Submitted:
18 July 2024
Posted:
19 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Absorption and Conductivity
1.2. The Characteristics of Silicon in Polymer
2. Materials and Methods
2.1. Elastic Silicon Polymers
2.2. The Structure of Elastic Silicon Polymers
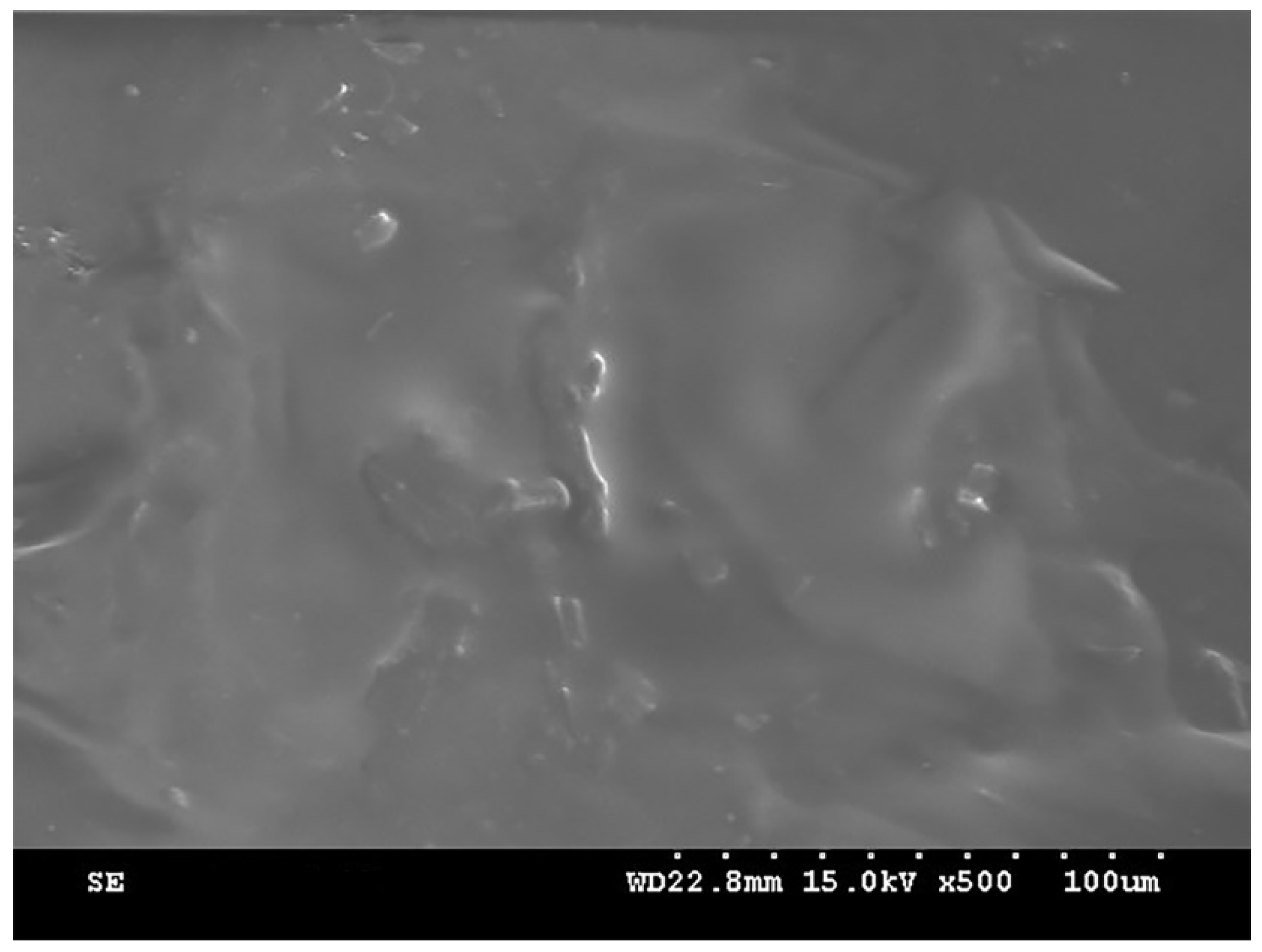
2.3. SEM Analysis
2.4. EDX Analysis
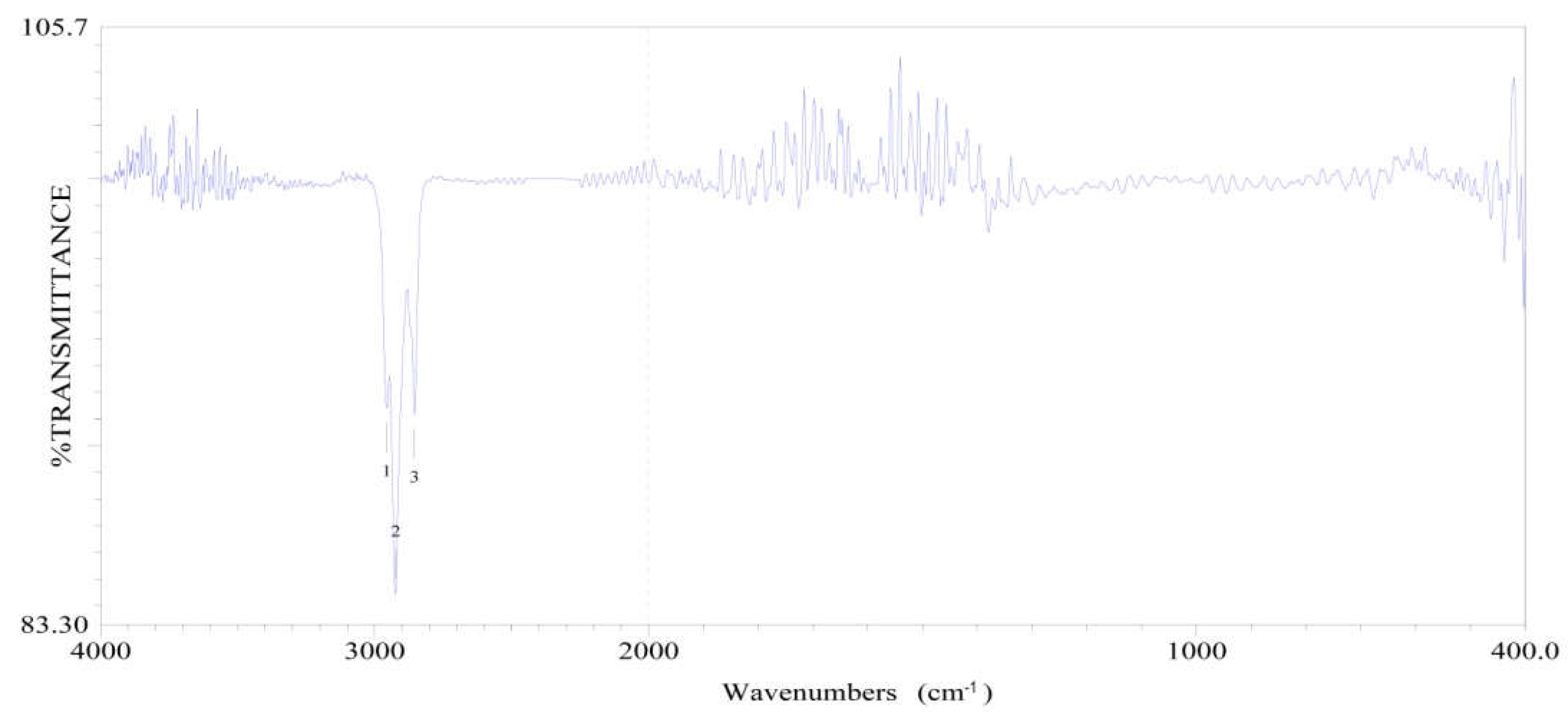
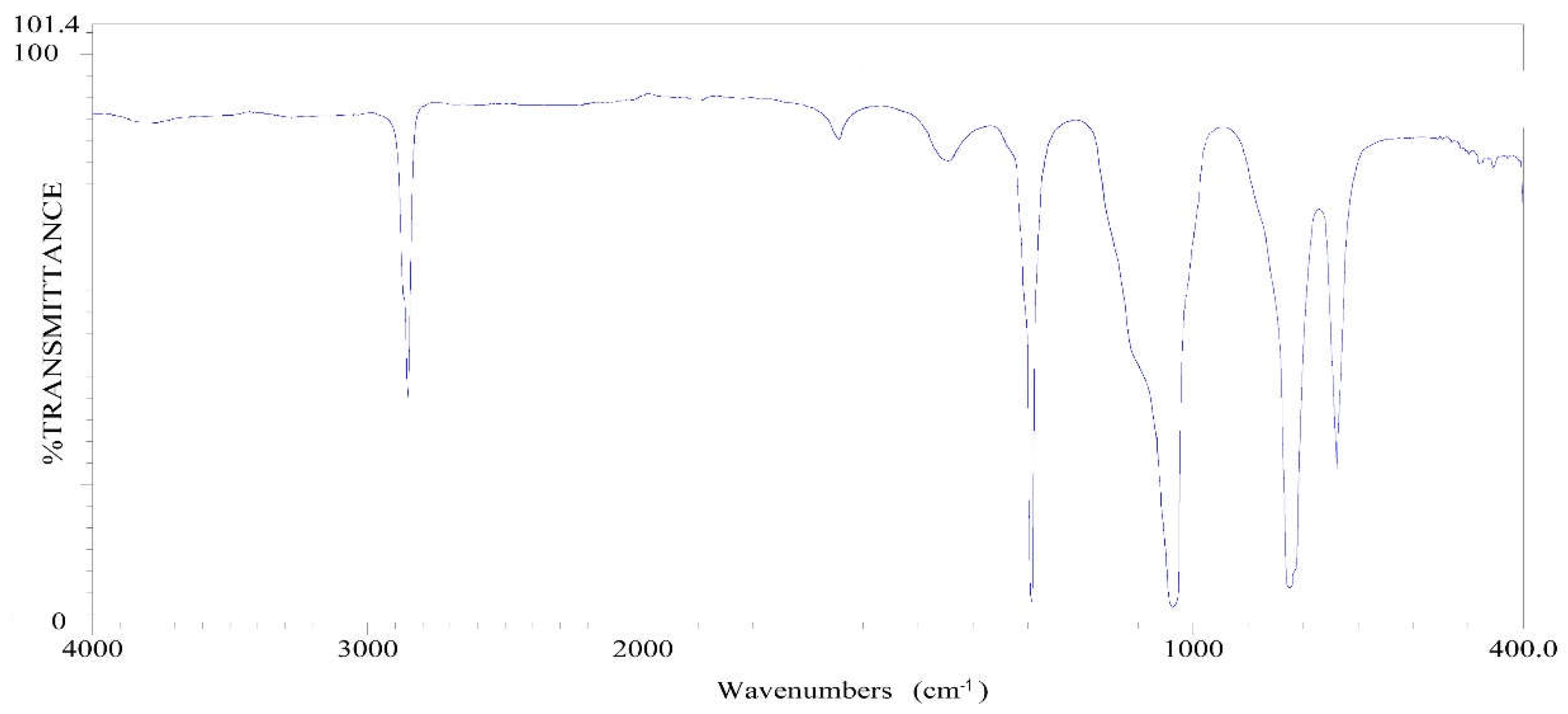
2.5. The Infrared Absorption Spectrum Analysis
2.5.1. The Infrared Spectra of Silicon-Oxygen Bond (Si-O)
