Submitted:
16 January 2026
Posted:
19 January 2026
You are already at the latest version
Abstract
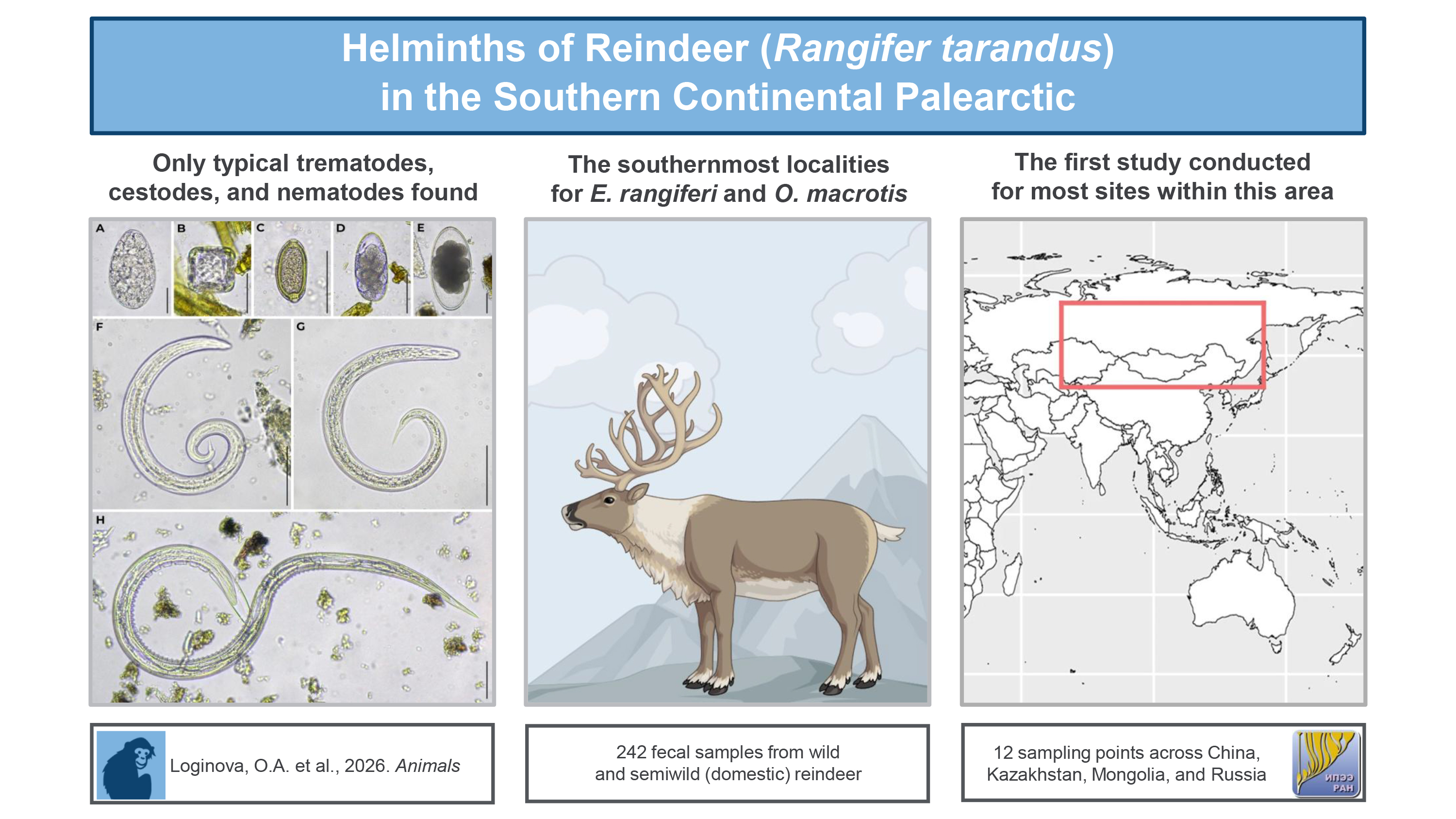
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fecal Sampling
2.2. Fecal Analysis
2.3. Helminth Identification
2.4. DNA Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic Acid |
| DSL | Dorsal-Spined Larva |
| E | East |
| ID | Identifier |
| IEE RAS | A. N. Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences |
| ITS | Internal Transcribed Spacer |
| GPT | Generative Pre-trained Transformer |
| L1 | First stage nematode larva |
| L3 | Third stage nematode larva |
| LLM | Large Language Model |
| N | North |
| NCBI | National Center for Biotechnology Information |
| PCR | Polymerase Chain Reaction |
| USA | United States of America |
| USSR | Union of Soviet Socialist Republics |
| WPI RAS | Water Problems Institute of the Russian Academy of Sciences |
References
- Kaltenborn, B.P.; Andersen, O.; Gundersen, V. The role of wild reindeer as a flagship species in new management models in Norway. Nor. J. Geogr. 2014, 68, 168–177. [Google Scholar] [CrossRef]
- Mysterud, A.; Tveraa, T.; Hansen, B.B.; Gundersen, V.; Tømmervik, H.; Erlandsson, R.; Røed, K.H; Våge, J.; Andersen, R.; Brænd, E.; Bøthun, S.W.; Elgaaen, M.; Holand, Ø.; Kjørstad, M.; Kvie, K.; Mossing, A.; Myren, I.S.; Panzacchi, M.; Peeters, B.; Punsvik, T.; Romtveit, L.; Skarin, A.; Moorter, B.V.; Veiberg, V.; Jaren, V.; Strand, O.; Rolandsen, C.M. A quality standard for conservation of wild reindeer. Wildl. Monogr. 2025, 219, e70005. [Google Scholar] [CrossRef]
- Gunn, A. Rangifer tarandus. The IUCN Red List of Threatened Species 2016 2016, e.T29742A22167140. [Google Scholar] [CrossRef]
- Panchenko, D.V.; Mizin, I.A.; Rozhnov, V.V. Severnyy olen’ Rangifer tarandus Linnaeus, 1758 (Reindeer Rangifer tarandus Linnaeus, 1758). In Krasnaya kniga Rossiyskoy Federatsii. Zhivotnyye (Red Data Book of the Russian Federation. Animals), (in Russian), 2nd ed.; Pavlov, D.S., Ed.; FGBU “VNII Ekologiya”: Moscow, Russia, 2021; pp. 1020–1025. ISBN 978-5-6047425-0-1. [Google Scholar]
- Stavridis, M.A.; Røed, S.B.; Hansen, B.B.; Mikkelsen, Ø.; Ciesielski, T.M.; Jenssen, B.M. Tracing the footprints of Arctic pollution: Spatial variations in toxic and essential elements in Svalbard reindeer (Rangifer tarandus platyrhynchus) faeces. Sci. Total Environ. 2024, 906, 167562. [Google Scholar] [CrossRef] [PubMed]
