Submitted:
15 September 2025
Posted:
16 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Preparation of Collagen-Coated Surfaces
2.3. Secretome Treatment
2.4. Cell Proliferation Assay
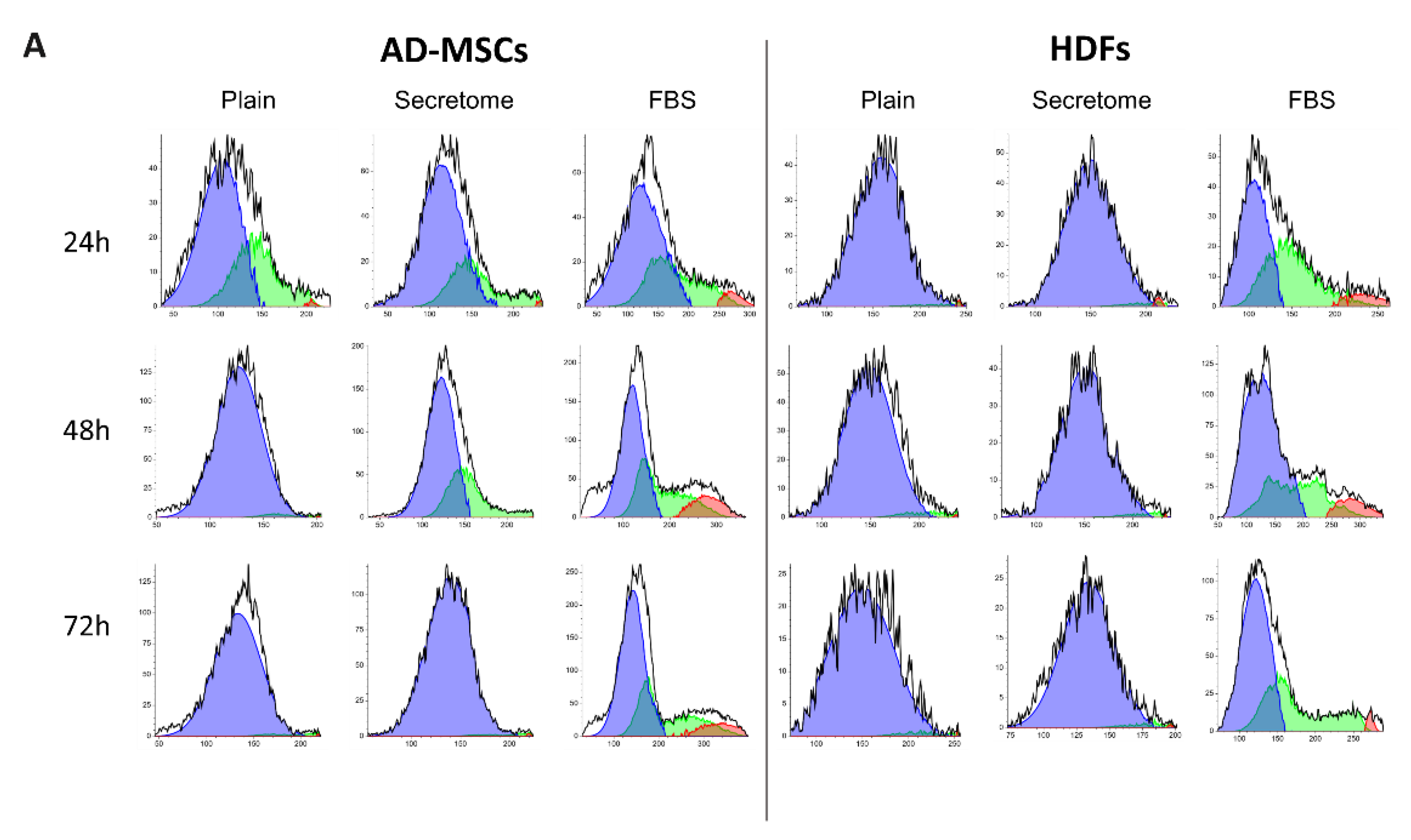
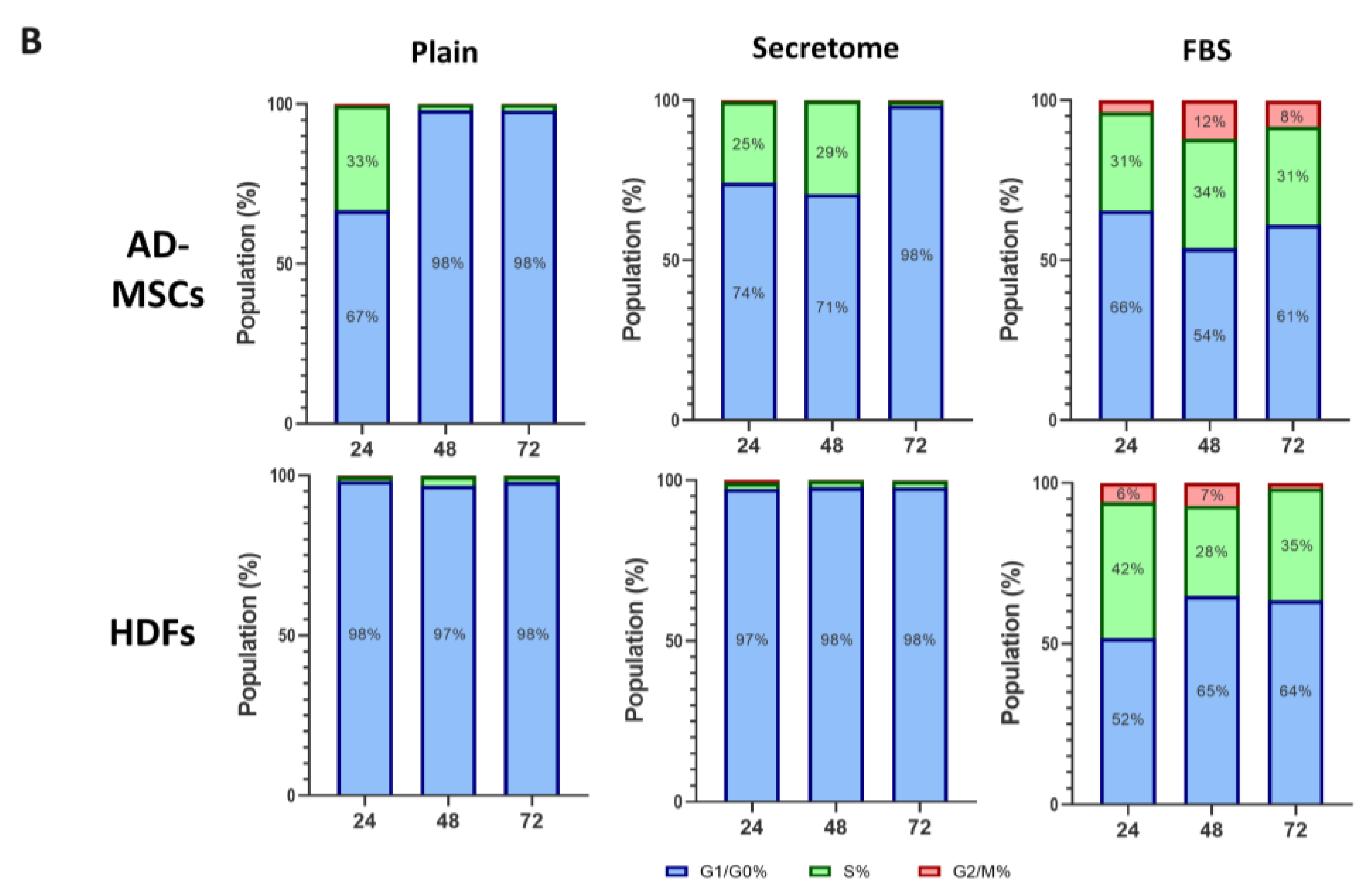
2.5. Cell Cycle Analysis
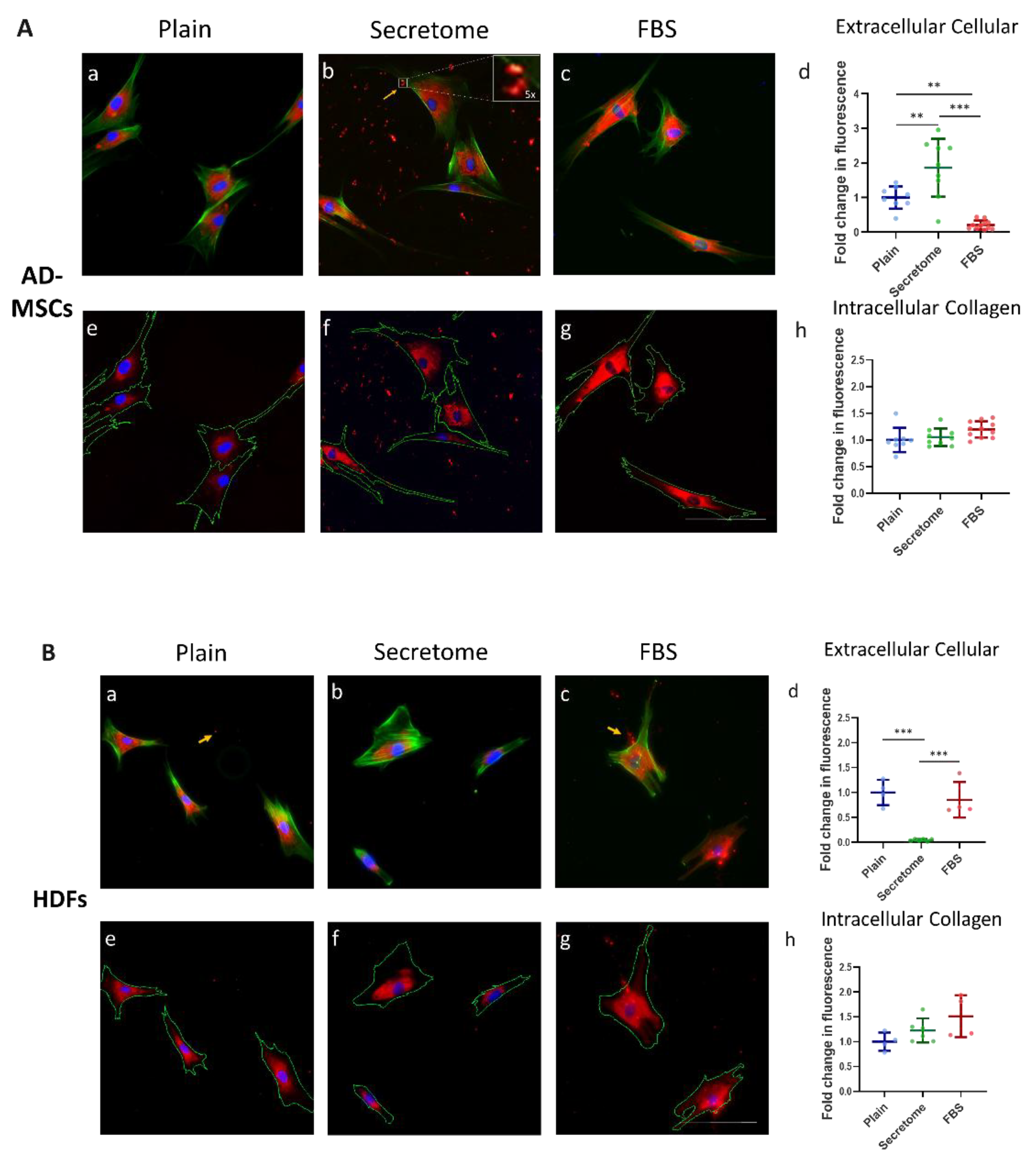
2.6. Morphological Analysis and Endogenous Collagen Production
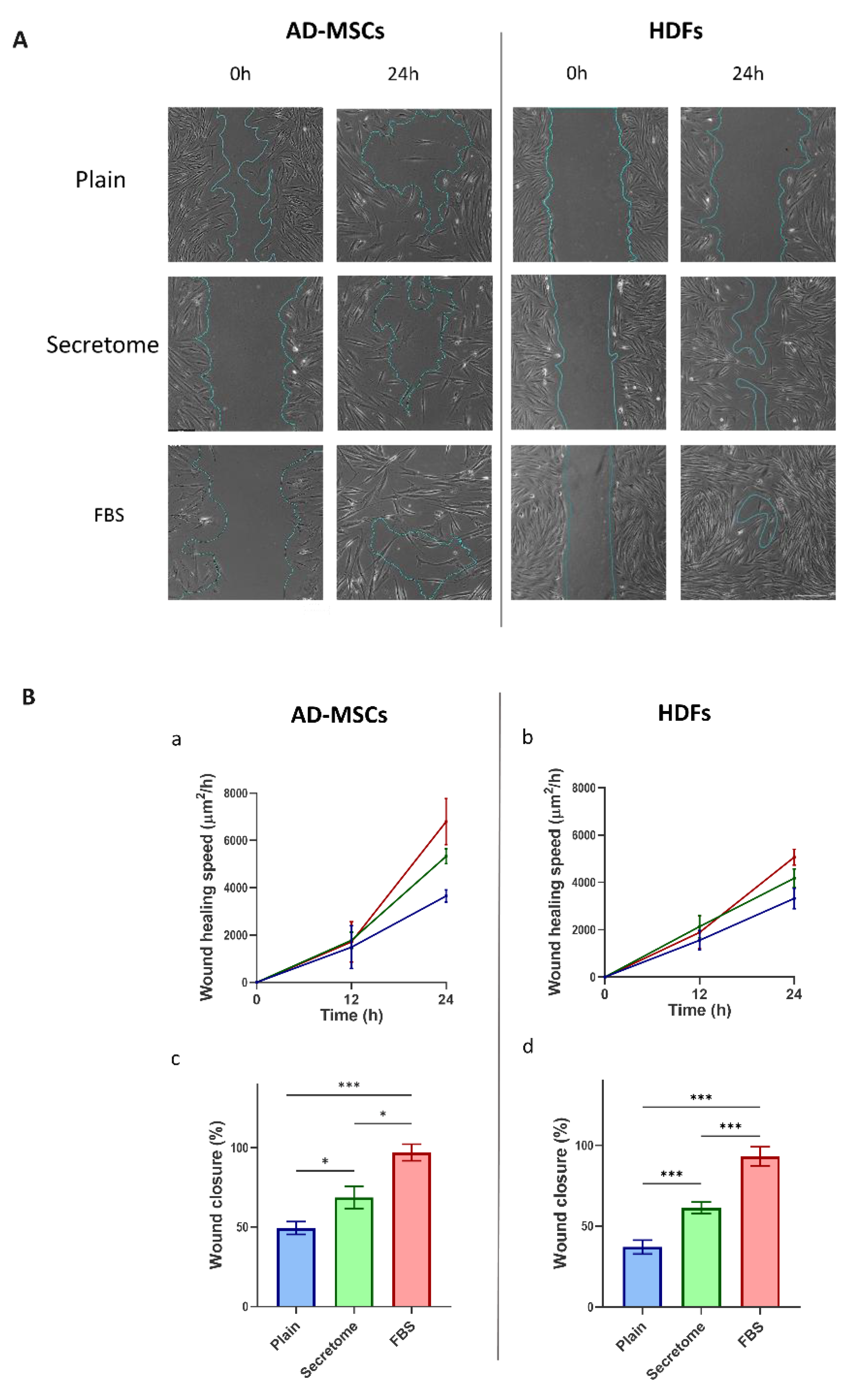
2.7. Artificial Wound Healing (Scratch) Assay
2.8. Statistical Analysis
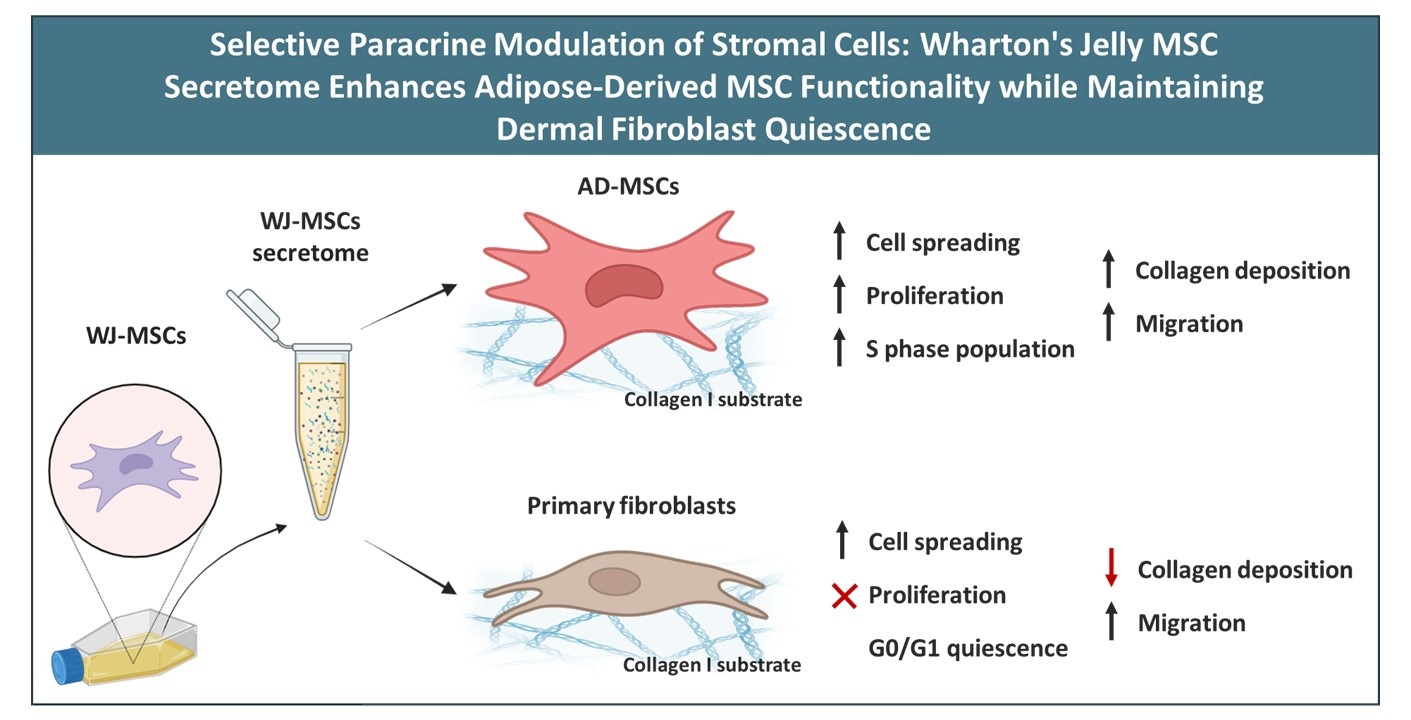
3. Results
3.1. Effect of the WJ-MSCs’ Secretome on the Morphology of AD-MSCs and Fibroblasts
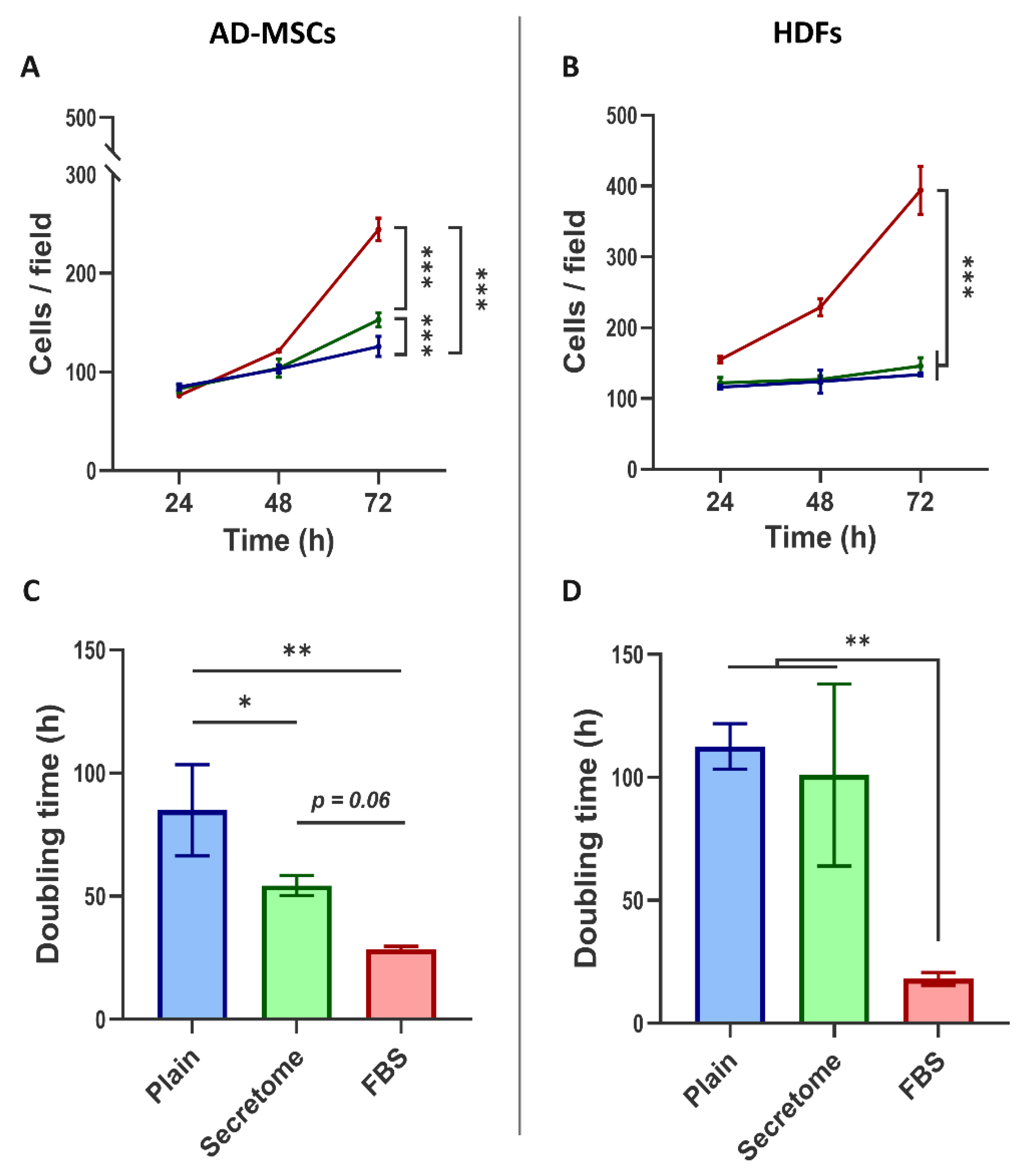
3.2. WJ-MSC Secretome Supports the Growth of MSCs, but not of Fibroblasts
3.3. Impact of Paracrine Signaling from WJ-MSCs on Cell Cycle Progression in Stem Cells and Fibroblasts
3.4. Paracrine Signaling from WJ-MSCs Modulates Extracellular Collagen Deposition
3.5. WJ-MSC Secretome Enhances Migration of both Stem Cells and Fibroblasts
4. Discussion
Insights into MSC–Fibroblast Interactions
Substrate Effects on Newly Secreted Collagen Organization
Dose Considerations for Clinical Translation
Therapeutic Implications of Combinatory MSC Strategies
Study Limitations
- In vitro constraints: Our experiments were conducted under controlled in vitro conditions, which do not fully replicate the complexity of in vivo tissue environments. Factors such as immune cell interactions, vascularization, mechanical stress, and systemic feedback loops are absent but play critical roles in regenerative outcomes. In vivo validation using relevant animal models is essential to assess therapeutic efficacy, biodistribution, and long-term safety.
