silva, A.C.; Souza, A.P. Estimating Emission of Leaves Seedlings Forest in Different Shading Levels, at Conditions of Transition Amazon-Cerrado, Brazil. American Journal of Plant Sciences 2014, 5, 2330–2341. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ribeiro, A.; Silva, C.R.A.; Xavier, A.; Freitas, A.F. Modeling the growth of eucalyptus seedlings based on thermal sum. Revista árvore 2017, 41, e410212. [CrossRef]
- Lima, P.R.; Horbach, M.A.; Dranski, J.A.L.; Ecco, M.; Malavasi, M.M.; Malavasi, U.C. Morphophysiological evaluation of Handroanthus impetiginosus (Mart. ex DC.) Mattos seedlings during hardening. Floresta e Ambiente 2014, 21, 316-326. [CrossRef]
- Augostinho, L.M.D.; Prado, R.M.; Rozane, D.E.; Freitas, N. Accumulation of dry mass and nutrient absorption rate in 'Pedro Sato' guava seedlings. Bragantia 2008, 67, 577-585, 2008. [CrossRef]
- Nogueira, R.C.; Paiva, R.; Lima, E.C.; Soares, G.A.; Oliveira, L.M.; Santos, B.R.; Emrich, E.B.; Castro, A.H.F. Curva de crescimento e análises bioquímicas de calos de murici-pequeno (Byrsonima intermedia A. Juss.). Rev. Bras. Pl. Med. 2008, 10, 44-48, 2008.
- César, F.R.C.F.; Matsumoto, S.N.; Viana, A.E.S.; Bonfim, J.A. Initial growth and quality of Pterogyne nitens Tull. seedling under artificial shading gradient. Ciência Florestal 2014, 24, 357-366. [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment 2007, 30, 1086-1106. [CrossRef]
- Marenco, R.A.; Nascimento, H.C.S.; Magalhães, N.S. Stomatal conductance in Amazonian tree saplings in response to variations in the physical environment. Phoyosynthetica 52, 493–500. [CrossRef]
- Kutschera, U. Review Cell Expansion in Plant Development. Brazilian Journal of Plant Physiology 2000, 12, 65–98. [Google Scholar]
- Bashline, L.; Lei, L.; Li, S.; Gu, Y. Cell wall, cytoskeleton, and cell expansion in higher plants. Mol Plant 2014, 7, 586–600. [Google Scholar] [CrossRef]
- Johnson, G.N. Physiology of PSI cyclic electron transport in higher plants. Biochimica et Biophysica Acta – Bioenergetics 2011, 1807, 384-389. [CrossRef]
- Duarte, D.M.; Rocha, G.T.F.B.L.; Matos, F.S.; Rodrigues, F. Responses of paricá seedlings to water stress. Revista Floresta 2016, 46, 405 – 412. [CrossRef]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annual Review of Plant Physiology 2016, 67, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, Y.; Zhou, M. Environmental explanation of maize specific leaf area under varying water stress regimes. Environmental and Experimental Botany 2020, 171, 1–10. [Google Scholar] [CrossRef]
- Santos, V.A.H.F.; Ferreira, M.J.; Rodrigues, J.V.F.C.; Garica, M.N.; Ceron, J.V.B.; Nelson, B.W.; Saleska, S.R. Causes of reduced leaf-level photosynthesis during strong El Niño drought in a Central Amazon forest. Global Change Biology 2018, 24, 4266–4279. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.E.; Ma, X.; Wagner, F.H.; Kim, H.; Oki, T.; Eamus, D.; Huete, A. Evapotranspiration seasonality across the Amazon Basin. Earth Syst. Dynam. 2017, 8, 439–454. [Google Scholar] [CrossRef]
