1. Introduction
Postoperative aneurysm sac poses a significant concern following endovascular aortic aneurysm repair (EVAR). The EVAR trial-1, which prospectively followed patients who underwent abdominal aortic aneurysm for 15 years postoperatively, found that secondary aneurysm rupture due to aneurysm enlargement occurred in the EVAR group [
1]. Microcirculatory disorder within the aneurysm wall, specifically, impaired vasa vasorum perfusion, may cause aneurysm wall enlargement [
2]. Within the aortic wall, the inner third is perfused by lumen-side blood flow, whereas the outer two-thirds rely on the vasa vasorum for blood supply [
2]. Endovascular treatment with a stent graft (SG) disrupts lumen-side perfusion. Consequently, the aortic wall inevitably becomes solely dependent on perfusion from the adventitial vasa vasorum. Thus, we propose that mitigating the microcirculatory impairment of the vasa vasorum is essential for preventing aneurysm enlargement after SG repair.
A regenerative approach involving the direct administration of human mesenchymal stromal cells (HMSCs) to the aneurysm sac has emerged as a promising option. HMSCs may promote aneurysm wall healing through several mechanisms: (1) suppression of inflammation via anti-inflammatory cytokines; (2) promotion of angiogenesis and extracellular matrix (ECM) organization through paracrine signaling; and (3) reconstruction of medial layers via differentiation into vascular smooth muscle cell-like phenotypes [
3,
4,
5,
6]. From a quantitative perspective, regeneration of aneurysmal tissue volume in the order of several cubic centimeters would require approximately 10
7–10
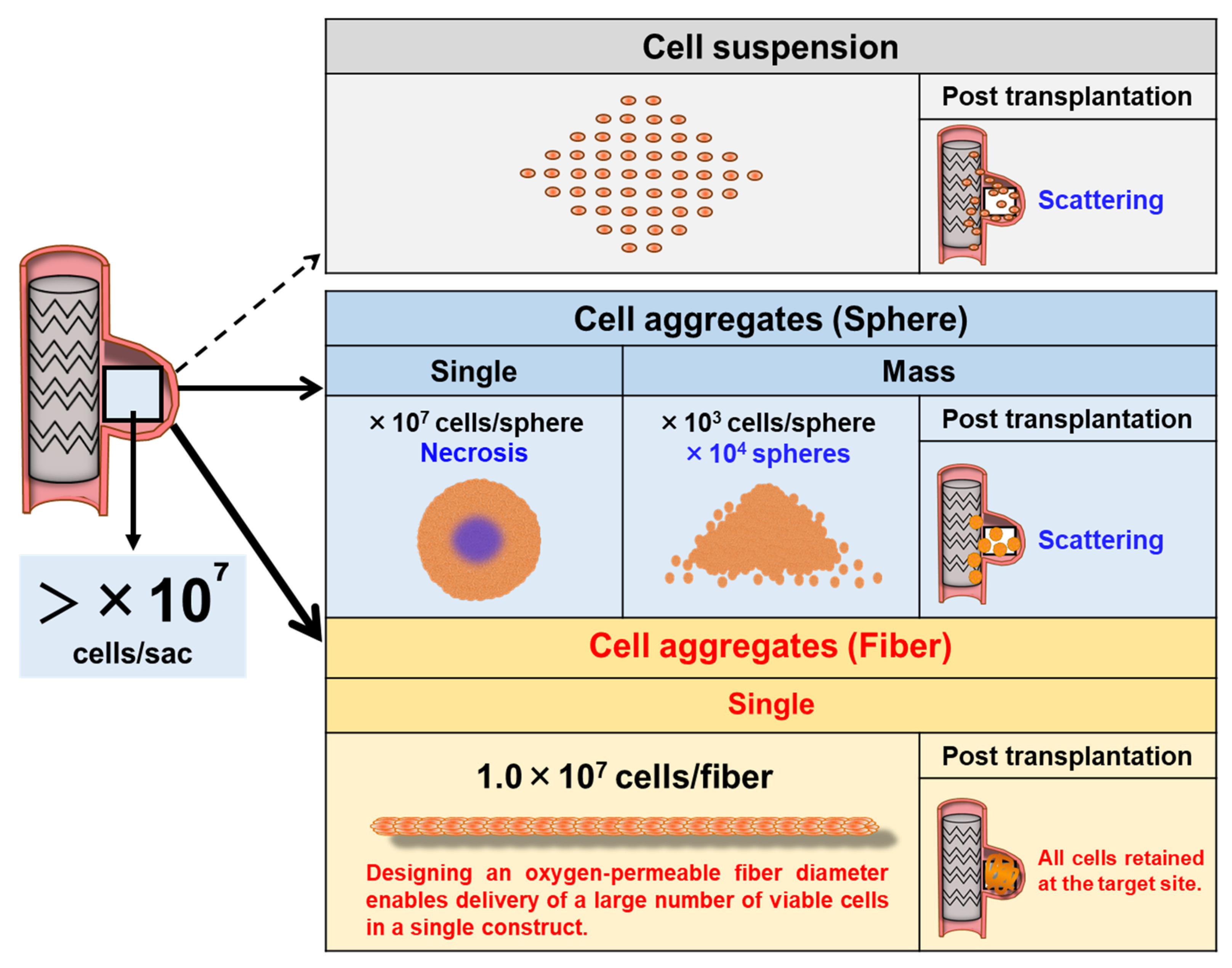
8 HMSCs, based on estimations of cellular volume and packing density. However, HMSC suspensions administered by direct injection are poorly retained and rapidly washed out under arterial blood flow (as observed in our preliminary investigations), leading to insufficient engraftment for therapeutic use (
Figure 1).
Although the delivery of spheroid-based HMSCs can improve cell survival and paracrine activity [
7], MSC spheroids are prone to developing central hypoxia and necrosis in a size-dependent manner, which may compromise cell viability and therapeutic function [
8,
9]. Furthermore, when numerous spheroids are administered, they tend to disperse within the aneurysm sac rather than remain concentrated in the target region, indicating insufficient localized cell retention (
Figure 1). This limitation mirrors that of single-cell suspension delivery, in which cells are similarly scattered and rapidly washed out under blood flow. Consequently, a catheter-deliverable cellular construct with structural stability and in situ retention capability must be developed to achieve endovascular regenerative therapy. To address this challenge, we employed the cell self-aggregation technique (CAT), a proprietary scaffold-free tissue fabrication method developed by our group [
10,
11]. CAT rapidly forms three-dimensional cellular constructs through patterned self-aggregation, which includes spheroids [
12,
13,
14], rings [
15,
16], fibers [
17,
18], and band-shaped tissues [
19] to develop multiple scaffold-free formats for regenerative applications. Especially designed for endovascular use, CAT enables the generation of contrast-agent–loaded HMSC aggregates suitable for radiographic visualization and catheter delivery [
18], making this platform feasible for regenerative catheter-based applications. Building on this platform, we developed fiber-shaped HMSC aggregates (HMSC fiber) as a catheter-deliverable, structurally stable alternative to spheroids. HMSC fiber retain strong cell–cell interactions while allowing oxygen and nutrient diffusion along the fiber axis, thereby reducing central necrosis (
Figure 1). Moreover, they can be aspirated, delivered, and positioned through catheters as a single continuous unit, minimizing uncontrolled dispersion and facilitating stable placement. Thus, HMSC fiber present a promising regenerative modality for definitive aneurysm wall reconstruction following endovascular treatment.
