Submitted:
22 April 2024
Posted:
24 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
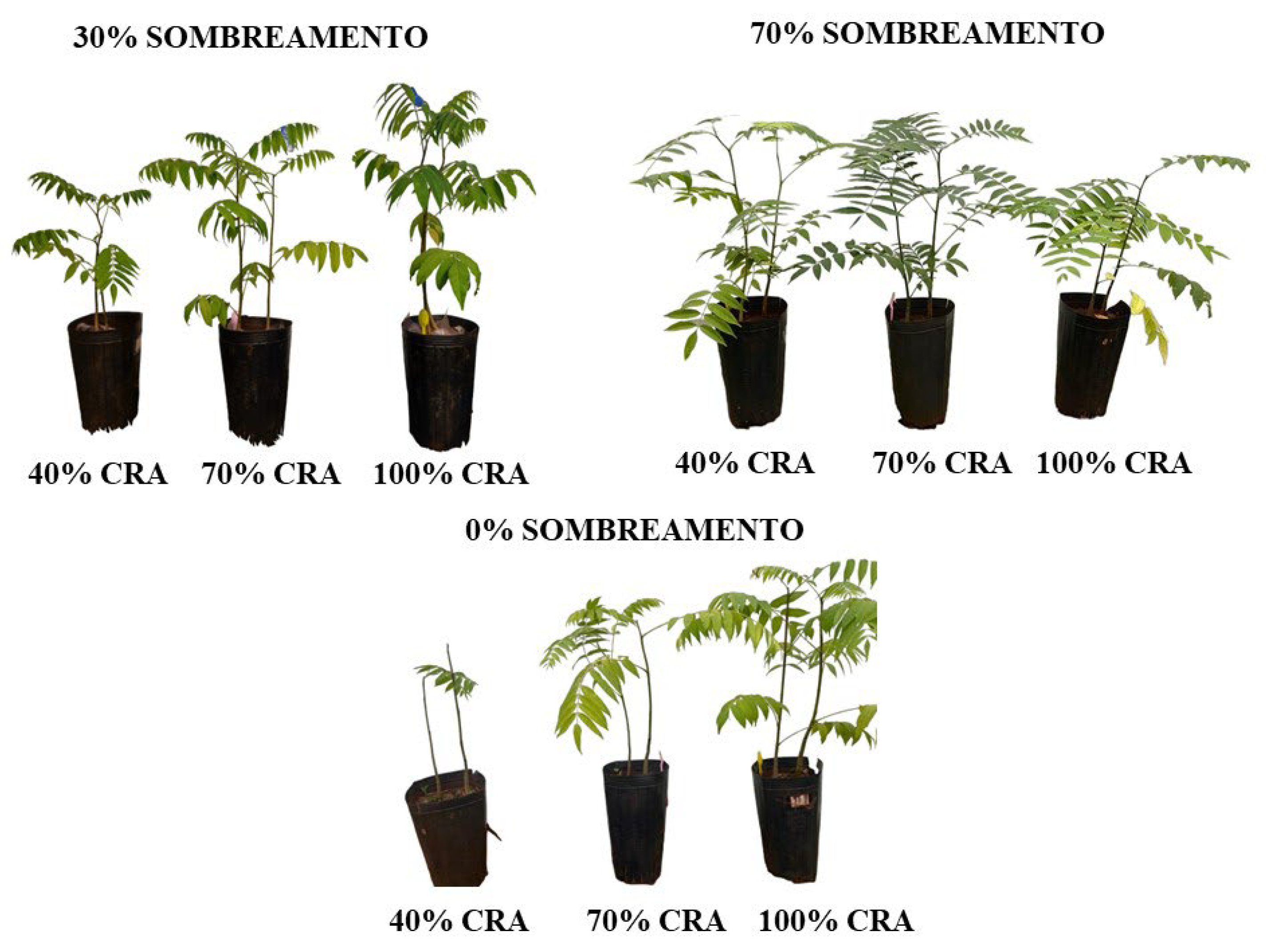
2.1. Visual Aspects
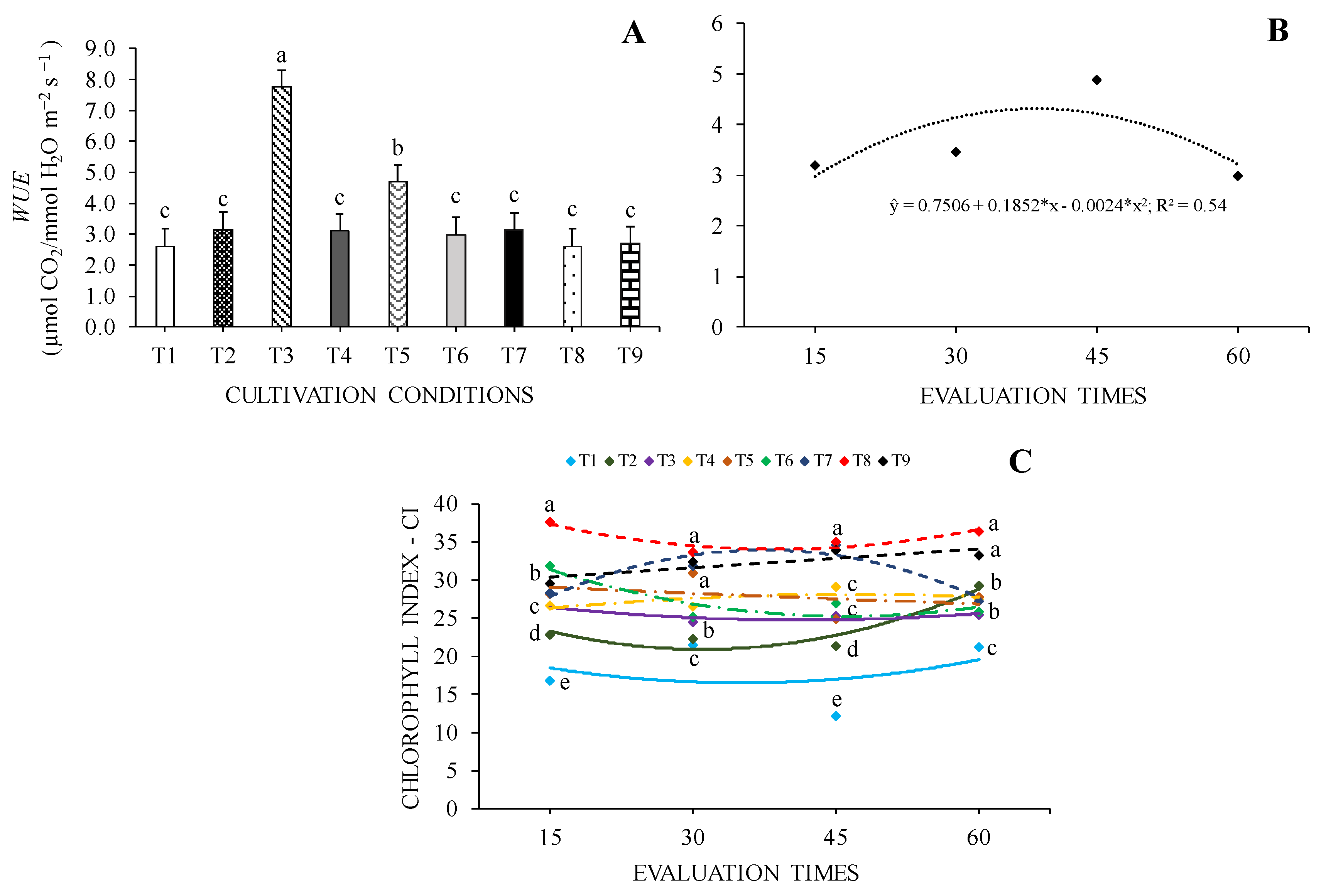
2.2. Gas Exchange and Chlorophyll Index
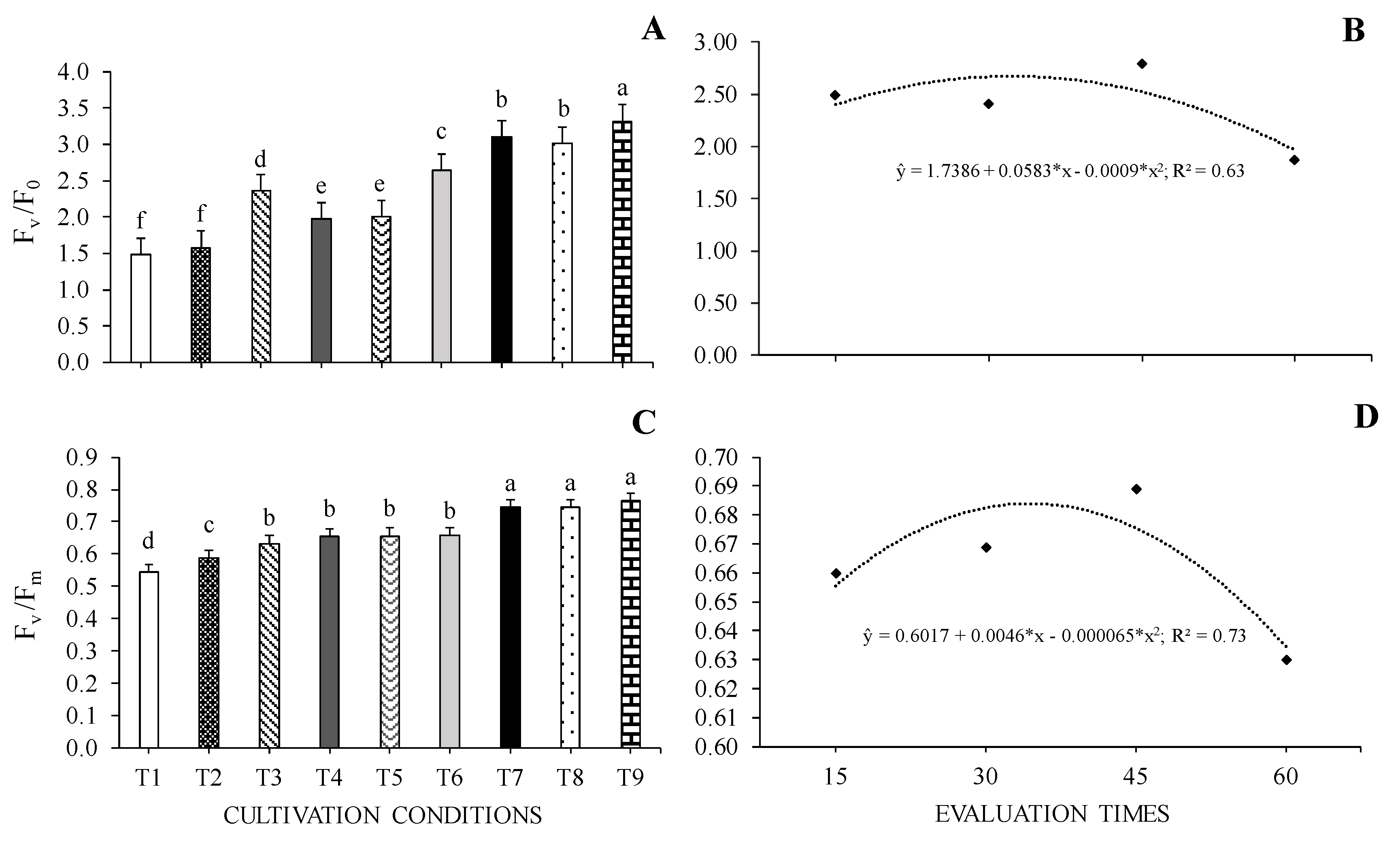
2.3. Chlorophyll a Fluorescence
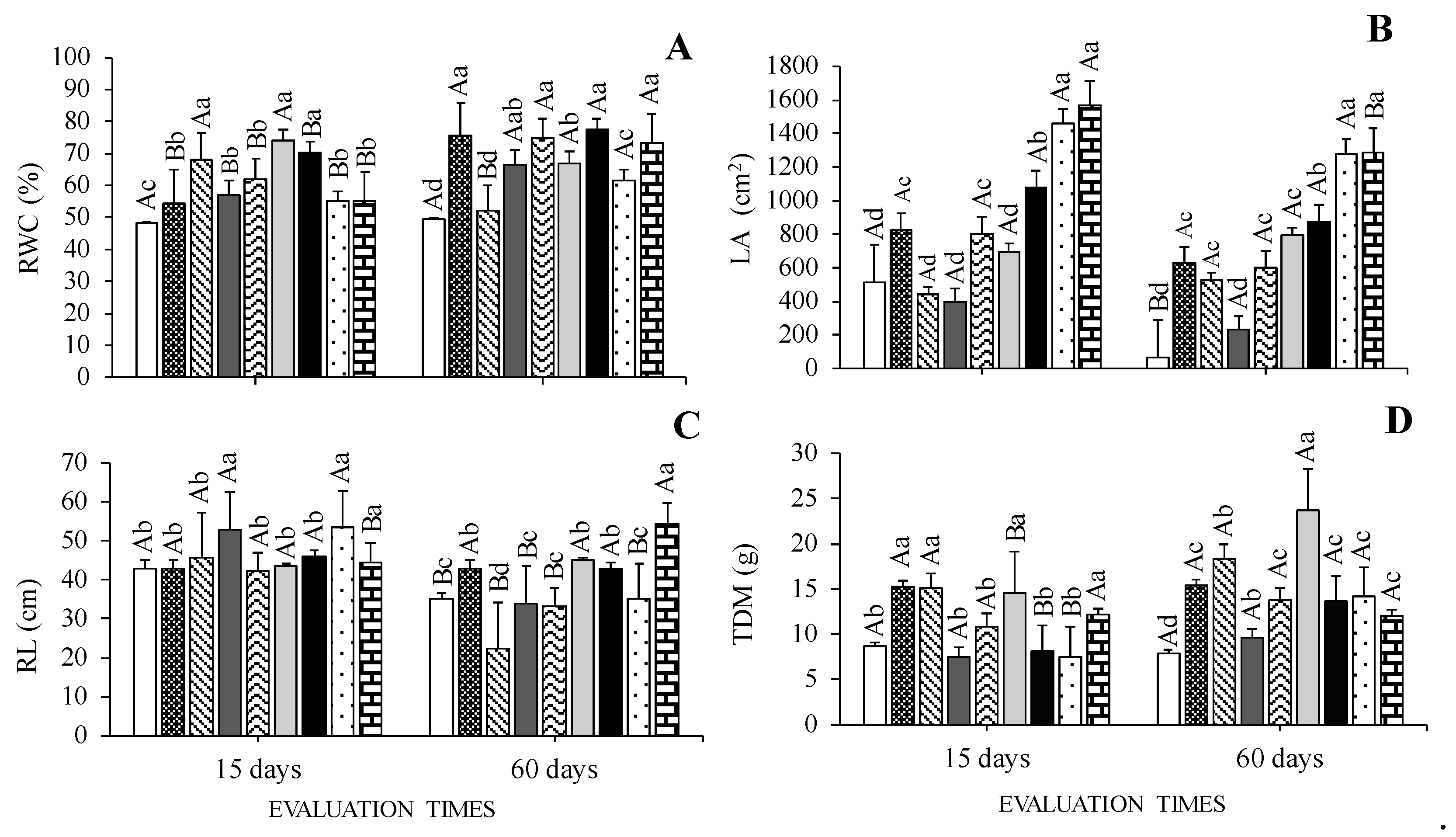
2.4. Relative Leaf Water Content and Growth
2.5. Phenotypic Plasticity
3. Discussion
4. Material and Methods
4.1. Experiment Location and Seedling Production
4.2. Shading Level and Water Regimes
4.3. Experimental Design
4.4. Evaluations
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, V.P.; Lima, R.A.F. de; Joner, F.; Siddique, I.; Raes, N.; Steege, H.T. Climate change threatens native potential agroforestry plant species in Brazil. Sci. Rep. 2022, 12, 2267. [Google Scholar] [CrossRef] [PubMed]
- Vourlitis, G.L.; Pinto Jr., O. B.; Dalmagro, H.J.; Arruda, P.E.Z.; Lobo, F.A.; Nogueira J.S. Tree growth responses to climate variation in upland and seasonally flooded forests and woodlands of the Cerrado-Pantanal transition of Brazil. Forest. Ecol. Manag. 2022, 505, 119917. [Google Scholar] [CrossRef]
- Wei, C.; Wang, Q.; Han, H.; Gan, X. Low soil moisture improved shading tolerance by regulating leaf functional traits in Tetracentron sinense Oliv. Seedlings. Glob. Ecol. Conserv. 2023, 48, e02757. [Google Scholar] [CrossRef]
- Salmona, Y.B.; Matricardi, E.A.T.; Skole, D.L.; Silva, J.F.A.; Coelho Filho, O.A.; Pedlowski, M.A.; Sampaio, J.M.; Castrillón, L.C.R.; Brandão, R.A.; Silva, A.L.; et al. A Worrying Future for River Flows in the Brazilian Cerrado Provoked by Land Use and Climate Changes. Sustainability 2023, 15, 4251. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Cerqueira, W.M.; Scalon, S.P.Q.; Santos, C.C.; Santiago, E.F.; Almeida, J.L.C.S.; Figueiredo, V.M.A.; Linné, J.A.; Silverio, J.M. Ecophysiological mechanisms and growth of Inga vera Willd. under different water and light availability. Braz. J. Biol. 2023, 83, e275378. [Google Scholar] [CrossRef]
