Submitted:
28 May 2024
Posted:
29 May 2024
Read the latest preprint version here
Abstract
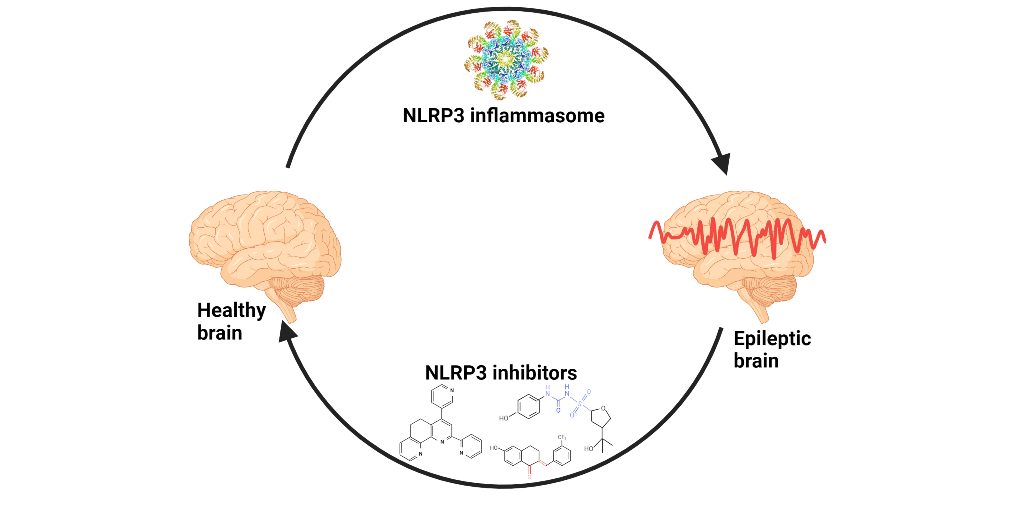
Keywords:
1. Introduction
2. Epileptogenesis
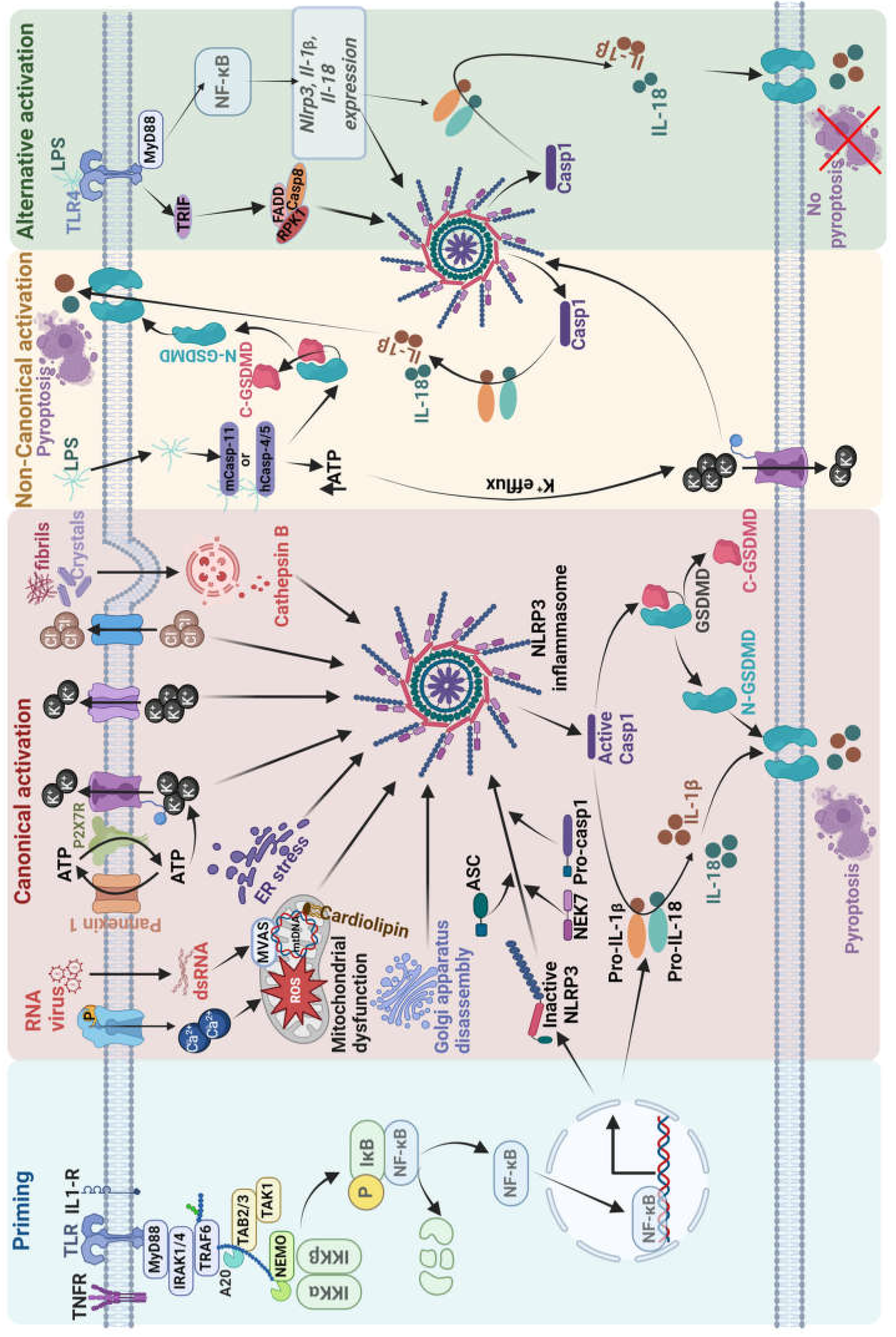
3. Inflammasomes
3. Activation of the NLRP3 Inflammasome
4. NLRP3 Inflammasome Involvement in Epilepsy
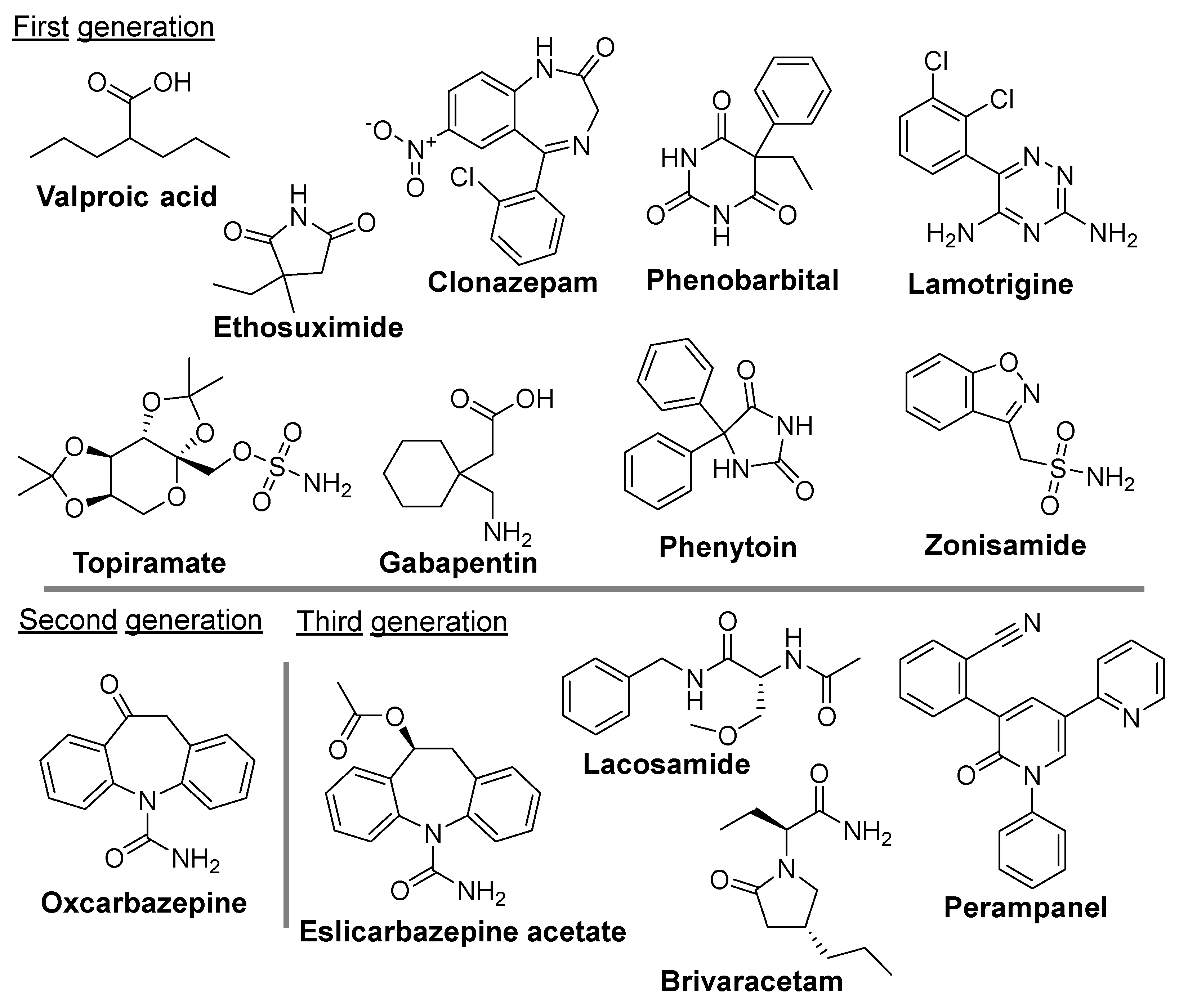
5. Current Antiseizure Medications for the Treatment of Epilepsy
5.1. Currently Preferred ASM Used to Treat Epilepsy
5.2. The Limitations of Current Clinical ASMs and Potential New Avenue for Drug Development in Epilepsy
6. NLRP3 Inflammasome in Epilepsy
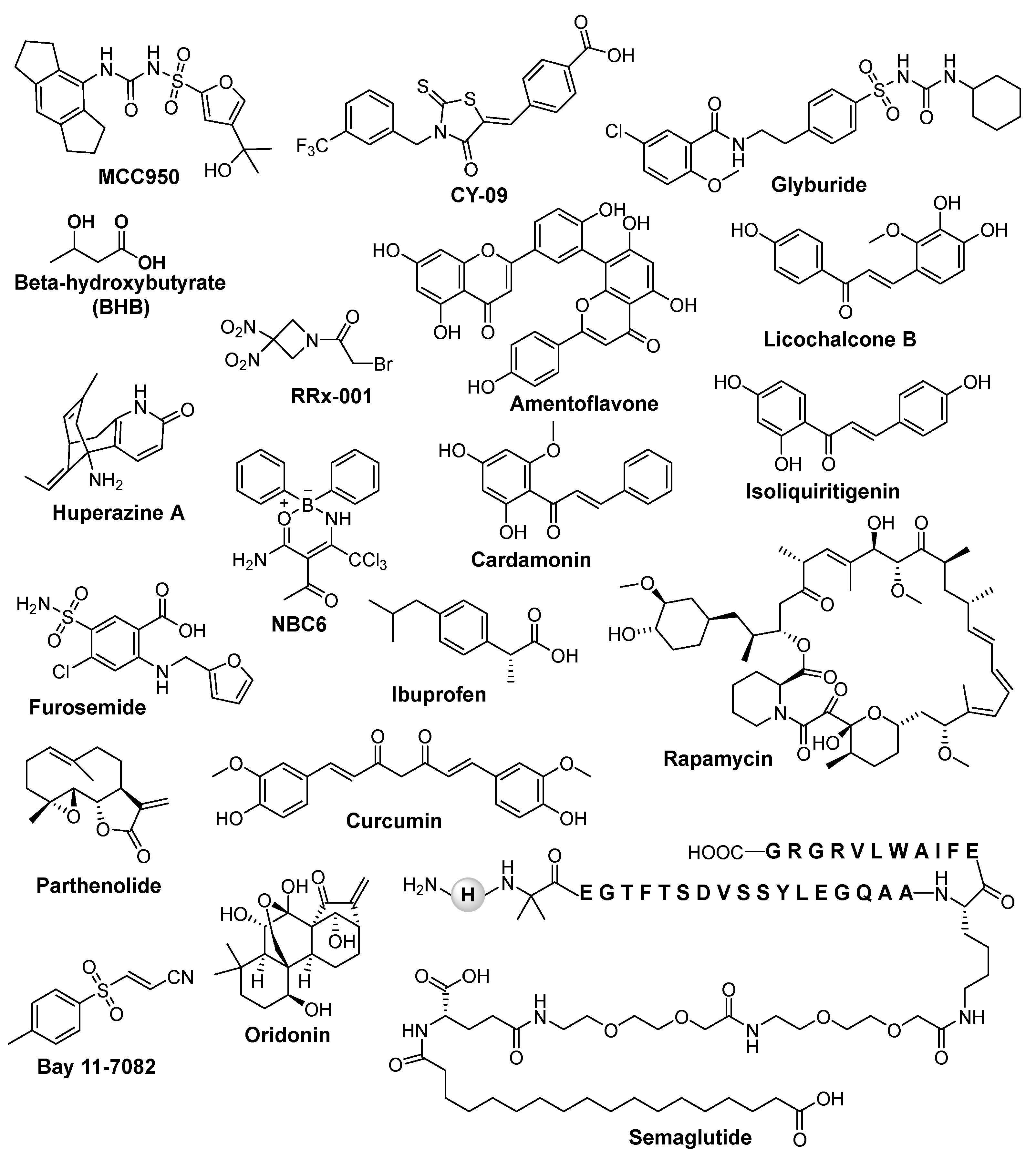
6.1. NLRP3 Inhibitors in Preclinical and Clinical Trial Phase
6.2. NLRP3 Inflammasome Inhibitors and Their Limitations as Remedial Strategies
7. Conclusions and Perspectives
| Compound | Nature of inhibitor | Mechanism of action | Disease model | References |
|---|---|---|---|---|
| MCC950 | Sulfonylurea | Directly interacts with Walker B motif of the NACHT domain, changes NLRP3 conformation. Blocks NLRP3-dependent ASC oligomerization and NLRP3 inflammasome activation Blocks ATPase activity |
In vitro SH-SY5Y model and in vivo model of cerebral trauma induced by PTZ Pilocarpine-induced SE mice KA-induced SE mice |
[72,73,85,126,127,131,137] |
| CY-09 | Glitazones | Directly binds to Cys172 residue in the Walker A motif of NLRP3 NACHT domain and inhibits NLRP3 ATPase activity | 0.83 mg/kg LPS-induced mice PTZ induced kindling mouse model |
[144,146,148,217] |
| Glyburide | Sulfonylurea | Suppresses KATP channels and inhibition of ASC agglomeration Blocks the assembly and activation of NLRP3 inflammasome and IL-1β release by dampening the binding of NEK7 to NLRP3 |
Pilocarpine-induced mouse model of SE Seizures induced by i.v. or i.p. PTZ models |
[151,156,157] |
| BHB | Natural products | Inhibition of K+ efflux and reduced ASC oligomerization and speck formation Inhibit NLRP3 inflammasome activation by modulating the production of ROS and by reducing the levels of ATP in the cell. |
Epileptic Kcna1-null mice 6-Hz induced seizure model of refractory epilepsy |
[163,164,165] |
| Amentoflavone | Naturally occurring bioflavonoid | Exerts neuroprotective effects by inhibiting the NLRP3 inflammasome | The chronic epilepsy model and BV2 microglial cellular inflammation model were established by PTZ kindling or LPS stimulation, respectively. | [78] |
| Semaglutide | Glucagon like peptide-1 | Decreases seizure severity, alleviated hippocampal neuronal apoptosis, ameliorated cognitive dysfunction by blocked ASC oligomerization and NLRP3 inflammasome activation | PTZ-kindled C57/BL6J mouse model and LPS induced inflammation in BV2 cells | [74] |
| Huperzine A | Naturally occurring sesquiterpene | Inhibits activation of NLRP3 inflammasome in a ROS-dependent manner |
Rat KA-induced model of epilepsy | [174] |
| Furosemide | Sulfonamide |
Increases the efficacy of valproic acid by inhibiting NLRP3 inflammasome activation | KA-induced epileptic rats | [175] |
| Ibuprofen | nonsteroidal anti-inflammatory drug (NSAID) | Exhibits antiepileptic and neuroprotective effects via inhibiting NLRP3 inflammasome activation | Rat model of PTZ-induced epilepsy | [176] |
| Rapamycin | Macrolide compound | Inhibits NLRP3inflammasome and ROS production | PTZ-kindled rats | [177] |
| Chaihu-Longgu-Muli decoction | Traditional Chinese medicine | Could significantly suppress the frequency and duration time of epileptic seizures via reducinge the expression of NLRP3, Caspase-1 TNF-α and IL-1β. | Rats with TLE | [178] |
| Parthenolide | Naturally occurring sesquiterpene lactone | Supresses NLRP3 ATPase activity by alkylating cysteine residues in ATPase domain of NLRP3 Inhibits protease activity of caspase 1 |
In vitro LPS and ATP induced NLRP3 stimulation | [180] |
| Bay 11-7082 | Sulfone | Blocks ATPase activity of NLRP3 (Juliana et al., 2010) | In vitro LPS and ATP induced NLRP3 stimulation | [180] |
| Oridonin | Natural terpenoids | Binds to Cys279 of NLRP3 NACHT domain and inhibits the interaction between NLRP3 and NEK7 thereby inhibiting the NLRP3 inflammasome activation | TBI mice | [179,218] |
| Curcumin | Natural polyphenolic compound | Inhibit IL-1b release and prevent inflammation via inhibition of NLRP3 | KA-induced epileptic syndrome in Sprague Dawley rats | [68,187] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASM | Antiseizure medication |
| SRS | Spontaneous recurrent seizure |
| BBB | Blood-brain barrier |
| TBI | Traumatic brain injury |
| DAMP | Damage-associated molecular pattern |
| PAMP | Pathogen-associated molecular pattern |
| ATP | Adenosine triphosphate |
| HMGB1 | High mobility group box1 |
| ROS | Reactive oxygen species |
| CARD | Caspase-recruitment domain |
| ASC | Apoptosis-associated speck-like protein containing a CARD. |
| PRR | Pattern-recognition receptor |
| NBD | Nucleotide binding domain |
| LRR-CR | Leucine-rich-repeat-containing receptor. |
| AIM2 | Absent-in-melanoma 2 |
| PYD | Pyrin domain |
| NOD | Nucleotide-binding oligomerization domain |
| NLR | Nod-like receptor |
| NLRP | Nod-like receptor protein |
| NLRP3 | NLR family pyrin domain containing 3. |
| NLRC4 | NLR family CARD domain-containing protein 4 |
| CNS | Central nervous system |
| NACHT | NAIP (neuronal apoptosis inhibitor protein), C2TA (MHC class 2 transcription activator), HET-E (incompatibility locus protein from Podospora anserina) and TP1 (telomerase-associated protein). |
| HD | Helical domain |
| WHD | Winged helix domain |
| NIMA | Never in mitosis A |
| NEK7 | NIMA related kinase 7 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| EM | Electron microscopy |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| miRNA | MicroRNA |
| P2RX7 | P2X purinoceptor 7 |
| ERS | Endoplasmic reticulum stress |
| GSDMD | Gasdermin D |
| TLR | Toll-like receptors |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| RIPK1 | Receptor-interacting serine/threonine-protein kinase 1 |
| FADD | Fas-associated protein with death domain |
| KA | Kainic acid |
| TLE | Temporal lobe epilepsy |
| SE | Status epilepticus |
| PTZ | Pentylenetetrazol |
| GABA | γ-aminobutyric acid |
| CAPS | Cryopyrin-associated periodic syndromes |
| MCC950 | 1-(1,2,3,5,6,7-Hexahydro-s-indacen-4-yl)-3-[4-(2-hydroxypropan-2-yl)furan-2-yl]sulfonylurea |
| BMDM | Bone marrow-derived macrophage |
| HMDM | Human monocyte derived macrophage |
| CY-09 | 4-[[4-Oxo-2-thioxo-3-[[3-(trifluoromethyl) phenyl] methyl] -5-thiazolidinylidene] methyl] benzoic acid |