- Reindeer and Caribou. Health and Disease; Tryland, M., Kutz, S.J., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA; London, UK; New York, NY, USA, 2019; p. 533. ISBN 978-1-4822-5068-8. [Google Scholar]
- Klein, D.R. Conflicts between domestic reindeer and their wild counterparts: a review of Eurasian and North American experience. Arctic 1980, 33, 739–756. Available online: https://www.jstor.org/stable/40509079. [CrossRef]
- Guillou, S. Le Renne: Habitant des régions froides (Reindeer: Inhabitant of cold regions); (In French). Éd. Atlas jeunesse: Paris, France, 2005; p. 29. ISBN 978-2-7312-3308-7. [Google Scholar]
- Kurtén, B. Pleistocene mammals in Europe; Weidenfeld and Nicolson: London, UK, 1968; p. 317. ISSN 0345-0074. [Google Scholar]
- Kurtén, B.; Anderson, E. Pleistocene mammals in North America; Columbia University Press: New York, USA, 1980; p. 442. ISBN 0-231-03733-3. [Google Scholar]
- Baskin, L.; Danell, K. Ecology of Ungulates: A Handbook of Species in Eastern Europe and Northern and Central Asia; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003; pp. 378–385. ISBN 3-540-43804-1. [Google Scholar]
- Zaplatin, M.A. Chara: With a movie camera across Transbaikalia (Chara: S kinoapparatom po Zabaykal’yu); (in Russian). Mysl’: Moscow, USSR, 1964; p. 142. [Google Scholar]
- Smirnov, M.N. Severnyy olen’ na yuge Sibiri (Reindeer in the South of Siberia); (in Russian). Siberian Federal University: Krasnoyarsk, Russia, 2016; p. 230. ISBN 978-5-7638-3461-1. [Google Scholar]
- Mizkewitsch, V.Y. Gel’minty Severnogo Olenya i Vyzyvayemyye Imi Zabolevaniya (Reindeer Helminths and the Diseases They Cause); 308p. (In Russian). Kolos: Leningrad, USSR, 1967. [Google Scholar]
- Aleuy, O.A.; Serrano, E.; Ruckstuhl, K.E.; Hoberg, E.P.; Kutz, S. Parasite intensity drives fetal development and sex allocation in a wild ungulate. Sci. Rep. 2020, 10, 15626. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.J.; Dobson, A.P.; Lafferty, K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006, 21, 381–385. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, K.; Abaunza, P. Parasites as Biological Tags for Stock Discrimination of Marine Fish: a Guide to Procedures and Methods. Fish. Res. 1998, 38, 45–56. [Google Scholar] [CrossRef]