In this study, we investigated the preparation and biological characteristics of CAT-derived HMSC fibers and evaluated their feasibility for catheter-mediated intraluminal transplantation into aortic aneurysm models following endovascular treatment using SGs. We sought to establish foundational evidence supporting HMSC fiber as a novel regenerative platform for aneurysm wall healing following endovascular repair.
2. Materials and Methods
2.1. Establishing the Swine Thoracic Aortic Aneurysm Model
This animal experimental study was approved by our institutional review board (IRB number: 2019-020). Previously, we developed a method of establishing a swine thoracic aortic aneurysm (TAA) model [
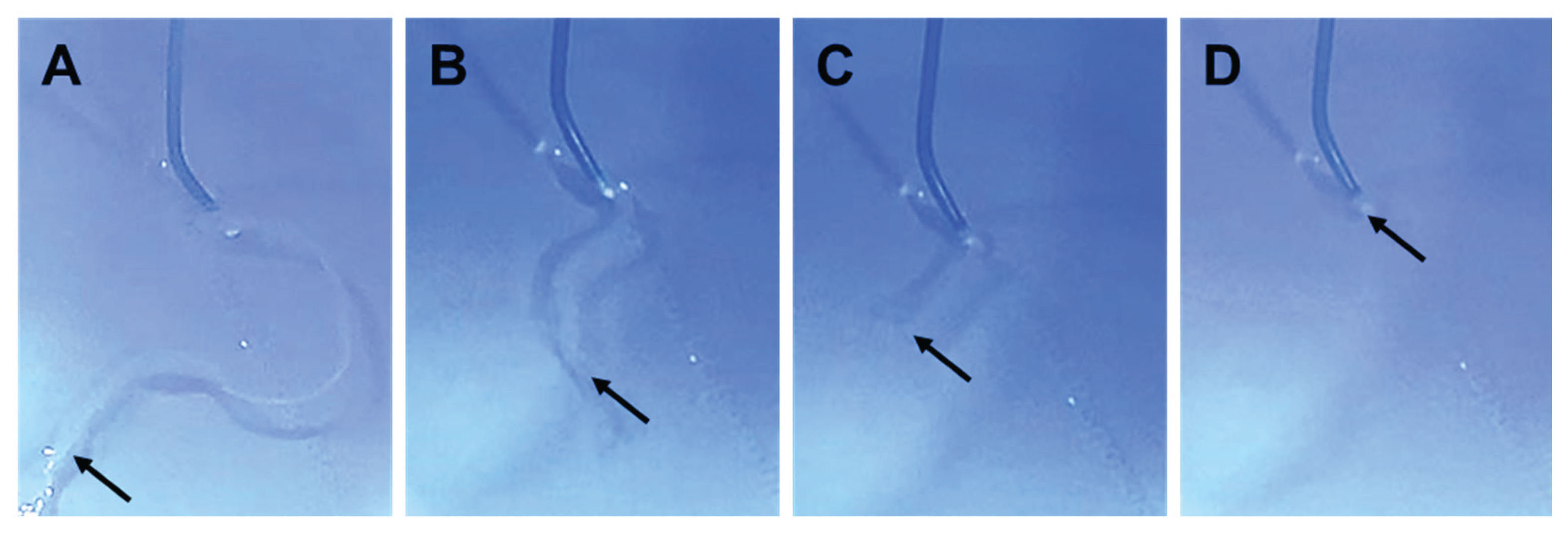
20]. This study also employed the same technique in swine weighing 35–40 kg. All animals underwent the same model induction procedures under sedation with subcutaneous medetomidine injection and general anesthesia with inhaled isoflurane. The animals were placed in the right lateral position, and left thoracotomy was performed to surgically expose the thoracic aorta. Heparin 4000 U was administered, followed by aortic clamping. A surgical incision was made around the anterior wall of the exposed aorta. A subintimal space was bluntly dissected at the incision site to create an aneurysm space. After suture closure for the adventitia, aortic clamping was released. The incision site was irrigated, hemostasis was confirmed, and the wound was closed. Fluoroquinolone was administered for wound prophylaxis. Levofloxacin 75 mg/day was administered orally for wound infection prophylaxis for 1 week postoperatively. Two weeks after model creation, angiography was performed under general anesthesia. After confirming the establishment of the TAA (
Figure 2), the model animals underwent endovascular repair. HMSC fiber therapy or saline was administered selectively into the target aneurysm cavity through a catheter.
2.2. Cell Culture and HMSC Fiber Preparation
Bone marrow-derived HMSCs were purchased from Promocell (Heidelberg, Germany) and cultured in mesenchymal stem cell growth medium 2 with 10% supplement (Promocell) and 1% penicillin streptomycin at 37 °C and 5% CO
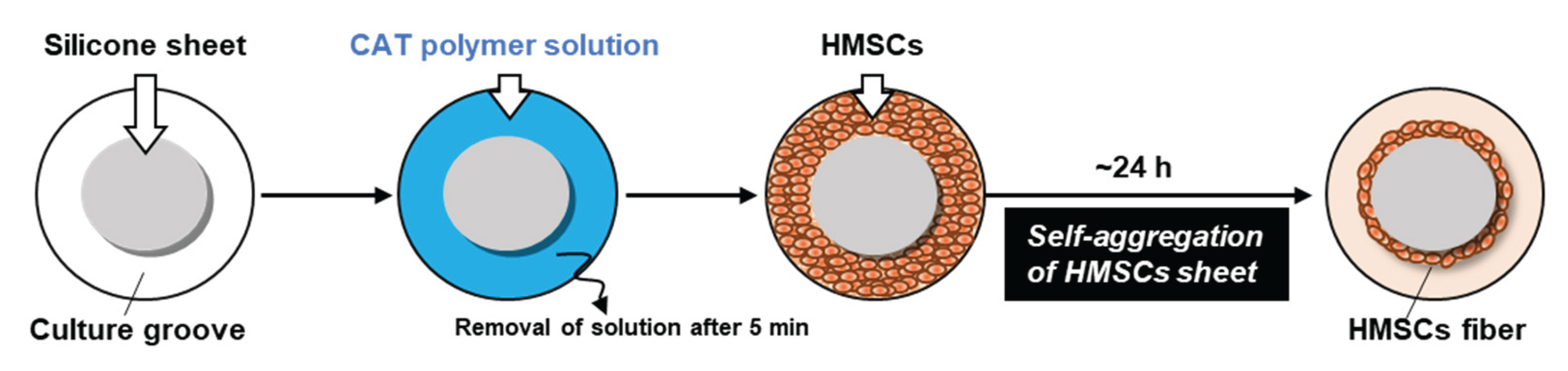
2. Third- or fourth-passage HMSCs were used for HMSC fiber preparation. HMSC fibers were prepared using a ring-shaped culture groove using the CAT (
Figure 3), as described previously [
18]. In detail, a 2-mm-thick silicone sheet (Togawa Rubber Co., Ltd., Osaka, Japan) was cut into a 60-mm-diameter disk and fixed to the surface of a 100-mm-diameter culture dish (AGC Techno Glass Co., Ltd., Shizuoka, Japan), thereby creating an annular culture groove in the peripheral region of the dish. The CAT polymer, poly(2-(dimethylamino) ethyl methacrylate)-
co-poly(methacrylic acid) (supplied by Nissan Chemical Corporation, Tokyo, Japan), was dissolved in UltraPure Water (Thermo Fisher Scientific Inc., MA, USA) at a concentration of 100 μg/mL, as described previously [
11]. A 5-mL portion of the polymer solution was poured into the culture groove and left to stand at room temperature for 5 min. Subsequently, the excess solution was removed by aspiration to produce a self-aggregation–inducing surface. HMSCs were seeded into the polymer-coated groove at a cell number of 4 × 10
6 to 12 × 10
6 cells/cm
2 and cultured overnight. During culture, the cells first formed a confluent monolayer sheet and subsequently underwent spontaneous self-aggregation, forming a ring-shaped cell aggregate along the groove (
Figure 3). Following culture completion, the silicone disk was removed using a forceps, and the ring-shaped aggregate was cut at a single point with scissors to produce one piece of HMSC fiber.
