Submitted:
30 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Characterization of Sugarcane bagasse ash
2.2. Sample preparation
2.3. Sodium silicate characterization
3. Results and discussions
4. Conclusions
Acknowledgments
References
- Kazmi, S.M.S.; Abbas, S.; Saleem, M.A.; Munir, M.J.; Khitab, A. Manufacturing of sustainable clay bricks: Utilization of waste sugarcane bagasse and rice husk ashes, Constr Build Mater. 120 (2016) 29–41. [CrossRef]
- James, J.; Pandian, P.K. A Short Review on the Valorisation of Sugarcane Bagasse Ash in the Manufacture of Stabilized / Sintered Earth Blocks and Tiles, Advances in Materials Science and Engineering. 2017 (2017) 15.
- Teixeira, S.R.; Souza, A.E.; Carvalho, C.L.; Reynoso, V.C.S.; Romero, M.; Rincón, J.M. Characterization of a wollastonite glass-ceramic material prepared using sugar cane bagasse ash (SCBA) as one of the raw materials, Mater Charact. 98 (2014) 209–214. [CrossRef]
- Souza, A.E.; Teixeira, S.R.; Santos, G.T.A.; Costa, F.B.; Longo, E. Reuse of sugarcane bagasse ash (SCBA) to produce ceramic materials, J Environ Manage. 92 (2011) 2774–2780. [CrossRef]
- Pérez, j. uso de la ceniza de bagazo de caña de azúcar como fuente de sio2 para síntesis de materiales vitrocerámicos con aplicaciones en la construcción, Universidad Autónoma de Nuevo León, 2018.
- Bernal, S.A.; Provis, J.L.; Rose, V.; De Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends, Cem Concr Compos. 33 (2011) 46–54. [CrossRef]
- Sodium and Potassium silicates Versatile compounds for your applications, (2004) 16.
- Mazrouei-Sebdani, Z.; Salimian, S.; Khoddami, A.; Shams-Ghahfarokhi, F. Sodium silicate based aerogel for absorbing oil from water: The impact of surface energy on the oil/water separation, Mater Res Express. 6 (2019). [CrossRef]
- Farid, R.; Rajan, K.; Sarkar, D.K. Surface & Coatings Technology Enhanced corrosion protection of aluminum by ultrasonically dip coated sodium silicate thin fi lms, Surf Coat Technol. 374 (2019) 355–361. [CrossRef]
- Liu, W.; Xi, W.; Hu, R.; Yue, M.; Yin, Y.; Guo, J. Preparation and characterization of sodium silicate / epoxy resin composite bonded Nd-Fe-B magnets with high performance *, Journal of Rare Earths. (2019). [CrossRef]
- Vinai, R.; Soutsos, M. Cement and Concrete Research Production of sodium silicate powder from waste glass cullet for alkali activation of alternative binders, Cem Concr Res. 116 (2019) 45–56. [CrossRef]
- Alam, Q.; Hendrix, Y.; Thijs, L.; Lazaro, A.; Schollbach, K.; Brouwers, H.J.H. Novel low temperature synthesis of sodium silicate and ordered mesoporous silica from incineration bottom ash, J Clean Prod. 211 (2019) 874–883. [CrossRef]
- Ahmad, N.; Ahmad, F. Green Chemistry: Principle and its Application, in: 2nd International Conference on Advancement in Engineering, Applied Science and Management, 2017. https://www.researchgate.net/publication/322078077.
- Abdallah, M.; Salem, M.M.; Zaafarany, I.A.; Fawzy, A.; Fattah, A.A.A. Corrosion performance of stainless steel and nickel alloys in aqueous sodium hydroxide as revealed from cyclic voltammetry and potentiodynamic anodic polarization, Oriental Journal of Chemistry. 33 (2017) 2875–2883. [CrossRef]
- Ingrao, C.; Saja, C.; Primerano, P. Application of Life Cycle Assessment to chemical recycling of post-use glass containers on the laboratory scale towards circular economy implementation, J Clean Prod. 307 (2021). [CrossRef]
- Zulfiqar, U.; Awais, M.; Hussain, S.Z.; Hussain, I.; Husain, S.W.; Subhani, T. Durable and self-healing superhydrophobic surfaces for building materials, Mater Lett. 192 (2017) 56–59. [CrossRef]
- Sultana, M.S.; Rahman, M.A.; Zaman, M.N.; Ahmed, A.N. Influence of Calcination on Different Properties of Sugarcane Bagasse and Waste Ash, Journal of Scientific Research. 7 (2015) 151–157.
