Submitted:
03 October 2025
Posted:
08 October 2025
You are already at the latest version
Abstract
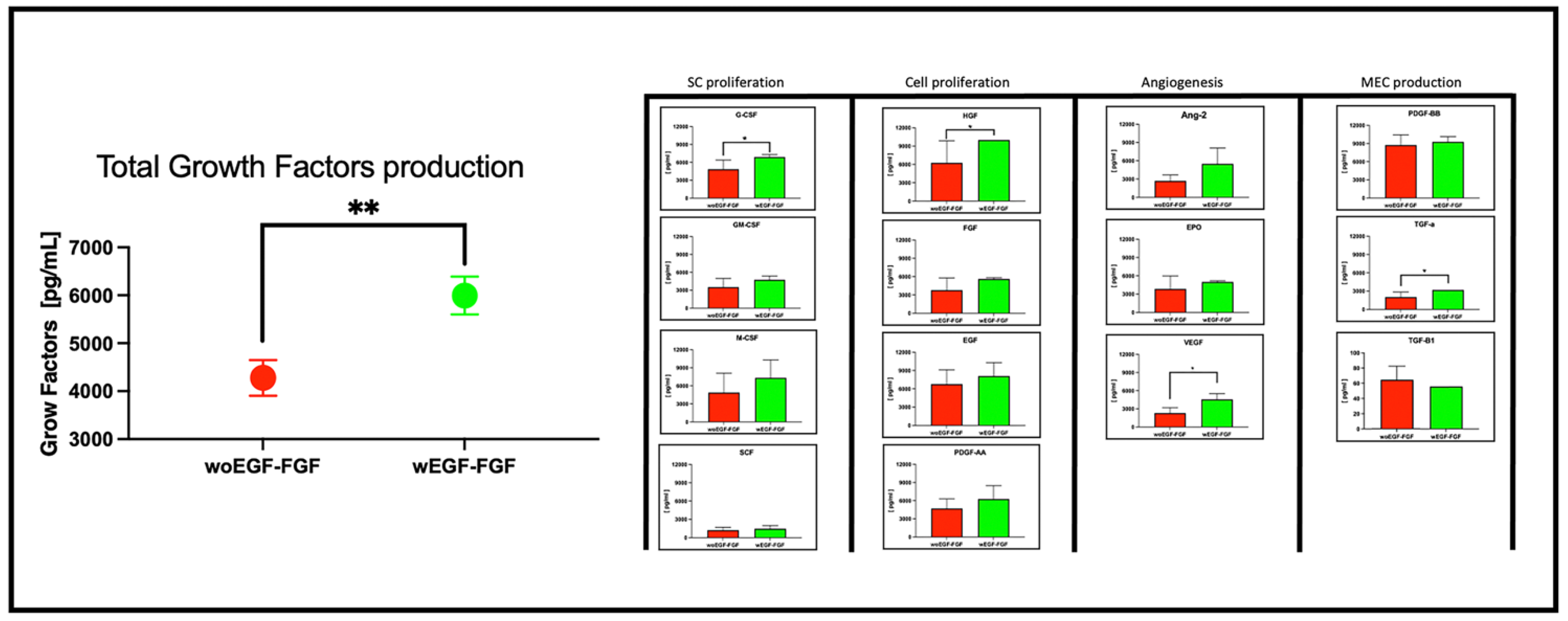
Background: In regenerative medicine, there is interest in using acellular therapy based on the secretome of mesenchymal stem cells (MSC) to promote wound healing. Wharton's jelly cells (WJ-MSCs) are a readily available source. Their secretion has been optimized when stimulated with bFGF and EGF to induce proliferation and prevent senescence. Therefore, evaluating the effect on proliferation and wound closure of human fibroblasts in vitro with different concentrations of the secretome of WJ-MSCs stimulated with growth factors is necessary to identify the most efficient work concentration. Methods: The secretome of human WJ-MSC was collected from passage 1 to passage 2 stimulated with bFGF and EGF (W bFGF/EGF) and the unstimulated secretome (WO bFGF/EGF). The immunophenotype of WJ-MSCs after stimulation was evaluated by flow cytometry for the markers: CD105+, CD73+, CD90+, HLA-ABC+, CD44+, HLA-DR-, CD34-, CD11b-, CD19-, and CD45-. The presence of 14 growth factors in the secretome was evaluated using LEGENDplex through flow cytometry. Fibroblasts were cultured, and their culture medium was supplemented with two different concentrations: one of 1.25 mg/ml and another of 6.25 mg/ml of both stimulated and unstimulated secretome. Proliferation, cellular metabolism, and wound closure were evaluated in vitro. Results: The immunophenotype of WJ-MSCs after stimulation remained unchanged, and the production of growth-assessed factors was increased in stimulated WJ-MSCs. The optimal concentration that induced proliferation and wound closure in vitro was 1.25mg/ml of stimulated WJ-MSC secretome. Conclusions: This study demonstrates that stimulation of WJ-MSCs with FGF and EGF enhances the secretion of growth factors, and that a concentration of 1.25 mg/ml of their secretome promotes optimal fibroblast proliferation and wound closure in vitro. These findings support the potential of optimized WJ-MSC secretome as a promising acellular strategy for regenerative medicine.