- Griebeler, A.M.; Araujo, M.M.; Barbosa, F.M.; Kettenhuber, P.L.; Nhantumbo, L.S.; Berghetti, A.L.P.; Denardi, L. Morphophysiological responses of forest seedling species subjected to different water regimes. J. For. Res. 2021, 32, 2099–2110. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A Review on potential plant-based water stress indicators for vegetable crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Bottero, A.; Forrester, D.I.; Cailleret, M.; Kohnle, U.; Gessler, A.; Michel, D.; Bose, A.K.; Bauhus, J.; Bugmann, H.; Cuntz, M; et al. Growth resistance and resilience of mixed silver fir and Norway spruce forests in central Europe: Contrasting responses to mild and severe droughts. Glob. Change Biol. 2021, 27, 4403–4419. [Google Scholar] [CrossRef]
- Croce, J.; Badano, E.I.; Trigo, C.B.; Martinez-Galvez, F.; Tálamo, A. Experimental approaches to select tree species for forest restoration: effects of light, water availability and interspecific competition in degraded areas. J. For. Res. 2022, v. 33, 1197–1207. [Google Scholar] [CrossRef]
- Pereira, G.A.; Barbosa, A.C.M.C.; Torbenson, M.C.A.; Stahle, D.W.; Granato-Souza, D.; Santos, R. M.; Barbosa, J.P.D. The Climate Response of Cedrela fissilis Annual Ring Width in the Rio São Francisco Basin, Brazil. Tree-Ring Res. 2018, 74, 162–171. [Google Scholar] [CrossRef]
- Prado, M.C.N.; Giuliani, G.K.F.; Ghiotto, T.C.; Carmo, J.B.; Guereiro, J.C.; Prado, E. P.; Pogetto, M.H.F.A.S.; Masson, M.V.; Tavares, W.S.; Wilcken, C.F.; et al. Detection and estimation of Mastigimas anjosi (Hemiptera: Calophyidae) populations on Cedrela fissilis trees. R. Soc. Open. Sci. 2022, 9, 211340. [Google Scholar] [CrossRef] [PubMed]