- Yahya, A.A.; Ali, N.; Kamal, N.L.M.; Shahidan, S.; Beddu, S.; Nuruddin, M.F.; Shafiq, N. Reducing Heavy Metal Element from Coal Bottom Ash by Using Citric Acid Leaching Treatment, MATEC Web of Conferences. 103 (2017) 0–6. [CrossRef]
- Pérez-Casas, J.A.; Zaldívar-Cadena, A.A.; Álvarez-Mendez, A.; Ruiz-Valdés, J.J.; de la Parra-Arciniega, S.M.; Sánchez-Vázquez, A.I. Synthesis of Silica Particles from Sugarcane Bagasse Ash for Its Application in Hydrophobic Coatings, Materials Proceedings. 2 (2020) 4. [CrossRef]
- Teresia. Möller, Selective crystalline inorganic materials as ion exchangers in the treatment of nuclear waste solutions, Academic Dissertation, Faculty of Science of the University of Helsinki, 2002.
- Stelte, W.; Holm, J.K.; Sanadi, A.R.; Barsberg, S.; Ahrenfeldt, J.; Henriksen, U.B. Fuel pellets from biomass: The importance of the pelletizing pressure and its dependency on the processing conditions, Fuel. 90 (2011) 3285–3290. [CrossRef]
- Lin, K.J.; Ding, H.; Demkowicz, M.J. Formation, migration, and clustering energies of interstitial He in α-quartz and β-cristobalite, Journal of Nuclear Materials. 479 (2016) 224–231. [CrossRef]