- Donor variability: The composition and potency of MSC secretomes are influenced by donor-specific factors including age, sex, metabolic status, and tissue origin. Proteomic profiling has revealed significant inter-donor variability in the abundance of key regenerative proteins such as VEGF, BDNF, and PDGF-AA [52,53]. This underscores the need for standardized donor selection and quality control protocols in secretome production.
- Time-limited assessment: Our analysis focused on cellular responses within a 72-hour window, capturing early proliferative, migratory, and ECM remodeling events. However, MSC secretome effects may evolve over longer durations, influencing differentiation, immunomodulation, and tissue integration. Time-course studies extending beyond 72 hours are needed to assess sustained activation, potential senescence, or feedback inhibition mechanisms.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WJ-MSCs | Wharton’s Jelly-Derived Mesenchymal Stem Cells |
| AD-MSCs | Adipose-Derived Mesenchymal Stem Cells |
| HDFs | Human Dermal Fibroblasts |
| RTC | Rat Tail Collagen |
| FBS | Fetal Bovine Serum |
| MSCs | Mesenchymal Stem Cells |
| ECM | Extracellular Matrix |
| PBS | Phosphate Buffered Saline |
| CSA | Cell Spreading Area |
| AR | Cell Aspect Ratio |
| FMT | Fibroblast-to-Myofibroblast Transition |
References
- Maldonado, V.V.; et al. Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy. J Biol Eng 2023, 17, 44. [Google Scholar] [CrossRef]