- Machulsky, S.N. Dikiye parnokopytnyye kak rezerventy gel’mintoznykh zabolevaniy dlya sel’skokhozyaystvennykh zhivotnykh Buryat-Mongol’skoy ASSR (Wild ungulates as reservoirs of Helminthic Diseases for Farm Animals of the Buryat-Mongolian ASSR). In Proceedings of the Buryat-Mongolian Zoological Veterinary Institute; (In Russian). BurGiz: Ulan-Ude, USSR, 1955; Volume 9, pp. 163–172. [Google Scholar]
- Sulimov, A.D. Gel’minty severnykh oleney Tuvy (Helminths of Reindeer in Tuva). In Proceedings of the Omsk Veterinary Institute; (In Russian). Zapadno-Sibirskoye knizhnoye Izdatel’stvo: Omsk, USSR, 1966; Volume 24, pp. 135–141. [Google Scholar]
- Sulimov, A.D. K gel’mintofaune dikikh zhvachnykh Tuvy (To the Helminth Fauna of Wild Ruminants of Tuva). In Proceedings of the Omsk Veterinary Institute; (In Russian). Zapadno-Sibirskoye knizhnoye izdatel’stvo: Omsk, USSR, 1968; Volume 25, pp. 238–242. [Google Scholar]
- Kadenatsi, A.N. Gel’minty severnogo olenya v Khabarovskom kray (Helminths of Reindeer in Khabarovsk Krai). In Proceedings of the All-Union Scientific Conference dedicated to the 90th anniversary of the Kazan Veterinary Institute; (In Russian). KVI: Kazan’, USSR, 1963; pp. 146–147. [Google Scholar]
- Zhaltsanova, D.S.D. Obnaruzheniye Fischoederius elongatus (Poirier, 1883) v Buryatskoy ASSR (Detection of Fischoederius elongatus (Poirier, 1883) in the Buryat ASSR). Epizootologiya, epidemiologiya i profilaktika gel’mintozov (In Russian). 1972, 24, 49–50. [Google Scholar]
- Loginova, O.A. Gel’minty severnykh oleney Kazakhstana (Helminths of Reindeer in Kazakhstan). In Proceedings of the ХVII All-Russian Scientific and Practical Conference “Modern Problems of General and Applied Parasitology”, (In Russian). Voronezh, Russia, 17–18 October 2024. [Google Scholar] [CrossRef]
- Loginova, O.A.; Sipko, T.P.; Hernandez-Blanco, J.A.; Plotnikova, Iu.K. Gel’mintokoproskopiya dikogo severnogo olenya i ovtsebyka v Magadanskoy oblasti (Helminthocoproscopy of Wild Reindeer and Muskoxen in the Magadan Region). In Proceedings of the International Scientific Conference “Biological problems of the North”, (In Russian). Magadan, Russia, 07–11 October 2024. [Google Scholar] [CrossRef]
- National Standard of the Russian Federation GOST R 54627-2011 Zhivotnyye sel’skokhozyaystvennyye zhvachnyye. Metody laboratornoy diagnostiki gel’mintozov (Agricultural Ruminant Animals. Methods of Laboratory Helminthological Diagnostics); Standartinform. 20p. (in Russian). Moscow, Russia, 2013.