- Sabonaro, D.Z.; Galbiatti, J.A. Effect of irrigation levels in substrates for “ipê-roxo” seedlings production. Scientia Forestalis 2007, 1, 95–102. [Google Scholar]
- Cunha, A.O.; Andrade, L.A.; Bruno, R.L.A.; Silva, J.A.L.; Souza, V.C. Efeitos de substratos e das dimensões dos recipientes na qualidade das mudas de Tabebuia impetiginosa (Mart. Ex D.C.) Standl. Revista Árvore 2005, 29, 507-516. [CrossRef]
- Carvalho, D.F.; Lima, M.E.; Oliveira, A.D.; Rocha, H.S.; Guerra, J.G.M. Crop coefficiente and water consumption of eggplant in no-tillage system and conventional soil preparation. Engenharia Agrícola 2012, 32, 784-793. [CrossRef]
- Pessoa, J.L.; Freire, A.L.O.; Costa, A.S. Gas exchange of Handroanthus impetiginosus (Mart. ex DC) Mattos plants under water stress and rehydration. Revista de Ciências Agroveterinárias 2017, 16, 269-276. [CrossRef]
- Bueno, M.M.; Leles, P.S.S.; Abreu, J.F.G.; Santos, J.J.S.; Carvalho, D.F. Water requirement and growth indicators of forest tree species seedlings produced with automated irrigation management. PLOS ONE 2020, 15, e0238677. [Google Scholar] [CrossRef]
- Melo, L.A.; Abreu, A.H.M.; Leles, P.S.S.; Oliveira, R.R.; Silva, D.T. Quality and initial growth of seedlings Mimosa caesalpiniifolia Benth. produced in different volumes of containers. Ciência Florestal 2018, 28, 47-55. [CrossRef]
- Silva, B.L.B.; Costa, E.; Binotti, F.F.S.; Benett, C.G.S.; Silva, A.G. Quality and growth of achachairu seedlings depending on substrate and shading. Pesquisa Agropecuária Tropical 2018, 48, 407-413.
- Esquivel-Muelbert, A.; Baker, T. R.; Dexter, K. G.; Lewis, S. L.; Brienen, R. J. W.; Feldpausch, T. R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E.; et al. Compositional response of Amazon forests to climate change. Global change biology 2018, 25, 39–56. [Google Scholar] [CrossRef]
- Ouzounis, T.; Parjikolaei, B.R.; Fretté, X.; Rosenqvist, E.; Ottosen, C.O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Frontiers in Plant Science 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Mira-García, A.B., Romero-Triguero, C., Gambín, J.M.B., Sáchez-Iglesias, M.P., Tortosa, P.A.N., Nicolás, E.N. Estimation of stomatal conductance by infra-red thermometry in citrus trees cultivated under regulated deficit irrigation and reclaimed water. Agr. Water Manag. 2020, 276, e108057. [CrossRef]
- Marenco, R.A.; Antezana-Vera, S.A.; Gouvêa, R.S.; Camargo, M.A.B.; Oliveira, M.F.; Santos, J.K.S. Physiology of Amazon tree species: photosynthesis, respiration and water relations. Ceres 61, 786–799. [CrossRef]
- Silva, A.M.L.; Costa, M.F.B.; Leite, V.G.; Rezende, A.A.; Teixeira, S.P. Leaf anatomy with taxonomic implications in ipe species. Hoehnea 2009, 36, 329–338. [Google Scholar] [CrossRef]
- Marenco, R.A.; Lopes, N.F. Fisiologia vegetal: fotossíntese, respiração, relações hídricas e nutrição mineral. 3. ed. Viçosa: Editora UFV, 2009; 486 p.
- Dombroski, J.L.D.; Freitas, R.M.O.; Tomczak, V.E.; Pinto, J.R.S.; Farias, R.M. Ecophysiology of water stressed Handroanthus impetiginosus (Mart. Ex. DC) Mattos) Seedlings. Scientia Forestalis 2014, 42, 155-163.