- Pînzariu, A.C.; et al. The therapeutic use and potential of MSCs: advances in regenerative medicine. Int J Mol Sci 2025, 26, 3084. [Google Scholar] [CrossRef]
- Vizoso, F.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Volarevic, V. The cross-talk between mesenchymal stem cells and immune cells in tissue repair and regeneration. Int J Mol Sci 2021, 22, 2472. [Google Scholar] [CrossRef]
- Sajjad, U.; et al. Exploring Mesenchymal Stem Cells Homing Mechanisms and Improvement Strategies. Stem Cells Transl Med 2024, 13, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Drobiova, H.; Sindhu, S.; Ahmad, R.; Haddad, D.; Al-Mulla, F.; Al Madhoun, A. Wharton’s jelly mesenchymal stem cells: a concise review of their secretome and prospective clinical applications. Front Cell Dev Biol 2023, 11. [Google Scholar] [CrossRef]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and Optimizing their therapeutic potential for clinical applications. Int J Mol Sci 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- Mendiratta, M.; et al. Concurrent hypoxia and apoptosis imparts immune programming potential in mesenchymal stem cells: Lesson from acute graft-versus-host-disease model. Stem Cell Research and Therapy 2024, 15. [Google Scholar] [CrossRef]
- Shivakumar, S.B.; et al. DMSO- and Serum-Free Cryopreservation of Wharton’s Jelly Tissue Isolated From Human Umbilical Cord. J Cell Biochem 2016, 2397–2412. [Google Scholar] [CrossRef]
- Wang, J.; Li, R. Effects, methods and limits of the cryopreservation on mesenchymal stem cells; BioMed Central Ltd., 2024. [Google Scholar] [CrossRef]
