Submitted:
15 January 2026
Posted:
16 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Stranding Epidemiologic Data and Autopsy Examination
2.2. Statistical Analysis
2.3. Histopathology and Pathologic Classification
2.4. Microbiology
2.5. Osmium Tetroxide (OsO4) and Chromic Acid (H2CrO4)
3. Results
3.1. Stranding epidemiology
3.2. Natural causes of death
3.3. Anthropogenic pathologic categories
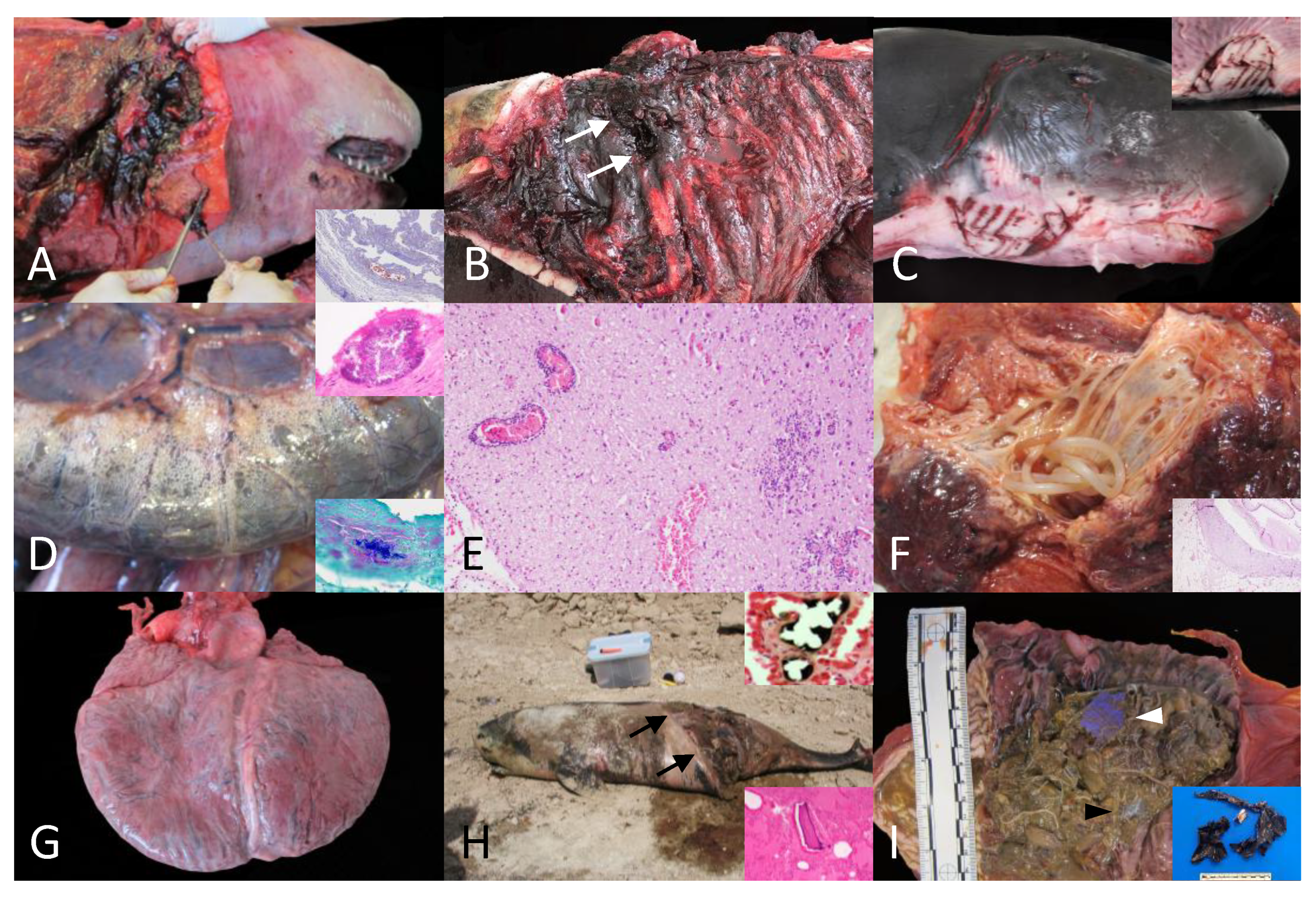
4. Discussion
Natural causes of death
Anthropogenic causes of death
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Committee on Taxonomy. List of marine mammal species and subspecies. Society for Marine Mammalogy. Available online: www.marinemammalscience.org (accessed on 29/05/2025).