- Souza, A.F.; Rocha Junior, E.O.; Laura, V.A. Early development and efficiency in water and nitrogen use by seedlings of Calophyllum brasiliense, Eucalyptus urograndis, Tabebuia impetiginosa and Toona ciliata. Ciência Florestal 2018, 28, 1465-1477. [CrossRef]
- Shimpl, F.C.; Ferreira, M.J.; Jaquetti, R.K.; Martins, S.C.V.; Gonçalves, J.F.C. Physiological responses of young Brazil nut (Bertholletia excelsa) plants to drought stress and subsequent rewatering. Flora 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Melo, H.F.; Souza, E.R.; Cunha, J.C. Fluorescence of chlorophyll a and photosynthetic pigments in Atriplex nummularia under abiotic stresses. Revista Brasileira de Engenharia Agrícola e Ambiental 2017, 21, 232-237. [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. Seedlings. Phoyosynthetica 2016, Czech Republic, 54, 524–531. [CrossRef]
- Marenco, R.A.; Neves, T.S.; Camargo, M.A.B.; Dias, D.P.; Costa, G.F.; Rodrigues, J.C. Dynamic photoinhibition of photosynthesis in canopy trees from the Central Amazon. Revista Brasileira de Biociências 2007, 5, 150-152. (Scientific Note).
- Dias, D.P.; Marenco, R.A. Photoinhibition of photosynthesis in Minquartia guianensis and Swietenia macrophylla inferred by monitoring the initial fluorescence. Phoyosynthetica 2006, 44, 255–240. [Google Scholar] [CrossRef]
- Dongsansuk, A.; Lütz, C.; Neuner, G. Effects of temperature and irradiance on quantum yield of PSII photochemistry and xanthophyll cycle in a tropical and a temperate species. Phoyosynthetica 2013, 51, 118–127. [Google Scholar] [CrossRef]
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annual Review of Plant Physiology 2006, 57, 521–565. [Google Scholar] [CrossRef]
- Liberato, M.A.R.; Gonçalves, J.F.C.; Chevreuil, L.R.; Nina Jr., A.R.; Fernandes, A.V.; Santos Jr., U.M. Leaf water potential, gas exchange and chlorophyll a fluorescence in acariquara seedlings (Minquartia guianensis Aubl.) under water stress and recovery. Brazilian Journal Plant Physiology 2006, 18, 315-323. [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e desenvolvimento vegetal. 6. ed. Porto Alegre: Artmed, 2017; 888 p.
- Thapper, A.; Mamedov, F.; Mokvist, F.; Hammarström, L.; Styring, S. Defining the Far-Red Limit of Photosystem II in Spinach. The Plant Cell 2009, 21, 2391–2401. [Google Scholar] [CrossRef]
- Franco, A.; Lüttge, U. Midday depression in savanna trees: coordinated adjustments in photochemical efficiency, photorespiration, CO2 assimilation and water use efficiency. Oecologia 2002, 31, 356–365. [Google Scholar] [CrossRef]