| FDA | Food and drug administration |
| BHB | Beta-hydroxybutyrate |
| STAT3 | Signal transducer and activator of transcription 3 |
References
- Loscher, W.; Klitgaard, H.; Twyman, R.E.; Schmidt, D. New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 2013, 12, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Lukawski, K.; Gryta, P.; Luszczki, J.; Czuczwar, S.J. Exploring the latest avenues for antiepileptic drug discovery and development. Expert Opin Drug Discov 2016, 11, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wei, C.; Fu, M.; Li, X.; Zhang, H.; Yao, B. MCC950 alleviates seizure severity and angiogenesis by inhibiting NLRP3/ IL-1beta signaling pathway-mediated pyroptosis in mouse model of epilepsy. Int Immunopharmacol 2024, 126, 111236. [Google Scholar] [CrossRef] [PubMed]
- Modglin, L. Epilepsy statistics 2022. Epilepsy statistics 2022 2022. [Google Scholar]
- Guiard, B.P.; Di Giovanni, G. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: the missing link? Front Pharmacol 2015, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Henning, O.; Medalen, T.E.M.; Nakken, K.O.; Lossius, M.I. How often do doctors discuss drug withdrawal with their seizure-free patients with epilepsy? Epilepsy Behav 2020, 108, 107095. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.; Klein, P. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs 2021, 35, 935–963. [Google Scholar] [CrossRef] [PubMed]
- Lerche, H. Drug-resistant epilepsy - time to target mechanisms. Nat Rev Neurol 2020, 16, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Alyu, F.; Dikmen, M. Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 2017, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.M.; Coulter, D.A. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci 2013, 14, 337–349. [Google Scholar] [CrossRef]
- Sung, P.S.; Lin, P.Y.; Liu, C.H.; Su, H.C.; Tsai, K.J. Neuroinflammation and Neurogenesis in Alzheimer's Disease and Potential Therapeutic Approaches. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Matanzo, A.; Llorens-Martin, M.; Hernandez, F.; Avila, J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: therapeutic approaches. Mediators Inflamm 2013, 2013, 260925. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol 2019, 16, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lin, L.; Li, H.; Wang, H.; Jiang, S.; Huang, P.; Lin, Q.; Chen, X.; Deng, Y. Melatonin Reduces Neuroinflammation and Improves Axonal Hypomyelination by Modulating M1/M2 Microglia Polarization via JAK2-STAT3-Telomerase Pathway in Postnatal Rats Exposed to Lipopolysaccharide. Mol Neurobiol 2021, 58, 6552–6576. [Google Scholar] [CrossRef] [PubMed]
- Bollaerts, I.; Van Houcke, J.; Andries, L.; De Groef, L.; Moons, L. Neuroinflammation as Fuel for Axonal Regeneration in the Injured Vertebrate Central Nervous System. Mediators Inflamm 2017, 2017, 9478542. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, B.; Li, L.; Wang, B.; Sun, M. Th17 cells and inflammation in neurological disorders: Possible mechanisms of action. Front Immunol 2022, 13, 932152. [Google Scholar] [CrossRef]
- Soltani Khaboushan, A.; Yazdanpanah, N.; Rezaei, N. Neuroinflammation and Proinflammatory Cytokines in Epileptogenesis. Mol Neurobiol 2022, 59, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 2019, 15, 459–472. [Google Scholar] [CrossRef]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J Neuroinflammation 2018, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Mahfoz, A.M.; Shahzad, N. Neuroinflammation impact in epileptogenesis and new treatment strategy. Behav Pharmacol 2019, 30, 661–675. [Google Scholar] [CrossRef]
- Banjara, M.; Ghosh, C. Sterile Neuroinflammation and Strategies for Therapeutic Intervention. Int J Inflam 2017, 2017, 8385961. [Google Scholar] [CrossRef] [PubMed]
- Relja, B.; Land, W.G. Damage-associated molecular patterns in trauma. Eur J Trauma Emerg Surg 2020, 46, 751–775. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 2020, 20, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M.; Frank, M.; Maier, S.F. Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 2017, 42, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- von Moltke, J.; Ayres, J.S.; Kofoed, E.M.; Chavarria-Smith, J.; Vance, R.E. Recognition of bacteria by inflammasomes. Annu Rev Immunol 2013, 31, 73–106. [Google Scholar] [CrossRef]
- Hoffmann, S.; Beyer, C. A Fatal Alliance between Microglia, Inflammasomes, and Central Pain. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Trendelenburg, G. Acute neurodegeneration and the inflammasome: central processor for danger signals and the inflammatory response? J Cereb Blood Flow Metab 2008, 28, 867–881. [Google Scholar] [CrossRef]
- Mortezaee, K.; Khanlarkhani, N.; Beyer, C.; Zendedel, A. Inflammasome: Its role in traumatic brain and spinal cord injury. J Cell Physiol 2018, 233, 5160–5169. [Google Scholar] [CrossRef]
- Slowik, A.; Lammerding, L.; Hoffmann, S.; Beyer, C. Brain inflammasomes in stroke and depressive disorders: Regulation by oestrogen. J Neuroendocrinol 2018, 30. [Google Scholar] [CrossRef]
- Walsh, J.G.; Muruve, D.A.; Power, C. Inflammasomes in the CNS. Nat Rev Neurosci 2014, 15, 84–97. [Google Scholar] [CrossRef]
- Yap, J.K.Y.; Pickard, B.S.; Chan, E.W.L.; Gan, S.Y. The Role of Neuronal NLRP1 Inflammasome in Alzheimer's Disease: Bringing Neurons into the Neuroinflammation Game. Mol Neurobiol 2019, 56, 7741–7753. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.C.; Zhang, J.G.; Tan, M.S.; Chen, H.; Meng, D.W.; Jiang, T.; Meng, X.F.; Li, Y.; Sun, Z.; Li, M.M.; et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model. J Neuroinflammation 2015, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- de Rivero Vaccari, J.P.; Lotocki, G.; Alonso, O.F.; Bramlett, H.M.; Dietrich, W.D.; Keane, R.W. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab 2009, 29, 1251–1261. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab 2014, 34, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, J.; de Rivero Vaccari, J.P.; Keane, R.W. Human astrocytes express a novel NLRP2 inflammasome. Glia 2013, 61, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Ducza, L.; Szucs, P.; Hegedus, K.; Bakk, E.; Gajtko, A.; Weber, I.; Hollo, K. NLRP2 Is Overexpressed in Spinal Astrocytes at the Peak of Mechanical Pain Sensitivity during Complete Freund Adjuvant-Induced Persistent Pain. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, P.; Huang, S.; Jia, Z.; Chen, T.; Liu, X.; Feng, H.; Chen, Y. Inhibiting Microglia-Derived NLRP3 Alleviates Subependymal Edema and Cognitive Dysfunction in Posthemorrhagic Hydrocephalus after Intracerebral Hemorrhage via AMPK/Beclin-1 Pathway. Oxid Med Cell Longev 2022, 2022, 4177317. [Google Scholar] [CrossRef]