- Plön, S. Pygmy Sperm Whale Kogia breviceps (de Blainville, 1838). In Handbook of the Mammals of Europe; Springer: Cham, Switzerland, 2023; pp. 1–27. [Google Scholar]
- McAlpine, D.F. Pygmy and dwarf sperm whales. In Encyclopedia of Marine Mammals, 3rd ed.; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, United Kingdom, 2018; pp. 786–788. [Google Scholar]
- McAlpine, D.F. Pygmy and dwarf sperm whales. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 936–938. [Google Scholar]
- West, K.L.; Walker, W.A.; Baird, R.W.; White, W.; Levine, G.; Brown, E.; et al. Diet of pygmy sperm whales (Kogia breviceps) in the Hawaiian Archipelago. Mar. Mamm. Sci. 2009, 25, 931–943. [Google Scholar] [CrossRef]
- Keenan-Bateman, T.F.; McLellan, W.A.; Costidis, A.M.; Harms, C.A.; Gay, D.M.; Rotstein, D.S.; et al. Habitat use pattern of the giant parasitic nematode Crassicauda magna within the pygmy sperm whale Kogia breviceps. Dis. Aquat. Organ. 2018, 127, 163–175. [Google Scholar] [CrossRef]
- Keenan, T.F.; McLellan, W.A.; Rommel, S.A.; Costidis, A.M.; Harms, C.A.; Thewissen, J.G.M.; et al. Gross and histological morphology of the cervical gill slit gland of the pygmy sperm whale (Kogia breviceps). Anat. Rec. 2022, 305. 688–703. [Google Scholar] [CrossRef]
- Keenan-Bateman, T.F.; McLellan, W.A.; Harms, C.A.; Piscitelli, M.A.; Barco, S.G.; Thayer, V.G.; et al. Prevalence and anatomic site of Crassicauda sp. infection, and its use in species identification, in kogiid whales from the mid-Atlantic United States. Mar. Mamm. Sci. 2016, 32, 868–883. [Google Scholar] [CrossRef]
- Bennati-Madureira, L.; Gomes, G.L.; Daros, K.A.C.; Casas, A.L. da S. Anatomical description of a pygmy sperm whale, Kogia breviceps (Cetacea: Kogiidae), pre-term calf using CT scan and 3D reconstructions. Anat. Rec. 2024, 307, 1043–1058. [Google Scholar] [CrossRef]
- Bloodworth, B.E.; Odell, D.K. Kogia breviceps (Cetacea: Kogiidae). Mamm. Species 2008, 819, 1–12. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; et al. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0193025. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, M.; de los Monteros, A.E.; Herráez, P.; Andrada, M.; Sierra, E.; Rodríguez, F.; et al. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999–2005). Dis. Aquat. Organ. 2013, 103, 87–99. [Google Scholar] [CrossRef]
- Stamper, M.A.; Whitaker, B.R.; Schofield, T.D. Case study: Morbidity in a pygmy sperm whale Kogia breviceps due to ocean-borne plastic. Mar. Mamm. Sci. 2006, 22, 719–722. [Google Scholar] [CrossRef]
- Shamsi, S.; Spröhnle-Barrera, C.; Hossen, M.S. Occurrence of Anisakis spp. (Nematoda: Anisakidae) in a pygmy sperm whale Kogia breviceps (Cetacea: Kogiidae) in Australian waters. Dis. Aquat. Organ. 2019, 134, 65–74. [Google Scholar] [CrossRef]
- Rousselet, E.; Stolen, M.; Durden, W.N.; Jablonski, T.; Stacy, N.I.; Rotstein, D.S. Bilateral polycystic kidneys and focal renal cystadenoma in a pygmy sperm whale (Kogia breviceps). J. Wildl. Dis. 2019, 55, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Plön, S.; Best, P.B.; Duignan, P.; Lavery, S.D.; Bernard, R.T.F.; Van Waerebeek, K.; et al. Population structure of pygmy (Kogia breviceps) and dwarf (Kogia sima) sperm whales in the Southern Hemisphere may reflect foraging ecology and dispersal patterns. In Advances in Marine Mammal Science; Springer: Cham, Switzerland, 2023; pp. 85–114. [Google Scholar]
- Bossart, G.D.; Hensley, G.; Goldstein, J.D.; Kroell, K.; Manire, C.A.; Defran, R.H.; et al. Cardiomyopathy and myocardial degeneration in stranded pygmy (Kogia breviceps) and dwarf (Kogia sima) sperm whales. Aquat. Mamm. 2007, 33, 113–122. [Google Scholar] [CrossRef]
- National Marine Fisheries Service (NMFS). Dwarf and pygmy sperm whale (Kogia spp.) best practices. 2021. [Google Scholar]
- Plön, S.; Andra, K.; Auditore, L.; Gegout, C.; Hale, P.J.; Hampe, O.; et al. Marine mammals as indicators of Anthropocene ocean health. npj Biodivers. 2024, 3, 24. [Google Scholar] [CrossRef]
- Li, W.T.; Chou, L.S.; Chiou, H.Y.; Chen, I.H.; Yang, W.C. Analyzing 13 years of cetacean strandings: Multiple stressors to cetaceans in Taiwanese waters and their implications for conservation and future research. Front. Mar. Sci. 2021, 8, 633056. [Google Scholar] [CrossRef]
- IJsseldijk, L.; Brownlow, A.C.; Mazzariol, S. Best practice on cetacean post mortem investigation and tissue sampling. In ACCOBANS–ASCOBANS Joint Guidelines; ACCOBANS: Monaco, 2019; pp. 1–73. [Google Scholar]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore: Baltimore, MD, USA, 2005. [Google Scholar]