- Bacarin, M.A.; Martinazzo, E.G.; Cassol, D.; Falqueto, A.R.; Silva, D.M. Daytime variations of chlorophyll a fluorescence in Pau d’alho seedlings. Revista Árvore 2016, 40, 1023-1030. [CrossRef]
- Weng, J.H.; Chen, Y.N.; Liao, T.S. Relationships between chlorophyll fluorescence parameters and photochemical reflectance index of tree species adapted to different temperature regimes. Plant function and evolutionary biology 2006, 33, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.K.S.; Alves, M.C.J.L.; Costa, R.N.; Silva, D.M.R.; Santos, J.C.C.S.; Moura, F.B.P.; Silva Jr., J. M.; Silva, J.V. Gas exchange and photochemical efficiency of Caatinga plants submitted to different water management strategies. Journal of Agricultura Science 2019, 11, 53–67. [Google Scholar] [CrossRef]
- Morais, R.R.; Gonçalvez, J.F.C.; Santos Jr., U.M.; Santos, A.L.W. Chloroplastid pigment contents and chlorophyll a fluorescence in Amazonian tropical three species. Revista Árvore 2007, 31, 959-966. [CrossRef]
- Ortiz, D.; Moreno, F.; Díez, M. C. Photosynthesis, growth, and survival in seedlings of four tropical fruit-tree species under intense radiation. Acta Amazonica 2021, 51, 1–9. [Google Scholar] [CrossRef]
- Reis, L.C.; Scalon, S.P.Q.; Dresch, D.M.; Foresti, A.C.; Santos, C.C.; Pereira, Z.V. Chlorophyll a fluorescence as an indicator of water stress in Calophyllum brasiliense. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2020, 48, 210-2020. [CrossRef]
- Reis, L.A.C.; Oliveira, J.A.; Farnese, F.S.; Rosado, A.M.; Reis, L.A.C. Chlorophyll fluorescence and water content parameters are good biomarkers for selecting drought tolerant eucalyptus clones. Forest Ecology and Management 2021, 481, 1–10. [Google Scholar] [CrossRef]

| Regression Fit | ||||||
|---|---|---|---|---|---|---|
| Height (cm) | ||||||
| LWR | B0 | B1 | B2 | R² | p-value | Fit |
| 25% | 3.6731 | 0.0040 | - | 0.9404 | < 0.0001 | Linear |
| 50% | -7.5659 | 0.0230 | - | 0.9399 | < 0.0001 | Linear |
| 75% | -15.7444 | 0.0370 | - | 0.9387 | < 0.0001 | Linear |
| 100% | -19.8946 | 0.0441 | - | 0.9529 | < 0.0001 | Linear |
| Basal stem diameter (mm) | ||||||
| LWR | B0 | B1 | B2 | R² | p-value | Fit |
| 25% | 0.8981 | 0.0019 | - | 0.9315 | < 0.0001 | Linear |
| 50% | -2.6150 | 0.0084 | - | 0.9848 | < 0.0001 | Linear |
| 75% | -3.8027 | 0.0111 | - | 0.9925 | < 0.0001 | Linear |
| 100% | -4.3303 | 0.0124 | - | 0.9950 | < 0.0001 | Linear |
| Number of leaves | ||||||
| LWR | B0 | B1 | B2 | R² | p-value | Fit |
| 25% | 6.0418 | -0.0031 | 3.00E-06 | 0.9835 | < 0.0001 | Quadratic |
| 50% | 0.1498 | 0.0108 | - | 0.9991 | < 0.0001 | Linear |
| 75% | 0.0777 | 0.0120 | - | 0.9949 | < 0.0001 | Linear |