- Krainovic, P.M.; Resende, A.F.; Amazonas, N.T.; Almeida, C.T.; Almeida, D.R.A.; Silva, C.C.; Andrade, H.S.F.; Rodrigues, R.R.; Brancalion, P.H.S. Potential native timber production in tropical forest restoration plantations. Perspect. Ecol. Conserv. 2023, 21, 294–301. [Google Scholar] [CrossRef]
- Santos, T.R.S.; Santos, J.A.S.; Pereira, E.G.; Garcia, Q.S. Revegetation of an area impacted by iron ore tailings: evaluating fertilization alternatives in native pioneer and secondary trees. Environ. Sci. Pollut. Res. Int. 2023, 30, 3760–3773. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.O.; Dresch, D.M.; Scalon, L.; Scalon, S.P.Q. Ecophysiological strategies of Cedrela fissilis Vell. seedlings under conditions of flooding and light availability. J. Sustain. For. 2021, 41, 783–798. [Google Scholar] [CrossRef]
- Rodriguez, D.R.O.; Sánchez-Salguero, R.; Hevia, A.; Bovi, R.C.; Ferreira, M. J.; Speer, J.H.; Tomazello-Filho, M. Does climate change alter the nutrient trends of Cedrela fissilis Vell. trees in the southern Brazilian Amazon? Ecol. Process. 2023, 12, 58. [Google Scholar] [CrossRef]
- Leonel, L.V.; Reis, F.O.; Figueiredo, F.A.M.M.A.; Ferraz, T.M.; Maia Júnior, S.O.; Silva, P.C.; Andrade, J.R. Light intensity and hydrogel soil amendment differentially affect growth and photosynthesis of successional tree species. J. For. Res. 2023, 34, 257–268 ttps://doiorg/101007/s11676. [Google Scholar] [CrossRef]
- Bartieres, E.M.M.; Dresch, D.M.; Reis, L.C.; Pereira, Z.V.; Mussury, R.M.; Scalon, S.P.Q. Shading minimizes the effects of water deficit in Campomanesia xanthocarpa (Mart.) O. Berg seedlings. Braz. J. Biol. 2023, 83, e244718. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dwivedi, S.; Bhagavatuba, L.; Datta, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Puértolas, J.; Dodd, I.C. Evaluating soil evaporation and transpiration responses to alternate partial rootzone drying to minimise water losses. Plant Soil 2022, 480, 473–489. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Thorpe, M.R.; Hocart, C.H.; Song, X. Stomatal and mesophyll conductance are dominant limitations to photosynthesis in response to heat stress during severe drought in a temperate and a tropical tree species. Trees 2021, 35, 1613–1626. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Stattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A. P. The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, S.; Yan, S.; Xu, W.; Chen, J. Light energy partitioning and photoprotection from excess light energy in shade-tolerant plant Amorphophallus xiei under steady-state and fluctuating high light. Acta Physiol. Plant 2021, 43, 125. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, J.; Wang, Y.; Xu, S.; Jiang, L.; Zhang, L.; Hou, W. Effects of exogenous NO on the growth and photosynthetic fluorescence characteristics of ryegrass seedlings under B[a]P stress. Acta Bot. Croat. 2023, 82, 71–79. [Google Scholar] [CrossRef]
- Eckert, D.; Jensen, A.M.; Gu, L. The maximum carboxylation rate of Rubisco affects CO2 refixation in temperate broadleaved forest trees. Plant Physiol. Biochem. 2020, 155, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Cummins, P. L. The coevolution of rubisco, photorespiration, and carbon concentrating mechanisms in higher plants. Front. Plant. Sci. 2021, 12, 662425. [Google Scholar] [CrossRef]
- Ye, X.; Gao, Z.; Xu, K.; Li, B.; Ren, T.; Li, X.; Cong, R.; Lu, Z.; Cakmak, I.; Lu, J. Photosynthetic plasticity aggravates the susceptibility of magnesium-deficient leaf to high light in rapeseed plants: the importance of Rubisco and mesophyll conductance. Plant J. 2023, 117, 483–497. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Fonseca, C.L.S.; Silva, D. M.; Gasparini, T.A.S.; Cuzzuol, G.R.F. Photosynthesis of plants of shade-tolerant ecotype of Paubrasilia echinata are more tolerance to drought than the sun-tolerant ecotype. Plant Stress 2023, 8, 100157. [Google Scholar] [CrossRef]