- Askeland, D.R.; Fulay, P. Ciencia e ingeniería de materiales, (2011) 197–247.
- Reddy, P.M.; Lakshmi, R.; Dass, F.P.; Sasikumar, S. Synthesis, characterization and formulation of sodium calcium silicate bioceramic for drug delivery applications, Science and Engineering of Composite Materials. 23 (2016) 375–380. [CrossRef]
- Medvedev, E.F.; Komarevskaya, A.S. IR spectroscopic study of the phase composition for sodium silicate synthesized in aqueous medium, Glass and Ceramics. 64 (2007) 7–11. [CrossRef]
- Rufino, E.S.; Monteiro, E.E.C. Characterisation of lithium and sodium salts of poly(methacrylic acid) by FTIR and thermal analyses, n.d.
- Vidal, L.; Joussein, E.; Colas, M.; Cornette, J.; Sanz, J.; Sobrados, I.; Gelet, J.L.; Absi, J.; Rossignol, S. Controlling the reactivity of silicate solutions: A FTIR, Raman and NMR study, Colloids Surf A Physicochem Eng Asp. 503 (2016) 101–109. [CrossRef]
- Maheshwari, A.; Wiemhöfer, H.D. Augmentation of grain boundary conductivity in Ca2+ doped ceria-carbonate-composite, Acta Mater. 103 (2016) 361–369. [CrossRef]
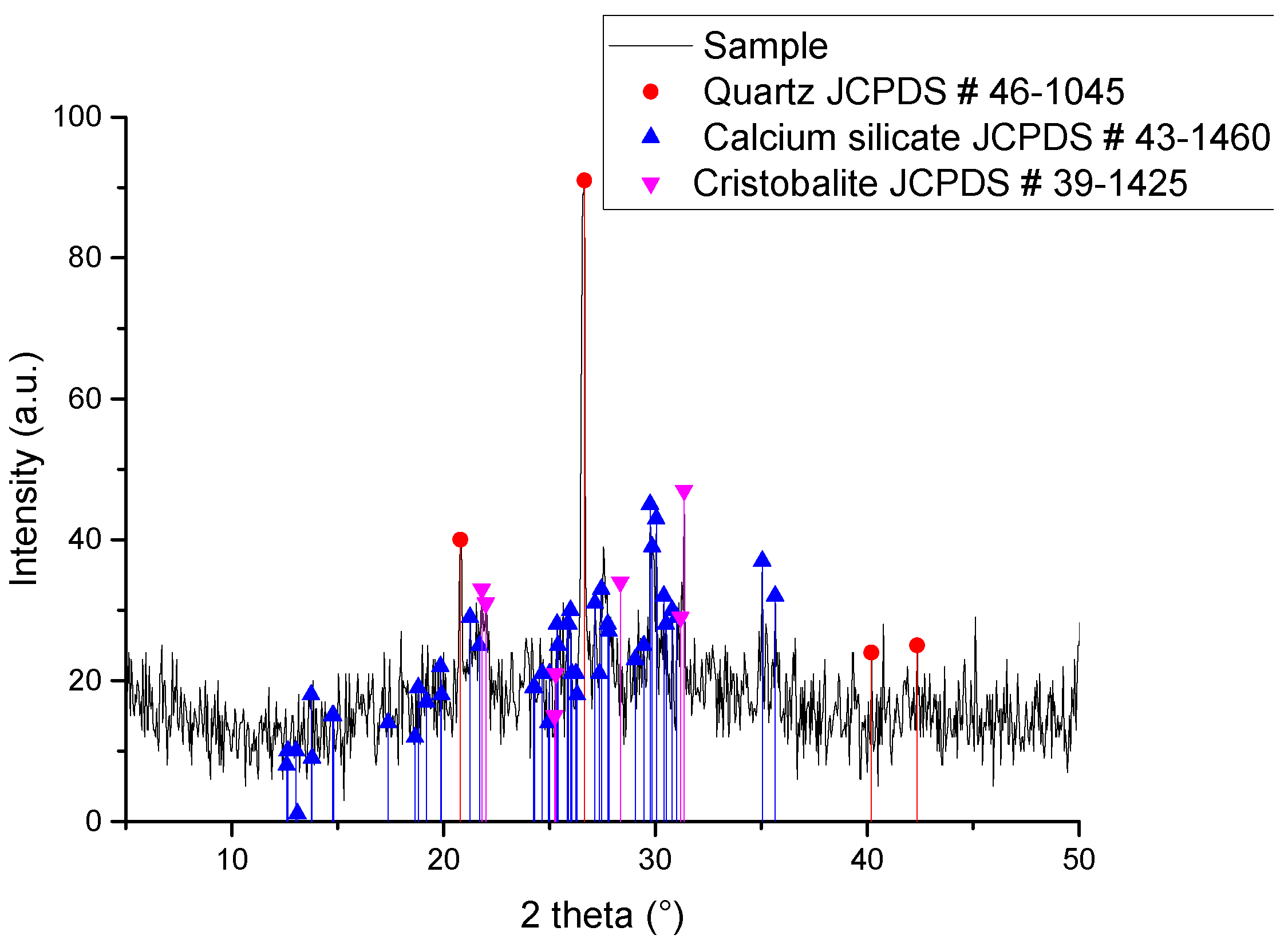
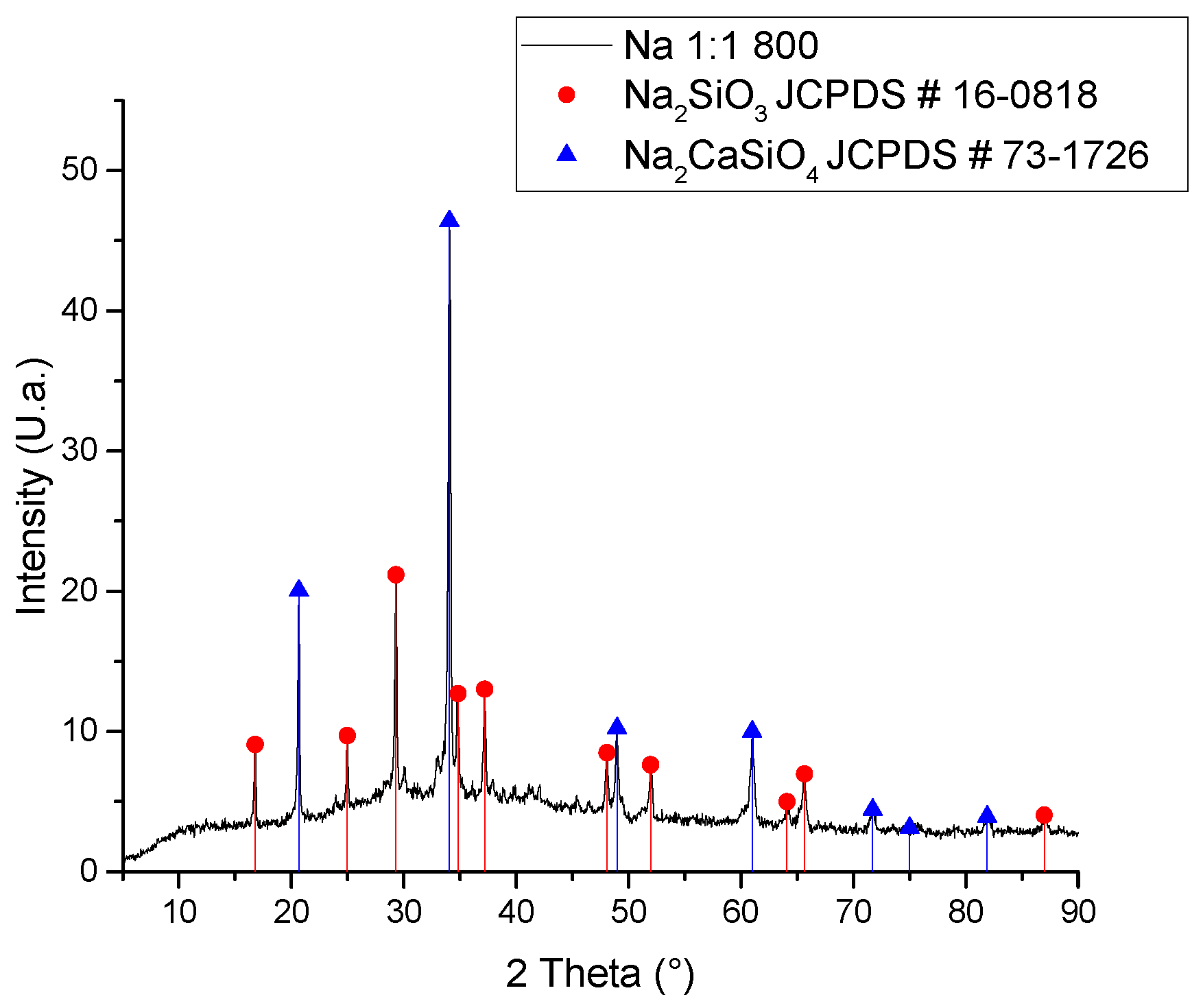
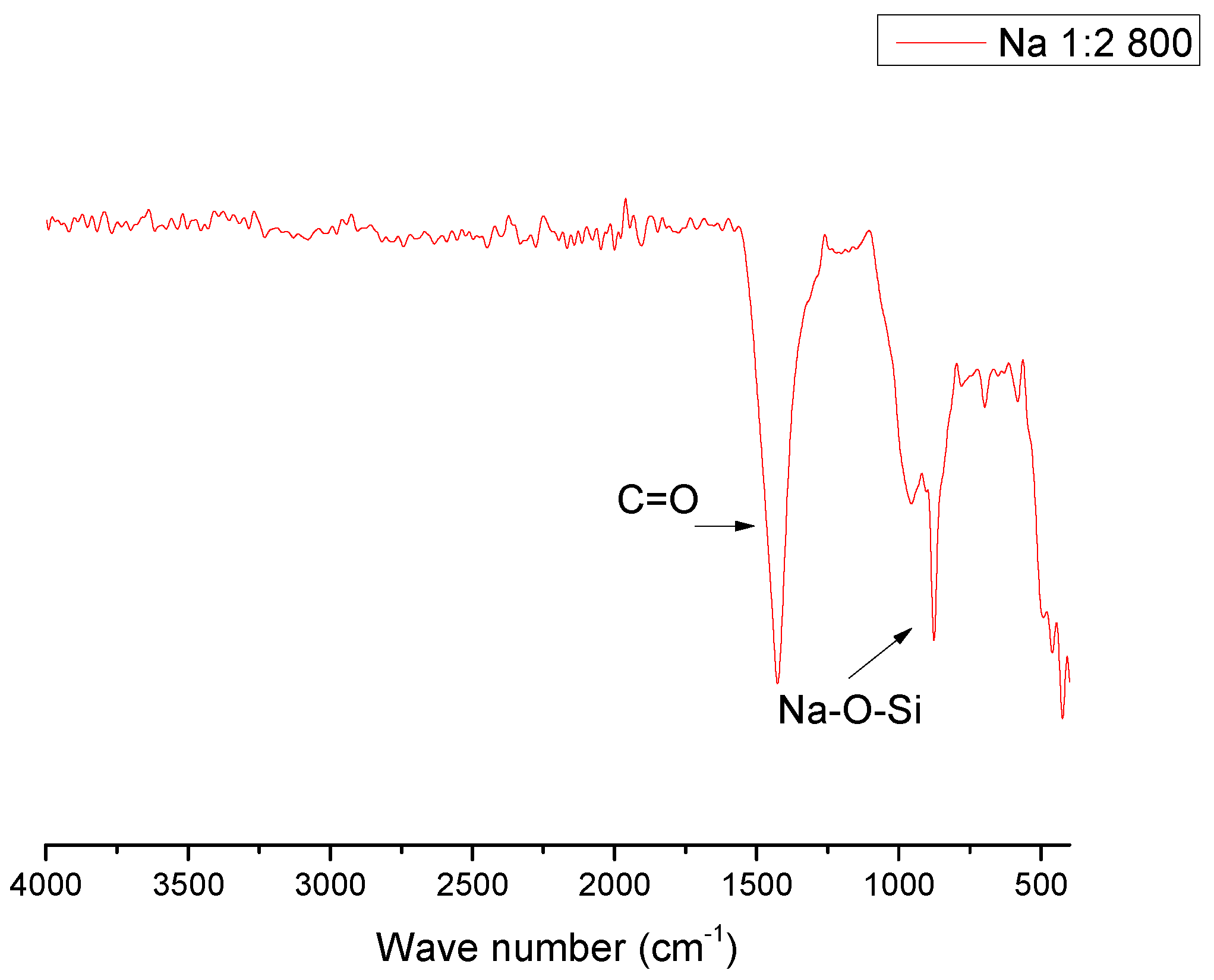