2.5.2. The Infrared Spectra of Silica
3. Results
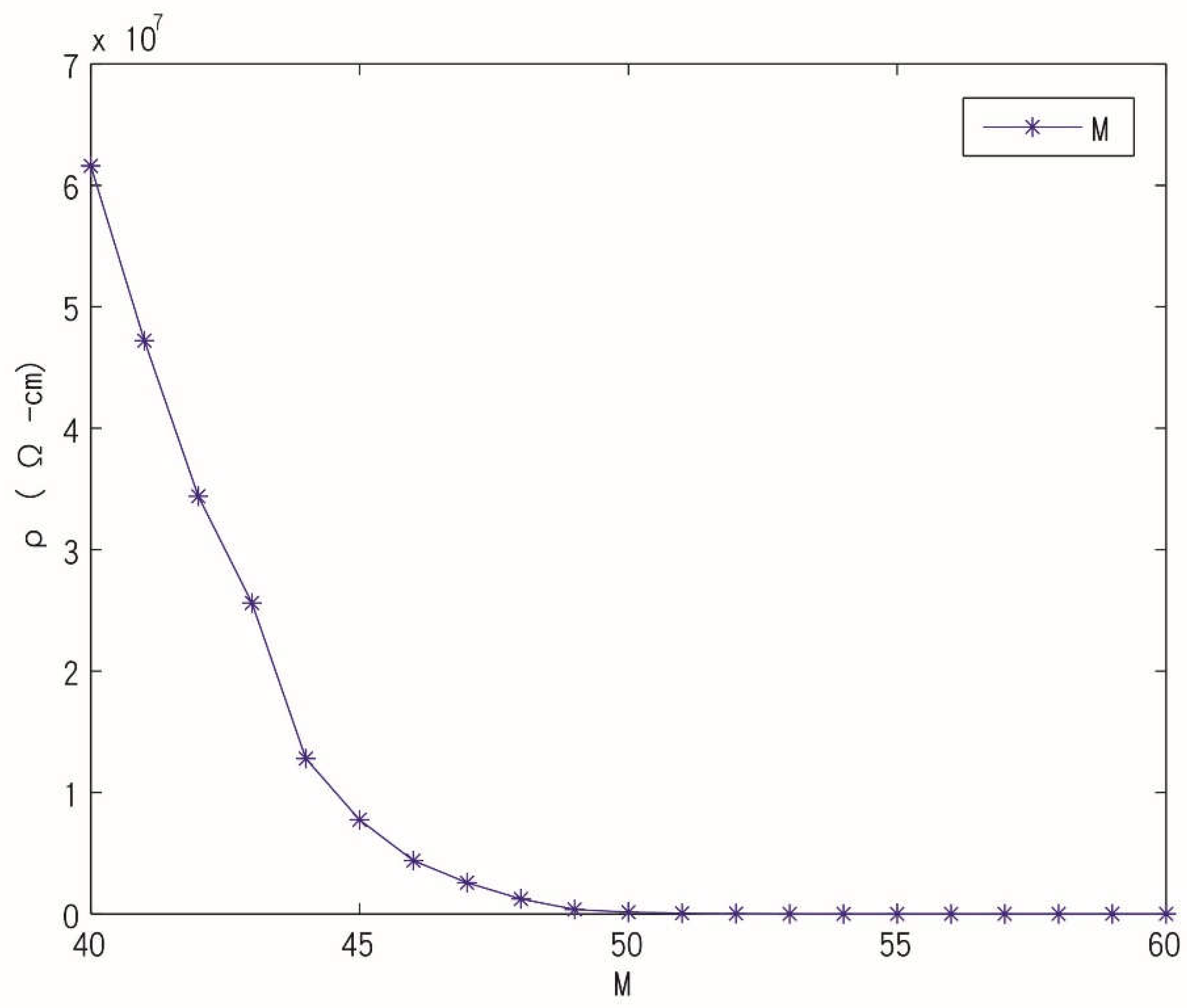
3.1. Conductive
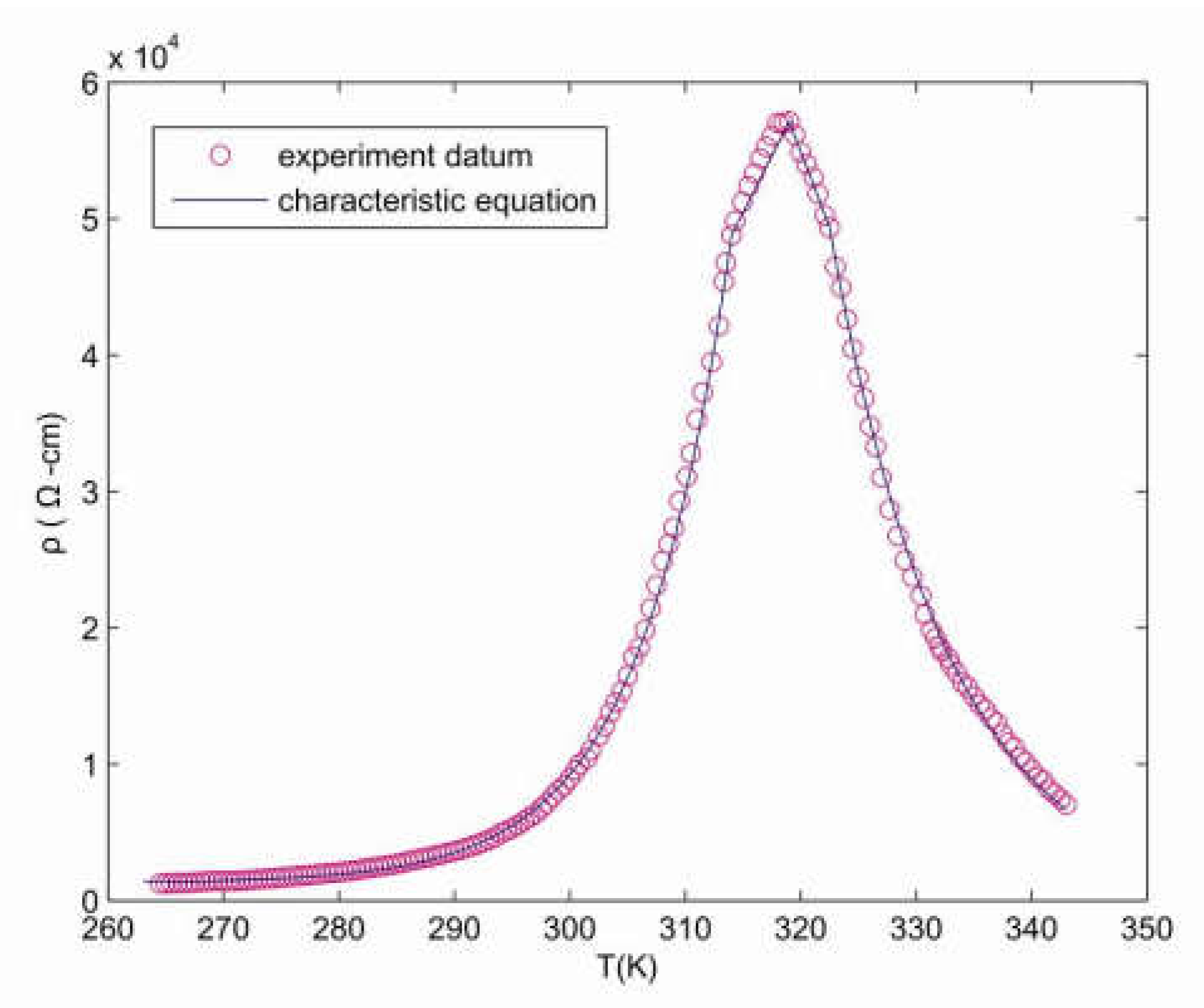
3.2. Resistance of Temperature Coefficient
4. Discussion
4.1. Conductive