- Vajda, T. Gyors eljárás a juhok tüdőférgességének megállapítására (Rapid method for diagnosing lung helminths in sheep). Állatorvosi Lapok (In Hungarian). 1931, 54, 187–190. [Google Scholar]
- Darling, S.T.; Barber, M.A.; Hacker, H.P. Hookworm and Malaria. Research in Malaya, Java, and the Fiji Islands (Report of Uncinariasis Commission to the Orient 1915–1917); The Rockefeller Foundation International Health Board: New York, USA, 1920; p. 25. [Google Scholar]
- Skrjabin, K.I. Trematody Zhivotnykh i Cheloveka. Osnovy Trematodologii (Trematodes of Animals and Man. Principles of Trematodology); (In Russian). Izdatelstvo Akademii Nauk SSSR: Moscow, USSR, 1949; Volume 3, p. 623. [Google Scholar]
- Spassky, A.A. Osnovy Tsestodologii. Anoplotsefalyaty – lentochnyye gel’minty domashnikh i dikikh zhivotnykh (Fundamentals of Cestodology. Anoplocephalates – tapeworms of domestic and wild animals); (In Russian). Izdatelstvo Akademii Nauk SSSR: Moscow, USSR, 1951; Volume 1, p. 735. [Google Scholar]
- Skrjabin, K.I.; Shikhobalova, N.P.; Schulz, R.S. Trikhostrongilidy Zhivotnykh i Cheloveka. Osnovy Nematodologii (Trichostrongylides of Animals and Man. Principles of Nematodology); (In Russian). Izdatelstvo Akademii Nauk SSSR: Moscow, USSR, 1954; Volume 3, p. 683. [Google Scholar]
- Boev, S.N. Prostrongilidy. Osnovy Nematodologii (Protostrongylides. Principles of Nematodology); (In Russian). Nauka SSSR: Moscow, USSR, 1975; Volume 25, p. 267. [Google Scholar]
- Kontrimavichus, V.L.; Delyamure, S.L.; Boev, S.N. Metastrongiloidei Zhivotnykh i Cheloveka. Osnovy Nematodologii (Metastrongyliodeas of Animals and Man. Principles of Nematodology); (In Russian). Nauka SSSR: Moscow, USSR, 1976; Volume 26, p. 239. [Google Scholar]
- Skrjabin, K.I.; Shikhobalova, N.P.; Orloff, I.W. Trikhotsefalidy i Kapillyariidy Zhivotnykh i Cheloveka i Vyzyvaemye Imi Zabolevaniya. Osnovy Nematodologii (Trichocephalides and Capillariides of Animals and Man and the Diseases They Cause. Principles of Nematodology); (In Russian). Izdatelstvo Akademii Nauk SSSR: Moscow, USSR, 1957; Volume 6, p. 587. [Google Scholar]
- Gasser, R.B.; Chilton, N.B.; Hoste, H.; Beveridge, I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 1993, 21, 2525–2526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2004, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database indexing for production MegaBLAST searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Blaker, H. Confidence curves and improved exact confidence intervals for discrete distributions. Can. J. Stat. 2000, 28, 783–798. [Google Scholar] [CrossRef]
- Skrjabin, K.I. Glistnyye invazii severnogo olenya (Worm infestations in reindeer); (In Russian). Sel’khozgiz: Moscow and Leningrad, USSR, 1930; p. 88. [Google Scholar]