- Shi, F.; Yang, Y.; Kouadir, M.; Fu, Y.; Yang, L.; Zhou, X.; Yin, X.; Zhao, D. Inhibition of phagocytosis and lysosomal acidification suppresses neurotoxic prion peptide-induced NALP3 inflammasome activation in BV2 microglia. J Neuroimmunol 2013, 260, 121–125. [Google Scholar] [CrossRef]
- Hanslik, K.L.; Ulland, T.K. The Role of Microglia and the Nlrp3 Inflammasome in Alzheimer's Disease. Front Neurol 2020, 11, 570711. [Google Scholar] [CrossRef]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS One 2015, 10, e0130624. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, M.J.; Park, Y.; Ahn, J.W.; Hwang, S.J.; Moon, J.S.; Cho, K.G.; Kwack, K. Upregulation of the NLRC4 inflammasome contributes to poor prognosis in glioma patients. Sci Rep 2019, 9, 7895. [Google Scholar] [CrossRef]
- Freeman, L.; Guo, H.; David, C.N.; Brickey, W.J.; Jha, S.; Ting, J.P. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med 2017, 214, 1351–1370. [Google Scholar] [CrossRef]
- Wu, P.J.; Liu, H.Y.; Huang, T.N.; Hsueh, Y.P. AIM 2 inflammasomes regulate neuronal morphology and influence anxiety and memory in mice. Sci Rep 2016, 6, 32405. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, S.E.; de Rivero Vaccari, J.P.; Dale, G.; Brand, F.J., 3rd; Nonner, D.; Bullock, M.R.; Dahl, G.P.; Dietrich, W.D.; Keane, R.W. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab 2014, 34, 621–629. [Google Scholar] [CrossRef]
- Sharif, H.; Wang, L.; Wang, W.L.; Magupalli, V.G.; Andreeva, L.; Qiao, Q.; Hauenstein, A.V.; Wu, Z.; Nunez, G.; Mao, Y.; et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 2019, 570, 338–343. [Google Scholar] [CrossRef]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Li, X.; Zhan, X.; Tang, M.; Fina, M.; Su, L.; Pratt, D.; Bu, C.H.; Hildebrand, S.; et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol 2016, 17, 250–258. [Google Scholar] [CrossRef]
- Liu, G.; Chen, X.; Wang, Q.; Yuan, L. NEK7: a potential therapy target for NLRP3-related diseases. Biosci Trends 2020, 14, 74–82. [Google Scholar] [CrossRef]
- Xu, J.; Lu, L.; Li, L. NEK7: a novel promising therapy target for NLRP3-related inflammatory diseases. Acta Biochim Biophys Sin (Shanghai) 2016, 48, 966–968. [Google Scholar] [CrossRef]
- El-Sharkawy, L.Y.; Brough, D.; Freeman, S. Inhibiting the NLRP3 Inflammasome. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 2018, 17, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, L.; Wu, T.; Xi, J.; Han, Y.; Yang, X.; Zhang, D.; Fang, Q.; Tang, B. NLRP3 Activation Was Regulated by DNA Methylation Modification during Mycobacterium tuberculosis Infection. Biomed Res Int 2016, 2016, 4323281. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, M.; O'Neil, J.D.; Clark, A.R.; Masters, S.L.; O'Neill, L.A.J. The RNA-binding protein Tristetraprolin (TTP) is a critical negative regulator of the NLRP3 inflammasome. J Biol Chem 2017, 292, 6869–6881. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.; Rieger, A.; Schildberg, F.A.; Knolle, P.A.; Schmid-Burgk, J.L.; Hornung, V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol 2012, 189, 4175–4181. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Regulation of the NLRP3 Inflammasome by Post-Translational Modifications and Small Molecules. Front Immunol 2020, 11, 618231. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, N.M.; Lamkanfi, M. Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis. Cold Spring Harb Perspect Biol 2020, 12. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Wu, J.; Yu, J.W.; Datta, P.; Miller, B.; Jankowski, W.; Rosenberg, S.; Zhang, J.; Alnemri, E.S. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 2007, 14, 1590–1604. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O'Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Caspase-11 Non-Canonical Inflammasome: Emerging Activator and Regulator of Infection-Mediated Inflammatory Responses. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Vigano, E.; Diamond, C.E.; Spreafico, R.; Balachander, A.; Sobota, R.M.; Mortellaro, A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun 2015, 6, 8761. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Dick, M.S.; Lagrange, B.; Degrandi, D.; Pfeffer, K.; Yamamoto, M.; Meunier, E.; Pelczar, P.; Henry, T.; Broz, P. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J 2018, 37. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Schmidt, T.; Schmid-Burgk, J.L.; Rapino, F.; Robertson, A.A.; Cooper, M.A.; Graf, T.; Hornung, V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 2016, 44, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Kanneganti, T.D. Novel roles for caspase-8 in IL-1beta and inflammasome regulation. Am J Pathol 2015, 185, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Jiang, L.; Man, S.; Wu, L.; Hu, Y.; Chen, W. Curcumin Reduces Neuronal Loss and Inhibits the NLRP3 Inflammasome Activation in an Epileptic Rat Model. Curr Neurovasc Res 2018, 15, 186–192. [Google Scholar] [CrossRef]
- Lin, W.S.; Hsu, T.R. Hypothesis: Febrile infection-related epilepsy syndrome is a microglial NLRP3 inflammasome/IL-1 axis-driven autoinflammatory syndrome. Clin Transl Immunology 2021, 10, e1299. [Google Scholar] [CrossRef]
- Meng, X.F.; Tan, L.; Tan, M.S.; Jiang, T.; Tan, C.C.; Li, M.M.; Wang, H.F.; Yu, J.T. Inhibition of the NLRP3 inflammasome provides neuroprotection in rats following amygdala kindling-induced status epilepticus. J Neuroinflammation 2014, 11, 212. [Google Scholar] [CrossRef]
- Xiang, T.; Luo, X.; Ye, L.; Huang, H.; Wu, Y. Klotho alleviates NLRP3 inflammasome-mediated neuroinflammation in a temporal lobe epilepsy rat model by activating the Nrf2 signaling pathway. Epilepsy Behav 2022, 128, 108509. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Mao, Q.; Yin, X.; Zhang, C.; Jin, Y.; Deng, A.; Gu, Z.; Chen, B. NLRP3 Inflammasome Activation Leads to Epileptic Neuronal Apoptosis. Curr Neurovasc Res 2018, 15, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Wei, Y.J.; Yang, X.L.; Liu, S.Y.; Yang, H.; Zhang, C.Q. NLRP3 inflammasome and endoplasmic reticulum stress in the epileptogenic zone in temporal lobe epilepsy: molecular insights into their interdependence. Neuropathol Appl Neurobiol 2020, 46, 770–785. [Google Scholar] [CrossRef]