- Fong, C.Y.; Subramanian, A.; Biswas, A.; Bongso, A. Freezing of fresh Wharton’s Jelly from human umbilical cords yields high post-thaw mesenchymal stem cell numbers for cell-based therapies. J Cell Biochem 2016, 117, 815–827. [Google Scholar] [CrossRef]
- Ghasemi, M.; Roshandel, E.; Mohammadian, M.; Farhadihosseinabadi, B.; Akbarzadehlaleh, P.; Shamsasenjan, K. Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: overview of clinical trials; BioMed Central Ltd., 2023. [Google Scholar] [CrossRef]
- Prado-Yupanqui, J.W.; et al. The Hidden Power of the Secretome: Therapeutic Potential on Wound Healing and Cell-Free Regenerative Medicine—A Systematic Review. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Trigo, C.M.; Rodrigues, J.S.; Camões, S.P.; Solá, S.; Miranda, J.P. Mesenchymal stem cell secretome for regenerative medicine: Where do we stand? J Adv Res 2025, 70, 103–124. [Google Scholar] [CrossRef]
- Cappe, B.; Vandenabeele, P.; Riquet, F.B. A guide to the expanding field of extracellular vesicles and their release in regulated cell death programs; John Wiley and Sons Inc., 01 May 2024. [Google Scholar] [CrossRef]
- Shin, S.; et al. Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and wharton’s jelly. Int J Mol Sci 2021, 22, 1–17. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, Y. Fibroblast activation and heterogeneity in fibrotic disease. Nat Rev Nephrol 2025. [Google Scholar] [CrossRef]
- Xu, R.; et al. Mesenchymal stem cells reversibly de-differentiate myofibroblasts to fibroblast-like cells by inhibiting the TGF-β-SMAD2/3 pathway. Molecular Medicine 2023, 29. [Google Scholar] [CrossRef]