| 100% | -4.9563 | 0.0237 | -6.00E-06 | 0.9979 | < 0.0001 | Quadratic |
| Number of leaflets | ||||||
| LWR | B0 | B1 | B2 | R² | p-value | Fit |
| 25% | 7.9715 | -0.0083 | 6.00E-06 | 0.9646 | < 0.0001 | Quadratic |
| 50% | 16.1861 | -0.0362 | 3.10E-05 | 0.9985 | < 0.0001 | Quadratic |
| 75% | 5.8707 | -0.0146 | 2.60E-05 | 0.9897 | < 0.0001 | Quadratic |
| 100% | -2.3092 | 0.0035 | 2.00E-05 | 0.9869 | < 0.0001 | Quadratic |
| LWR | Etr1 | Etm2 | (1-ETr/ETp) | Yr3 | Ym4 | (1-Yr/Yp) | Ky5 |
|---|---|---|---|---|---|---|---|
| (mm) | (g m-2) | ||||||
| 75% | 465.41 | 593.2 | 0.22 | 802.47 | 1121.32 | 0.28 | 1.32 |
| 50% | 346.15 | 593.2 | 0.42 | 364.27 | 1121.32 | 0.68 | 1.62 |
| 25% | 212.99 | 593.2 | 0.64 | 34.4 | 1121.32 | 0.97 | 1.51 |
| Hour | Level water replacement | |||
|---|---|---|---|---|
| 25% | 50% | 75% | 100% | |
| Net photosynthesis rate – µmol m-2 s-1 | ||||
| 09:30 | 6.11 Ab | 12.54 Aa | 11.12 Aa | 10.1 Aa |
| 12:30 | 5.36 Ab | 6.92 Bb | 10.30 Aa | 10.41 Aa |
| 13:30 | 4.92 Aa | 7.15 Ba | 6.75 Ba | 8.02 Aa |
| 16:00 | 3.50 Ab | 6.28 Bab | 6.56 Bab | 8.05 Aa |
| Transpiration rate – mol m-2 s-1 | ||||
| 09:30 | 2.95 Ab | 5.47 Aa | 4.88 Aa | 5.03 Aa |
| 12:30 | 2.65 ABc | 3.93 BAcb | 4.92 Aab | 5.81 Aa |
| 13:30 | 2.43 ABb | 3.48 BCab | 3.28 Bab | 4.51 ABa |
| 16:00 | 1.26 Bb | 2.22 Cab | 2.23 Bab | 3.09 Ba |
| Water use efficiency – µmol CO2 mol-1 H20 | ||||
| 09:30 | 2.21 Ba | 2.29 Ba | 2.29 Ba | 1.94 Ba |
| 12:30 | 2.26 ABa | 1.82 Ba | 2.16 Ba | 1.81 Ba |
| 13:30 | 1.97 Ba | 2.12 Ba | 2.06 Ba | 1.78 Ba |
| 16:00 | 2.75 Aa | 2.83 Aa | 3.15 Aa | 2.77 Aa |
| Stomatal conductance – mol m-2 s-1 | ||||
| 09:30 | 0.10 Ab | 0.23 Aa | 0.19 Aa | 0.21 Aa |
| 12:30 | 0.07 Ab | 0.10 Bb | 0.14 ABab | 0.19 Aa |
| 13:30 | 0.06 Ab | 0.09 Bab | 0.09 Bab | 0.16 Aa |
| 16:00 | 0.06 Ab | 0.13 Bb | 0.13 ABb | 0.24 Aa |
| Internal carbon to atmospheric carbon ratio | ||||
| 09:30 | 0.64 Bb | 0.71 Aab | 0.68 ABab | 0.72 ABa |
| 12:30 | 0.50 Cc | 0.64 Bab | 0.57 Cb | 0.66 Ba |
| 13:30 | 0.62 Bb | 0.59 Bb | 0.63 BCb | 0.71 ABa |
| 16:00 | 0.69 Aa | 0.67 Aa | 0.76 Aa | 0.78 Aa |
| Leaf temperature – °C | ||||
| 09:30 | 40.37 Ba | 40.03 Ba | 40.40 Ba | 40.81 Aa |
| 12:30 | 41.94 Aa | 42.02 Aa | 41.97 Aa | 41.90 Aa |
| 13:30 | 39.53 Ba | 40.41 Ba | 39.47 Ba | 39.43 Ba |
| 16:00 | 33.63 Ca | 33.47 Ca | 33.83 Ca | 34.19 Ca |
| 100% of level water replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TM | dPv | A | E | EUA | gs | Ci/Ca | Tf | ||
| TM | 1.00 | 1.00** | 0.19NS | 0.37** | -0.52** | -0.08NS | -0.51** | 0.78** | |
| dPv | 1.00 | 0.19NS | 0.37** | -0.52** | -0.06NS | -0.50** | 0.77** | ||