- Cowan, D.F.; Curry, B.E. Histopathology of the alarm reaction in small odontocetes. J. Comp. Pathol. 2008, 139, 24–33. [Google Scholar] [CrossRef]
- Herráez, P.; Sierra, E.; Arbelo, M.; Jaber, J.R.; Espinosa de los Monteros, A.; Fernández, A. Rhabdomyolysis and myoglobinuric nephrosis (capture myopathy) in a striped dolphin. J. Wildl. Dis. 2007, 43, 770–774. [Google Scholar] [CrossRef]
- Herráez, P.; Espinosa de los Monteros, A.; Fernández, A.; Edwards, J.F.; Sacchini, S.; Sierra, E. Capture myopathy in live-stranded cetaceans. Vet. J. 2013, 196, 181–188. [Google Scholar] [CrossRef]
- Câmara, N.; Sierra, E.; Fernández, A.; Suárez-Santana, C.M.; Puig-Lozano, R.; Arbelo, M.; et al. Skeletal and cardiac rhabdomyolysis in a live-stranded neonatal Bryde’s whale with fetal distress. Front. Vet. Sci. 2019, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Sierra, E.; Zucca, D.; Arbelo, M.; García-Álvarez, N.; Andrada, M.; Déniz, S.; et al. Fatal systemic morbillivirus infection in bottlenose dolphin, Canary Islands, Spain. Emerg. Infect. Dis. 2014, 20, 269–271. [Google Scholar] [CrossRef]
- Sierra, E.; Sánchez, S.; Saliki, J.T.; Blas-Machado, U.; Arbelo, M.; Zucca, D.; et al. Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the Canary Islands. J. Clin. Microbiol. 2014, 52, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Sierra, E.; Fernández, A.; Zucca, D.; Câmara, N.; Felipe-Jiménez, I.; Suárez-Santana, C.; et al. Morbillivirus infection in Risso’s dolphin Grampus griseus: A phylogenetic and pathological study of cases from the Canary Islands. Dis. Aquat. Organ. 2018, 128, 123–136. [Google Scholar] [CrossRef]
- Vandevanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; et al. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef]
- Sierra, E.; Fernández, A.; Espinosa de los Monteros, A.; Arbelo, M.; Díaz-Delgado, J.; Andrada, M.; et al. Histopathological muscle findings may be essential for a definitive diagnosis of suspected sharp trauma associated with ship strikes in stranded cetaceans. PLoS ONE 2014, 9, e88780. [Google Scholar] [CrossRef]
- Arregui, M.; Fernández, A.; Paz-Sánchez, Y.; Santana, Á.; Sacchini, S.; Sierra, E.; et al. Comparison of three histological techniques for fat emboli detection in lung cetacean tissue. Sci. Rep. 2020, 10, 18518. [Google Scholar] [CrossRef] [PubMed]
- Ressel, L.; Hetzel, U.; Ricci, E. Blunt force trauma in veterinary forensic pathology. Vet. Pathol. 2016, 53, 941–961. [Google Scholar] [CrossRef]
- West, K.; Silva-Krott, I.; Rotstein, D.; Levine, G. Causes of mortality and pathologic findings in Pacific Island cetaceans: A review of strandings from 2006–2022. In Annual Marine Species Monitoring Report for the Pacific; U.S. Navy Marine Species Monitoring Program: San Diego, CA, USA, 2022. [Google Scholar]
- Cipriani, P.; Palomba, M.; Giulietti, L.; Aco-Alburqueque, R.; Andolfi, R.; Ten Doeschate, M.; et al. Anisakid parasite diversity in a pygmy sperm whale, Kogia breviceps (Cetacea: Kogiidae), stranded at the edge of its distribution range in the Northeast Atlantic Ocean. Parasite 2024, 31, 43. [Google Scholar] [CrossRef]
- Moore, M.; van der Hoop, J.; Barco, S.; Costidis, A.; Gulland, F.; Jepson, P.; et al. Criteria and case definitions for serious injury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Dis. Aquat. Organ. 2013, 103, 229–264. [Google Scholar] [CrossRef] [PubMed]
- Puig-Lozano, R.; Fernández, A.; Saavedra, P.; Tejedor, M.; Sierra, E.; de la Fuente, J.; et al. Retrospective study of traumatic intra-interspecific interactions in stranded cetaceans, Canary Islands. Front. Vet. Sci. 2020, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.D.; Selbmann, A.; Middel, H.; Schulze, J.; Bellon, G.; Samarra, F.I.P. Interactions between killer whales (Orcinus orca) and neonate long-finned pilot whales (Globicephala melas) off South Iceland. Ecol. Evol. 2025, 15, e11023. [Google Scholar] [CrossRef]
- Ochoa, G.M.; Ramos, E.A.; Gonzalez-Socoloske, D. Predation of a sperm whale (Physeter macrocephalus) by killer whales (Orcinus orca) in Guanaja, Honduras. Aquat. Mamm. 2025, 51, 31–36. [Google Scholar] [CrossRef]
- Ferguson, S.H.; Higdon, J.W.; Chmelnitsky, E.G. The rise of killer whales as a major Arctic predator. In A Little Less Arctic; Springer: Dordrecht, The Netherlands, 2010; pp. 117–136. [Google Scholar]
- Ferguson, S.H.; Kingsley, M.C.S.; Higdon, J.W. Killer whale (Orcinus orca) predation in a multi-prey system. Popul. Ecol. 2012, 54, 31–41. [Google Scholar] [CrossRef]