- Dyachkova, U.; Vigovskiy, M.; Basalova, N.; Efimenko, A.; Grigorieva, O. M2-macrophage-induced chronic inflammation promotes reversible mesenchymal stromal cell senescence and reduces their anti-fibrotic properties. Int J Mol Sci 2023, 24, 17089. [Google Scholar] [CrossRef]
- Desai, V.D.; Hsia, H.C.; Schwarzbauer, J.E. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Konala, V.B.R.; Bhonde, R.; Pal, R. Secretome studies of mesenchymal stromal cells (MSCs) isolated from three tissue sources reveal subtle differences in potency. In Vitro Cell Dev Biol Anim 2020, 56, 689–700. [Google Scholar] [CrossRef]
- Akhir, H.M.; Teoh, P.L. Collagen type I promotes osteogenic differentiation of amniotic membrane-derived mesenchymal stromal cells in basal and induction media. Biosci Rep 2020, 40. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Best, S.M.; Cameron, R.E. Collagen: a network for regenerative medicine. J Mater Chem B 2016, 4, 6484–6496. [Google Scholar] [CrossRef]
- Komsa-Penkova, R.; Stavreva, G.; Belemezova, K.; Kyurkchiev, S.; Todinova, S.; Altankov, G. Mesenchymal stem-cell remodeling of adsorbed type-I collagen—The effect of collagen oxidation. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Somaiah, C.; et al. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS One 2015, 10, e0145068. [Google Scholar] [CrossRef]
- Abedi, M.; Shafiee, M.; Afshari, F.; Mohammadi, H.; Ghasemi, Y. Collagen-based medical devices for regenerative medicine and tissue engineering. Appl Biochem Biotechnol 2024, 196, 5563–5603. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv Drug Deliv Rev 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb Perspect Biol 2011, 3, 1–19. [Google Scholar] [CrossRef]