2.3. Endovascular HMSC Fiber Treatment
The pre-established swine TAA model was divided into two intervention groups: A control group undergoing SG placement followed by saline infusion, and a HMSC fiber group receiving SG placement followed by intraluminal transplantation of HMSC fibers. Under isoflurane inhalation anesthesia, a 5-Fr sheath (Boston Scientific, Marlborough, MA, USA) was inserted retrogradely through the right common carotid artery (CCA). Next, a pigtail catheter (TERUMO Medical Corporation, Somerset, NJ, USA) was positioned in the ascending aorta for angiography, performed to confirm the presence of the TAA. Thereafter, to facilitate SG delivery and placement, the abdominal aorta was surgically exposed through a midline abdominal incision.
First, a 5-Fr sheath was inserted into the abdominal aorta, after which an Amplatz super stiff guidewire (Boston Scientific Japan, Tokyo, Japan) was advanced into the ascending aorta. The 5-Fr sheath was then replaced with a 12-Fr DrySeal sheath (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) for SG delivery. We used the Excluder iliac extender (W. L. Gore & Associates, Inc.) as a treatment device, which has a zero-porosity structure and is clinically used in the abdominal–iliac artery region; its diameter is compatible with the normal swine thoracic aorta (approximately 13–14 mm). In accordance with clinical practice, a sealing length of 2 cm was secured on either the proximal or distal side of the aorta during SG placement. Prior to SG deployment, a 4-Fr angiographic catheter (Tempo®, Miami Lakes, FL, USA) was preplaced into the aneurysm sac via a 5-Fr sheath inserted through the right CCA. After deploying the SG, either HMSC fiber or an equal volume (0.7 mL) of saline was administered into the aneurysm sac through the catheter. After administration, the infusion catheter was removed promptly from the aneurysm space. After confirmation of accurate SG deployment by angiography, both sheaths inserted into the right CCA and abdominal aorta were removed, and the access site was closed using 6-0 Prolene sutures. Following wound closure, fluoroquinolone topical medication was applied to the neck and abdominal incision sites, which were then covered with gauze dressings to prevent contamination. Postoperatively, oral levofloxacin (75 mg/day) was administered for 7 days for infection control.
2.4. Follow-Up Angiography
Two weeks after the treatment, follow-up angiography was performed under general anesthesia with isoflurane inhalation. First, a 5-Fr sheath was inserted through the femoral artery. After confirming the absence of an endoleak for the aneurysm and the absence of complications, such as thrombosis or embolism in any organs, the animal was euthanized via intravenous administration of 20 mg potassium chloride according to the animal experiment protocol. The thoracic aorta was harvested, preserved in 4% paraformaldehyde, and evaluated by histological analysis.
2.5. Histological Analysis
HMSC fibers prepared at defined cell numbers per unit length were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 4- to 5-μm thickness. Consecutive sections were stained routinely with hematoxylin–eosin (HE) and Masson’s trichrome stain. For immunofluorescence analysis, sections were deparaffinized and rehydrated following standard procedures. Antigen retrieval was performed by immersing the sections in an antigen activator immunosaver (Nisshin-EM, Tokyo, Japan) heated to 90 °C for 40 min. Blocking was performed using 1% bovine serum albumin in phosphate-buffered saline at room temperature for 1 h. The sections were incubated with primary antibodies of anti-fibronectin mouse monoclonal (1:100; ab6328; Abcam, Cambridge, UK), anti-CD44 mouse monoclonal (1:100; ab6124; Abcam), and anti-CD105 rabbit polyclonal (1:100; ab107595; Abcam) at 4 °C for 15 h, followed by secondary antibodies of rabbit anti-mouse IgG H&L (Alexa Fluor® 594; 1:1000; ab150128; Abcam) for fibronectin and CD44 and goat anti-rabbit IgG H&L (Alexa Fluor® 594; 1:1000; ab150080; Abcam) for CD105 at room temperature for 2 h. The sections were mounted on the ProLong Diamond Antifade Mountant with 4′,6-diamidino-2-phenylindole (P36971; Thermo Fisher Scientific) for nuclear staining. The sections were observed under an inverted fluorescence microscope (Eclipse Ti2; Nikon Corporation, Tokyo Japan) equipped with a camera (DS-Fi3; Nikon corporation) and proprietary imaging software (NIS-Elements; Nikon corporation). The fluorescence colors shown in the images were assigned as pseudocolors using imaging software. Aneurysm specimens harvested 2 weeks after HMSC fiber transplantation were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 4- to 5-μm thickness. Serial sections were stained with Masson’s trichrome stain. Immunofluorescence staining was performed by incubating the sections with antibodies of anti-α-smooth muscle actin (α-SMA) mouse monoclonal (1:100; 904601; BioLegend, CA, USA) and anti-CD31 rabbit polyclonal (1:50; ab28364; Abcam). Subsequently, the sections were incubated with secondary antibodies of rabbit anti-mouse IgG H&L (Alexa Fluor® 594; 1:1000; ab150128; Abcam) for α-SMA and goat anti-rabbit IgG H&L (Alexa Fluor® 488; 1:1000; ab150077; Abcam) for CD31 at room temperature for 2 h. Fluorescence images were obtained using the same aforementioned microscope system.