- Pitman, R.L.; Ballance, L.T.; Mesnick, S.I.; Chivers, S.J. Killer whale predation on sperm whales: Observations and implications. Mar. Mamm. Sci. 2001, 17, 494–507. [Google Scholar] [CrossRef]
- Seyboth, E.; Bassoi, M.; de Lima, R.C.; do Prado, J.H.F. Killer whale predation on an Antarctic minke whale in the northern Antarctic Peninsula. An. Acad. Bras. Cienc. 2024, 96, e20231345. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.A.; Stacey, P.J.; Baird, R.W. A review of killer whale interactions with other marine mammals: Predation to co-existence. Mamm. Rev. 1991, 21, 151–180. [Google Scholar] [CrossRef]
- Olson, P.A.; Hancock-Hanser, B.L.; McCullough, J.L.K.; Dahlheim, M.; Schulman-Janiger, A.; Black, N.; et al. Killer whale predation on a pygmy sperm whale in an oceanic habitat. Mar. Mamm. Sci. 2023, 39, 281–288. [Google Scholar] [CrossRef]
- Dunphy-Daly, M.M.; Heithaus, M.R.; Claridge, D.E. Temporal variation in dwarf sperm whale (Kogia sima) habitat use and group size off Great Abaco Island, Bahamas. Mar. Mamm. Sci. 2008, 24, 171–184. [Google Scholar] [CrossRef]
- Martín, V.; Tejedor, M.; Carrillo, M.; Pérez-Gil, M.; Arbelo, M.; Servidio, A.; et al. Strandings and at-sea observations reveal the Canary Archipelago as an important habitat for pygmy and dwarf sperm whales. In Advances in Marine Mammal Research; Springer: Cham, Switzerland, 2024; pp. 21–64. [Google Scholar]
- Fernández, A.; Câmara, N.; Sierra, E.; Arbelo, M.; Bernaldo de Quirós, Y.; Jepson, P.D.; et al. Cetacean intracytoplasmic eosinophilic globules: A cytomorphological, histological, histochemical, immunohistochemical, and proteomic characterization. Animals 2023, 13, 2034. [Google Scholar] [CrossRef]
- Fernández, A.; Sierra, E.; Díaz-Delgado, J.; Sacchini, S.; Sánchez-Paz, Y.; Suárez-Santana, C.; et al. Deadly acute decompression sickness in Risso’s dolphins. Sci. Rep. 2017, 7, 17254. [Google Scholar] [CrossRef]
- de Quirós, Y.B.; González-Díaz, O.; Arbelo, M.; Sierra, E.; Sacchini, S.; Fernández, A. Decompression vs. decomposition: Distribution, amount, and gas composition of bubbles in stranded marine mammals. Front. Physiol. 2012, 3, 177. [Google Scholar] [CrossRef]
- Pons-Bordas, C.; Hazenberg, A.; Hernandez-Gonzalez, A.; Pool, R.V.; Covelo, P.; Sánchez-Hermosin, P.; et al. Recent increase of ulcerative lesions caused by Anisakis spp. in cetaceans from the north-east Atlantic. J. Helminthol. 2020, 94, e127. [Google Scholar] [CrossRef]
- Motta, M.R.A.; Pinheiro, D.C.S.N.; Carvalho, V.L.; Viana, D.A.; Vicente, A.C.P.; Iñiguez, A.M. Gastric lesions associated with the presence of Anisakis spp. Dujardin, 1845 (Nematoda: Anisakidae) in cetaceans stranded on the coast of Ceará, Brazil. Biota Neotrop. 2008, 8, 91–95. [Google Scholar] [CrossRef]
- Hrabar, J.; Bočina, I.; Gudan Kurilj, A.; Đuras, M.; Mladineo, I. Gastric lesions in dolphins stranded along the eastern Adriatic coast. Dis. Aquat. Organ. 2017, 125, 125–139. [Google Scholar] [CrossRef]
- Nakagun, S.; Kobayashi, Y. Histochemical and immunohistochemical characterizations of hepatic trematodiasis in odontocetes. Front. Vet. Sci. 2020, 7, 375. [Google Scholar] [CrossRef]
- Jaber, J.R.; Pérez, J.; Arbelo, M.; Andrada, M.; Hidalgo, M.; Gómez-Villamandos, J.C.; et al. Hepatic lesions in cetaceans stranded in the Canary Islands. Vet. Pathol. 2004, 41, 147–153. [Google Scholar] [CrossRef]
- Segura-Göthlin, S.; Fernández, A.; Arbelo, M.; Andrada Borzollino, M.A.; Felipe-Jiménez, I.; Colom-Rivero, A.; et al. Viral skin diseases in odontocete cetaceans: Gross, histopathological, and molecular characterization of selected pathogens. Front. Vet. Sci. 2023, 10, 1169301. [Google Scholar] [CrossRef] [PubMed]
- Martineau, D.; Lagacé, A.; Béland, P.; Higgins, R.; Armstrong, D.; Shugart, L.R. Pathology of stranded beluga whales (Delphinapterus leucas) from the St. Lawrence Estuary, Québec, Canada. J. Comp. Pathol. 1988, 98, 287–310. [Google Scholar] [CrossRef]
- Sacristán, C.; Esperón, F.; Ewbank, A.C.; Díaz-Delgado, J.; Ferreira-Machado, E.; Costa-Silva, S.; et al. Novel herpesviruses in riverine and marine cetaceans from South America. Acta Trop. 2019, 190, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Manire, C.A.; Smolarek, K.A.; Romero, C.H.; Kinsel, M.J.; Clauss, T.M.; Byrd, L. Proliferative dermatitis associated with a novel alphaherpesvirus in an Atlantic bottlenose dolphin (Tursiops truncatus). J. Zoo Wildl. Med. 2006, 37, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Felipe-Jiménez, I.; Fernández, A.; Andrada, M.; Arbelo, M.; Segura-Göthlin, S.; Colom-Rivero, A.; et al. Contribution to herpesvirus surveillance in beaked whales stranded in the Canary Islands. Animals 2021, 11, 3170. [Google Scholar] [CrossRef]