- Weeks, B.S.; Fu, R.; Zaidi, M. Vitamin C promotes wound healing: The use of in vitro scratch assays to assess re-epithelialization. Cell Physiology 2023, 2023, Available: www. [Google Scholar]
- Alexandrova-Watanabe, A.; et al. Assessment of Red Blood Cell Aggregation in Preeclampsia by Microfluidic Image Flow Analysis—Impact of Oxidative Stress on Disease Severity. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J Cell Sci 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The Extracellular Matrix as a Multitasking Player in Disease. FEBS Journal 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Ningsih, S.S.; Jusman, S.W.A.; Syaidah, R.; Nauli, R.; Fadilah, F. Efficient protocol for isolating human fibroblast from primary skin cell cultures: application to keloid, hypertrophic scar, and normal skin biopsies. Biol Methods Protoc 2024, 9. [Google Scholar] [CrossRef]
- Mikulíková, K.; Eckhardt, A.; Pataridis, S.; Mikšík, I. Study of posttranslational non-enzymatic modifications of collagen using capillary electrophoresis/mass spectrometry and high performance liquid chromatography/mass spectrometry. J Chromatogr A 2007, 1155, 125–133. [Google Scholar] [CrossRef]
- Komsa-Penkova, R.; Spirova, R.; Bechev, B. Modification of Lowry’s method for collagen concentration measurement. J Biochem Biophys Methods 1996, 32, 33–43. [Google Scholar] [CrossRef]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: improvements in speed, utility and usability. BMC Bioinformatics 2021, 22, 433. [Google Scholar] [CrossRef]