- Wang, L.; Ding, J.; Zhu, C.; Guo, B.; Yang, W.; He, W.; Li, X.; Wang, Y.; Li, W.; Wang, F.; et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazolekindled mice. Int J Mol Med 2021, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, L.; An, D.; Xu, W.; Wu, C.; Sha, S.; Li, Y.; Zhu, Y.; Chen, A.; Du, Y.; et al. TRPV4-induced inflammatory response is involved in neuronal death in pilocarpine model of temporal lobe epilepsy in mice. Cell Death Dis 2019, 10, 386. [Google Scholar] [CrossRef]
- Magalhaes, D.M.; Pereira, N.; Rombo, D.M.; Beltrao-Cavacas, C.; Sebastiao, A.M.; Valente, C.A. Ex vivo model of epilepsy in organotypic slices-a new tool for drug screening. J Neuroinflammation 2018, 15, 203. [Google Scholar] [CrossRef]
- Jiang, Q.; Tang, G.; Zhong, X.M.; Ding, D.R.; Wang, H.; Li, J.N. Role of Stat3 in NLRP3/caspase-1-mediated hippocampal neuronal pyroptosis in epileptic mice. Synapse 2021, 75, e22221. [Google Scholar] [CrossRef]
- Rong, S.; Wan, D.; Fan, Y.; Liu, S.; Sun, K.; Huo, J.; Zhang, P.; Li, X.; Xie, X.; Wang, F.; et al. Amentoflavone Affects Epileptogenesis and Exerts Neuroprotective Effects by Inhibiting NLRP3 Inflammasome. Front Pharmacol 2019, 10, 856. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, J.; Xia, Z.; Zhang, A.; Chao, J.; Yao, H. Repeated restraint stress increases seizure susceptibility by activation of hippocampal endoplasmic reticulum stress. Neurochem Int 2017, 110, 25–37. [Google Scholar] [CrossRef]
- Yue, J.; He, J.; Wei, Y.; Shen, K.; Wu, K.; Yang, X.; Liu, S.; Zhang, C.; Yang, H. Decreased expression of Rev-Erbalpha in the epileptic foci of temporal lobe epilepsy and activation of Rev-Erbalpha have anti-inflammatory and neuroprotective effects in the pilocarpine model. J Neuroinflammation 2020, 17, 43. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, G.; Chen, L.; Kim, S.; Yu, J.; Hu, G.; Chen, J.; Huang, Y.; Zheng, G.; Huang, S. The Role of NLRP3 and IL-1β in Refractory Epilepsy Brain Injury. Front Neurol. 2020, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Cristina de Brito Toscano, E.; Leandro Marciano Vieira, E.; Boni Rocha Dias, B.; Vidigal Caliari, M.; Paula Goncalves, A.; Varela Giannetti, A.; Mauricio Siqueira, J.; Kimie Suemoto, C.; Elaine Paraizo Leite, R.; Nitrini, R.; et al. NLRP3 and NLRP1 inflammasomes are up-regulated in patients with mesial temporal lobe epilepsy and may contribute to overexpression of caspase-1 and IL-beta in sclerotic hippocampi. Brain Res 2021, 1752, 147230. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, L.; Wang, S.; Li, Y.; Liu, L; Chen, G.; Wang, S.; Zhou, S. Negative Regulation of Autophagy in Activating Nucleotide-Binding Oligomerization Domain-Like Receptor Family Pyrin Domain-Containing 3 Inflammasomes in the Hippocampus of an Epilepsy Rat Model. Nanosci. Nanotechnol. Lett. 2019, 11, 947–959. [Google Scholar] [CrossRef]
- Liu, Z.; Xian, H.; Ye, X.; Chen, J.; Ma, Y.; Huang, W. Increased levels of NLRP3 in children with febrile seizures. Brain Dev 2020, 42, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, S.; Xia, L.; Peng, X.; Wang, S.; Yao, B. NLRP3 Inflammasome Activation Enhances ADK Expression to Accelerate Epilepsy in Mice. Neurochem Res 2022, 47, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Pohlentz, M.S.; Muller, P.; Cases-Cunillera, S.; Opitz, T.; Surges, R.; Hamed, M.; Vatter, H.; Schoch, S.; Becker, A.J.; Pitsch, J. Characterisation of NLRP3 pathway-related neuroinflammation in temporal lobe epilepsy. PLoS One 2022, 17, e0271995. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.B.; Banerjee, J.; Srivastava, A.; Tripathi, M.; Sarkar, C.; Kakkar, A.; Jain, M.; Chandra, P.S. RNA-seq analysis of hippocampal tissues reveals novel candidate genes for drug refractory epilepsy in patients with MTLE-HS. Genomics 2016, 107, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Wu, Y.; Yang, Y.J.; Li, W.Z.; Dong, K.; Zhou, J.; Yin, Y.Y.; Huang, D.K.; Wu, W.N. Sinomenine exerts anticonvulsant profile and neuroprotective activity in pentylenetetrazole kindled rats: involvement of inhibition of NLRP1 inflammasome. J Neuroinflammation 2018, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Samadianzakaria, A.; Abdolmaleki, Z.; Faedmalekia, F. The effect of valproic acid and furosemide on the regulation of the inflammasome complex (NLRP1 and NLRP3 mRNA) in the brain of epileptic animal model. Brain Research Bulletin 2022, 191, 20–29. [Google Scholar] [CrossRef]
- Cosford, N.D.; Meinke, P.T.; Stauderman, K.A.; Hess, S.D. Recent advances in the modulation of voltage-gated ion channels for the treatment of epilepsy. Curr Drug Targets CNS Neurol Disord 2002, 1, 81–104. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef] [PubMed]
- Treiman, D.M. GABAergic mechanisms in epilepsy. Epilepsia 2001, 42 Suppl 3, 8–12. [Google Scholar] [CrossRef]
- Romoli, M.; Mazzocchetti, P.; D'Alonzo, R.; Siliquini, S.; Rinaldi, V.E.; Verrotti, A.; Calabresi, P.; Costa, C. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Curr Neuropharmacol 2019, 17, 926–946. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, C.U.; Johannessen, S.I. Valproate: past, present, and future. CNS Drug Rev 2003, 9, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.A.; Cnaan, A.; Shinnar, S.; Hirtz, D.G.; Dlugos, D.; Masur, D.; Clark, P.O.; Adamson, P.C.; Childhood Absence Epilepsy Study, T. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia 2013, 54, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Brigo, F.; Igwe, S.C.; Bragazzi, N.L.; Lattanzi, S. Clonazepam monotherapy for treating people with newly diagnosed epilepsy. Cochrane Database Syst Rev 2022, 2, CD013028. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Pratt, J.A.; Marsden, C.D. Mechanism of action of clonazepam in myoclonus in relation to effects on GABA and 5-HT. Adv Neurol 1986, 43, 629–643. [Google Scholar] [PubMed]
- Nardou, R.; Yamamoto, S.; Chazal, G.; Bhar, A.; Ferrand, N.; Dulac, O.; Ben-Ari, Y.; Khalilov, I. Neuronal chloride accumulation and excitatory GABA underlie aggravation of neonatal epileptiform activities by phenobarbital. Brain 2011, 134, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zeng, L.N.; Li, Y.P. Side effects of phenobarbital in epilepsy: a systematic review. Epileptic Disord 2011, 13, 349–365. [Google Scholar] [CrossRef]
- Contreras-Garcia, I.J.; Cardenas-Rodriguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gomez-Manzo, S.; Hernandez-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macias, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef]