Keywords:
1. Introduction
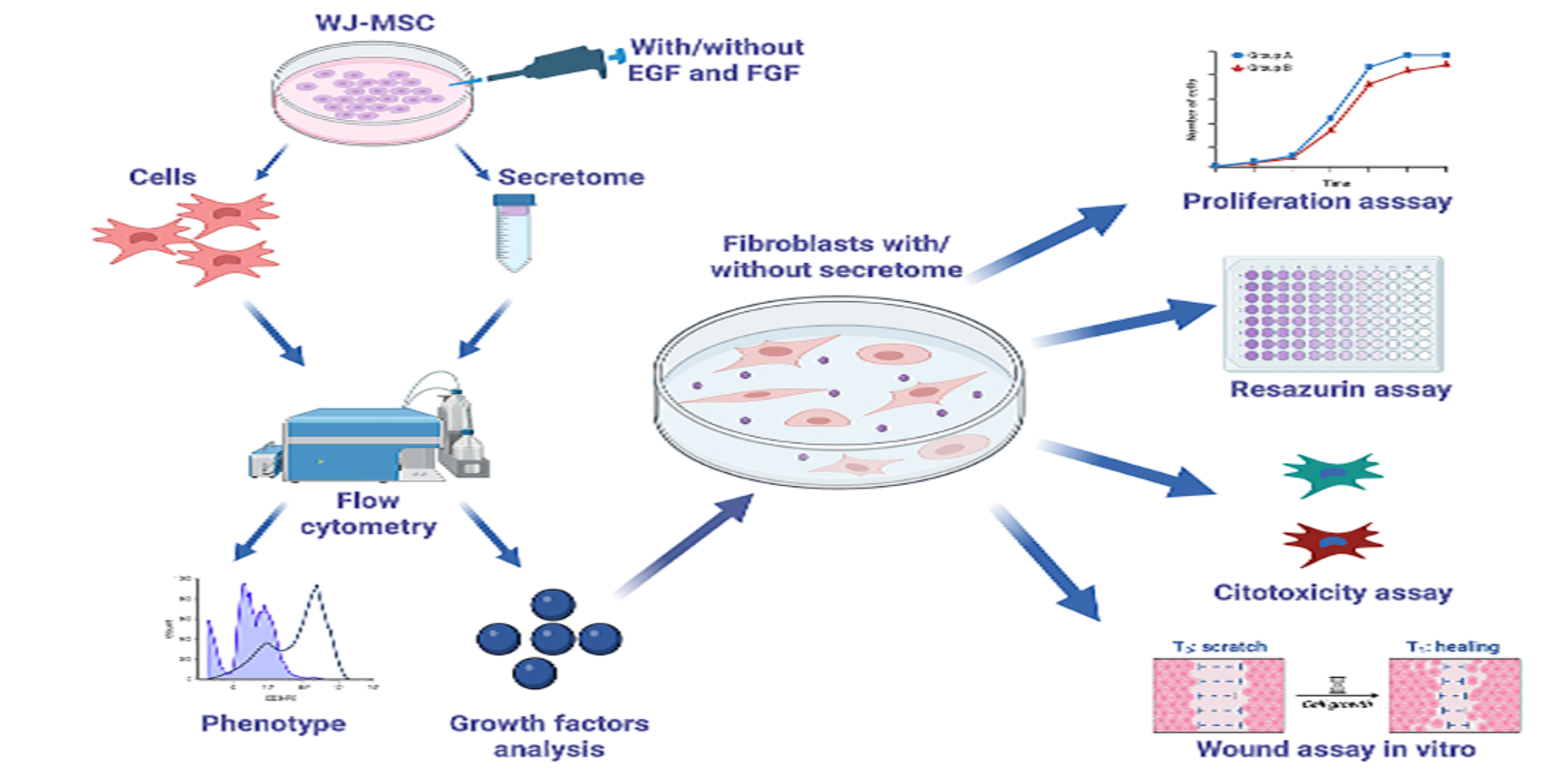
2. Materials and Methods
Sample Collection and Cord Processing
Stimulation and Secretome Collection from WJ-MSC Cultures
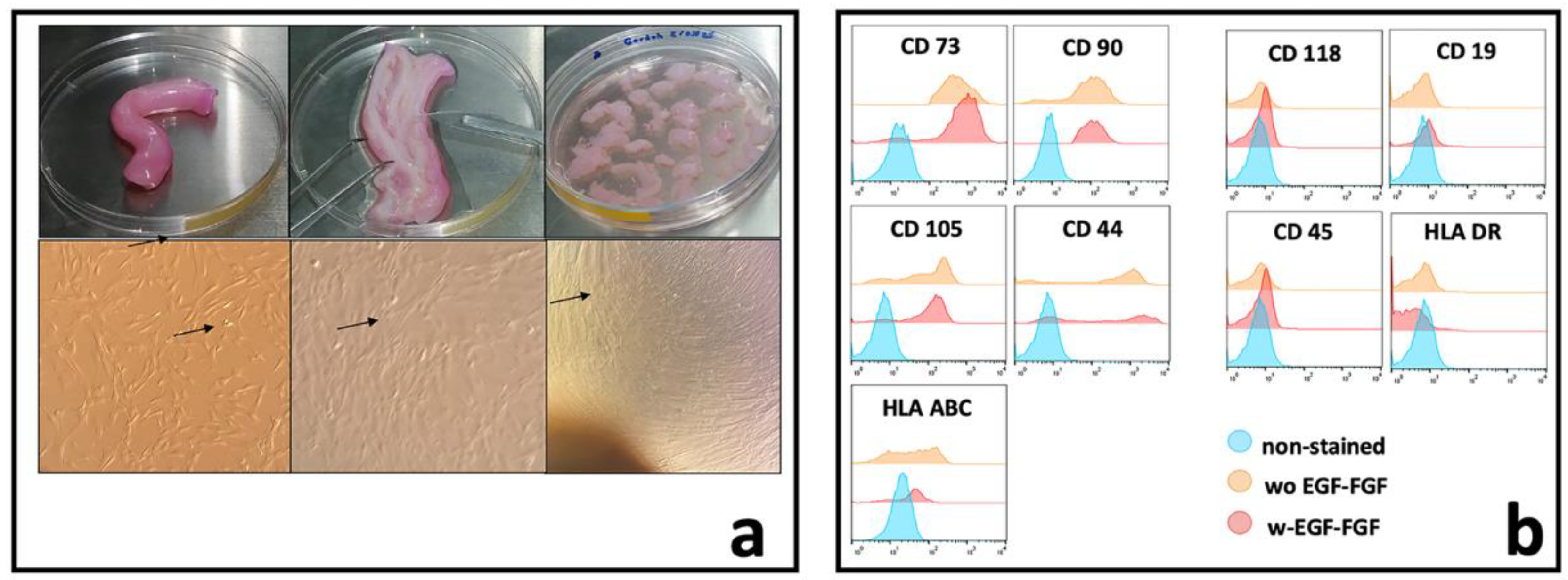
Flow Cytometry Assay
Multiplex Analysis of WJ-MSC Secretome