- Sacristán, C.; Ewbank, A.C.; Duarte-Benvenuto, A.; Sacristán, I.; Zamana-Ramblas, R.; Costa-Silva, S.; et al. Survey of selected viral agents (herpesvirus, adenovirus and hepatitis E virus) in liver and lung samples of cetaceans, Brazil. Sci. Rep. 2024, 14, 2689. [Google Scholar] [CrossRef]
- Smolarek Benson, K.A.; Manire, C.A.; Ewing, R.Y.; Saliki, J.T.; Townsend, F.I.; Ehlers, B.; et al. Identification of novel alpha- and gammaherpesviruses from cutaneous and mucosal lesions of dolphins and whales. J. Virol. Methods 2006, 136, 261–266. [Google Scholar] [CrossRef]
- Uzal, F.A.; Navarro, M.A.; Asin, J.; Henderson, E.E. Clostridial diseases of horses: A review. Vaccines 2022, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Uzal, F.A.; Songer, J.G. Clostridial diseases. In Diseases of Swine, 11th ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 792–806. [Google Scholar]
- Buck, J.D.; Shepard, L.L.; Spotte, S. Clostridium perfringens as the cause of death of a captive Atlantic bottlenosed dolphin (Tursiops truncatus). J. Wildl. Dis. 1987, 23, 488–491. [Google Scholar] [CrossRef]
- Driscoll, P.; Pierce, V.; Whitaker, B.; Kelly, K. Multi-agency case report: Clostridium perfringens disseminated infection in a live-stranded bottlenose dolphin (Tursiops truncatus) in Maryland. In Proceedings of the International Association for Aquatic Animal Medicine; IAAM: Seattle, WA, USA, 2007. [Google Scholar]
- Danil, K.; St. Leger, J.; Dennison, S.; Bernaldo de Quirós, Y.; Scadeng, M.; Nilson, E.; et al. Clostridium perfringens septicemia in a long-beaked common dolphin Delphinus capensis: An etiology of gas bubble accumulation in cetaceans. Dis. Aquat. Organ. 2014, 111, 183–190. [Google Scholar] [CrossRef]
- Gulland, F.; Dierauf, L.; Whitman, K. (Eds.) Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- St. Leger, J.; Raverty, S.; Mena, A. Cetacea. In Pathology of Wildlife and Zoo Animals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 461–514. [Google Scholar]
- Hutchinson, K.M.; Tart, K.; Anderson, K.L.; Powell, L.L. Pneumatosis of the intestines, colon and liver in a young cat. Vet. Med. Sci. 2018, 4, 150–158. [Google Scholar] [CrossRef]
- Kiu, R.; Shaw, A.G.; Sim, K.; Acuna-Gonzalez, A.; Price, C.A.; Bedwell, H.; et al. Particular genomic and virulence traits associated with preterm infant-derived toxigenic Clostridium perfringens strains. Nat. Microbiol. 2023, 8, 1160–1175. [Google Scholar] [CrossRef]
- Neto, G.G.; Bueno, M.G.; Silva, R.O.S.; Lobato, F.C.F.; Guimarães, J.P.; Bossart, G.D.; et al. Acute necrotizing colitis with pneumatosis intestinalis in an Amazonian manatee calf. Dis. Aquat. Organ. 2016, 120, 189–194. [Google Scholar] [CrossRef]
- Rich, A.F.; Zendri, F.; Costa, T.; Timofte, D.; Drake, G.J.; Rowland, H.; et al. Nectarivorous bird emphysematous ingluvitis (NBEI): A novel disease in Loriinae birds associated with Clostridium perfringens infection. Front. Vet. Sci. 2020, 7, 606112. [Google Scholar] [CrossRef] [PubMed]
- Šeol, B.; Gomerčić, M.Đ.; Naglić, T.; Gomerčić, T.; Galov, A.; Gomerčić, H. Isolation of Clostridium tertium from a striped dolphin (Stenella coeruleoalba) in the Adriatic Sea. J. Wildl. Dis. 2006, 42, 709–711. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Fernández, A.; Xuriach, A.; Sierra, E.; Bernaldo de Quirós, Y.; Mompeo, B.; et al. Verminous arteritis due to Crassicauda sp. in Cuvier’s beaked whales (Ziphius cavirostris). Vet. Pathol. 2016, 53, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Beveridge, I.; Bryant, M.S. Morphological and molecular observations on the status of Crassicauda magna, a parasite of the subcutaneous tissues of the pygmy sperm whale, with a re-evaluation of the systematic relationships of the genus Crassicauda. Parasitol. Res. 2015, 114, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Di Guardo, G.; Pozzi-Mucelli, R.; Scaravelli, D.; Francese, M. Use of computer tomography for imaging of Crassicauda grampicola in a Risso’s dolphin (Grampus griseus). J. Zoo Wildl. Med. 2004, 35, 391–394. [Google Scholar] [CrossRef]