| A | 1.00 | 0.93** | 0.07NS | 0.86** | 0.13NS | 0.28* | |||
| E | 1.00 | -0.28* | 0.75** | 0.02NS | 0.56** | ||||
| EUA | 1.00 | 0.15NS | 0.24NS | -0.78** | |||||
| gs | 1.00 | 0.55** | -0.03NS | ||||||
| Ci/Ca | 1.00 | -0.61** | |||||||
| Tf | 1.00 | ||||||||
| 75% of level water replacement | |||||||||
| TM | dPv | A | E | EUA | gs | Ci/Ca | Tf | ||
| TM | 1.00 | 1.00** | 0.51** | 0.61** | -0.39** | 0.24NS | -0.45** | 0.76** | |
| dPv | 1.00 | 0.52** | 0.61** | -0.37** | 0.25NS | -0.45** | 0.75** | ||
| A | 1.00 | 0.85** | 0.00NS | 0.80** | -0.22NS | 0.41** | |||
| E | 1.00 | -0.48** | 0.73** | -0.19NS | 0.70** | ||||
| EUA | 1.00 | -0.09NS | 0.05NS | -0.68** | |||||
| gs | 1.00 | 0.28* | 0.09NS | ||||||
| Ci/Ca | 1.00 | -0.58** | |||||||
| Tf | 1.00 | ||||||||
| 50% of level water replacement | |||||||||
| TM | dPv | A | E | EUA | gs | Ci/Ca | Tf | ||
| TM | 1.00 | 1.00** | 0.57** | 0.67** | -0.65** | 0.43** | -0.15NS | 0.75** | |
| dPv | 1.00 | 0.55** | 0.66** | -0.63** | 0.41** | -0.16NS | 0.74** | ||
| A | 1.00 | 0.83** | -0.24NS | 0.86** | 0.13NS | 0.41** | |||
| E | 1.00 | -0.68** | 0.77** | 0.11NS | 0.71** | ||||
| EUA | 1.00 | -0.22NS | 0.13NS | -0.87** | |||||
| gs | 1.00 | 0.57** | 0.17NS | ||||||
| Ci/Ca | 1.00 | -0.49** | |||||||
| Tf | 1.00 | ||||||||
| 25% of level water replacement | |||||||||
| TM | dPv | A | E | EUA | gs | Ci/Ca | Tf | ||
| TM | 1.00 | 1.00** | 0.13NS | 0.28* | -0.37** | 0.14NS | -0.29* | 0.74** | |
| dPv | 1.00 | 0.13NS | 0.26* | -0.36** | 0.13NS | -0.30* | 0.73** | ||
| A | 1.00 | 0.94** | 0.11NS | 0.95** | -0.01NS | 0.26* | |||
| E | 1.00 | -0.21NS | 0.94** | 0.10NS | 0.45** | ||||
| EUA | 1.00 | -0.05NS | -0.42** | -0.57** | |||||
| gs | 1.00 | 0.23NS | 0.19NS | ||||||
| Ci/Ca | 1.00 | -0.38** | |||||||
| Tf | 1.00 | ||||||||
| Solar time | 28 DAT (464 DDA) | 66 DAT (1046 DDA) | 90 DAT (1400 DDA) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% | |
| Maximum quantum yield of PSII (Fv/Fm) | ||||||||||||
| 07:00 | 0.799 | 0.797 | 0.800 | 0.800 | 0.722 | 0.799 | 0.809 | 0.810 | 0.761 | 0.819 | 0.825 | 0.828 |
| 09:00 | 0.759 | 0.763 | 0.753 | 0.755 | 0.663 | 0.765 | 0.767 | 0.770 | 0.731 | 0.800 | 0.804 | 0.813 |
| 11:00 | 0.735 | 0.742 | 0.736 | 0.736 | 0.634 | 0.684 | 0.729 | 0.748 | 0.734 | 0.805 | 0.810 | 0.819 |
| 13:00 | 0.723 | 0.740 | 0.740 | 0.734 | 0.658 | 0.749 | 0.765 | 0.781 | 0.730 | 0.805 | 0.806 | 0.815 |
| 15:00 | 0.757 | 0.763 | 0.762 | 0.749 | - | - | - | - | - | - | - | - |
| 17:00 | 0.799 | 0.799 | 0.792 | 0.789 | - | - | - | - | - | - | - | - |
| Effective quantum yield of PSII (ΦPSII) | ||||||||||||