- Roukos, V.; Pegoraro, G.; Voss, T.C.; Misteli, T. Cell cycle staging of individual cells by fluorescence microscopy. Nat Protoc 2015, 10, 334–348. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS One 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res Ther 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays—State of the art. BioNanoMaterials 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Kehl, D.; et al. Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential. NPJ Regen Med 2019, 4. [Google Scholar] [CrossRef]
- Chang, Y.; Lee, J.W.N.; Holle, A.W. The mechanobiology of fibroblast activation in disease. APL Bioeng 2025, 9, 021505. [Google Scholar] [CrossRef]
- Canty, E.G.; Kadler, K.E. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 2005, 118, 1341–1353. [Google Scholar] [CrossRef]
- González-Masís, J.; et al. Self-assembly study of type I collagen extracted from male Wistar Hannover rat tail tendons. Biomater Res 2020, 24. [Google Scholar] [CrossRef]
- Gwam, C.; Mohammed, N.; Ma, X. Stem cell secretome, regeneration, and clinical translation: a narrative review. Ann Transl Med 2021, 9, 70–70. [Google Scholar] [CrossRef]
- Chouaib, B.; Haack-Sørensen, M.; Chaubron, F.; Cuisinier, F.; Collart-Dutilleul, P.Y. Towards the standardization of mesenchymal stem cell secretome-derived product manufacturing for tissue regeneration. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry Part A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Hafez, A. Efficacy and safety of Wharton’s jelly-derived mesenchymal stem cell exosomes in the treatment of diabetic foot ulcers: a double-blinded randomized controlled clinical trial (WJ-MSC). 2025. [Google Scholar]