- Angadi, S.V. Umesh, M.R.; Begna, S.; Gowda, P. Light interception, agronomic performance, and nutritive quality of annual forage legumes as affected by shade. Field Crops Res. 2022, 275, 108358. [Google Scholar] [CrossRef]
- Genesio, L.; Bassi, R.; Miglietta, F. Plants with less chlorophyll: A global change perspective. Glo. Change Biol. 2020, 27, 959–967. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol 2021, 185, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Zhang, Z.; Sui, Y.; Zhu, H.; Shi, Y.; Sui, X.; Li, Y.; Jiang, X. Insights into the changes in leaf functional traits of Aralia elata grown under different shading treatments. Plant Growth Regul. 2023, 101, 679–691. [Google Scholar] [CrossRef]
- Vetoshkina, D.; Balashov, N.; Ivanov, B.; Ashikhmin, A.; Borisova-Mubarakshina, M. Light harvesting regulation: A versatile network of key components operating under various stress conditions in higher plants. Plant Physiol. Biochem. 2023, 194, 576–588. [Google Scholar] [CrossRef]
- Xu, R.; Wang, L.; Zhang, J.; Zhou, J.; Cheng, S.; Tigabu, M.; Ma, X.; Wu, P.; Li, M. Growth Rate and Leaf Functional Traits of Four Broad-Leaved Species Underplanted in Chinese Fir Plantations with Different Tree Density Levels. Forests 2022, 13, 308. [Google Scholar] [CrossRef]
- Luo, D.; Huang, G.; Zhang, Q.; Zhou, G.; Peng, S.; Li, Y. Plasticity of mesophyll cell density and cell wall thickness and composition play a pivotal role in regulating plant growth and photosynthesis under shading in rapeseed. Ann. Bot. 2023, 132, 963–978. [Google Scholar] [CrossRef]
- Yu, X.F.; Ming, X.Y.; Xiong, M.; Zhang, C.; Yue, L.J.; Yang, L.; Fan, C.Y. Partial shade improved the photosynthetic capacity and polysaccharide accumulation of the medicinal plant Bletilla ochracea Schltr. Photosynthetica 2022, 60, 168–178 1032615/ps2021064. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, T.; Guo, S.; Liu, R.; Li, M. Leaf anatomy, photosynthesis and chlorophyll fluorescence of lettuce as influenced by arbuscular mycorrhizal fungi under high temperature stress. Sci. Hortic. 2021, 280, 109933. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmulling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2020, 44, 645–664. [Google Scholar] [CrossRef]