4.2. Temperature Coefficient
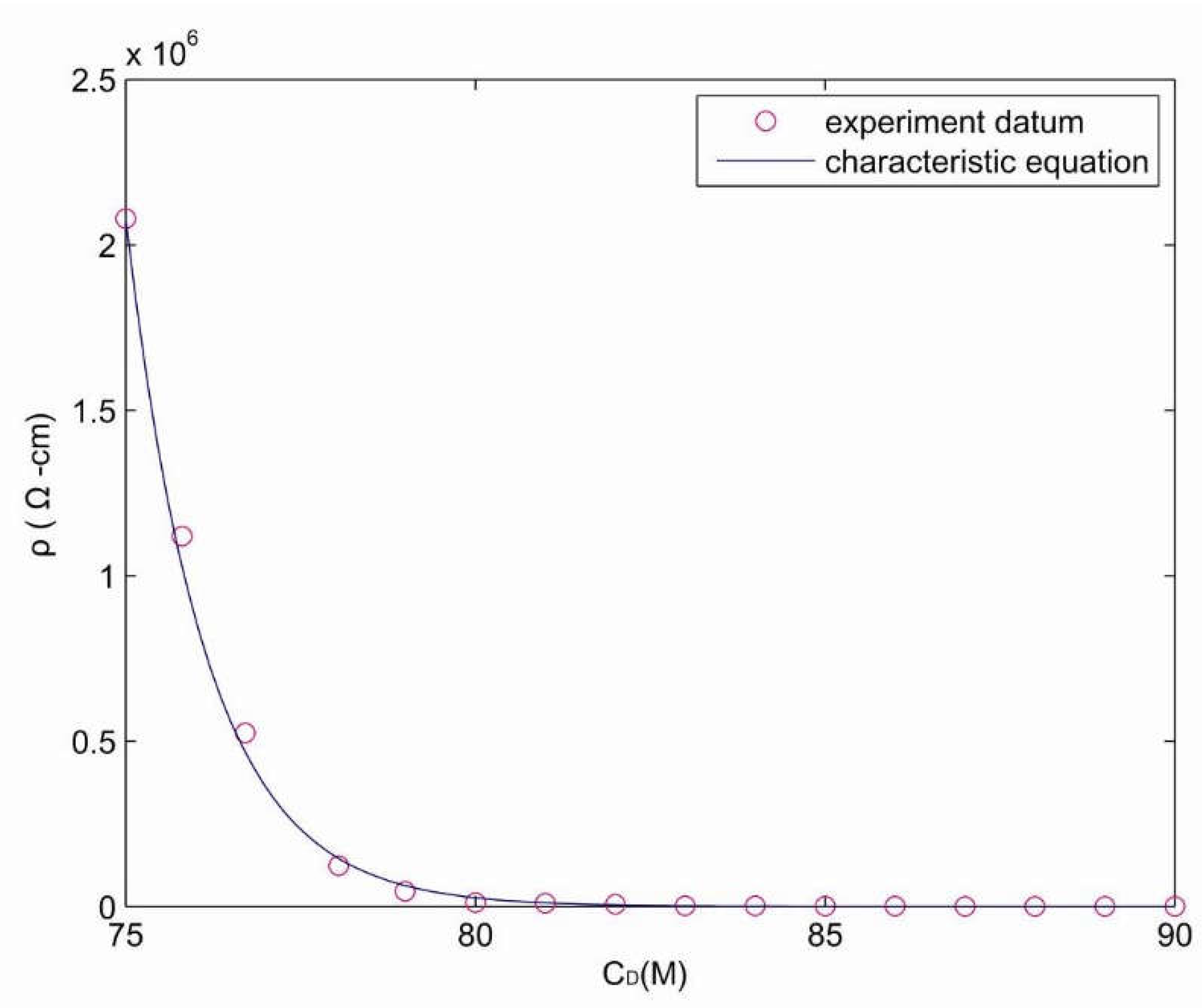
4.3. The Characteristic Equation of Carbon Concentration and Resistance
4.4. The Characteristic Equation of Resistance and Temperature Coefficient
5. Conclusions
5.1. Silicon Atomic Structure Effect the Adsorption Conductive of Elastic Polymers
5.2. Applicability of The Characteristic Equation
5.3. Physisorption Effect the Elastic Polymers
References
- N. K. Guimard, N. Gomez, and C. E. Schmidt, Prog. Polym. Sci. 2007, 32, 876.