- Itoh, K.; Ishihara, Y.; Komori, R.; Nochi, H.; Taniguchi, R.; Chiba, Y.; Ueno, M.; Takata-Tsuji, F.; Dohgu, S.; Kataoka, Y. Levetiracetam treatment influences blood-brain barrier failure associated with angiogenesis and inflammatory responses in the acute phase of epileptogenesis in post-status epilepticus mice. Brain Res 2016, 1652, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.A.; Ebrahimi, F. The effect of lamotrigine on epilepsy. Iran J Neurol 2012, 11, 162–163. [Google Scholar] [PubMed]
- Goldsmith, D.R.; Wagstaff, A.J.; Ibbotson, T.; Perry, C.M. Lamotrigine: a review of its use in bipolar disorder. Drugs 2003, 63, 2029–2050. [Google Scholar] [CrossRef] [PubMed]
- Marson, A.; Burnside, G.; Appleton, R.; Smith, D.; Leach, J.P.; Sills, G.; Tudur-Smith, C.; Plumpton, C.; Hughes, D.A.; Williamson, P.; et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet 2021, 397, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R.; Parmeggiani, L. Topiramate and its clinical applications in epilepsy. Expert Opin Pharmacother 2006, 7, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A. Mechanisms of action of antiepileptic drugs. Seizure 1995, 4, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.R.; Chuang, H.Y.; Chen, N.C.; Chen, S.F.; Hsu, C.Y.; Chuang, Y.C. Zonisamide Therapy Reduces Metabolic Consequences and Diminishes Nonalcoholic Fatty Liver Disease in Patients with Epilepsy. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Bang, L.M.; Goa, K.L. Spotlight on oxcarbazepine in epilepsy. CNS Drugs 2004, 18, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.C.; Yuan, H.; Wang, Y.; Xu, H.Q.; Hong, W.K.; Zheng, R.Y. Eslicarbazepine acetate add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev 2017, 10, CD008907. [Google Scholar] [CrossRef]
- Landmark, C.J.; Rektorli, L.; Burns, M.L.; Revdal, E.; Johannessen, S.I.; Brodtkorb, E. Pharmacokinetic data on brivaracetam, lacosamide and perampanel during pregnancy and lactation. Epileptic Disord 2021, 23, 426–431. [Google Scholar] [CrossRef]
- D'Andrea Meira, I.; Romao, T.T.; Pires do Prado, H.J.; Kruger, L.T.; Pires, M.E.P.; da Conceicao, P.O. Ketogenic Diet and Epilepsy: What We Know So Far. Front Neurosci 2019, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.F.J.; Yengo-Kahn, A.; Englot, D.J. Vagus Nerve Stimulation for the Treatment of Epilepsy. Neurosurg Clin N Am 2019, 30, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Ohemeng, K.K.; Parham, K. Vagal Nerve Stimulation: Indications, Implantation, and Outcomes. Otolaryngol Clin North Am 2020, 53, 127–143. [Google Scholar] [CrossRef]
- Wallace, S.J. Newer antiepileptic drugs: advantages and disadvantages. Brain Dev 2001, 23, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Farhat, G.; Yamout, B.; Mikati, M.A.; Demirjian, S.; Sawaya, R.; El-Hajj Fuleihan, G. Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology 2002, 58, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Paulson, O.B.; Gyory, A.; Hertz, M.M. Blood-brain barrier transfer and cerebral uptake of antiepileptic drugs. Clin Pharmacol Ther 1982, 32, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Hung, C.Y.; Chiu, K.M.; Lee, M.Y.; Lu, C.W.; Wang, S.J. Neferine, an Alkaloid from Lotus Seed Embryos, Exerts Antiseizure and Neuroprotective Effects in a Kainic Acid-Induced Seizure Model in Rats. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for Parkinson disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Satija, G.; Madan, A.; Garg, M.; Alam, M.M.; Shaquiquzzaman, M.; Khanna, S.; Tiwari, P.; Parvez, S.; Iqubal, A.; et al. Role of NLRP3 Inflammasome and Its Inhibitors as Emerging Therapeutic Drug Candidate for Alzheimer's Disease: a Review of Mechanism of Activation, Regulation, and Inhibition. Inflammation 2022, 1–32. [Google Scholar] [CrossRef]
- Tezcan, G.; Garanina, E.E.; Alsaadi, M.; Gilazieva, Z.E.; Martinova, E.V.; Markelova, M.I.; Arkhipova, S.S.; Hamza, S.; McIntyre, A.; Rizvanov, A.A.; et al. Therapeutic Potential of Pharmacological Targeting NLRP3 Inflammasome Complex in Cancer. Front Immunol 2020, 11, 607881. [Google Scholar] [CrossRef]
- Wu, A.G.; Zhou, X.G.; Qiao, G.; Yu, L.; Tang, Y.; Yan, L.; Qiu, W.Q.; Pan, R.; Yu, C.L.; Law, B.Y.; et al. Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Res Rev 2021, 65, 101202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Yin, H.R.; He, Q.Y.; Wang, Y. Targeting the NLRP3 inflammasome as new therapeutic avenue for inflammatory bowel disease. Biomed Pharmacother 2021, 138, 111442. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Schroder, K.; Pelegrin, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci 2022, 43, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Schwaid, A.G.; Spencer, K.B. Strategies for Targeting the NLRP3 Inflammasome in the Clinical and Preclinical Space. J Med Chem 2021, 64, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015, 21, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Hill, J.R.; Day, C.J.; Zamoshnikova, A.; Boucher, D.; Massey, N.L.; Chitty, J.L.; Fraser, J.A.; Jennings, M.P.; Robertson, A.A.B.; et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol 2019, 15, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Abellan, A.; Angosto-Bazarra, D.; Martinez-Banaclocha, H.; de Torre-Minguela, C.; Ceron-Carrasco, J.P.; Perez-Sanchez, H.; Arostegui, J.I.; Pelegrin, P. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat Chem Biol 2019, 15, 560–564. [Google Scholar] [CrossRef]
- Umiker, B.; Lee, H.H.; Cope, J.; Ajami, N.J.; Laine, J.P.; Fregeau, C.; Ferguson, H.; Alves, S.E.; Sciammetta, N.; Kleinschek, M.; et al. The NLRP3 inflammasome mediates DSS-induced intestinal inflammation in Nod2 knockout mice. Innate Immun 2019, 25, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; El-Kader, E.M.A. Novel complementary coloprotective effects of metformin and MCC950 by modulating HSP90/NLRP3 interaction and inducing autophagy in rats. Inflammopharmacology 2021, 29, 237–251. [Google Scholar] [CrossRef]