| 07:00 | 0.378 | 0.423 | 0.423 | 0.417 | 0.188 | 0.444 | 0.503 | 0.405 | 0.298 | 0.357 | 0.366 | 0.356 |
| 09:00 | 0.281 | 0.352 | 0.328 | 0.284 | 0.159 | 0.273 | 0.351 | 0.385 | 0.281 | 0.426 | 0.453 | 0.497 |
| 11:00 | 0.327 | 0.335 | 0.335 | 0.364 | 0.106 | 0.294 | 0.366 | 0.385 | 0.288 | 0.438 | 0.410 | 0.396 |
| 13:00 | 0.338 | 0.358 | 0.366 | 0.375 | 0.155 | 0.410 | 0.430 | 0.513 | 0.278 | 0.468 | 0.480 | 0.506 |
| 15:00 | 0.383 | 0.352 | 0.382 | 0.349 | - | - | - | - | - | - | - | - |
| 17:00 | 0.235 | 0.228 | 0.211 | 0.185 | - | - | - | - | - | - | - | - |
| Electron transport rate (µmol m-2 s-1) | ||||||||||||
| 07:00 | 89.27 | 100.01 | 99.84 | 98.40 | 44.32 | 104.86 | 118.90 | 95.57 | 70.48 | 84.28 | 86.45 | 84.14 |
| 09:00 | 66.50 | 83.10 | 77.47 | 67.09 | 37.67 | 64.45 | 83.034 | 90.91 | 66.43 | 100.69 | 107.10 | 117.42 |
| 11:00 | 77.36 | 79.13 | 79.24 | 85.90 | 25.12 | 69.46 | 86.499 | 91.00 | 68.13 | 103.48 | 96.76 | 93.59 |
| 13:00 | 79.90 | 84.51 | 86.42 | 88.59 | 36.56 | 96.97 | 101.67 | 121.21 | 65.69 | 110.58 | 113.49 | 119.46 |
| 15:00 | 90.47 | 83.05 | 90.19 | 82.37 | - | - | - | - | - | - | - | - |
| 17:00 | 55.48 | 53.85 | 49.92 | 43.64 | - | - | - | - | - | - | - | - |
| Maximum efficiency of PSII (Fv’/Fm’) | ||||||||||||
| 07:00 | 0.511 | 0.496 | 0.501 | 0.545 | 0.422 | 0.644 | 0.719 | 0.623 | 0.575 | 0.637 | 0.733 | 0.674 |
| 09:00 | 0.484 | 0.509 | 0.479 | 0.469 | 0.341 | 0.465 | 0.508 | 0.529 | 0.475 | 0.599 | 0.685 | 0.678 |
| 11:00 | 0.479 | 0.492 | 0.495 | 0.524 | 0.296 | 0.428 | 0.479 | 0.497 | 0.497 | 0.697 | 0.672 | 0.635 |
| 13:00 | 0.463 | 0.475 | 0.490 | 0.528 | 0.339 | 0.526 | 0.561 | 0.611 | 0.466 | 0.656 | 0.660 | 0.702 |
| 15:00 | 0.525 | 0.497 | 0.527 | 0.505 | - | - | - | - | - | - | - | - |
| 17:00 | 0.584 | 0.606 | 0.581 | 0.605 | - | - | - | - | - | - | - | - |
| Non-photochemical dissipation (NPQ) | ||||||||||||
| 07:00 | 3.242 | 3.281 | 3.172 | 2.383 | 2.637 | 1.200 | 0.67431 | 1.638 | 1.432 | 1.611 | 0.742 | 1.356 |
| 09:00 | 2.379 | 2.155 | 2.366 | 2.523 | 2.939 | 2.774 | 2.268 | 2.070 | 2.109 | 1.698 | 0.967 | 1.086 |
| 11:00 | 2.069 | 2.015 | 1.897 | 1.646 | 3.390 | 2.050 | 2.005 | 2.046 | 1.873 | 0.859 | 1.123 | 1.681 |
| 13:00 | 2.116 | 2.208 | 2.017 | 1.519 | 2.892 | 1.932 | 1.601 | 1.346 | 2.274 | 1.188 | 1.206 | 0.874 |
| 15:00 | 1.903 | 2.285 | 1.965 | 1.998 | - | - | - | - | - | - | - | - |
| 17:00 | 1.876 | 1.642 | 1.807 | 1.494 | - | - | - | - | - | - | - | - |
| Fraction of open reaction centers of the PSII (qL) | ||||||||||||
| 07:00 | 0.600 | 0.764 | 0.757 | 0.608 | 0.317 | 0.444 | 0.401 | 0.416 | 0.326 | 0.324 | 0.22 | 0.280 |