- Suárez-González, Z.; Fernández, A.; Colom-Rivero, A.; García-Álvarez, N.; González, J.F.; Sierra, E. Morphological and molecular characterization of Crassicauda anthonyi in Cuvier’s beaked whales from the Canary Islands. BMC Vet. Res. 2025, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Lempereur, L.; Delobelle, M.; Doom, M.; Haelters, J.; Levy, E.; Losson, B.; et al. Crassicauda boopis in a fin whale (Balaenoptera physalus) ship-struck in the eastern North Atlantic Ocean. Parasitol. Open 2017, 3, e12. [Google Scholar] [CrossRef]
- Kot, B.C.; Ho, H.H.; Leung, E.K.; Chung, T.Y.; Tsui, H.C. Characterisation of Crassicauda fuelleborni nematode infection in Indo-Pacific finless porpoises (Neophocaena phocaenoides) using postmortem computed tomography. Int. J. Parasitol. Parasites Wildl. 2022, 18, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.C.W.; Ho, H.H.N.; Leung, E.K.C.; Chung, T.YT.; Tsui, H.C.L. Characterisation of Crassicauda fuelleborni nematode infection in Indo-Pacific finless porpoises (Neophocaena phocaenoides) using postmortem computed tomography. Int. J. Parasitol. Parasites Wildl. 2022, 18, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, R.H. Taxonomy and distribution of a Crassicauda species (Nematoda: Spirurida) infecting the kidney of the common fin whale (Balaenoptera physalus Linné, 1758). J. Parasitol. 1985, 71, 485–488. [Google Scholar] [CrossRef]
- Robinson, W.F.; Robinson, N.A. Cardiovascular system. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; Vol. 3, pp. 1–101.e1. [Google Scholar]
- Bossart, G.D.; Odell, D.K.; Altman, N.H. Cardiomyopathy in stranded pygmy and dwarf sperm whales. J. Am. Vet. Med. Assoc. 1985, 187, 1137–1140. [Google Scholar] [CrossRef]
- Bryan, C.E.; Bossart, G.D.; Christopher, S.J.; Davis, W.C.; Kilpatrick, L.E.; McFee, W.E.; et al. Selenium protein identification and profiling by mass spectrometry: A tool to assess progression of cardiomyopathy in a whale model. J. Trace Elem. Med. Biol. 2017, 44, 40–49. [Google Scholar] [CrossRef]
- Sierra, E.; de los Monteros, A.E.; Fernández, A.; Arbelo, M.; Caballero, M.J.; Rivero, M.; et al. Sarcoplasmic masses in the skeletal muscle of a stranded pigmy sperm whale (Kogia breviceps). J. Wildl. Dis. 2013, 49, 679–683. [Google Scholar] [CrossRef]
- Abbas, A.; Kumar, V.; Aster, J. Environmental and nutritional diseases. In Robbins & Cotran Pathologic Basis of Disease, 10th ed.; Elsevier: Philadelphia, PA, USA, 2021; pp. 391–436. [Google Scholar]
- IJsseldijk, L.L.; Hessing, S.; Mairo, A.; ten Doeschate, M.T.I.; Treep, J.; van den Broek, J.; et al. Nutritional status and prey energy density govern reproductive success in a small cetacean. Sci. Rep. 2021, 11, 19201. [Google Scholar] [CrossRef]
- Puig-Lozano, R.; Bernaldo de Quirós, Y.; Díaz-Delgado, J.; García-Álvarez, N.; Sierra, E.; de la Fuente, J.; et al. Retrospective study of foreign body-associated pathology in stranded cetaceans, Canary Islands (2000–2015). Environ. Pollut. 2018, 243, 1840–1852. [Google Scholar] [CrossRef]
- Pallin, L.J.; Garrigue, C.; Kellar, N.M.; Baker, C.S.; Bonneville, C.D.; Derville, S.; et al. Demographic and physiological signals of reproductive events in humpback whales on a southwest Pacific breeding ground. Conserv. Physiol. 2024, 12, coae002. [Google Scholar] [CrossRef]
- Raverty, S.; St. Leger, J.; Noren, D.P.; Burek Huntington, K.; Rotstein, D.S.; Gulland, F.M.D.; et al. Pathology findings and correlation with body condition index in stranded killer whales (Orcinus orca) in the northeastern Pacific and Hawaii from 2004 to 2013. PLoS ONE 2020, 15, e0242505. [Google Scholar] [CrossRef]
- Albrecht, S.; Minto, C.; Rogan, E.; Deaville, R.; O’Donovan, J.; Daly, M.; et al. Emaciated enigma: Decline in body conditions of common dolphins in the Celtic Seas ecoregion. Ecol. Evol. 2024, 14, e70325. [Google Scholar] [CrossRef] [PubMed]
- Kebke, A.; Samarra, F.; Derous, D. Climate change and cetacean health: Impacts and future directions. Philos. Trans. R. Soc. B 2022, 377, 20210147. [Google Scholar] [CrossRef]
- Owen, K.; Thompson, R.; Donnelly, D.; Noad, M.; Bury, S.; Pinkerton, M.; et al. Southern Ocean humpback whale trophic ecology. II. Influence of fasting and opportunistic feeding on skin stable isotope values of migrating whales. Mar. Ecol. Prog. Ser. 2024, 734, 157–171. [Google Scholar] [CrossRef]
- Van Aswegen, M.; Szabo, A.; Currie, J.J.; Stack, S.H.; West, K.L.; Hofmann, N.; et al. Energetic cost of gestation and prenatal growth in humpback whales. J. Physiol. 2025, 603, 529–550. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, F.; Madsen, P.T.; Andrews-Goff, V.; Double, M.C.; How, J.R.; Clapham, P.; et al. Extreme capital breeding for giants: Effects of body size on humpback whale energy expenditure and fasting endurance. Ecol. Modell. 2025, 501, 110994. [Google Scholar] [CrossRef]