- Sanches, M.C.; Marzinek, J.; Bragiola, N.G.; Nascimento, A.R.T. Morpho-physiological responses in Cedrela fissilis Vell. submitted to changes in natural light conditions: implications for biomass accumulation. Trees 2017, 31, 215–227. [Google Scholar] [CrossRef]
- Xingyang, S.; Guangsheng, Z.; Qijing, H.; Huailin, Z. Stomatal limitations to photosynthesis and their critical Water conditions in different growth stages of maize under water stress. Agric. Water Manag. 2020, 241, 106330. [Google Scholar] [CrossRef]
- Granata, M.U.; Bracco, F.; Catoni, R. C. Phenotypic plasticity of two invasive alien plant species inside a deciduous forest in a strict nature reserve in Italy. J. Sustain. For. 2019, 39, 346–364. [Google Scholar] [CrossRef]
- Olguin, F.Y.; Moretti, A.P.; Pinazo, M.; Gortari, F.; Bahima, J.V.; Graciano, C. Morphological and physiological plasticity in seedlings of Araucaria angustifolia and Cabralea canjerana is related to plant establishment performance in the rainforest. For. Ecol. Manag. 2020, 460, 117867. [Google Scholar] [CrossRef]
- Souza, C.C.; Oliveira, F.A.; Silva, I.F.; Amorim Neto, M.S. Avaliação de métodos de determinação de água disponível e manejo da irrigação em terra roxa sob cultivo de algodoeiro herbáceo. Rev. Bras. Eng. Agric. 2000, 4, 338–342. [Google Scholar] [CrossRef]
- Bolhàr-Nordenkampf, H.R.; Long, S.P.; Baker, N.R. Fluorescência da clorofila como sonda da competência fotossintética de folhas no campo: uma revisão do instrumento atual. Funct. Ecol. 1989, 3, 497–514. [Google Scholar] [CrossRef]
- Slavick, B. Methods of Studying Plant Water Relations. Springer- Verlag, New York, 1979. ISBN 978-3-642-65834-1.