- Bravo-Grimaldo E, Hachey S, Cameron CG, Freund MS, Metastable reaction mixtures for the in situ polymerization of conducting polymers. Macromolecules. 2007, 40:7166–7170. [CrossRef]
- Zhou Y, et al. Investigation on polymer anode design for flexible polymer solar cells. 2008,Appl Phys Lett 92:233308. [CrossRef]
- Zaumseil J, Friend RH, Sirringhaus H. Spatial control of the recombination zone in an ambipolar light-emitting organic transistor. Nat Mater. 2006, 5:69–74. [CrossRef]
- Julio, M. D’Arcya, Henry D. Tranb, Vincent C. Tungc, Alexander K. Tucker-Schwartza, Rain P. Wonga, Yang Yangc, and Richard B. Kaner , Versatile solution for growing thin films of conducting polymers. Edited by Noel A. Clark, University of Colorado, Boulder, CO, and approved September 14, 2010. [CrossRef]
- Yeon, H. Y.; Byung K. L.; Ji S. C.; Sungwon K.; Bongyoung Y.; Yong S. K.; Kinam P.; Yong W. C., A Glucose Sensor Fabricated by Piezoelectric Inkjet Printing of Conducting Polymers and Bienzymes; 2011 c The Japan Society for Analytical Chemistry, Analytical Sciences. 2011, Vol 27.
- Rouquerol F, Rouquerol J, Sing K: Adsorption by powders and porous solids London: Academic Press. 1999, 1-447.
- Li D, Kaner RB. Processable stabilizer-free polyaniline nanofiber aqueous colloids. Chem Commun. 2005, 26:3286–3288. [CrossRef]
- Chaudhury MK, Whitesides GM: Direct measurement of interfacial interactions between semispherical lenses and flat sheets of poly (dimethylsiloxane) and their chemical derivatives. Langmuir. 1991, 7:1013.
- Hossein Ghanbari, Achala de Mel, Alexander M Seifalian, Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: a glimpse into prospective horizons, International Journal of Nanomedicine, April 2011 Volume. 2011, 6, 775 – 786. [CrossRef]
- Kannan RY, Salacinski HJ, Edirisinghe MJ, Hamilton G, Seifalian AM. Polyhedral oligomeric silsequioxane- polyurethane nanocomposite microvessels for an artificial capillary bed. Biomaterials. 2006, 27(26):4618–4626. [CrossRef]
- Li GZ, Yamamoto T, Nozaki K, Hikosaka M. Crystallization of ladderlike poly phenyl silsesquioxane (PPSQ)/isotactic polystyrene (i-PS) blends. Polymer. 2001, 42(20):8435–8441.
- Maciel GE, Sullivan MJ, Sindorf DW. C-13 and Si-29 nuclear magnetic-resonance spectra of solid poly (methylsiloxane) polymers. Macromolecules. 1981, 14(5): 1607 –1608.
- Frye CL, Collins WT. Oligomeric silsequioxanes, (Hsio3/2)N. J Am Chem Soc. 1970, 92(19):5586–5588.
- Brook, MA. Silicon in Organic, Organometallic, and Polymer Chemistry. New York: John Wiley. and referenced cited therein. 2000.
- Scott E. Denmark, The Interplay of Invention, Discovery, Development and Application in Organic Synthetic Methodology: A Case Study, J Org Chem. 2009, 74(8): 2915–2927. [CrossRef]
- Denmark SE, Tymonko SA. J. Org. Chem 2003;70:9151–9154. [PubMed: 14604401] (b) Lee M, Ko S, Chang S. J. Am. Chem. Soc. 2000, 122:12011–12012.
- Ojima, I.; Li, Z.; Zhu, J. The chemistry of organic silicon compounds. Rappoport, Z.; Apeloig, Y., editors. Vol. Vol. 2. Great Britain: John Wiley & Sons. 1998, 1687-1792.
- Denmark SE, Kallemeyn JM. Org. Lett, 2003; 5: 3483–3486. [PubMed: 12967305].
- Lickiss PD. Adv. Inorg. Chem, 1995, 42: 147–262.
- Teh-Hua Tsai, Chen-Yu Wang, “A Study of Ammonium Bifluoride as an Agent for Cleaning Silicon Contamination in the Wafer Dicing Process”, Applied Sciences. 2023, 13(9), 5294, 13p. [CrossRef]
- Teh-Hua Tsai, Chen-Yu Wang, “Metal Corrosion Protection in Ammonium Bifluoride-Base Cleaning Agent for Si Contaminants”, Aspects in Mining & Mineral Science, 11(4), AMMS. 000767, 2023.
- Teh-Hua Tsai, Chen-Yu Wang, “A New Quick Sieve Method for Etchant Evaluation and UBM Cu Undercut Improvement”, American Journal of Biomedical Science & Research, 19(6) AJBSR.MS.ID.002657, 2023. [CrossRef]
- Jager EWH, Smela E, Inganas O, Microfabricating conjugated polymer actuators. Science. 2000, 290:1540–1545. [CrossRef]
- Amara JP, Swager TM. Macromolecules, 2005, 38:9091–9094.