- Dempsey, C.; Rubio Araiz, A.; Bryson, K.J.; Finucane, O.; Larkin, C.; Mills, E.L.; Robertson, A.A.B.; Cooper, M.A.; O'Neill, L.A.J.; Lynch, M.A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun 2017, 61, 306–316. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, L.; Luo, X.; Liang, J.; Sun, E.; He, Y. Protection of MCC950 against Alzheimer's disease via inhibiting neuronal pyroptosis in SAMP8 mice. Exp Brain Res 2020, 238, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Fu, R.; Wang, S.; Huang, Y.; Li, X.; Zhou, M.; Zhao, J.; Yang, N. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin Exp Immunol 2018, 194, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Wang, Q.; Cheng, M.; Lin, B. TPM1 mediates inflammation downstream of TREM2 via the PKA/CREB signaling pathway. J Neuroinflammation 2022, 19, 257. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shi, H.; Chang, S.; Gao, Y.; Li, X.; Lv, C.; Yang, H.; Xiang, H.; Yang, J.; Xu, L.; et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol 2019, 74, 105575. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, C.; He, D.; Jiang, N.; Bai, Y.; Xin, Y. Rapamycin and MCC950 modified gut microbiota in experimental autoimmune encephalomyelitis mouse by brain gut axis. Life Sci 2020, 253, 117747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, A.; Lv, J.; Zhang, Q.; Ran, Y.; Wei, C.; Wu, J. Development of small molecule inhibitors targeting NLRP3 inflammasome pathway for inflammatory diseases. Eur J Med Chem 2020, 185, 111822. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Nasoohi, S.; Ishrat, T. MCC950, the Selective Inhibitor of Nucleotide Oligomerization Domain-Like Receptor Protein-3 Inflammasome, Protects Mice against Traumatic Brain Injury. J Neurotrauma 2018, 35, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Sanz-Rodgriguez, A.; Jimenez-Mateos, E.M.; Concannon, C.G.; Jimenez-Pacheco, A.; Moran, C.; Mesuret, G.; Petit, E.; Delanty, N.; Farrell, M.A.; et al. CHOP regulates the p53-MDM2 axis and is required for neuronal survival after seizures. Brain 2013, 136, 577–592. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Paul, R.; Choudhury, A.; Chandra Boruah, D.; Devi, R.; Bhattacharya, P.; Choudhury, M.D.; Borah, A. Hypercholesterolemia causes psychomotor abnormalities in mice and alterations in cortico-striatal biogenic amine neurotransmitters: Relevance to Parkinson's disease. Neurochem Int 2017, 108, 15–26. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, J.; Han, B.; Huang, R.; Zhang, A.; Xia, Z.; Chang, H.; Chao, J.; Yao, H. Neuronal Nitric Oxide Synthase Contributes to PTZ Kindling Epilepsy-Induced Hippocampal Endoplasmic Reticulum Stress and Oxidative Damage. Front Cell Neurosci 2017, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 2018, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, H.; Chen, Y.; Huang, W.; Cheng, J.; Ye, J.; Wang, A.; Tao, J.; Wang, C.; Liu, Q.; et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med 2017, 214, 3219–3238. [Google Scholar] [CrossRef]
- Fan, Y.; Xue, G.; Chen, Q.; Lu, Y.; Dong, R.; Yuan, H. CY-09 Inhibits NLRP3 Inflammasome Activation to Relieve Pain via TRPA1. Comput Math Methods Med 2021, 2021, 9806690. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wu, X.; Luo, Q.; Wei, G.; Xu, M.; Wu, Y.; Liu, Y.; Li, X.; Zi, J.; Ju, W.; et al. NLRP3 regulates platelet integrin alphaIIbbeta3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica 2018, 103, 1568–1576. [Google Scholar] [CrossRef]
- Shen, K.; Jiang, W.; Zhang, C.; Cai, L.; Wang, Q.; Yu, H.; Tang, Z.; Gu, Z.; Chen, B. Molecular Mechanism of a Specific NLRP3 Inhibitor to Alleviate Seizure Severity Induced by Pentylenetetrazole. Curr Mol Pharmacol 2021, 14, 579–586. [Google Scholar] [CrossRef]
- Zhu, Q.; Zheng, F.; You, W.; Kang, X.; Chen, C.; Pan, Z.; Zhou, J.; Hu, W. Expression of Histone H1 in Rats with Traumatic Brain Injury and the Effect of the NLRP3 Inflammasome Pathway. World Neurosurg 2023, 171, e286–e290. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.J.; Zhang, M.M.; Zhou, H.; Gao, Y.H.; Cheng, W.J.; Ye, Z.W.; Yuan, Z.Y.; Xu, G.H.; Li, C.F.; et al. CY-09 Alleviates the Depression-like Behaviors via Inhibiting NLRP3 Inflammasome-Mediated Neuroinflammation in Lipopolysaccharide-Induced Mice. ACS Chem Neurosci 2022, 13, 3291–3302. [Google Scholar] [CrossRef]
- Riddle, M.C. Editorial: sulfonylureas differ in effects on ischemic preconditioning--is it time to retire glyburide? J Clin Endocrinol Metab 2003, 88, 528–530. [Google Scholar] [CrossRef]
- Perregaux, D.G.; McNiff, P.; Laliberte, R.; Hawryluk, N.; Peurano, H.; Stam, E.; Eggler, J.; Griffiths, R.; Dombroski, M.A.; Gabel, C.A. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J Pharmacol Exp Ther 2001, 299, 187–197. [Google Scholar]
- Coelho, F.R.; Cavriani, G.; Soares, A.L.; Teixeira, S.A.; Almeida, P.C.; Sudo-Hayashi, L.S.; Muscara, M.N.; Oliveira-Filho, R.M.; Vargaftig, B.B.; Tavares-de-Lima, W. Lymphatic-borne IL-1beta and the inducible isoform of nitric oxide synthase trigger the bronchial hyporesponsiveness after intestinal ischema/reperfusion in rats. Shock 2007, 28, 694–699. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Mueller, J.L.; Vitari, A.C.; Misaghi, S.; Fedorova, A.; Deshayes, K.; Lee, W.P.; Hoffman, H.M.; Dixit, V.M. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 2009, 187, 61–70. [Google Scholar] [CrossRef]
- Masters, S.L.; Dunne, A.; Subramanian, S.L.; Hull, R.L.; Tannahill, G.M.; Sharp, F.A.; Becker, C.; Franchi, L.; Yoshihara, E.; Chen, Z.; et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol 2010, 11, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, F.; Berishvili, E.; Parnaud, G.; Rouget, C.; Bosco, D.; Berney, T.; Lavallard, V. NLRP3 inflammasome is expressed and regulated in human islets. Cell Death Dis 2018, 9, 726. [Google Scholar] [CrossRef]
- Liu, H.; Gu, C.; Liu, M.; Liu, G.; Wang, Y. NEK7 mediated assembly and activation of NLRP3 inflammasome downstream of potassium efflux in ventilator-induced lung injury. Biochem Pharmacol 2020, 177, 113998. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, J.; Chang, Y.; Lin, Z.; Shi, Z.; Li, X.; Chen, X.; Lin, C.; Pan, S.; Huang, K. Attenuation of cerebral edema facilitates recovery of glymphatic system function after status epilepticus. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Shafaroodi, H.; Barati, S.; Ghasemi, M.; Almasirad, A.; Moezi, L. A role for ATP-sensitive potassium channels in the anticonvulsant effects of triamterene in mice. Epilepsy Res 2016, 121, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.L.; Asher, J.L.; Molony, R.D.; Shaw, A.C.; Zeiss, C.J.; Wang, C.; Morozova-Roche, L.A.; Herzog, R.I.; Iwasaki, A.; Dixit, V.D. beta-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep 2017, 18, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yin, X.; Wang, M.; Wang, Y.; Li, F.; Gao, Y.; Han, G.; Gao, Z.; Wang, Z. beta-Hydroxybutyrate alleviates pyroptosis in MPP(+)/MPTP-induced Parkinson's disease models via inhibiting STAT3/NLRP3/GSDMD pathway. Int Immunopharmacol 2022, 113, 109451. [Google Scholar] [CrossRef] [PubMed]