- McHuron, E.A.; Adamczak, S.; Costa, D.P.; Booth, C. Estimating reproductive costs in marine mammal bioenergetic models: A review of current knowledge and data availability. Conserv. Physiol. 2023, 11, coac080. [Google Scholar] [CrossRef]
- Martínez-Burnes, J.; Mota-Rojas, D.; Villanueva-García, D.; Ibarra-Ríos, D.; Lezama-García, K.; Barrios-García, H.; et al. Meconium aspiration syndrome in mammals. CAB Rev. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Colegrove, K.M.; Venn-Watson, S.; Litz, J.; Kinsel, M.J.; Terio, K.A.; Fougeres, E.; et al. Fetal distress and in utero pneumonia in perinatal dolphins during the Northern Gulf of Mexico unusual mortality event. Dis. Aquat. Organ. 2016, 119, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Groch, K.; Catão-Dias, J.; Kolesnikovas, C.; de Castilho, P.; Moreira, L.; et al. Pathologic findings and causes of death in southern right whales Eubalaena australis, Brazil. Dis. Aquat. Organ. 2019, 137, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Laist, D.W.; Knowlton, A.R.; Mead, J.G.; Collet, A.S.; Podesta, M. Collisions between ships and whales. Mar. Mamm. Sci. 2001, 17, 35–75. [Google Scholar] [CrossRef]
- Ritter, F.; Panigada, S. Collisions of vessels with cetaceans—The underestimated threat. In World Seas: An Environmental Evaluation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 531–547. [Google Scholar]
- Schoeman, R.P.; Patterson-Abrolat, C.; Plön, S. A global review of vessel collisions with marine animals. Front. Mar. Sci. 2020, 7, 292. [Google Scholar] [CrossRef]
- Manuel, C.; Ritter, F. Increasing numbers of ship strikes in the Canary Islands: Proposals for immediate action to reduce risk of vessel–whale collisions. J. Cetacean Res. Manag. 2010, 11, 131–136. [Google Scholar]
- Arregui, M.; de Quirós, Y.B.; Saavedra, P.; Sierra, E.; Suárez-Santana, C.M.; Arbelo, M.; et al. Fat embolism and sperm whale ship strikes. Front. Mar. Sci. 2019, 6, 379. [Google Scholar] [CrossRef]
- Peel, D.; Smith, J.N.; Childerhouse, S. Vessel strike of whales in Australia: The challenges of analysis of historical incident data. Front. Mar. Sci. 2018, 5, 69. [Google Scholar] [CrossRef]
- Izadi, S.; Johnson, M.; de Soto, N.A.; Constantine, R. Night-life of Bryde’s whales: Ecological implications of resting in a baleen whale. Behav. Ecol. Sociobiol. 2018, 72, 130. [Google Scholar] [CrossRef]
- Neilson, J.L.; Gabriele, C.M.; Jensen, A.S.; Jackson, K.; Straley, J.M. Summary of reported whale-vessel collisions in Alaskan waters. J. Mar. Biol. 2012, 2012, 106282. [Google Scholar] [CrossRef]
- Winkler, C.; Murphy, S.; Ritter, F.; Panigada, S.; Murphy, S. Global numbers of ship strikes: An assessment of collisions between vessels and cetaceans using available data in the IWC Ship Strike Database. Report to the International Whaling Commission, 2020. Available online: www.m-e-e-r.org (accessed on 12 January 2026).
- Fais, A.; Lewis, T.P.; Zitterbart, D.P.; Álvarez, O.; Tejedor, A.; Soto, N.A. Erratum: Abundance and distribution of sperm whales in the Canary Islands: Can sperm whales in the archipelago sustain the current level of ship-strike mortalities? PLoS ONE 2016, 11, e0150660. [Google Scholar]
- Constantine, R.; Johnson, M.; Riekkola, L.; Jervis, S.; Kozmian-Ledward, L.; Dennis, T.; et al. Mitigation of vessel-strike mortality of endangered Bryde’s whales in the Hauraki Gulf, New Zealand. Biol. Conserv. 2015, 186, 149–157. [Google Scholar] [CrossRef]
- Campbell-Malone, R.; Barco, S.G.; Daoust, P.-Y.; Knowlton, A.R.; McLellan, W.A.; Rotstein, D.S.; et al. Gross and histologic evidence of sharp and blunt trauma in North Atlantic right whales (Eubalaena glacialis) killed by vessels. J. Zoo Wildl. Med. 2008, 39, 37–55. [Google Scholar] [CrossRef]
- Moore, M.J.; van der Hoop, J.; Barco, S.G.; Costidis, A.M.; Gulland, F.M.; Jepson, P.D.; et al. Criteria and case definitions for serious injury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Dis. Aquat. Organ. 2013, 103, 229–264. [Google Scholar] [CrossRef]
- De Siqueira, A.; Cuevas, S.E.C.; Salvagni, F.A.; Maiorka, P.C. Forensic veterinary pathology. Vet. Pathol. 2016, 53, 979–987. [Google Scholar] [CrossRef]
- International Whaling Commission. Bycatch and entanglement of cetaceans in fishing. Available online: https://iwc.int/bycatch (accessed on 12 January 2026).