Isolation and Primary Culture of Human Dermal Fibroblasts
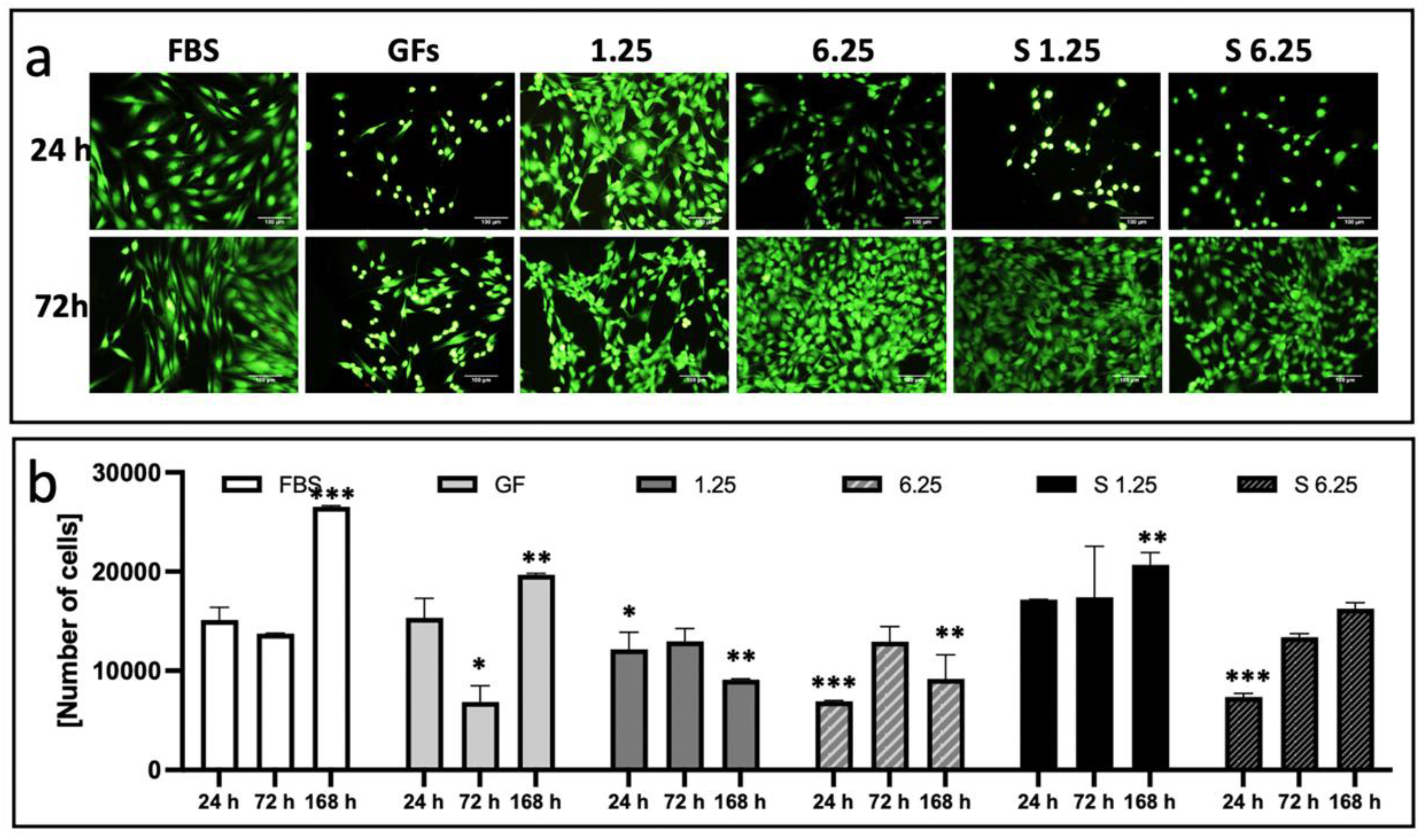
Effect of WJ-MSC Secretome on Cytotoxicity and Proliferation of Human Fibroblasts
In Vitro Wound-Healing Assay
Effect of the WJ-MSC Secretome on Procollagen I Production and α-Smooth Muscle Actin Expression in Human Fibroblasts.