3. Results
3.1. Preparation and Characteristics of the HMSC Fibers
Within approximately 1 h of culture, the HMSCs seeded into the ring-shaped culture groove for HMSCs fiber preparation formed a confluent cell monolayer sheet without intercellular gaps, a result consistent with our previous report [
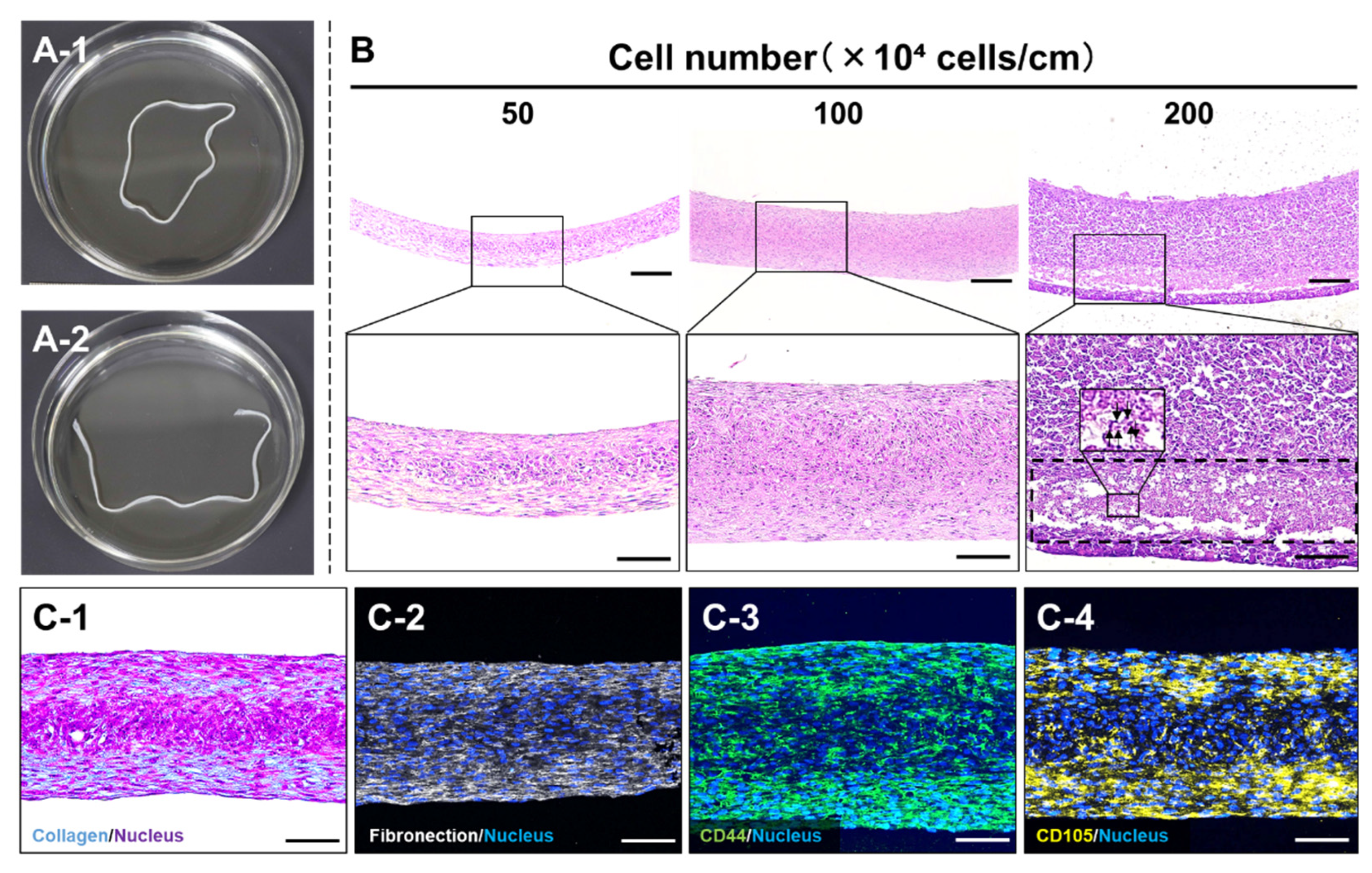
18]. Subsequently, the cell sheet detached from the culture surface from the outer edge toward the inner region and underwent spontaneous aggregation, forming a ring-shaped HMSC aggregate around the silicone disk at 1 day after seeding. When the silicone disk was detached from the culture surface, the ring-shaped HMSC aggregate floated freely in the culture medium (
Figure 4A-1). By cutting the aggregate at a single point, a fiber-shaped HMSC aggregate with a length of approximately 18 cm, demarcated by the circumference of the silicone disk, was obtained as the HMSC fibers (
Figure 4A-2). The resulting HMSC fibers could be lifted and handled with forceps without rupture (Supplementary Movie S1). When the number of seeded cells was adjusted to achieve cell numbers per unit length ranging from 50 × 10
4 to 200 × 10
4 cells/cm, HMSC fibers were successfully prepared irrespective of the cell number per unit length (
Figure 4B). Histological analysis of longitudinal sections revealed that the thickness of HMSC fibers increased with cell number per unit length, reaching a length of approximately 100–500 μm across this range (
Figure 4B). Notably, HMSC fibers prepared at 200 × 10
4 cells/cm contained internal regions with indistinct and fragmented nuclei (dashed boxes and arrows), indicating the occurrence of cell necrosis likely caused by insufficient oxygen and nutrient supply. For biological characterization, HMSC fibers prepared at a cell number per unit length of 100 × 10
4 cells/cm were subjected to histological and immunohistochemical analyses. Masson’s trichrome and immunofluorescence staining for ECM components (collagen and fibronectin;
Figure 4C-1, 4C-2) and mesenchymal cell markers (CD44 and CD105;
Figure 4C-3, 4C-4) revealed the widespread distribution of these markers throughout the HMSC fibers. These results indicate that most HMSCs constituting the fiber structure retained MSC characteristics while being surrounded by ECM.
3.2. HMSC Fiber Manipulation
When negative pressure was applied to the catheter, the HMSC fiber was smoothly aspirated from the catheter tip without structural collapse and was completely accommodated within the catheter lumen (
Figure 5A and Supplementary Movie S2). The HMSC fiber remained intact during aspiration and was stored completely inside the catheter along its entire length (
Figure 5B–5D).
Sequential images showing the process of loading an HMSC fiber into a catheter lumen. The HMSC fiber was aligned at the catheter tip (A), subsequently accommodated within the catheter lumen (B, C), and fully contained inside the catheter prior to delivery (D).