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
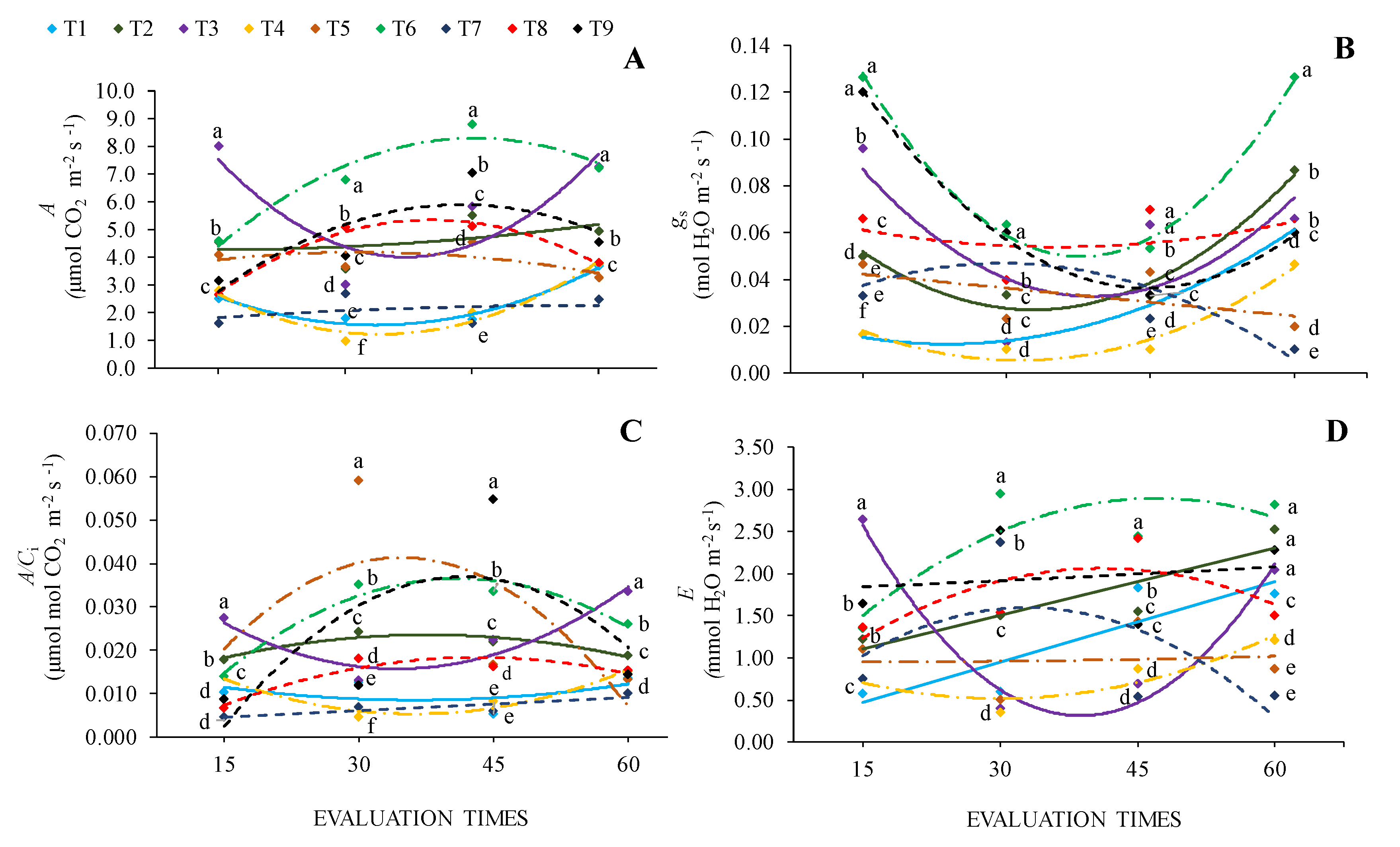

| CHARACTERISTICS EVALUATED | ||||
|---|---|---|---|---|
| A (µmol CO2 m-2 s-1) | gs (mol H2O m-2 s-1) | A/Ci (µmol mol CO2 m-2 s-1) | ||
| T1 | ŷ = 4.8925 - 0.1979*x + 0.0029*x2; R2; = 0.96 |
ŷ = 0.0333 – 0.0017*x + 0.000037*x2; R2; = 0.97 |
No adjustment | |
| T2 | No adjustment | ŷ = 0.1108 – 0. 0051*x + 0.000078*x2 R2; = 0.96 |
ŷ = 0.0091 + 0.00076*x – 0.000010*x2; R2; = 0.88 |
|
| T3 | ŷ = 13.8925 - 0.5310*x + 0.0071*x2; R2; = 0.70 |
No adjustment | ŷ = 0.0492 – 0.00195*x + 0.000028*x2; R2; = 0.89 |
|
| T4 | ŷ = 5.825 - 0.2688x + 0.0039*x2; R2; = 0.94 |
ŷ = 0.0525 – 0.0030*x + 0.000048*x2; R2; = 0.95 |
ŷ = 0.0289 – 0.0013*x + 0.000018*x2; R2; = 0.93 |
|
| T5 | No adjustment | No adjustment | No adjustment | |
| T6 | ŷ= -0.3825 + 0.3827*x - 0.0042*x2; R2; = 0.93 |
ŷ = 0.2658 – 0.0114*x + 0.000152*x2; R2; = 0.99 |
ŷ = -0.0152 + 0.0024*x – 0.000029*x2; R2; = 0.98 |
|
| T7 | No adjustment | ŷ = 0.0083 + 0.0026*x – 0.000044*x2; R2; = 0.72 |
ŷ = 0.0032 + 0.000099*x; R2; = 0.73 | |
| T8 | ŷ= -1.3425 + 0.3626*x - 0.0041*x2; R2; = 0.98 |
No adjustment | ŷ = -0.0072 + 0.0011*x – 0.000014*x2; R2; = 0.86 |
|
| T9 | ŷ = -1.3275 + 0.3303*x - 0.0037x2; R2; = 0.65 |
ŷ = 0.2283 – 0.0086*x + 0.000096*x2; R2; = 0.99 |