Statistical Analysis
3. Results
3.1. Isolation and Characterization of WJ-MSCs.
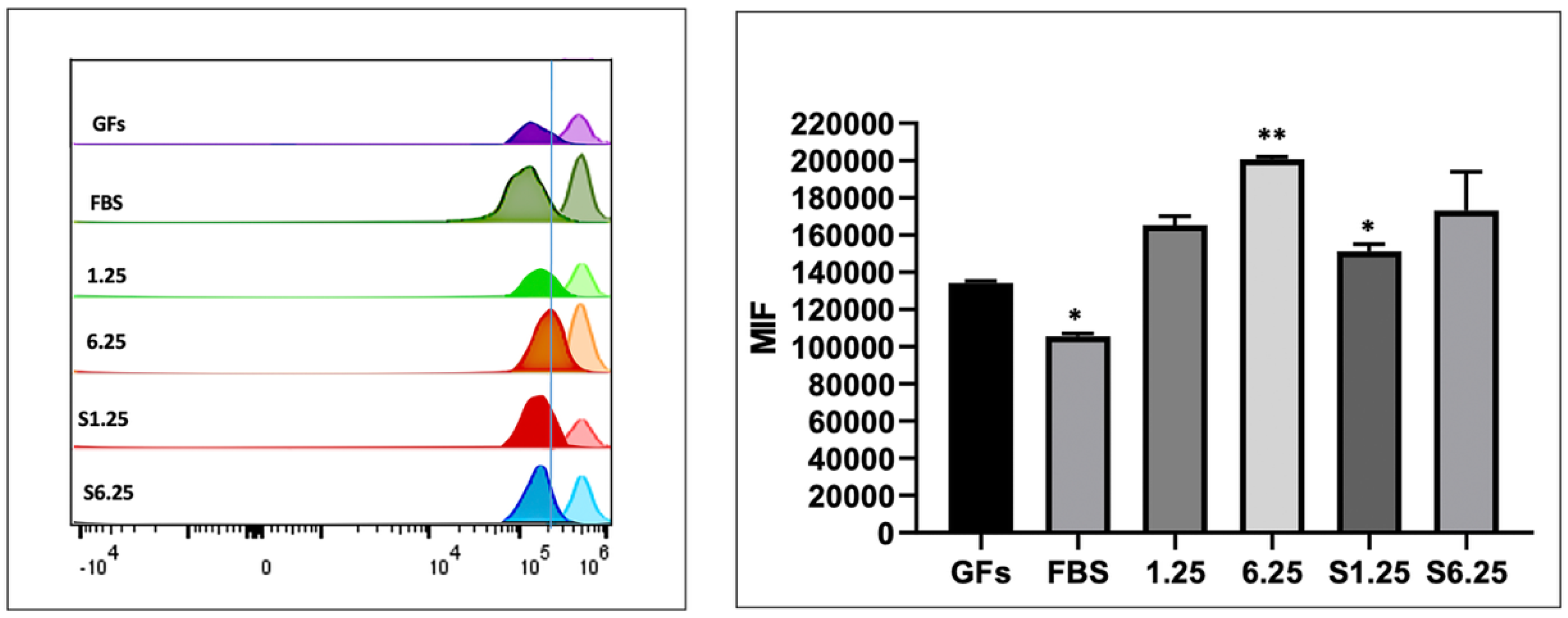
3.3. Secretome Preserves Fibroblast Viability and Enhances Proliferation
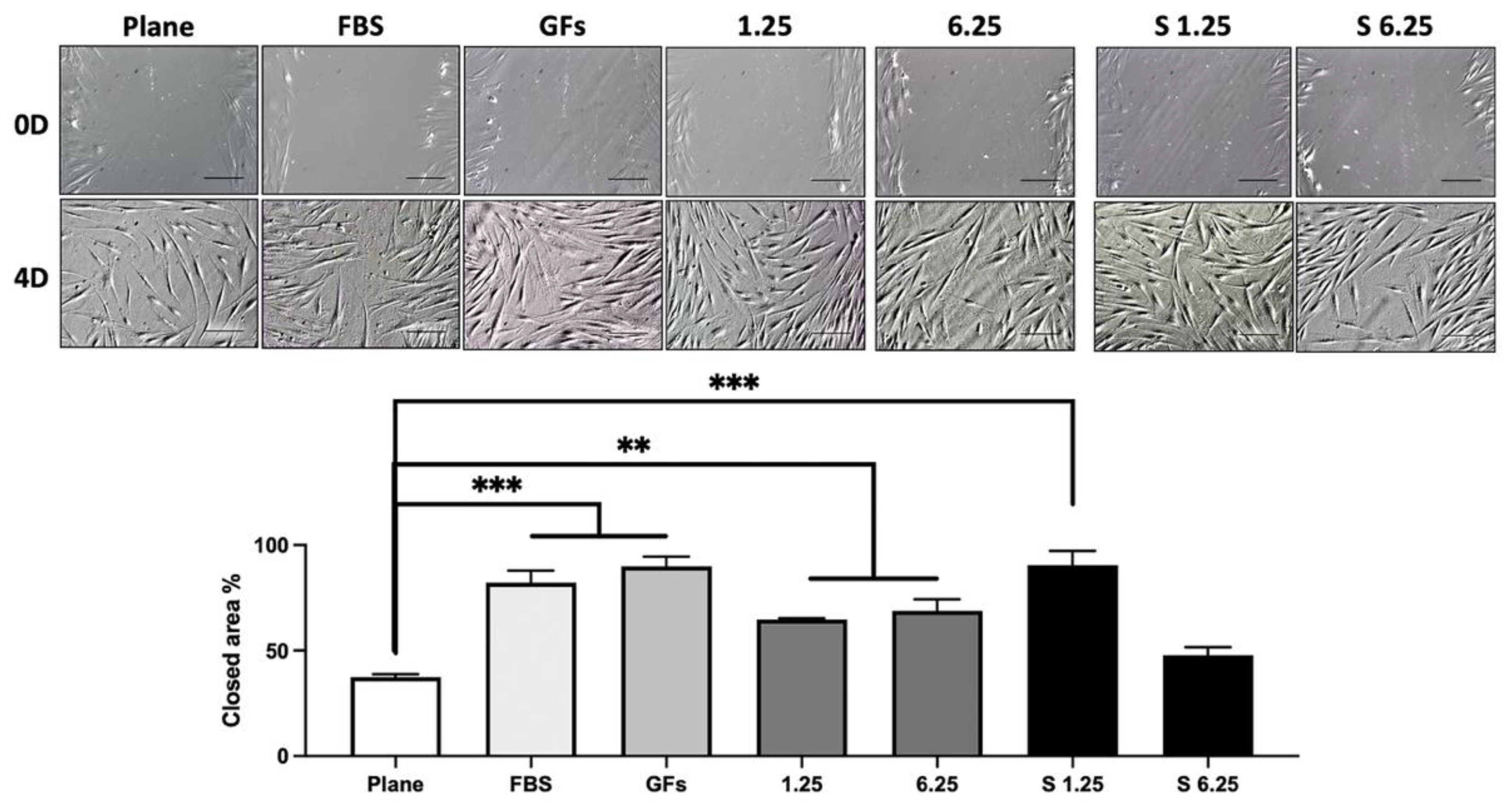
3.4. WJ-MSC Secretome Enhances Fibroblast In Vitro Wound Healing.
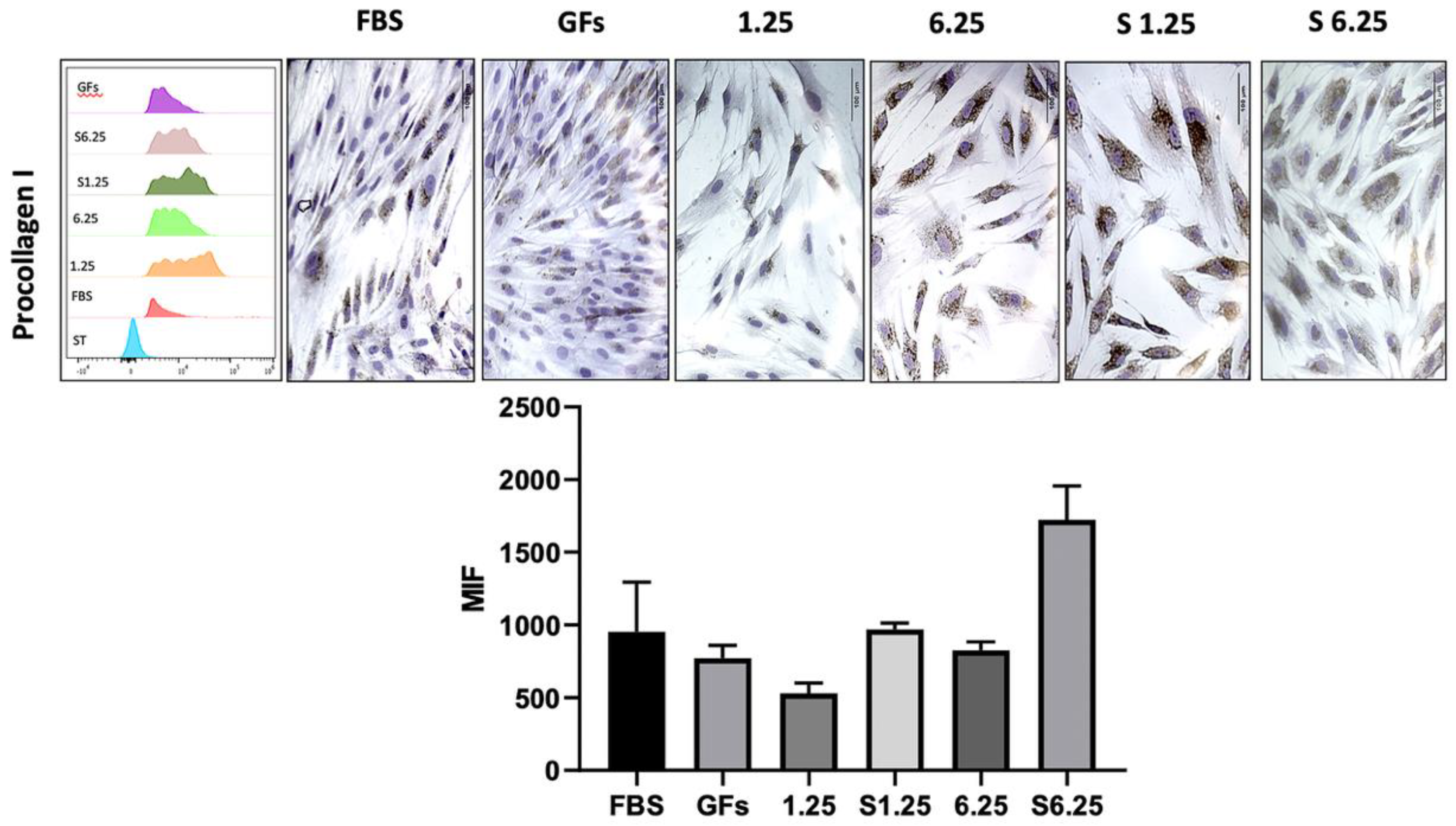
3.5. WJ-MSC Secretome Promotes Type I Collagen Expression
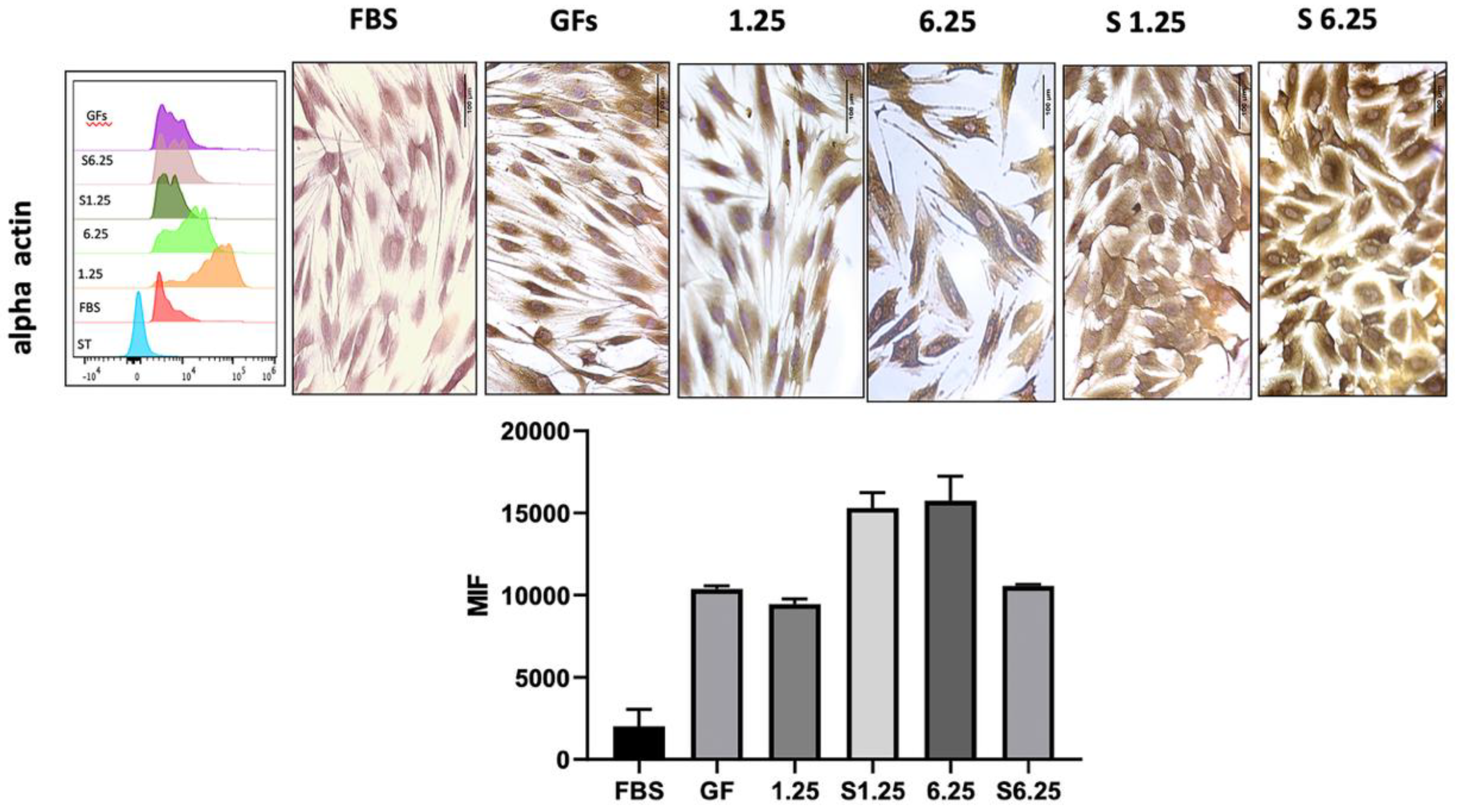
3.6. Secretome-Mediated Upregulation of Alpha-Actin in Human Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal stem cells |
| WJ-MSC | Wharton’s jelly-derived Mesenchymal Stem Cells |
| bFGF | basic Fibroblast Growth Factor |
| EGF | Epidermal Growth Factor |
| W bFGF/EGF | With bFGF/EGF |
| WO bFGF/EGF | Without bFGF/EGF |
| CD105 | Cluster of differentiation 105 |
| CD73 | Cluster of differentiation 73 |
| CD90 | Cluster of differentiation 90 |
| CD44 | Cluster of differentiation 44 |
| CD34 | Cluster of differentiation 34 |
| CD11b | Cluster of differentiation 11b |
| CD19 | Cluster of differentiation 19 |
| CD45 | Cluster of differentiation 45 |
| HLA-ABC | Human Leukocyte Antigen-ABC |
| HLA-DR | Human Leukocyte AntigeN-DR |
| MHC-I | Major Histocompatibility Complex Class I |
| MHC-II | Major Histocompatibility Complex Class II |
| DMEM/F12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| FBS | Fetal Bovine Serum |
| EDTA | Ethylenediaminetetraacetic acid |
| P0 | Passage 0 |
| P1 | Passage 1 |
| P2 | Passage 2 |
| PBS | Phosphate-Buffered Saline |
| EPO | Erythropoietin |
| bFGF | Basic Fibroblast Growth Factor |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HGF | Hepatocyte Growth Factor. |
| M-CSF | Macrophage Colony-Stimulating Factor |
| PDGF-AA | Platelet-Derived Growth Factor AA |
| PDGF-BB | Platelet-Derived Growth Factor BB |
| SCF | Stem Cell Factor |
| TGF-α | Transforming Growth Factor-alpha |