3.3. Transplantation
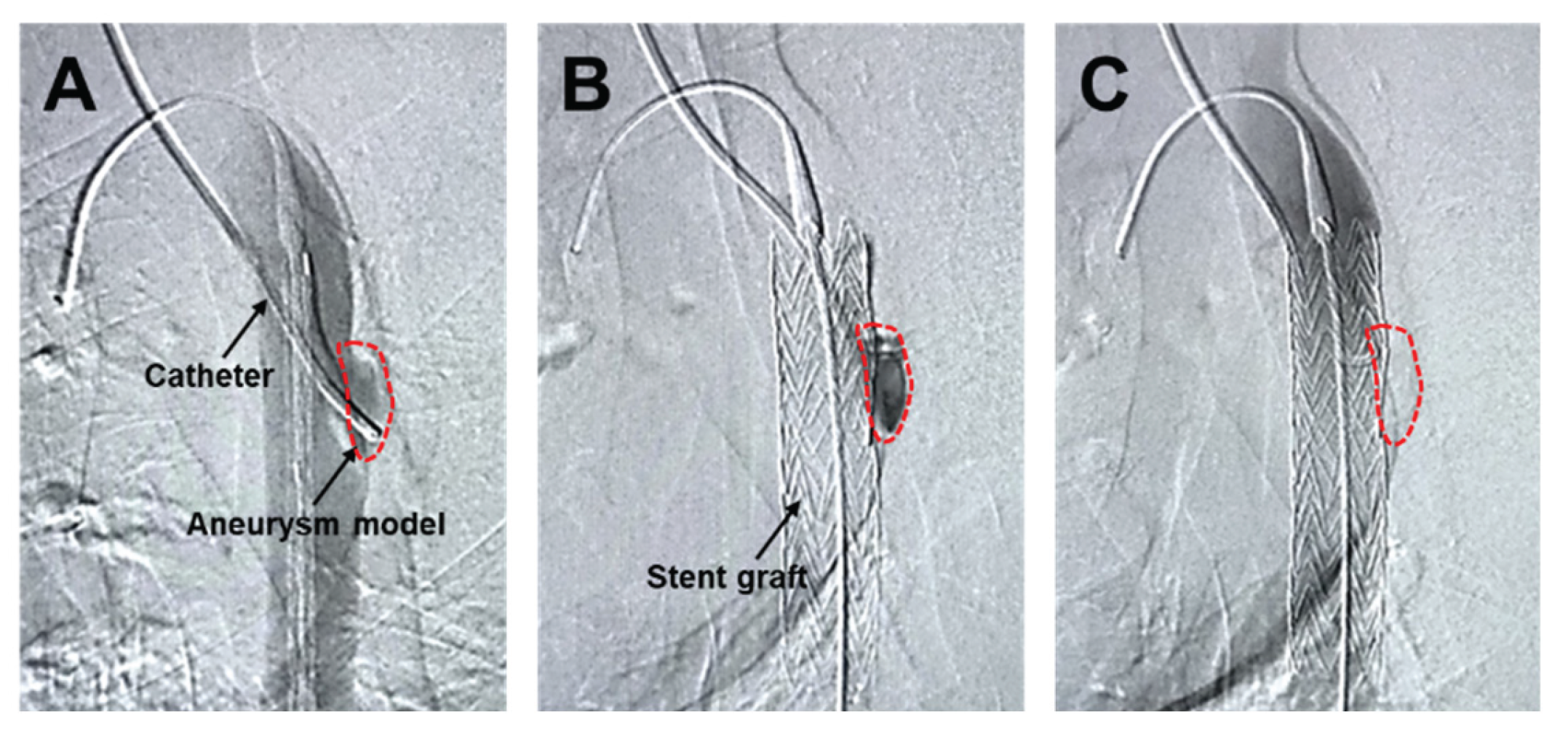
The angiographic catheter prepositioned within the aneurysm model (
Figure 6A) was maintained in situ during and after SG deployment (
Figure 6B), creating a condition that minimized intraluminal blood washout within the aneurysm sac. Under these conditions, the HMSC fiber was transplanted through the catheter and into the aneurysm sac. Although the HMSC fiber itself was not directly visualized by angiography, no residual fibers were observed within the catheter lumen, and the catheter was removed smoothly without resistance. Furthermore, follow-up angiography performed using a pigtail catheter demonstrated sustained aneurysm sealing after the procedure (
Figure 6C). Collectively, these observations are consistent with the successful intraluminal delivery of the HMSC fiber into the aneurysm sac.
Angiographic imaging of the aneurysm model with a catheter prepositioned within the aneurysm sac (demarcated by the dashed line) (A). SG deployment covering the aneurysm while maintaining the catheter position within the aneurysm sac, enabling the administration of the HMSC fibers under reduced blood washout conditions (B). Follow-up angiography using a pigtail catheter after the procedure, confirming sustained aneurysm sealing (C).
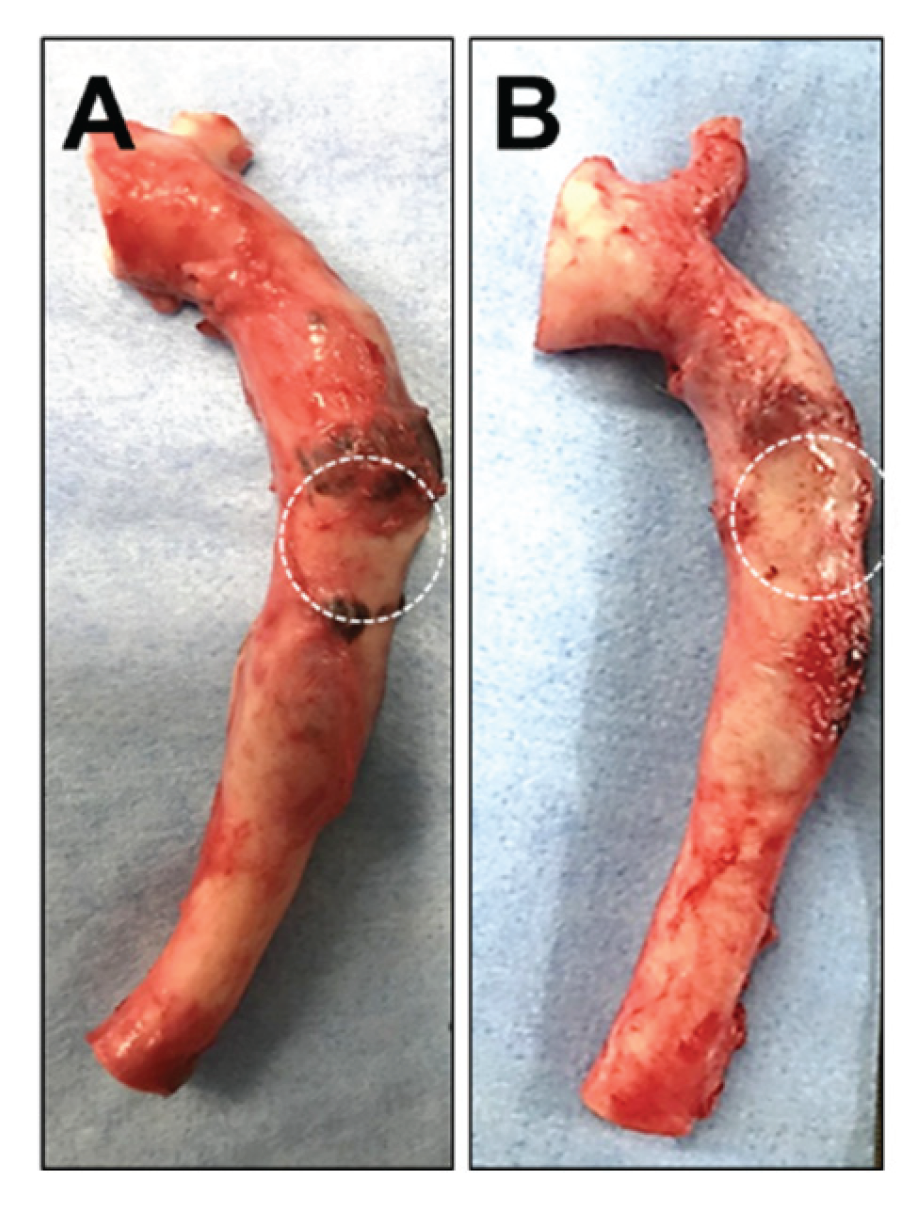
Two weeks after the administration of either saline or the HMSC fiber, the thoracic aorta specimens were harvested and grossly examined. No significant macroscopic differences were observed in the aneurysm region between the two intervention groups (
Figure 7A, 7B), indicating that any potential effects of the interventions were not readily detectable by gross inspection at this time point.