No adjustment | |
| E (mmol H2O m-2 s-1) | CI (Chlorophyll index) | |||
| T1 | ŷ = -0.0033 + 0.0318*x; R2; = 0.78 | No adjustment | ||
| T2 | ŷ = 0.7100 + 0.0264*x; R2; = 0.80 | ŷ = 29.8666 – 0.5777*x + 0.0093*x2; R2; = 0.88 | ||
| T3 | ŷ = 6.330 – 0.3102*x + 0.0040*x2; R2; = 0.96 | No adjustment | ||
| T4 | ŷ = 1.2658 – 0.0499*x + 0.00083*x2; R2; = 0.83 | No adjustment | ||
| T5 | No adjustment | No adjustment | ||
| T6 T7 |
ŷ = -0.1050 + 0.1275*x – 0.00135*x2; R2; = 0.71 | ŷ = 38.6916 – 0.5836*x + 0.0063*x2; R2; = 0.76 | ||
| No adjustment | ŷ = 16.8666 + 0.9144*x – 0.0122*x2; R2; = 0.87 | |||
| T8 | No adjustment | ŷ = 43.0416 – 0.4765*x + 0.0061*x2; R2; = 0.79 | ||
| T9 | No adjustment | ŷ = 29.200 + 0.0824*x; R2; = 0.69 | ||
|
Shading level |
PPI (0.00 to 1.00) | |||
|---|---|---|---|---|
| A | Fv/Fm | LA | TDM | |
| 0% | 0.49 | 0.16 | 0.88 | 0.42 |
| 30% | 0.54 | 0.04 | 0.70 | 0.48 |
| 70% | 0.45 | 0.02 | 0.30 | 0.38 |
| Evaluation Period | Shading Level | PAR | Leaf T. (°C) |
|---|---|---|---|
| Days | (μmol m-2 s-1) | (°C) | |
|
15 |
0% 30% 70% |
1159 750 58 |
38.0 33.0 32.0 |
|
30 |
0% 30% 70% |
2038 714 140 |
33.0 31.7 32.0 |
|
45 |
0% 30% 70% |
1934 688 79 |
42.0 36.8 36.1 |
|
60 |
0% 30% 70% |
1212 960 87 |
40.3 38.2 31.5l |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