| TGF-β | Transforming Growth Factor-beta |
| VEGF | Vascular Endothelial Growth Factor |
| HBSS | Hanks’ Balanced Salt Solution |
| DMEM | Dulbecco’s Modified Eagle Medium/Nutrient Mixture |
| CFSE | Carboxyfluorescein diacetate succinimidyl ester |
| Calcein AM | Calcein acetoxymethyl ester |
| GFs | Growth Factors |
| S1.25 | Stimulated secretome concentration 1.25 mg/mL |
| S6.25 | Stimulated secretome concentration 6.25 mg/mL |
| 1.25 | Unstimulated secretome concentration 1.25 mg/mL |
| 6.25 | Unstimulated secretome concentration 6.25 mg/mL |
| SD | Standard deviation |
| MFI | Mean fluorescence intensity |
| ANOVA | Analysis of Variance |
| ns | not significant |
| IMH | Immunohistochemistry |
| RNA | Ribonucleic acid |
| PMA | Phorbol ester 12-myristate 13-acetate |
References
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- Saleh, M.; Fotook Kiaei, S.Z.; Kavianpour, M. Application of Wharton jelly-derived mesenchymal stem cells in patients with pulmonary fibrosis. Stem Cell Res Ther 2022, 13, 71. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; et al. Stem cells: past, present, and future. Stem Cell Res Ther 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.J.; Jahnavi, V.S. Wharton’s jelly mesenchymal stem cells as off-the-shelf cellular therapeutics: a closer look into their regenerative and immunomodulatory properties. Open Tissue Eng Regen Med J 2011, 4, 28–38. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.j.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. IJMS 2017, 18, 1852. [Google Scholar] [CrossRef]
- Asserson, D.B. Allogeneic Mesenchymal Stem Cells After In Vivo Transplantation: A Review. Cell Reprogram 2023, 25, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ruiz, V.; Blanco, J.F.; Villarón, E.M.; Fidalgo, H.; López-Parra, M.; Sánchez-Guijo, F. Autologous mesenchymal stem cell transplantation for spinal fusion: 10 years follow-up of a phase I/II clinical trial. Stem Cell Res Ther 2023, 14, 78. [Google Scholar] [CrossRef]