Representative gross images of thoracic aorta specimens transplanted into the aneurysm model without (A) or with (B) HMSC fiber administration. The dashed circles indicate the aneurysm region.
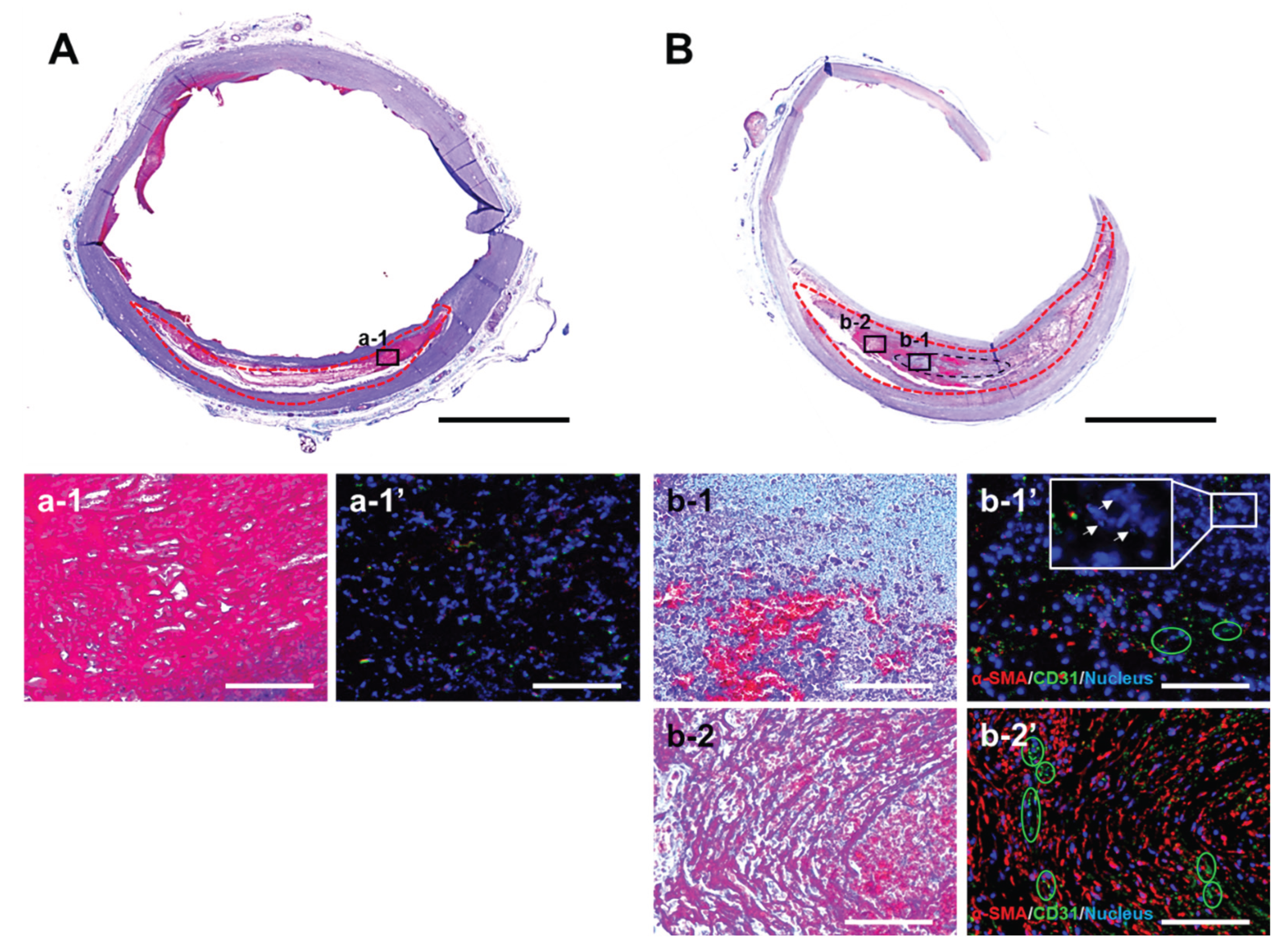
Masson’s trichrome staining of cross-sectional slices of the aneurysm model revealed the formation of a reddish-purple thrombus within the aneurysmal lumen (red dashed outline), regardless of whether HMSC fibers were implanted (
Figure 8A, 8B). At higher magnification, aneurysms without HMSC fiber implantation contained only a thrombus and exhibited minimal additional tissue formation (Figure 8a-1). In contrast, in aneurysms implanted with HMSC fibers, a thin, light-blue region with high cellular density was observed within the thrombus (Figure 8b-1), indicating tissue derived from the implanted HMSC fiber (black dashed outline). Furthermore, a region located several millimeters away from the HMSC fiber contained areas within the thrombus showing very faint, minimal collagen deposition (Figure 8b-2). Immunofluorescence analysis revealed abundant α-SMA–positive fibroblasts and CD31-positive endothelial cells forming capillary structures (green circles) within and near the HMSC fiber-derived tissue (Figure 8b-1,’ b-2’). In contrast, fibroconnective tissue formation was scarce in aneurysms without HMSC fiber implantation (Figure 8a-1’). α-SMA–positive cells were present within the HMSC fiber-derived tissue; however, most cells were α-SMA–negative and contained small, fragmented nuclei (white arrows), indicating cell death (Figure 8b-1’).
4. Discussion
The fundamental concept of EVAR is to prevent aneurysm rupture by stimulating thrombus formation within the aneurysm sac. However, thrombus formation alone does not facilitate the biological repair of the degenerated aneurysm wall. Previous cell therapy studies of aortic aneurysms have mostly been limited to small-animal models, in which the administration of MSCs via intravenous delivery or direct seeding has elicited aneurysm enlargement attenuation through humoral and anti-inflammatory mechanisms [
21,
22,
23]. While these findings support the therapeutic potential of MSCs, these studies evaluated cell therapy alone, overlooking the mechanisms of how MSC-based therapy might be functionally integrated via a type of endovascular treatment, which currently represents the clinical standard.