- Sun, H.; et al. Clinical outcomes of autologous adipose-derived mesenchymal stem cell combined with high tibial osteotomy for knee osteoarthritis are correlated with stem cell stemness and senescence. J Transl Med 2024, 22. [Google Scholar] [CrossRef]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J Stem Cells 2020, 12, 1439–1690. [Google Scholar] [CrossRef]
- Koczkowska, M.; et al. Identifying differentiation markers between dermal fibroblasts and adipose-derived mesenchymal stromal cells (AD-MSCs) in human visceral and subcutaneous tissues using single-cell transcriptomics. Stem Cell Research and Therapy 2025, 16. [Google Scholar] [CrossRef]
- Nováková, S.; et al. Proteomic study revealed a distinction between human dermal Fibroblasts and Mesenchymal Stem Cells from Different Sources. Stem Cell Rev Rep 2025. [CrossRef]
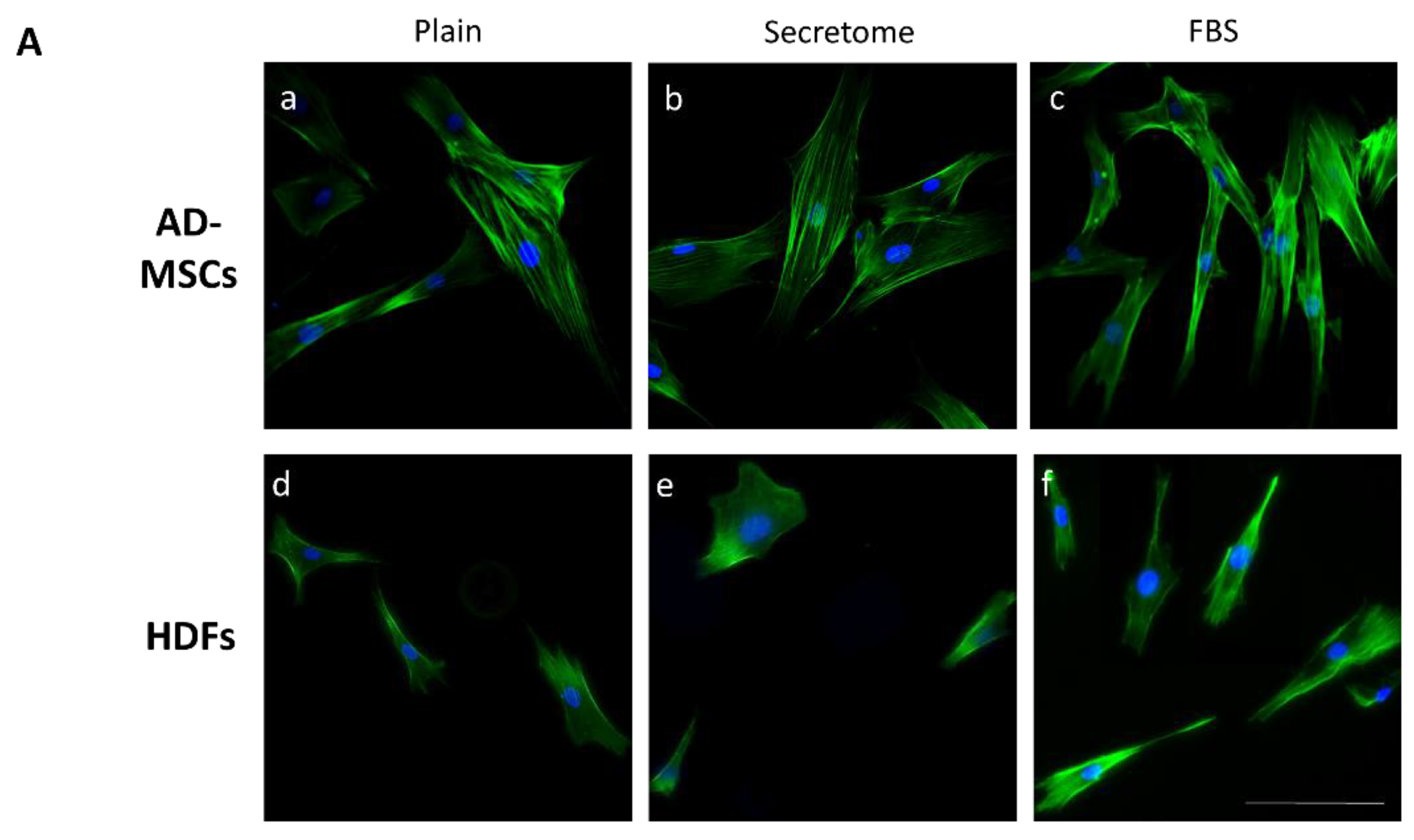
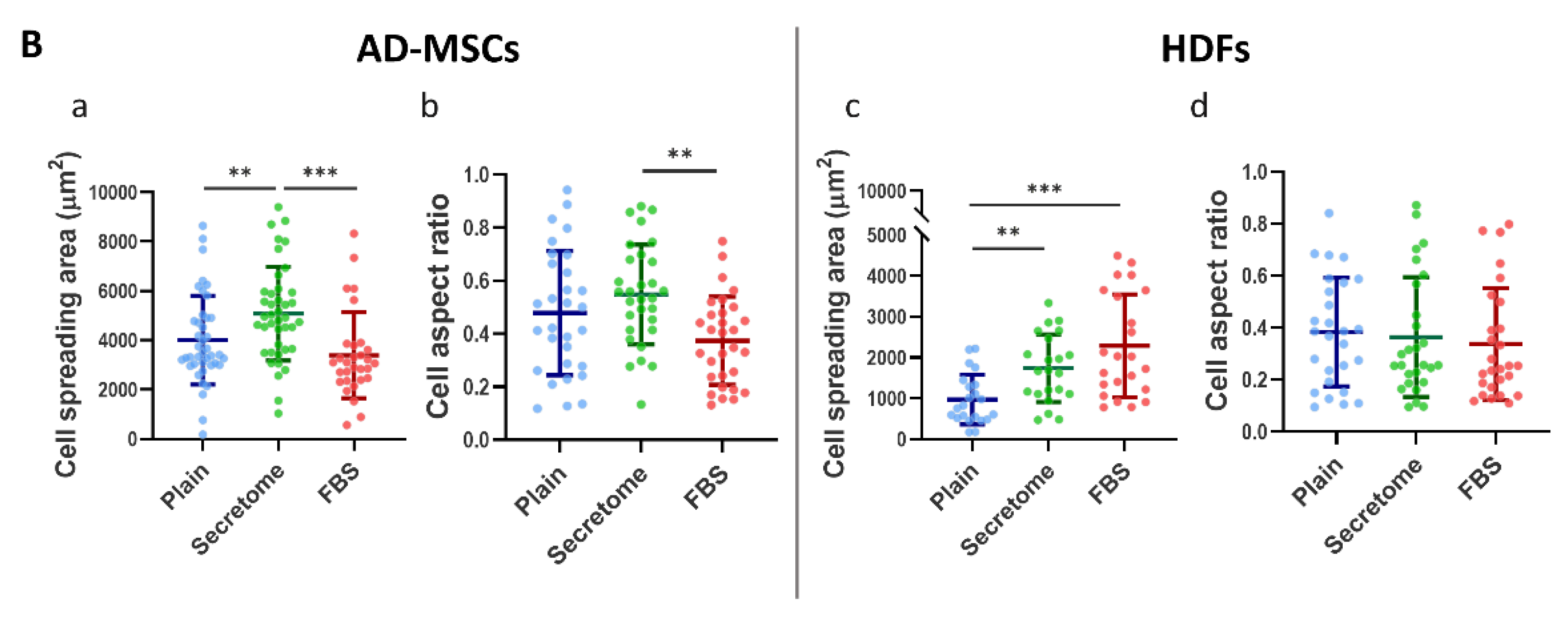
| Condition | Average Cell Spreading Area (CSA) µm2 | Deviation from the Control of CSA (%) | Average Aspect Ratio (AR) | Deviation from the Control of AR (%) | |
|---|---|---|---|---|---|
| AD- MSCs | Plain (Control) |
4007 | - | 0.4781 | - |
| Secretome | 5081 | 26.8 | 0.548 | 14.6 | |
| FBS | 3389 | -15.4 | 0.3736 | -21.9 | |
| HDFs | Plain (Control) |
971.6 | - | 0.3825 | - |
| Secretome | 1737 | 78.8 | 0.3634 | -5.0 | |
| FBS | 2289 | 135.6 | 0.337 | -11.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