- Skrjabin, K.I. Metod polnykh gel’mintologicheskikh vskrytiy pozvonochnykh, vklyuchaya cheloveka. (Method of complete helminthological dissections of vertebrates, including humans.); (In Russian). Izdaniye 1-go Moskovskogo gosudarstvennogo Universiteta: Moscow, USSR, 1928; p. 45. [Google Scholar]
- Rehbein, S.; Vymyslická, P.J.; Peterka, T.; Strube, C.; Visser, M.; Mayr, S.; Lackerschmid, J. Calicophoron daubneyi (Paramphistomidae) in deer of the Šumava National Park, Czech Republic – Consequence of Prevalent Rumen Fluke Infection in Cattle. Vet. Parasitol.: Reg. St. Rep. 2024, 50, art. 101012. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, S.L. The taxonomy of the family Paramphistomidae Fischoeder, 1901 with special reference to the morphology of species occurring in ruminants. I. General considerations. Syst. Parasitol. 1982, 4, 7–57. [Google Scholar] [CrossRef]
- Haukisalmi, V.; Laaksonen, S.; Oksanen, A.; Beckmen, K.; Halajian, A.; Yanagida, T.; Nakao, M. Molecular taxonomy and subgeneric classification of tapeworms of the genus Moniezia Blanchard, 1891 (Cestoda, Anoplocephalidae) in northern cervids (Alces and Rangifer). Parasitol. Int. 2018, 67, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Dróżdż, J. Polymorphism in the Ostertagiinae Lopez-Neyra, 1947 and Comments on the Systematics of These Nematodes. Syst. Parasitol. 1995, 32, 91–99. [Google Scholar] [CrossRef]
- Loginova, O.A.; Rozenfeld, S.B.; Sipko, T.P.; Mizin, I.A.; Panchenko, D.V.; Laishev, K.A.; Bondar, M.G.; Kolpashchikov, L.A.; Gruzdev, A.R.; Kulemeev, P.S.; et al. Diversity and Distribution of Helminths in Wild Ruminants of the Russian Arctic: Reindeer (Rangifer tarandus), Muskoxen (Ovibos moschatus), and Snow Sheep (Ovis nivicola). Diversity 2023, 15, 672. [Google Scholar] [CrossRef]
- Loginova, O.A.; Kolpashchikov, L.A.; Spiridonov, S.E. First report of Orthostrongylus sp. (Nematoda: Protostrongylidae) in wild reindeer (Rangifer tarandus) from the Taimyr, Russia: Nearctic parasites in a Palearctic host. Parasitol. Res. 2023, 122, 685–689. [Google Scholar] [CrossRef] [PubMed]



| Sample Set ID | Map Reference |
Type of Reindeer | Number of Fecal Samples |
Location (Country, Administrative Unit) |
Protected Area |
Coordinates (Decimal Degrees) | Altitude (Meters) |
Date Collected |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | semiwild | 2 | Kazakhstan, Akmola Region |
— | 52.93877 70.24527 |
450 | April 2025 |
| 2 | 2 | wild | 8 | Russia, Novosibirsk Oblast |
Vasyugansky State Nature Reserve | 56.636553 79.981242 |
129 | February 2025 |
| 3 | 3 | wild | 3 | Russia, Altai Republic |
Altai State Nature Reserve |
51.38385 88.98008 |
1671 | February 2024 |
| 4 | 4 | semiwild | 20 | Mongolia, Khövsgöl Province |
— | 50.566855 100.127792 |
1915 | August 2023 |
| 5 | 5 | wild | 20 | Russia, Buryatia |
Baikal State Nature Reserve |
51.30777 105.24722 |
1370 | February 2012 |
| 6 | 5 | wild | 35 | Russia, Buryatia |
Baikal State Nature Reserve |
51.30777 105.24722 |
1370 | July 2023 |