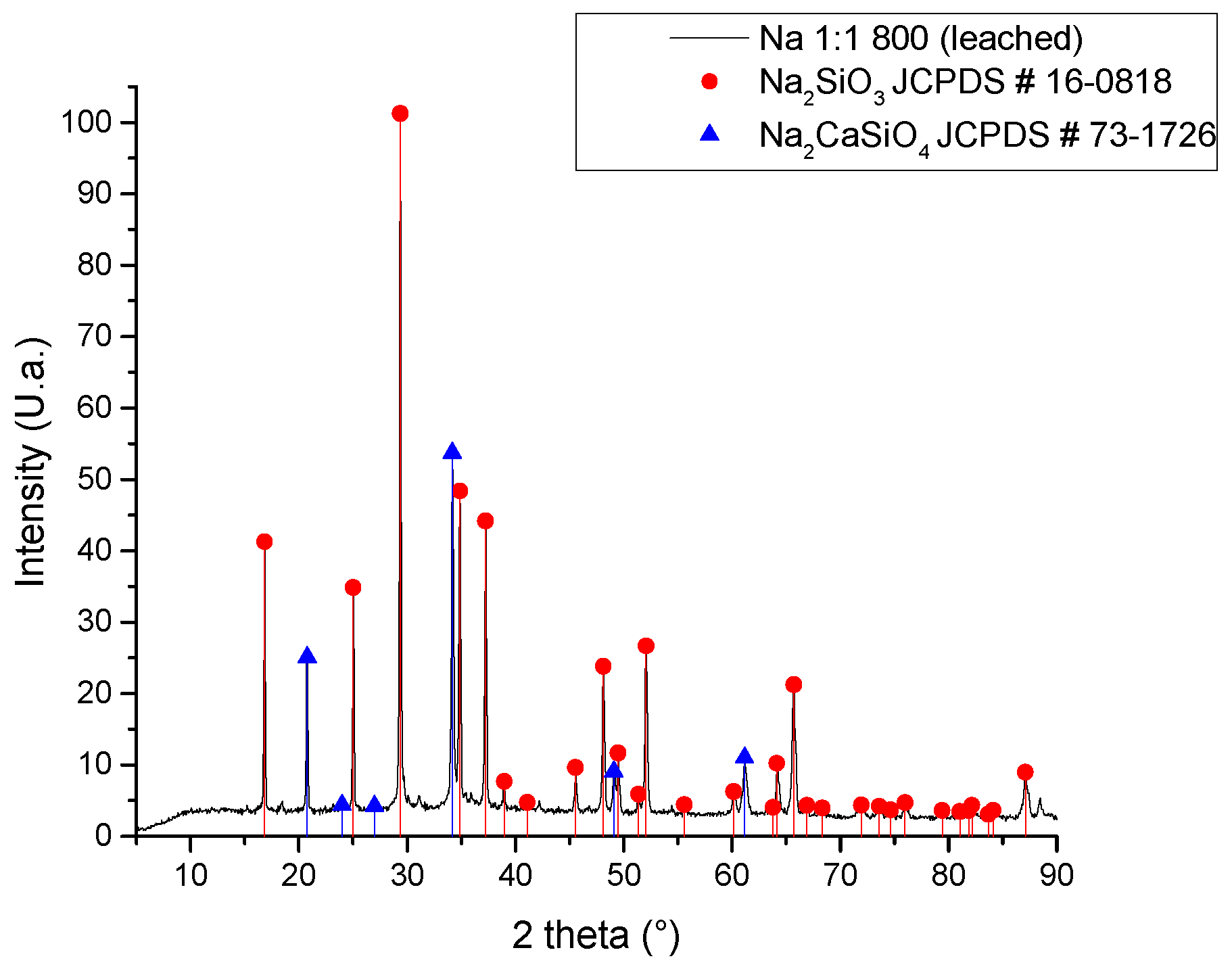
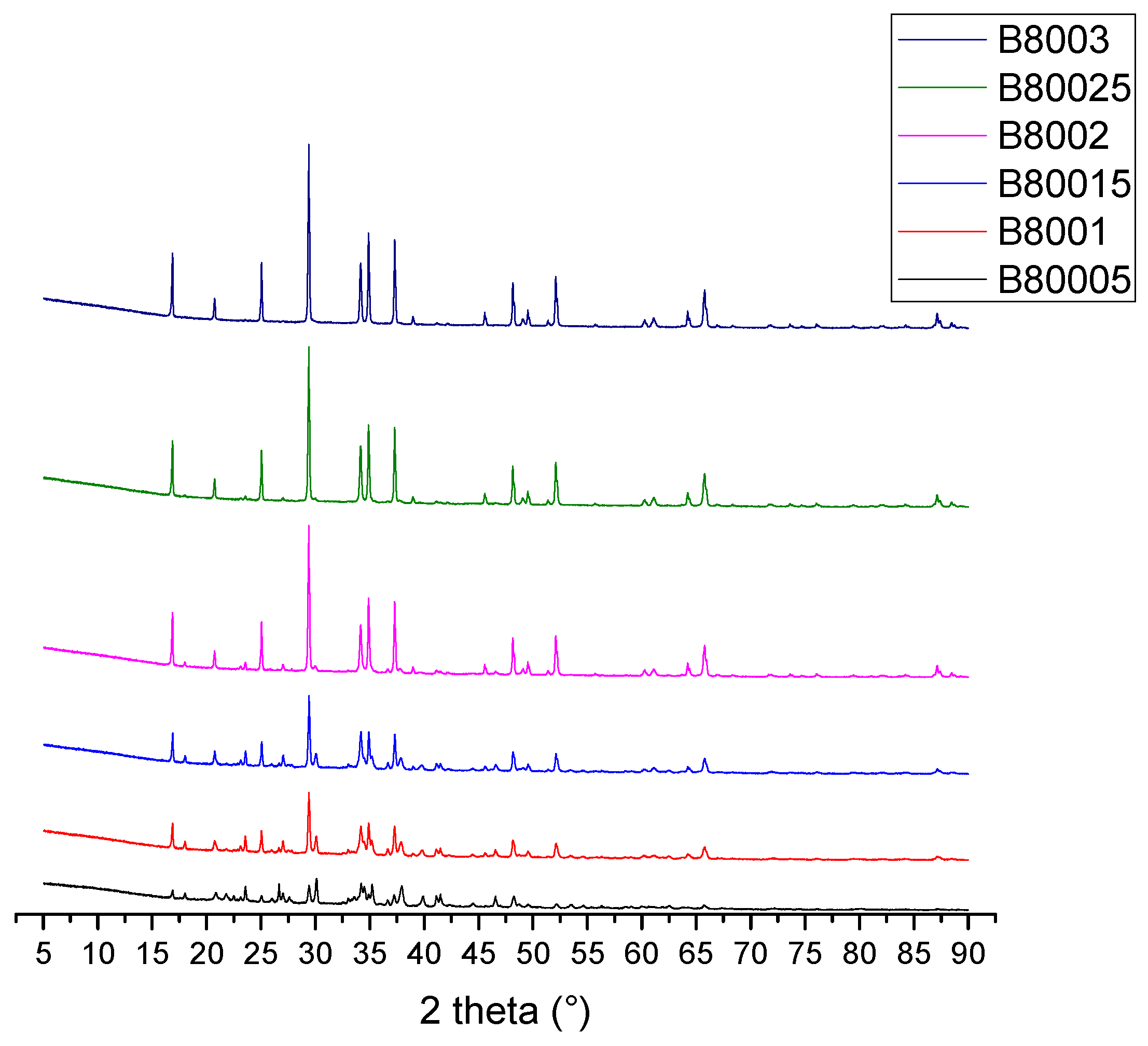
| Compound | %wt/wt. | Leached ash %wt/wt. |
|---|---|---|
| Na2O | 0.48 | 0.00 |
| MgO | 0.92 | 0.30 |
| Al2O3 | 2.21 | 1.70 |
| SiO2 | 70.85 | 78.61 |
| P2O5 | 1.15 | 1.28 |
| SO3 | 1.46 | 0.25 |
| K2O | 4.34 | 5.61 |
| CaO | 12.73 | 7.45 |
| TiO2 | 0.44 | 0.47 |
| MnO | 0.13 | 0.11 |
| Fe2O3 | 4.82 | 4.05 |
| CuO | 0.03 | 0.02 |
| ZnO | 0.06 | 0.03 |
| SrO | 0.13 | 0.05 |
| ZrO2 | 0.02 | 0.01 |
| Ag2O | 0.18 | 0.06 |
| BaO | 0.04 | 0.00 |
| Total | 100 | 100 |
| Phase | CIF |
|---|---|
| Na2CO3 | 96-210-6298 |
| Na2SiO3 | 96-231-0859 |
| Na2CaSiO4 | 96-101-0112 |
| Quartz | 96-901-5023 |
| SiO2 (no polymorph identified) | 96-900-6299 |
| NaO2 | 96-412-4632 |
| Cristobalite | 96-901-4487 |
| Sample | SiO2 | Na2CO3 | NaO2 | Na2SiO3 | Na2CaSiO4 |
|---|---|---|---|---|---|
| B0.5 | 0.42 | 0.31 | 0.10 | 0.12 | 0.045 |
| B1.0 | 0.39 | 0.27 | 0.00 | 0.27 | 0.072 |
| B1.5 | 0.34 | 0.27 | 0.00 | 0.28 | 0.10 |
| B2.0 | 0.23 | 0.20 | 0.00 | 0.44 | 0.13 |
| B2.5 | 0.24 | 0.00 | 0.00 | 0.62 | 0.14 |
| B3.0 | 0.00 | 0.00 | 0.00 | 0.81 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