- Ata S. Coalescence of bubbles covered by particles. Langmuir. 2008, 24:6085–6091. [CrossRef]
- BestehornM, Pototsky A, Thiele U. 3d large scale Marangoni convection in liquid films. Eur Phys J B, 2003, 33:457–467. [CrossRef]
- Iwasita, T.; Schmickler, W. Ber. Bunsen-Ges. 1985, 89, 138–42.
- Royea, W. J.; Hamann, T. W.; Brunschwig, B. S.; Lewis, N. S. J. Phys. Chem. 2006, 110, 19433–19442.
- Jason C. Sanchez, Antonio G. DiPasquale, Anthony A. Mrse, William C. Trogler Received, Lewis acid–base interactions enhance explosives sensing in silacycle polymers, Anal Bioanal Chem. 2009, 395:387–392. [CrossRef]
- Yamaguchi S, Tamao K. Bull Chem Soc Jpn. 1996, 69:2327–2334.
- Yamaguchi, Y. Yamaguchi Y. Synth Met. 1996, 82:149–153.



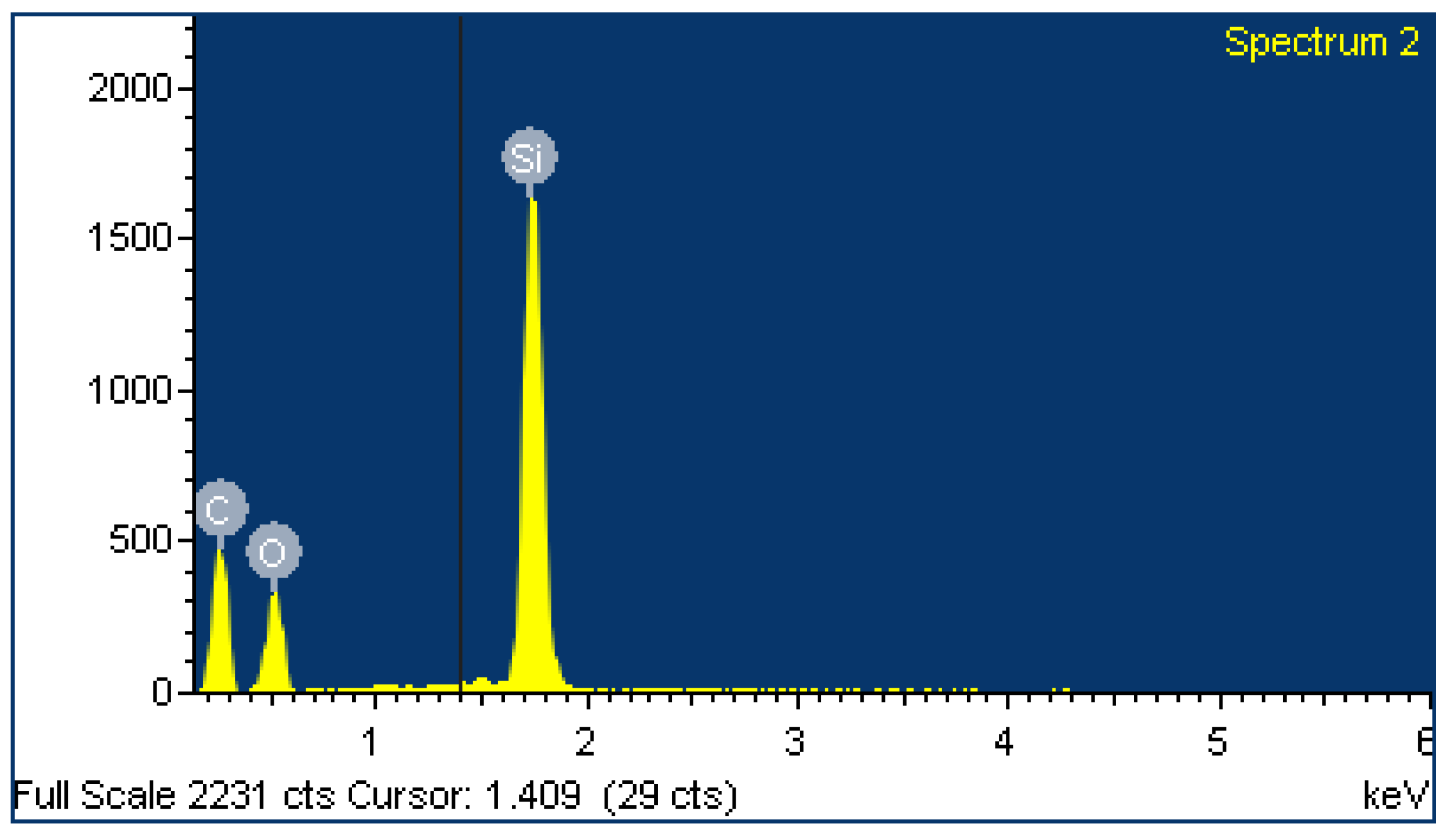
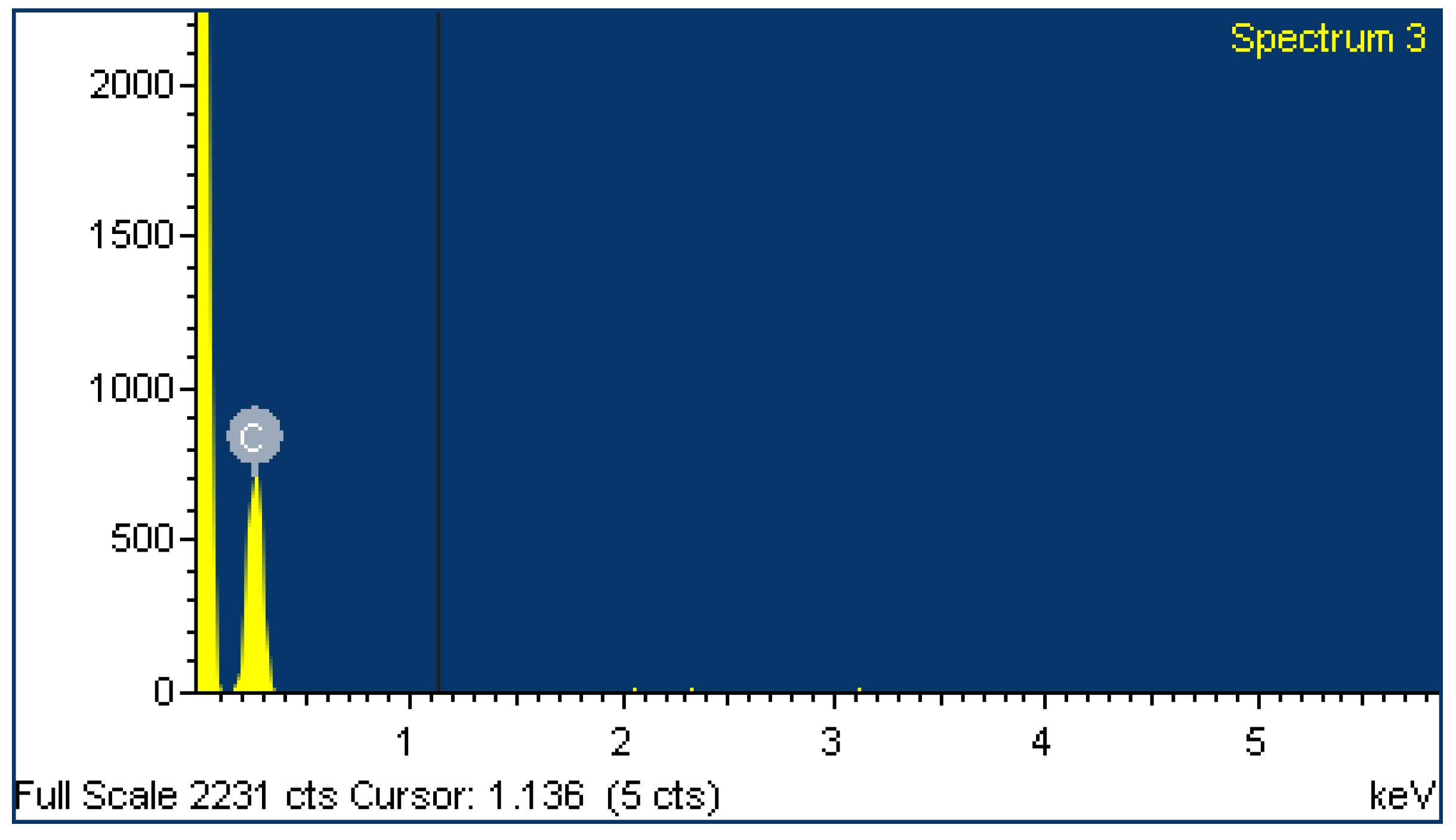

| Sample | A | B | ||
| Element | Weight% | Atomic% | Weight% | Atomic% |
| C | 49.64 | 62.58 | 95.29 | 96.42 |
| O | 25.21 | 23.86 | 4.71 | 3.58 |
| Si | 25.15 | 13.56 | ||
| S | ||||
| Totals | 100 | 100 | ||
| Chemical groups | Peak / cm-1 | The characteristics of peak |
| Si—H | 2250~2100 950~800 |
Si—H Extension vibrating absorption, strong Si—H Bending vibrating absorption, strong |
| Si—C | 890~690 | Si—C Extension vibrating absorption, strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