- Krupa, P.; Vackova, I.; Ruzicka, J.; Zaviskova, K.; Dubisova, J.; Koci, Z.; Turnovcova, K.; Urdzikova, L.M.; Kubinova, S.; Rehak, S.; Jendelova, P. The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. Int J Mol Sci 2018, 19, 1503. [Google Scholar] [CrossRef]
- Liang, H.; Suo, H.; Wang, Z.; Feng, W. Progress in the treatment of osteoarthritis with umbilical cord stem cells. Hum Cell 2020, 33, 470–475. [Google Scholar] [CrossRef]
- Kalaszczynska, I.; Ferdyn, K. Wharton’s jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Yang, Q.; Pinto, V.M.R.; Duan, W.; Paxton, E.E.; Dessauer, J.H.; Ryan, W.; Lopez, M.J. In vitro characteristics of heterogeneous equine hoof progenitor cell isolates. Front. Bioeng. Biotechnol 2019, 7, 155. [Google Scholar] [CrossRef]
- Thompson, P.A.; Perera, T.; Marin, D.; Oran, B.; Popat, U.; Qazilbash, M.; Hosing, C.M. Double umbilical cord blood transplant is effective therapy for relapsed or refractory Hodgkin lymphoma. Leukemia & Lymphoma 2015, 57, 1607–1615. [Google Scholar] [CrossRef]
- Zhang, Y.; Na, T.; Zhang, K.; Yang, Y.; Xu, H.; Wei, L.; Du, Y. GMP-grade microcarrier and automated closed industrial scale cell production platform for culture of MSCs. J Tissue Eng Regen 2022, 16, 934–944. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, W.; Jiang, X.; Liu, Y. Mesenchymal stem cell-derived exosomes in cardiovascular and cerebrovascular diseases: From mechanisms to therapy. Biomedecine & Pharmacotherapie 2023, 163, 114817. [Google Scholar] [CrossRef]
- Maguire, G. Stem cell therapy without the cells. Communicative & Integrative Biology 2013, 6. [Google Scholar] [CrossRef]