This study addressed translational gap arising from the absence of a catheter-deliverable cellular construct that can be stably retained within the aneurysm sac. To this end, we designed and engineered HMSC fiber as a scaffold-free cellular biomaterial that can retain endogenous ECM and enable precise geometric control. Using our CAT-based tissue fabrication technology [
18], HMSC fibers were prepared to satisfy biological requirements related to cell viability, as well as procedural requirements associated with intravascular manipulation. CAT enables the spontaneous delamination and self-aggregation of cell monolayers without the use of exogenous scaffold materials, thereby facilitating the formation of three-dimensional constructs while preserving endogenous ECM. Consistent with this principle, HMSC fibers prepared in the present study retained ECM-rich, three-dimensional architectures, as shown in
Figure 4C-1 and C-2. Preservation of endogenous ECM represents a critical feature for regenerative applications, as it contributes to cell–cell adhesion, structural cohesion, and maintenance of the native cellular microenvironment. In cell sheet engineering, temperature-responsive culture surfaces allow the recovery of cell sheets without enzymatic treatment [
24] while preserving endogenous ECM. Furthermore, immunohistochemical analyses have confirmed the retention of ECM components in the harvested sheets [
25]. Such ECM-retaining cell sheets can improve post-transplant cell survival and tissue engraftment [
26] and have already been translated into clinical applications targeting tissues such as the cornea and myocardium [
27,
28].
From a procedural perspective, HMSC fibers maintained their morphology during forceps handling, as well as during aspiration into a catheter, without fragmentation or structural collapse (
Figure 5, Supplementary Movies S1 and S2). This structural stability is attributable to strong cell–cell adhesion mediated by retained endogenous ECM, clearly distinguishing HMSC fibers from conventional cell suspension–based delivery approaches, which must be prone to dispersion and washout under arterial blood flow. Delivery of HMSC fiber as a single, continuous, shape-retaining construct enables localized and controllable cell placement within the aneurysm sac, thereby overcoming a major limitation of existing intravascular cell delivery strategies. Within the context of endovascular delivery, such scaffold-free and ECM-retaining architecture provides a structural basis for ensuring mechanical stability during catheter manipulation and for achieving high early-stage engraftment without introducing foreign biomaterials. Furthermore, by precisely controlling the number of cells per unit fiber length, we systematically investigated the relationship between fiber diameter and intra-fiber cell viability and identified a structural range that maintained sufficient cellular density while avoiding central necrosis (
Figure 4B). These findings indicate that HMSC fiber possess a defined design window in which biological constraints related to oxygen and nutrient diffusion and mechanical requirements for intravascular handling can be simultaneously satisfied. From a biomaterials perspective, this result highlights the importance of treating geometric structure itself as a key design parameter rather than merely considering cellular aggregates as size-variable assemblies.
The histological findings in the present study indicate an early regenerative response that could be mediated, at least in part, by the paracrine activity of the transplanted HMSCs (Figures 8b-1‘, b-2‘), consistent with previous reports demonstrating that HMSCs promote angiogenesis via vascular endothelial growth factor and platelet-derived growth factor and contribute to tissue formation and ECM deposition through factors such as fibroblast growth factor and transforming growth factor [
7,
29,
30]. This interpretation is further supported by previous studies showing that MSCs can modulate inflammatory pathways associated with aneurysm progression, including attenuation of IL-17–related immune signaling and reduction of inflammatory mediators such as MMP-2, MMP-9, and TNF-α [
3,
31,
32]. A key limitation of this study is that transplantation was performed under a xenogeneic condition, where immune-mediated elimination of transplanted cells is unavoidable. Accordingly, sustained structural integration and long-term contribution to aneurysm wall reconstruction could not be fully evaluated. Future studies using allogeneic and longer-term models will be essential to clarify the relative contributions of paracrine signaling and direct tissue construction and to determine whether HMSC fiber delivery can contribute to durable aneurysm stabilization.
5. Conclusions
In this study, we synthesized fiber-shaped HMSC aggregates that formed HMSC fibers optimized for an endovascular procedure by controlling the cell number per unit length. The resulting HMSC fibers retained MSC characteristics and endogenous ECM, facilitating stable handling and catheter-based implantation. Following transplantation into a swine aortic aneurysm model, HMSC fibers induced fibroconnective tissue formation accompanied by neovascularization within the aneurysm sac. The results of this study demonstrate that HMSC fiber provide a controllable and stable platform for localized endovascular cell delivery, extending the concept of cell therapy beyond simple cell suspension injection. Further validation is required regarding the safety and efficacy of cell therapy for aortic diseases.
Author Contributions
Conceptualization, S.F. and I.R.; methodology, S.F. and I.R.; validation, S.F., M.K., I.R., and L.T.; formal analysis, S.F. and L.T.; writing—original draft preparation, S.F., I.R., and L.T.; writing—review and editing, T.O., H.O., M.H., and H.O. Supervision, R.I., T.O., and H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI (Grant Number 24K11753) and Yoshimi Memorial T.M.P. Grant 2021.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board of Animal experiment (IRB number: 2019-020).
Conflicts of Interest
The authors declare no conflicts of interest in this study.
List of Abbreviations
| α-SMA |
Alfa-smooth muscle actin |
| CAT |
Cell self-aggregation technique |
| CCA |
Common carotid artery |
| ECM |
Extracellular matrix |
| EVAR |
Endovascular aortic aneurysm repair |
| HMSC |
Human mesenchymal stromal cell |
| SG |
Stent graft |
| TAA |
Thoracic aortic aneurysm |
References
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M.; EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016, 388(10058), 2366–2374. [Google Scholar] [CrossRef]
- Tanaka, H; Unno, N; Suzuki, Y; Sano, H; Yata, T; Urano, T. Hypoperfusion of the Aortic Wall Secondary to Degeneration of Adventitial Vasa Vasorum Causes Abdominal Aortic Aneurysms. Curr Drug Targets 2018, 19(11), 1327–1332. [Google Scholar] [CrossRef]