- Lutas, A.; Yellen, G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci 2013, 36, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Uchida, Y.; Kadono, K.; Hirao, H.; Kawasoe, J.; Watanabe, T.; Ueda, S.; Okajima, H.; Terajima, H.; Uemoto, S. Up-regulation of FOXO1 and reduced inflammation by beta-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc Natl Acad Sci U S A 2019, 116, 13533–13542. [Google Scholar] [CrossRef]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. beta-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer's disease pathology. J Neuroinflammation 2020, 17, 280. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D'Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Kim, D.Y.; Simeone, K.A.; Simeone, T.A.; Pandya, J.D.; Wilke, J.C.; Ahn, Y.; Geddes, J.W.; Sullivan, P.G.; Rho, J.M. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol 2015, 78, 77–87. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741. [Google Scholar] [CrossRef]
- Oronsky, B.; Takahashi, L.; Gordon, R.; Cabrales, P.; Caroen, S.; Reid, T. RRx-001: a chimeric triple action NLRP3 inhibitor, Nrf2 inducer, and nitric oxide superagonist. Front Oncol 2023, 13, 1204143. [Google Scholar] [CrossRef]
- Chen, Y.; He, H.; Lin, B.; Chen, Y.; Deng, X.; Jiang, W.; Zhou, R. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell Mol Immunol 2021, 18, 1425–1436. [Google Scholar] [CrossRef]
- Reid, T.; Oronsky, B.; Caroen, S.; Cabrales, P. The direct NLRP3 inhibitor and Phase 3 small molecule anticancer agent, RRx-001, protects aged triple transgenic Alzheimer’s disease model mice from CNS degeneration and cognitive decline. Alzheimer's Dement 2022, 18. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 inhibits interleukin-1beta production and inflammasome activation of microglia in epileptic seizures. J Neuroinflammation 2019, 16, 66. [Google Scholar] [CrossRef]
- Li, Q.; Feng, H.; Wang, H.; Wang, Y.; Mou, W.; Xu, G.; Zhang, P.; Li, R.; Shi, W.; Wang, Z.; et al. Licochalcone B specifically inhibits the NLRP3 inflammasome by disrupting NEK7-NLRP3 interaction. EMBO Rep 2022, 23, e53499. [Google Scholar] [CrossRef]
- Honda, H.; Nagai, Y.; Matsunaga, T.; Okamoto, N.; Watanabe, Y.; Tsuneyama, K.; Hayashi, H.; Fujii, I.; Ikutani, M.; Hirai, Y.; et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol 2014, 96, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, Y.; Ding, R.; Feng, L.; Fu, Z.; Yang, S.; Deng, X.; Xie, Z.; Zheng, S. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-kappaB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J Neuroinflammation 2017, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lv, Q.; Miao, Y.M.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol 2018, 155, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Mohseni-Moghaddam, P.; Sadr, S.S.; Roghani, M.; Arabzadeh, S.; Khamse, S.; Zamani, E.; Hosseini, M.; Moradi, F. Huperzine A ameliorates cognitive dysfunction and neuroinflammation in kainic acid-induced epileptic rats by antioxidant activity and NLRP3/caspase-1 pathway inhibition. Clin Exp Pharmacol Physiol 2019, 46, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Samadianzakaria, A.; Abdolmaleki, Z.; Faedmaleki, F. The effect of valproic acid and furosemide on the regulation of the inflammasome complex (NLRP1 and NLRP3 mRNA) in the brain of epileptic animal model. Brain Res Bull 2022, 191, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, S.; Guo, C.; Hu, Z.; Peng, J.; Guo, K.; Zhang, X.; Li, J. Ibuprofen Exerts Antiepileptic and Neuroprotective Effects in the Rat Model of Pentylenetetrazol-Induced Epilepsy via the COX-2/NLRP3/IL-18 Pathway. Neurochem Res 2020, 45, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, F.; Moradifar, F.; Hosseini, A. Rapamycin attenuates depression and anxiety-like behaviors through modulation of the NLRP3 pathway in pentylenetetrazole-kindled male Wistar rats. Fundam Clin Pharmacol 2021, 35, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yang, P.; Li, F.; Yu, Q.; Kuang, W.; Zhu, Y.; Lu, J.; Wu, H.; Li, L.; Huang, H. Chaihu-Longgu-Muli Decoction exerts an antiepileptic effect in rats by improving pyroptosis in hippocampal neurons. J Ethnopharmacol 2021, 270, 113794. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun 2018, 9, 2550. [Google Scholar] [CrossRef]
- Juliana, C.; Fernandes-Alnemri, T.; Wu, J.; Datta, P.; Solorzano, L.; Yu, J.W.; Meng, R.; Quong, A.A.; Latz, E.; Scott, C.P.; et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem 2010, 285, 9792–9802. [Google Scholar] [CrossRef]
- Wu, L.; Chen, M.; Li, M.; Wang, Y.; Li, Y.; Zheng, L.; Ke, Z.; Liu, K.; Qiao, Y.; Shi, X. Oridonin alleviates kanamycin-related hearing loss by inhibiting NLRP3/caspase-1/gasdermin D-induced inflammasome activation and hair cell pyroptosis. Mol Immunol 2022, 149, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.B.; Jain, S.; Nagpal, D.; Agarwal, N.K.; Mediratta, P.K.; Sharma, K.K. Liposomal formulation of curcumin attenuates seizures in different experimental models of epilepsy in mice. Fundam Clin Pharmacol 2013, 27, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Bharal, N.; Sahaya, K.; Jain, S.; Mediratta, P.K.; Sharma, K.K. Curcumin has anticonvulsant activity on increasing current electroshock seizures in mice. Phytother Res 2008, 22, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, K.M.; Mishra, A.; Poroikov, V.V.; Goel, R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur J Pharmacol 2013, 704, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.K.; Briyal, S.; Sharma, M. Protective effect of curcumin against kainic acid induced seizures and oxidative stress in rats. Indian J Physiol Pharmacol 2009, 53, 39–46. [Google Scholar] [PubMed]
- Hashemian, M.; Anissian, D.; Ghasemi-Kasman, M.; Akbari, A.; Khalili-Fomeshi, M.; Ghasemi, S.; Ahmadi, F.; Moghadamnia, A.A.; Ebrahimpour, A. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog Neuropsychopharmacol Biol Psychiatry 2017, 79, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1beta Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J Immunol 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhong, L.; Xian, R.; Yuan, B. MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol Immunol 2015, 65, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, M.; Gerlic, M.; Kurowska-Stolarska, M.; Rainey, A.A.; Pich, D.; McInnes, I.B.; Hammerschmidt, W.; O'Neill, L.A.; Masters, S.L. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol 2012, 189, 3795–3799. [Google Scholar] [CrossRef]