| 7 | 5 | wild | 35 | Russia, Buryatia |
Baikal State Nature Reserve |
51.30777 105.24722 |
1370 | July 2024 |
| 8 | 5 | wild | 35 | Russia, Buryatia |
Baikal State Nature Reserve |
51.30777 105.24722 |
1370 | July 2025 |
| 9 | 6 | wild | 1 | Russia, Irkutsk Oblast |
— | 58.609750 114.980806 |
884 | September 2021 |
| 10 | 7 | semiwild | 30 | Russia, Zabaykalsky Krai |
— | 56.579214 118.886167 |
882 | February 2024 |
| 11 | 8 | wild | 7 | Russia, Amur Oblast |
— | 56.73530 121.50033 |
1396 | February 2023 |
| 12 | 9 | semiwild | 10 | China, Inner Mongolia |
— | 51.35080 121.50146 |
788 | August 2023 |
| 13 | 10 | wild | 17 | Russia, Amur Oblast |
— | 55.142893 128.850543 |
653 | February 2023 |
| 14 | 11 | wild | 9 | Russia, Amur Oblast |
— | 55.679389 129.329878 | 1064 | February 2023 |
| 15 | 12 | wild | 10 | Russia, Amur Oblast |
Tokinsko-Stanovoy National Park | 55.44280 130.48006 |
1518 | July 2021 |
| Sample Set ID | Species | GenBank 1 | Voucher 2 |
|---|---|---|---|
| 12 | Elaphostrongylus rangiferi | PX720411 | IPEE_Parasites 14460 |
| 15 | Orthostrongylus macrotis | PX720450 | IPEE_Parasites 14459 |
| 15 | Ostertagia gruehneri | PX716654 | IPEE_Parasites 14458 |
| Map Site | Trematodes | Cestodes | Nematodes | ||||
|---|---|---|---|---|---|---|---|
| Paramphistomoidea | Moniezia | Strongyle-type | Nematodirus | Elaphostrongylus rangiferi | Orthostrongylus macrotis | Capillaria | |
| 1 | – | – | – | – | – | – | – |
| 2 | – | 5 (63%); 1.2±0.3 | 1(13%); 0.3±0.0 | – | 6 (75%); 0.6±0.1 | – | – |
| 3 | – | – | – | – | – | – | – |
| 4 | 2 (10%); 0.5±0.2 | 1 (5%); 0.3±0.0 | 9 (45%); 1.0±0.4 | – | – | – | 2 (10%); 0.3±0.0 |
| 5 | 2 (2%); 0.3±0.0 | 7 (6%); 0.6±0.1 | – | – | 1 (1%); 2.0±0.0 | – | – |
| 6 | – | – | – | – | – | – | – |
| 7 | – | – | – | 3 (10%); 0.6±0.3 | – | – | – |
| 8 | – | 1 (14%); 0.3±0.0 | – | – | 3 (43%); 1.0±0.3 | – | – |
| 9 | – | – | 3 (30%); 1.1±0.5 | – | 2 (20%); 36.2±20.3 | – | 1(10%); 0.7±0.0 |
| 10 | – | 5 (29%); 0.7±1.2 | – | – | – | – | – |
| 11 | – | – | – | – | – | – | – |
| 12 | 3 (30%); 0.6±0.1 | – | 5 (50%)*; 2.6±1.2 | 1 (10%); 0.3±0.0 | 1 (10%); 1.3±0.0 | 1 (10%); 4.0±0.0 | – |
| Helminths | Intensity of Invasion Depending on the Number of Found Helminth Eggs and Larvae, Specimens per 1 g of Feces | |||
|---|---|---|---|---|
| Low | Medium | High | Very High | |
| Nematodes, Cestodes | 1–100 | 101–500 1 | 501–1000 | >1000 |
| GINs (eggs + L3s) 2 | 0.3–6.7 (sites 2, 4 ,9, 12) | – | – | – |
| Nematodirus (eggs) | 0.3–1.3 (sites 7, 12) | – | – | – |
| E. rangiferi (L1s) | 0.3–75.3 (sites 2, 5, 8, 9, 12) | – | – | – |
| O. macrotis (L1s) | 4.0 (site 12) | – | – | – |
| Capillaria (eggs) | 0.3–0.7 (sites 4, 9) | – | – | – |
| Moniezia (eggs) | 0.3–2.0 (sites) | – | – | – |
| Trematodes | 1–10 | 11–100 | >100 | – |
| Paramphistomoidea (eggs) | 0.3–0.7 (sites 4, 5, 12) | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