- Duan, L.; Li, X.; Xu, X.; Xu, L.; Wang, D.; Ouyang, K.; Liang, Y. Large-scale Preparation of Synovial Fluid Mesenchymal Stem Cell-Derived Exosomes by 3D Bioreactor Culture. J Vis Exp 2022, 26, 185. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Yu, Y.; Wang, L.; Zhao, M. Umbilical cord-derived mesenchymal stem cell secretome promotes skin regeneration and rejuvenation: From mechanism to therapeutics. Cell Proliferation 2024, 57. [Google Scholar] [CrossRef]
- Trotzier, C.; Bellanger, C.; Abdessadeq, H.; Delannoy, P.; Mojallal, A.; Auxenfans, C. Deciphering influence of donor age on adipose-derived stem cells: in vitro paracrine function and angiogenic potential. Scientific Reports 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Miclau, K.; Hambright, W.S.; Huard, J.; Stoddart, M.J.; Bahney, C.S. Cellular expansion of MSCs: Shifting the regenerative potential. Aging Cell 2023, 22. [Google Scholar] [CrossRef]
- Turlo, A.J.; Hammond, D.E.; Ramsbottom, K.A.; Soul, J.; Gillen, A.; McDonald, K.; Peffers, M.J. Mesenchymal stromal cell secretome is affected by tissue source and donor age. Stem Cells 2023, 41, 1047–1059. [Google Scholar] [CrossRef]
- Ionescu, L.; Byrne, R.N.; van Haaften, T.; Vadivel, A.; Alphonse, R.S.; Rey-Parra, G.J.; Weissmann, G.; Hall, A.; Eaton, F.; Thébaud, B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 2012, 303, 967–977. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ruan, J.; Zhong, B. Progress in human epidermal growth factor research. Sheng wu gong cheng xue bao [Chinese journal of biotechnology] 2020, 36, 2813–2823. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Shimazu, A.; Miyazaki, K.; Pan, H.; Koike, C.; Yoshida, E. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to bFGF. Biochemistry Biography. Common Res 2001, 288, 413–419. [Google Scholar] [CrossRef]
- Castell-Rodríguez, A.E.; Herrera-Enríquez, M.A.; Piñón-Zárate, G.; Jarquín-Yáñez, K.; Chaires-Rosas, C.P.; Arellano-Olivares, R.M. (2019). Method for Preparing a Supplement from Mesenchymal Cell Cultures of Wharton’s Jelly and Uses of Same (Patent No. EP 3517606 A1).