- Yamawaki-Ogata, A.; Mutsuga, M.; Narita, Y. A review of current status of cell-based therapies for aortic aneurysms. Inflamm. Regen. 2023, 43, 40. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liao, R.; Li, X.; Zhang, C.; Huo, S.; Qin, L.; Xiong, Y.; He, T.; Xiao, G.; Zhang, T. Mesenchymal stem cells in treating human diseases: Molecular mechanisms and clinical studies. Signal Transduct. Target. Ther. 2025, 10, 262. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bendeck, M.P.; Simmons, C.A.; Santerre, J.P. Deriving vascular smooth muscle cells from mesenchymal stromal cells: Evolving differentiation strategies and current understanding of their mechanisms. Biomaterials 2017, 145, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Yen, B.L.; Hsieh, C.-C.; Hsu, P.-J.; Chang, C.-C.; Wang, L.-T.; Yen, M.-L. Three-dimensional spheroid culture of human mesenchymal stem cells: Offering therapeutic advantages and in vitro glimpses of in vivo state. Stem Cells Transl. Med. 2023, 12, 235–244. [Google Scholar] [CrossRef]
- Murphy, K.C.; Hung, B.P.; Browne-Bourne, S.; Zhou, D.; Yeung, J.; Genetos, D.C.; Leach, J.K. Measurement of oxygen tension within mesenchymal stem cell spheroids. J. R. Soc. Interface 2017, 14, 20160851. [Google Scholar] [CrossRef]
- Murphy, K.C.; Fang, S.Y.; Leach, J.K. Hypoxia and necrosis in MSC spheroid cores are associated with spheroid size and diffusive limitations. Stem Cells Transl. Med. 2016, 5, 773–779. [Google Scholar]
- Iwai, R.; Nemoto, Y.; Nakayama, Y. The effect of electrically charged polyion complex nanoparticle-coated surfaces on adipose-derived stromal progenitor cell behaviour. Biomaterials 2013, 34, 9096–9102. [Google Scholar] [CrossRef]
- Iwai, R.; Haruki, R.; Nemoto, Y.; Nakayama, Y. Induction of cell self-organization on weakly positively charged surfaces prepared by the deposition of polyion complex nanoparticles of thermoresponsive, zwitterionic copolymers. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Iwai, R.; Nemoto, Y.; Nakayama, Y. Preparation and characterization of directed, one-day self-assembled millimeter-size spheroids of adipose-derived mesenchymal stem cells. J. Biomed. Mater. Res. A 2016, 104, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Nakamura, M.; Takao, T.; Takihira, S.; Yoshida, A.; Miura, A.; Ming, L.; Yoshitomi, H.; Gozu, M.; Okamoto, K.; Hojo, H.; Kusaka, N.; Iwai, R.; Nakata, E.; Ozaki, T.; Toguchida, J.; Takarada, T. Induction and expansion of human PRRX1+ limb-bud-like mesenchymal cells from pluripotent stem cells. Nat. Biomed. Eng. 2021, 5, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, S.; Yamada, D.; Takao, T.; Iwai, R.; Takarada, T. Efficient production of chondrocyte particles from human iPSC-derived chondroprogenitors using a plate-based cell self-aggregation technique. Int. J. Mol. Sci. 2024, 25, 22. [Google Scholar] [CrossRef]
- Hiwatashi, S.; Iwai, R.; Nakayama, Y.; Moriwaki, T.; Okuyama, H. Successful tracheal regeneration using biofabricated autologous analogues without artificial supports. Sci. Rep. 2022, 12, 20279. [Google Scholar] [CrossRef]
- Ota, T.; Takao, T.; Iwai, R.; Moriwaki, T.; Kitaguchi, Y.; Fujisawa, Y.; Yamada, D.; Kimata, Y.; Takarada, T. Fabrication of shape-designable cartilage from human induced pluripotent stem cell-derived chondroprogenitors using a cell self-aggregation technique. Biomed. Mater. 2023, 18, 055012. [Google Scholar] [CrossRef]
- Hashimoto, S.; Sugiyama, A.; Ota, T.; Matsumoto, H.; Kimata, Y.; Iwai, R. Development of a unique tissue-engineered in vitro vascular model with endothelial layer-inverted vascular tissue structure using a cell self-aggregation technique. J. Biosci. Bioeng. 2024, 138, 235–243. [Google Scholar] [CrossRef]
- Teng, L.; Fukushima, S.; Koizumi, M.; Okano, H.J.; Ohki, T.; Matsuura, K.; Iwai, R. Successful preparation of contrast particle-loaded human mesenchymal stem cell aggregates using adherent cell self-aggregation technique. J. Biomed. Mater. Res. A 2025, 113, e37964. [Google Scholar] [CrossRef]
- Ota, T.; Iwai, R.; Kitaguchi, Y.; Moriwaki, T.; Takarada, T.; Kimata, Y. Fabrication of scaffold-free mesenchyme tissue bands by cell self-aggregation technique for potential use in tissue regeneration. Biomed. Mater. 2022, 17, 065003. [Google Scholar] [CrossRef]
- Fukushima, S; Ohki, T; Koizumi, M; Ohta, H; Takahashi, T; Okano, H.J. A reproducible swine model of a surgically created saccular thoracic aortic aneurysm. Exp Anim. 2021, 70(2), 257–263. [Google Scholar] [CrossRef]
- Sharma, A.K.; Lu, G.; Jester, A.; Johnston, W.F.; Zhao, Y.; Hajzus, V.A.; Saadatzadeh, M.R.; Su, G.; Bhamidipati, C.M.; Mehta, G.S.; Kron, I.L.; Laubach, V.E.; Murphy, M.P.; Ailawadi, G.; Upchurch, G.R., Jr. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation 2012, 126, S38–45. [Google Scholar] [CrossRef]
- Hashizume, R; Yamawaki-Ogata, A; Ueda, Y; Wagner, W.R.; Narita, Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J Vasc Surg. 2011, 54, 1743–1752. [Google Scholar] [CrossRef]
- Hosoyama, K; Wakao, S; Kushida, Y; Ogura, F; Maeda, K; Adachi, O.; Kawamoto, S.; Dezawa, M.; Saiki, Y. Intravenously injected human multilineage-differentiating stress-enduring cells selectively engraft into mouse aortic aneurysms and attenuate dilatation by differentiating into multiple cell types. J Thorac Cardiovasc Surg. 2018, 155(6), 2301–2313. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef]
- Ide, T.; Sakamoto, K.; Hamuro, Y.; Sugiyama, Y.; Tsuchiya, K.; Tano, Y.; Matsuki, M.; Ito, M.; Akimoto, R. Structural characterization of bioengineered human corneal endothelial cell sheets. Biomaterials 2006, 27, 607–614. [Google Scholar] [CrossRef]
- Hamdi, H.; Lu, Z.; Su, H.; Chen, J.; Miyagi, Y.; Ishikawa, K.; Caiazzo, M.; Abdel-Latif, A.; Ashraf, M. Epicardial adipose stem cell sheets result in greater post-infarction survival. Cardiovasc. Res. 2011, 91, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Sawa, Y.; Yoshikawa, Y.; Toda, K.; Fukushima, S.; Yamazaki, K.; Ono, M.; Sakata, Y.; Hagiwara, N.; Kinugawa, K.; Miyagawa, S. Safety and Efficacy of Autologous Skeletal Myoblast Sheets (TCD-51073) for the Treatment of Severe Chronic Heart Failure Due to Ischemic Heart Disease. Circ. J. 2015, 79, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashi, R.; Watanabe, K.; Yamamoto, K.; Adachi, W.; Kikuchi, A.; Okano, T. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004, 94, 678–685. [Google Scholar] [CrossRef]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef]
- Wen, H.; Wang, M.; Gong, S.; Li, X.; Meng, J.; Wen, J.; Wang, Y.; Zhang, S.; Xin, S. Human umbilical cord mesenchymal stem cells attenuate abdominal aortic aneurysm progression in Sprague–Dawley rats: Implication of vascular smooth muscle cell phenotypic modulation. Stem Cells Dev. 2020, 29, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Ashida, S.; Yamawaki-Ogata, A.; Tokoro, M.; Mutsuga, M.; Usui, A.; Narita, Y. Administration of anti-inflammatory M2 macrophages suppresses progression of angiotensin II-induced aortic aneurysm in mice. Sci Rep. 2023, 13(1), 1380. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).