- Masere, C.; Baird, K. An initial exploration of cetacean bycatch and interactions in the WCPFC; Western and Central Pacific Fisheries Commission (WCPFC).
- Read, A.J.; Drinker, P.; Northridge, S. Bycatch of marine mammals in U.S. and global fisheries. Conserv. Biol. 2006, 20, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Puig-Lozano, R.; Fernández, A.; Sierra, E.; Saavedra, P.; Suárez-Santana, C.M.; de la Fuente, J.; et al. Retrospective study of fishery interactions in stranded cetaceans, Canary Islands. Front. Vet. Sci. 2020, 7, 571. [Google Scholar] [CrossRef]
- Reeves, R.; McClellan, K.; Werner, T. Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endang. Species Res. 2013, 20, 71–97. [Google Scholar] [CrossRef]
- Martino, L.; Leiva Forns, M.; Cid Cañete, M.; Pérez, L.; Pradas, C.; Domingo, M. Bycatch in cetaceans from the north-western Mediterranean Sea: Retrospective study of lesions and utility of bycatch criteria. Vet. Sci. 2025, 12, 711. [Google Scholar] [CrossRef]
- Elliott, B.; Kiszka, J.J.; Bonhommeau, S.; Shahid, U.; Nelson, L.; Read, A.J. Bycatch in drift gillnet fisheries: A sink for Indian Ocean cetaceans. Indian Ocean Tuna Commission (IOTC), 1992. Available online: https://iotc.org/cmms (accessed on 12 January 2026).
- Basran, C.J.; Sigurðsson, G.M. Using case studies to investigate cetacean bycatch/interaction under-reporting in countries with reporting legislation. Front. Mar. Sci. 2021, 8, 788562. [Google Scholar] [CrossRef]
- Segniagbeto, G.H.; Van Waerebeek, K.; Bowessidjaou, J.E.; Ketoh, K.; Kpatcha, T.K.; Okoumassou, K.; et al. Annotated checklist and fisheries interactions of cetaceans in Togo, with evidence of Antarctic minke whale in the Gulf of Guinea. Integr. Zool. 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Moura, J.F.; Acevedo-Trejos, E.; Tavares, D.C.; Meirelles, A.C.O.; Silva, C.P.N.; Oliveira, L.R.; et al. Stranding events of Kogia whales along the Brazilian coast. PLoS ONE 2016, 11, e0146108. [Google Scholar] [CrossRef]
- Cardona-Maldonado, M.A.; Mignucci-Giannoni, A. Pygmy and dwarf sperm whales in Puerto Rico and the Virgin Islands, with a review of Kogia in the Caribbean. Caribb. J. Sci. 1999, 35, 29–37. [Google Scholar]
- Eisfeld-Pierantonio, S.M.; Pierantonio, N.; Simmonds, M.P. The impact of marine debris on cetaceans with consideration of plastics generated by the COVID-19 pandemic. Environ. Pollut. 2022, 300, 118967. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.; Silva, M.A.; Pham, C.K.; Duncan, E.M. Cetaceans playing with single-use plastics (SUPs): A widespread interaction with likely severe impacts. Mar. Pollut. Bull. 2023, 194, 115428. [Google Scholar] [CrossRef] [PubMed]
- Alexiadou, P.; Foskolos, I.; Frantzis, A. Ingestion of macroplastics by odontocetes of the Greek Seas, Eastern Mediterranean: Often deadly! Mar. Pollut. Bull. 2019, 146, 67–75. [Google Scholar] [CrossRef]
- Zimmer-Correa, M.; Carneiro Proietti, M.; Couto Di Tullio, J.; Rodrigues, L.S.; Quadro Oreste, E.; Kessler, F.; et al. Plastic ingestion by odontocetes from the Western South Atlantic: A particular concern to a threatened species. Environ. Pollut. 2024, 360, 124659. [Google Scholar] [CrossRef]
- Redaelli, L.; Alcázar-Treviño, J.; Escánez, A.; Fernandez, M.; Aguilar de Soto, N.; Alves, F.; et al. Acoustic mimicry of plastics with the prey of deep-cetaceans: An experimental approach. In Proceedings of the International Conference on Underwater Acoustics, 2024; Institute of Acoustics. [Google Scholar]
- Whitaker, B.R.; Geraci, J.R.; St. Aubin, D.J. The near-fatal ingestion of plastic by a pygmy sperm whale, Kogia breviceps. VIN document, 1994. Available online: https://www.vin.com/doc/?id=3864102 (accessed on 12 January 2026).
- West, K.; Silva-Krott, I.; Rotstein, D.; Levine, G. U.S. Navy Marine Species Monitoring Program. Causes of mortality and pathologic findings in Pacific Island cetaceans. 2023. Available online: https://www.navymarinespeciesmonitoring.us/index.php/download_file/view/3092/ (accessed on 12 January 2026).
- Knowlton, A.R.; Kraus, S.D. Mortality and serious injury of northern right whales (Eubalaena glacialis) in the western North Atlantic Ocean. J. Cetacean Res. Manag. 2020, 21, 193–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