- Aguilar-Sandoval, D.M. Modulation of growth factor production in stem cells. B.Sc., UNAM, Faculty of Science, 2024.
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.j.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–7. [Google Scholar] [CrossRef]
- Buyl, K.; Vanhaecke, T.; Desmae, T.; Lagneaux, L.; Rogiers, V.; Najar, M.; De Kock, J. Evaluation of a new standardized enzymatic isolation protocol for human umbilical cord-derived stem cells. Toxicol In Vitro 2015, 29, 1254–62. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, H.; Yu, Y.; Lu, Y.; He, B.; Liu, M.; Zhuang, L.; Xu, Y.; Li, W. In Situ Rapid-Formation Sprayable Hydrogels for Challenging Tissue Injury Management. Adv Mater 2024, 36. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Giudice, V.; D’Esposito, V.; Prevete, N.; Scala, P.; de Paulis, A.; Selleri, C.; Formisano, P.; Rossi, F.W.; Montuori, N. Cell-free regenerative medicine: identifying the best source of mesenchymal stem cells for skin therapy in Systemic Sclerosis. Front. Cell Dev. Biol 2025, 13. [Google Scholar] [CrossRef]
- Riekstina, U.; Muceniece, R.; Cakstina, I.; Muiznieks, I.; Ancans, J. Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology 2008, 58, 153–162. [Google Scholar] [CrossRef]
- Marino, L.; Castaldi, M.A.; Fulgione, C.; Castaldi, S.G.; Manzo, P.; Giudice, V.; et al. Reduced proliferative potential with conserved stem/stromal phenotype of human umbilical cord mesenchymal stem cells in placental syndromes: a prospective cohort study. Clin. Exp. Obstet. Gynecol 2023, 50, 196. [Google Scholar] [CrossRef]
- Salehinejad, P.; Alitheen, N.B.; Mandegary, A.; Nematollahi-Mahani, S.N.; Janzamin, E. Effect of EGF and bFGF on the expansion properties of human umbilical cord mesenchymal cells. In Vitro Cell Dev Biol Anim 2013, 49, 515–523. [Google Scholar] [CrossRef]
- Nie, W.B.; Zhang, D.; Wang, L.S. Growth Factor Gene-Modified Mesenchymal Stem Cells in Tissue Regeneration. Drug Des Devel Ther 2020, 14, 1241–1256. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned medium of mesenchymal stromal cells: A new class of therapeutics. Biochemistry. Biokhimiia 2019, 84, 1375–1389. [Google Scholar] [CrossRef]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Research International 2014, 1–14. [Google Scholar] [CrossRef]
- Yamada, M.; Tatsumi, R.; Yamanouchi, K.; Hosoyama, T.; Shiratsuchi, S.; Sato, A.; Mizunoya, W.; Ikeuchi, Y.; Furuse, M.; Allen, R.E. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol 2010, 298, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, J.; Cai, X.; Yin, T.; Zhang, Y.; Yang, C.; Yang, J. Granulocyte colony-stimulating factor in reproductive-related disease: Function, regulation and therapeutic effect. Biomedicine & Pharmacotherapy 2022, 150. [Google Scholar] [CrossRef]
- Forte, G.; Minieri, M.; Cossa, P.; Antenucci, D.; Sala, M.; Gnocchi, V.; Fiaccavento, R.; Carotenuto, F.; De Vito, P.; Baldini, P.M. Effects of hepatocyte growth factor on mesenchymal stem cells: proliferation, migration and differentiation. Stem Cells 2006, 24, 23–33. [Google Scholar] [CrossRef]
- Meng, H.F.; Jin, J.; Wang, H.; Wang, L.S.; Wu, C.T. Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings. J. Cell Mol. Med 2022, 26, 4745–4755. [Google Scholar] [CrossRef]
- Qin, P.; Kurpakus, M.A. The role of laminin-5 in TGFα/EGF-mediated corneal epithelial cell motility. Experimental Eye Research 1998, 66, 569–579. [Google Scholar] [CrossRef] [PubMed]
- L, P.K.; Kandoi, S.; Misra, R.; SV; KR; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front Immunol 2018, 9, 2837. [Google Scholar] [CrossRef]
- Stolfi, C.; Troncone, E.; Marafini, I.; Monteleone, G. Role of TGF-Beta and Smad7 in Gut Inflammation, Fibrosis and Cancer. Biomolecules 2020, 11, 17. [Google Scholar] [CrossRef]
- Tutuianu, R.; Rosca, A.M.; Iacomi, D.M.; Simionescu, M.; Titorencu, I. Human Mesenchymal Stromal Cell-Derived Exosomes Promote In Vitro Wound Healing by Modulating the Biological Properties of Skin Keratinocytes and Fibroblasts and Stimulating Angiogenesis. Int J Mol Sci 2021, 22, 623. [Google Scholar] [CrossRef]
- Schuster, R.; Younesi, F.; Ezzo, M.; Hinz, B. The Role of Myofibroblasts in Physiological and Pathological Tissue Repair. Cold Spring Harb Perspect Biol 2023, 15. [Google Scholar] [CrossRef]
- Aw, Y.B.; Chen, S.; Yeo, A.; Dangerfield, J.A.; Mok, P. Development and functional testing of a novel in vitro delayed scratch closure assay. Histochem Cell Biol 2024, 162, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.S.; Tan, M.L.L.; Lim, P.L.K.; Sharma, B.; Yeo, A.; Aw, Y.B.; Ng, Y.Z.; Bonnard, C.; Becker, D.L.; Mok, P. Secretome from prolonged high-density human Wharton’s jelly stem cell culture accelerates wound healing in both in vitro and in vivo models. Int Wound J 2025, 22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