- Meng, Q. , Huo, X., Sun, H., Wang, H., Luan, Z. and Wang, S. miR-223 regulates myocardial ischemia-reperfusion damage via targeting NLRP3 in vitro and in vivo. Int J Clin Exp Med 2018, 11. [Google Scholar]
- Wang, R.; Li, Q.; He, Y.; Yang, Y.; Ma, Q.; Li, C. miR-29c-3p inhibits microglial NLRP3 inflammasome activation by targeting NFAT5 in Parkinson's disease. Genes Cells 2020, 25, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dixon, B.J.; Doycheva, D.M.; Li, B.; Zhang, Y.; Hu, Q.; He, Y.; Guo, Z.; Nowrangi, D.; Flores, J.; et al. IRE1alpha inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic-ischemic brain injury in rats. J Neuroinflammation 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Han, Y.; Chen, R.; Yang, P.; Zhang, C. LncRNA ZNF883-Mediated NLRP3 Inflammasome Activation and Epilepsy Development Involve USP47 Upregulation. Mol Neurobiol 2022, 59, 5207–5221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lu, Y.; Yuan, L.; Zhang, P.; Zou, D.; Wei, F.; Chen, X. miR-29a-5p Alleviates Traumatic Brain Injury- (TBI-) Induced Permeability Disruption via Regulating NLRP3 Pathway. Dis Markers 2021, 2021, 9556513. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Oskuee, R.K.; Atkin, S.L.; Navashenaq, J.G.; Sahebkar, A. MicroRNAs as important regulators of the NLRP3 inflammasome. Prog Biophys Mol Biol 2020, 150, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.R.; Goya Grocin, A.; Kovacic, T.; Singh, R.; Ward, J.A.; Shenoy, A.R.; Tate, E.W. A Probe for NLRP3 Inflammasome Inhibitor MCC950 Identifies Carbonic Anhydrase 2 as a Novel Target. ACS Chem Biol 2021, 16, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Saeed, M.; Kuete, V.; Greten, H.J.; Efferth, T. Oridonin Targets Multiple Drug-Resistant Tumor Cells as Determined by in Silico and in Vitro Analyses. Front Pharmacol 2018, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Pethani, J.P.; Shah, H.A.; Vyas, V.; Sasane, S.; Bhavsar, H.; Bandyopadhyay, D.; Giri, P.; Viswanathan, K.; Jain, M.R.; et al. Identification of a novel orally bioavailable NLRP3 inflammasome inhibitor. Bioorg Med Chem Lett 2020, 30, 127571. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sasane, S.; Shah, H.A.; Pethani, J.P.; Deshmukh, P.; Vyas, V.; Iyer, P.; Bhavsar, H.; Viswanathan, K.; Bandyopadhyay, D.; et al. Discovery of N-Cyano-sulfoximineurea Derivatives as Potent and Orally Bioavailable NLRP3 Inflammasome Inhibitors. ACS Med Chem Lett 2020, 11, 414–418. [Google Scholar] [CrossRef]
- Smolak, P.; Nguyen, M.; Diamond, C.; Wescott, H.; Doedens, J.R.; Schooley, K.; Snouwaert, J.N.; Bock, M.G.; Harrison, D.; Watt, A.P.; et al. Target Cell Activation of a Structurally Novel NOD-Like Receptor Pyrin Domain-Containing Protein 3 Inhibitor NT-0796 Enhances Potency. J Pharmacol Exp Ther 2024, 388, 798–812. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Citterio, L.A.; Agostini, S.; Marventano, I.; La Rosa, F.; Re, F.; Seneci, P.; Saresella, M.; Clerici, M. Glibenclamide-Loaded Nanoparticles Reduce NLRP3 Inflammasome Activation and Modulate miR-223-3p/miR-7-1-5p Expression in THP-1 Cells. Pharmaceuticals (Basel) 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Debnath, M.; Forster, J., 3rd; Pandey, A.; Bharadwaj, H.; Patel, R.; Kulkarni, A. Nanoparticle-mediated co-delivery of inflammasome inhibitors provides protection against sepsis. Nanoscale 2024, 16, 4678–4690. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, T.; Xiong, X.; Yang, Q.; Su, Z.; Zheng, M.; Chen, Q. Colchicine delivered by a novel nanoparticle platform alleviates atherosclerosis by targeted inhibition of NF-kappaB/NLRP3 pathways in inflammatory endothelial cells. J Nanobiotechnology 2023, 21, 460. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol Pharm 2019, 16, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, X.; Yu, H.; Shi, X.; Zhou, Y.; Alvarez, S.; Naldrett, M.J.; Kachman, S.D.; Ro, S.H.; Sun, X.; et al. Therapeutic potential of garlic chive-derived vesicle-like nanoparticles in NLRP3 inflammasome-mediated inflammatory diseases. Theranostics 2021, 11, 9311–9330. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, W.; Zhu, J.; Zhai, Z.; Xie, J.; Zhou, J.; Feng, X.; Feng, B.; Pan, Q.; Li, S.; Venkatesan, R.; et al. ROS-responsive polymer nanoparticles with enhanced loading of dexamethasone effectively modulate the lung injury microenvironment. Acta Biomater 2022, 148, 258–270. [Google Scholar] [CrossRef]
- Liu, X.; Lu, B.; Fu, J.; Zhu, X.; Song, E.; Song, Y. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells. J Hazard Mater 2021, 404, 124050. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; McLeland, C.B.; Potter, T.M.; Stern, S.T.; Adiseshaiah, P.P. Assessing NLRP3 Inflammasome Activation by Nanoparticles. Methods Mol Biol 2018, 1682, 135–147. [Google Scholar] [CrossRef]
- Shirasuna, K.; Karasawa, T.; Takahashi, M. Exogenous nanoparticles and endogenous crystalline molecules as danger signals for the NLRP3 inflammasomes. J Cell Physiol 2019, 234, 5436–5450. [Google Scholar] [CrossRef]
- Wang, X.; Lin, J.; Wang, Z.; Li, Z.; Wang, M. Possible therapeutic targets for NLRP3 inflammasome-induced breast cancer. Discov Oncol 2023, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Forster, J., 3rd; Ramesh, A.; Kulkarni, A. Protein Corona Formation on Lipid Nanoparticles Negatively Affects the NLRP3 Inflammasome Activation. Bioconjug Chem 2023, 34, 1766–1779. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer's disease. Bioorg Chem 2021, 108, 104681. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Upadhyay, S.P.; Singh, V.; Boinpelly, V.C.; Zhou, J.; Johnson, D.K.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone: A potential scaffold for NLRP3 inflammasome inhibitors. Eur J Med Chem Rep 2023, 7. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, H.; Sun, P.; Hua, L.; Liang, S.; Ou, Y.; Wu, D.; Wu, X.; Chen, H.; Hao, Y.; et al. Discovery of chalcone analogues as novel NLRP3 inflammasome inhibitors with potent anti-inflammation activities. Eur J Med Chem 2021, 219, 113417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, M.; He, F.; Zhou, S.; Zhu, L. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J Neuroinflammation 2017, 14, 207. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhu, H.Y.; Wang, X.L.; Lu, X.W.; Pan, C.L.; Xu, L.; Liu, X.; Xu, N.; Zhang, Z.Y. Oridonin Ameliorates Traumatic Brain Injury-Induced Neurological Damage by Improving Mitochondrial Function and Antioxidant Capacity and Suppressing Neuroinflammation through the Nrf2 Pathway. J Neurotrauma 2022, 39, 530–543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