| 09:00 | 0.418 | 0.525 | 0.533 | 0.449 | 0.367 | 0.439 | 0.530 | 0.558 | 0.425 | 0.496 | 0.378 | 0.474 |
| 11:00 | 0.535 | 0.52 | 0.518 | 0.515 | 0.296 | 0.551 | 0.630 | 0.636 | 0.410 | 0.346 | 0.342 | 0.377 |
| 13:00 | 0.604 | 0.618 | 0.597 | 0.542 | 0.356 | 0.629 | 0.597 | 0.670 | 0.440 | 0.460 | 0.471 | 0.438 |
| 15:00 | 0.566 | 0.552 | 0.554 | 0.544 | - | - | - | - | - | - | - | - |
| 17:00 | 0.239 | 0.232 | 0.233 | 0.167 | - | - | - | - | - | - | - | - |
| 100% Level water replacement | ||||||||
|---|---|---|---|---|---|---|---|---|
| TM | DPV | Fv/Fm | ΦPSII | NPQ | ETR | Fv’/Fm’ | qL | |
| TM | 1,00 | 0,99** | -0,87** | -0,04NS | 0,46** | -0,04NS | -0,72** | 0,82** |
| DPV | 1,00 | -0,91** | -0,10NS | 0,45** | -0,10NS | -0,76** | 0,83** | |
| Fv/Fm | 1,00 | 0,17NS | -0,50** | 0,17NS | 0,80** | -0,84** | ||
| ΦPSII | 1,00 | -0,68** | 1,00** | 0,55** | 0,18NS | |||
| NPQ | 1,00 | -0,68** | -0,91** | 0,48** | ||||
| ETR | 1,00 | 0,55** | 0,18NS | |||||
| Fv’/Fm’ | 1,00 | -0,71** | ||||||
| qL | 1,00 | |||||||
| 75% Level water replacement | ||||||||
| TM | DPV | Fv/Fm | ΦPSII | NPQ | ETR | Fv’/Fm’ | qL | |
| TM | 1.00 | 0.99** | -0.82** | -0.12NS | 0.57** | -0.12NS | -0.74** | 0.84** |
| DPV | 1.00 | -0.85** | -0.17NS | 0.59** | -0.17NS | -0.77** | 0.84** | |
| Fv/Fm | 1.00 | 0.27NS | -0.56** | 0.27NS | 0.82** | -0.81** | ||
| ΦPSII | 1.00 | -0.70** | 1.00** | 0.60** | 0.08NS | |||
| NPQ | 1.00 | -0.70** | -0.93** | 0.54** | ||||
| ETR | 1.00 | 0.60** | 0.08NS | |||||
| Fv’/Fm’ | 1.00 | -0.73** | ||||||
| qL | 1.00 | |||||||
| 50% Level water replacement | ||||||||
| TM | DPV | Fv/Fm | ΦPSII | NPQ | ETR | Fv’/Fm’ | qL | |
| TM | 1.00 | 0.98** | -0.79** | -0.36* | 0.42** | -0.36* | -0.70** | 0.62** |
| DPV | 1.00 | -0.83** | -0.39* | 0.42** | -0.39* | -0.72** | 0.62** | |
| Fv/Fm | 1.00 | 0.50** | -0.38* | 0.50** | 0.79** | -0.57** | ||
| ΦPSII | 1.00 | -0.76** | 1.00** | 0.78** | 0.07NS | |||
| NPQ | 1.00 | -0.76** | -0.86** | 0.37* | ||||
| ETR | 1.00 | 0.78** | 0.07NS | |||||
| Fv’/Fm’ | 1.00 | -0.55** | ||||||
| qL | 1.00 | |||||||
| 25% Level water replacement | ||||||||
| TM | DPV | Fv/Fm | ΦPSII | NPQ | ETR | Fv’/Fm’ | qL | |
| TM | 1.00 | 0.99** | -0.72** | -0.77** | 0.58** | -0.77** | -0.80** | -0.20NS |
| DPV | 1.00 | -0.73** | -0.78** | 0.57** | -0.78** | -0.79** | -0.24NS | |
| Fv/Fm | 1.00 | 0.79** | -0.35* | 0.79** | 0.79** | 0.34** | ||
| ΦPSII | 1.00 | -0.65** | 1.00** | 0.88** | 0.51** | |||
| NPQ | 1.0 | -0.65** | -0.84** | 0.20NS | ||||
| ETR | 1.00 | 0.88** | 0.51** | |||||
| Fv’/Fm’ | 1.00 | 0.06NS | ||||||
| qL | 1.00 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





