Submitted:
29 December 2025
Posted:
30 December 2025
You are already at the latest version
Abstract
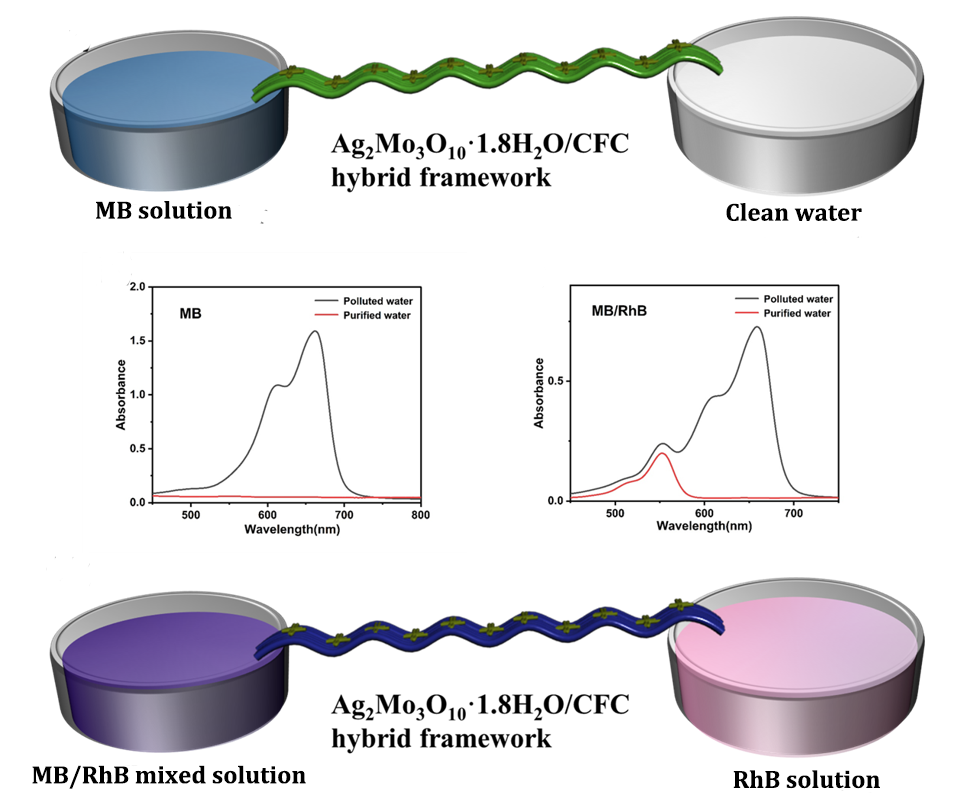
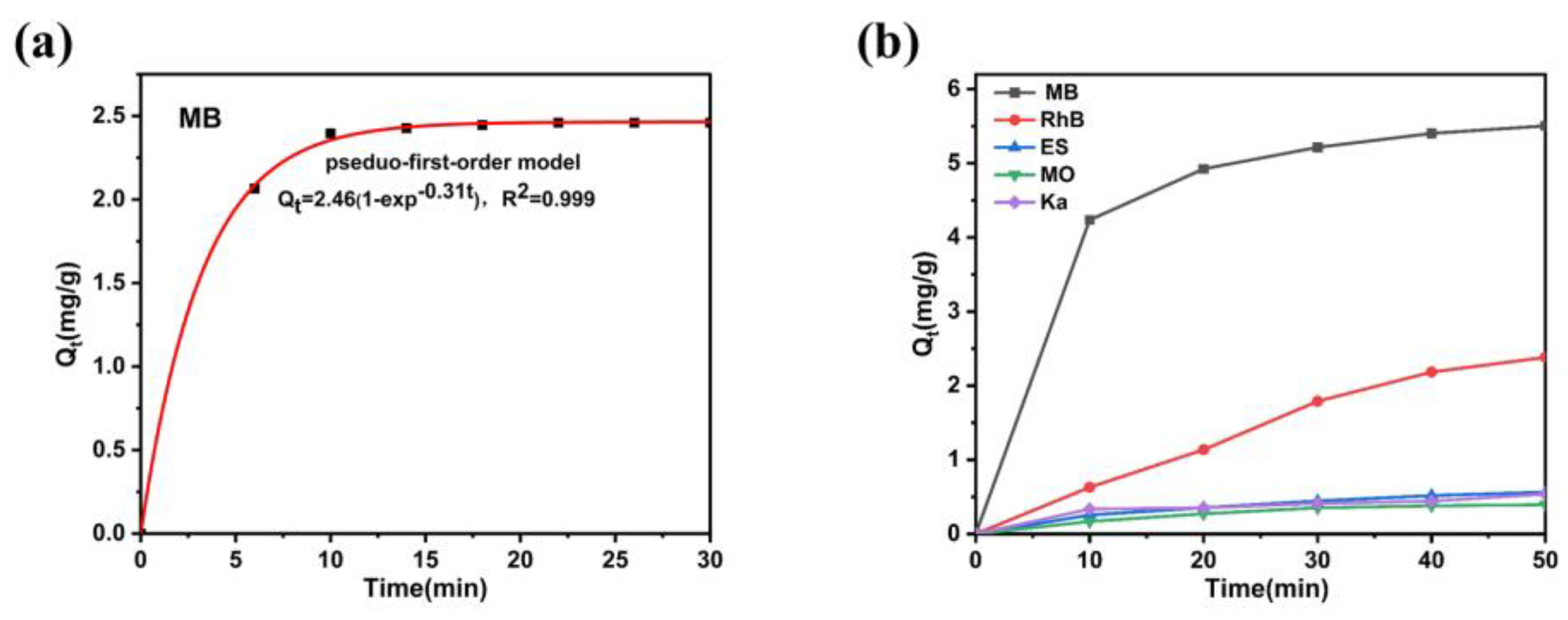
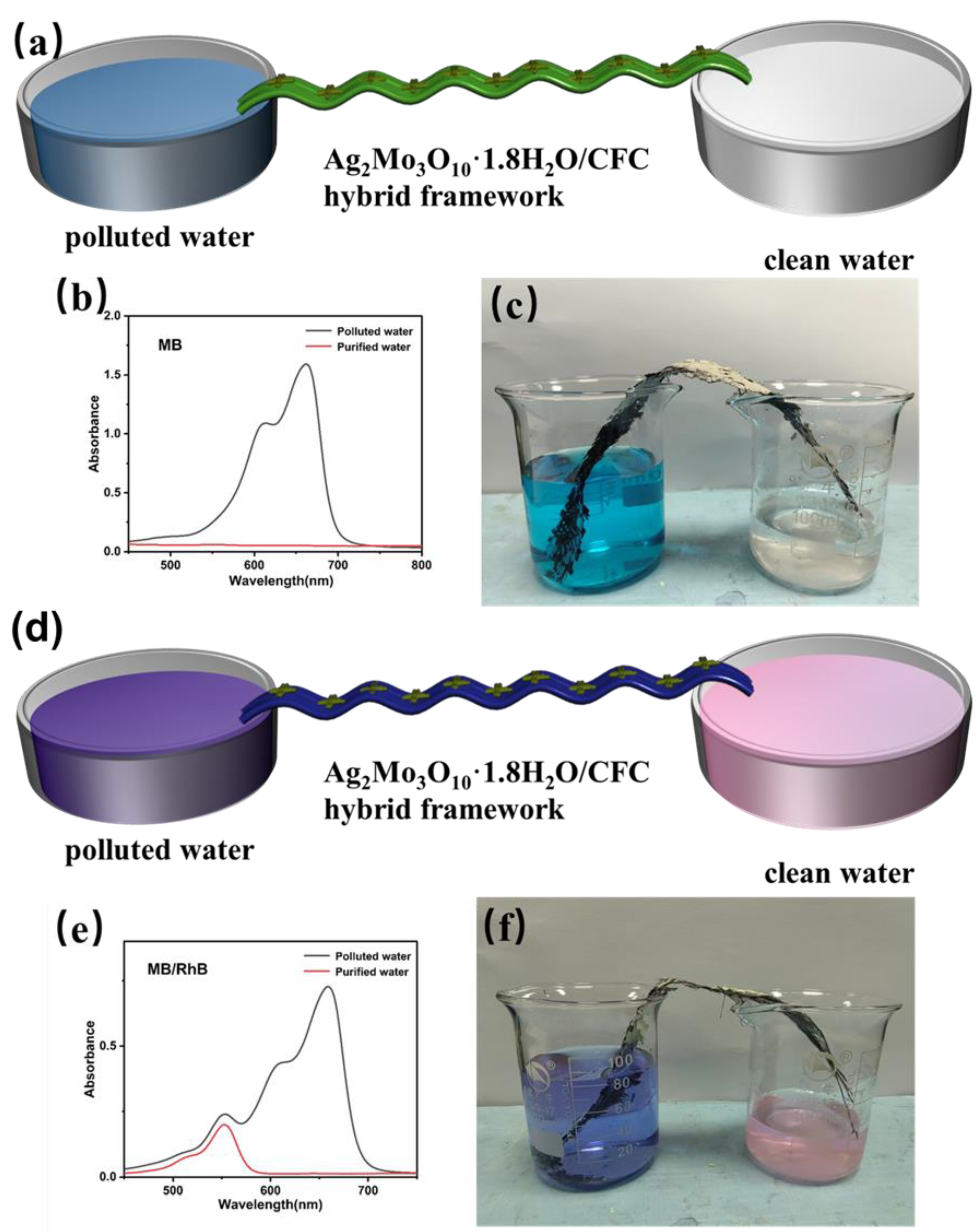
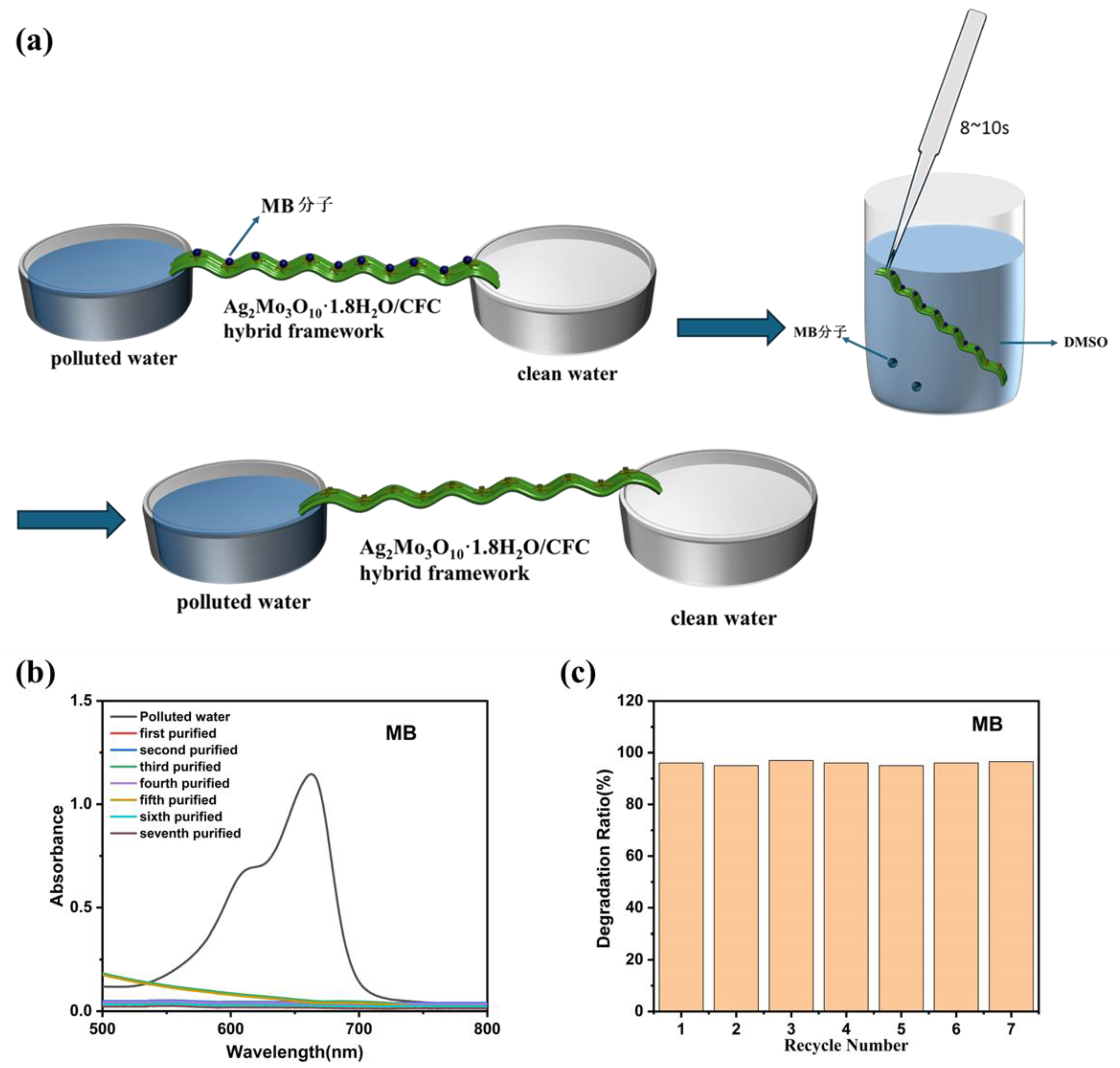
This study presents a novel membrane-inspired Ag₂Mo₃O₁₀·1.8H₂O/carbon fiber cloth (CFC) hybrid framework designed for the continuous and selective recovery of high-value sulfur-containing molecules from organic wastewater. The framework was fabricated by uniformly growing Ag₂Mo₃O₁₀·1.8H₂O nanowires on CFC membrane, forming a hierarchical porous network with abundant micro-nano channels that facilitate efficient, capillary-driven water transport. Owing to its mesoporous structure and specific Ag-S coordination affinity, the material exhibits excellent selectivity for sulfur-containing dyes, achieving rapid adsorption (>94% removal of methylene blue within 10 minutes) and high specificity in mixed solutions. Moreover, the hybrid framework demonstrates outstanding reusability, retaining high recovery efficiency over multiple cycles. A continuous-flow system based on this framework operates without external pressure and achieves a water transport rate of 1875 mL·h-1·m-2. These results underscore the potential of the Ag₂Mo₃O₁₀·1.8H₂O/CFC system as an efficient, scalable, and sustainable platform for industrial wastewater resource recovery.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Details Materials
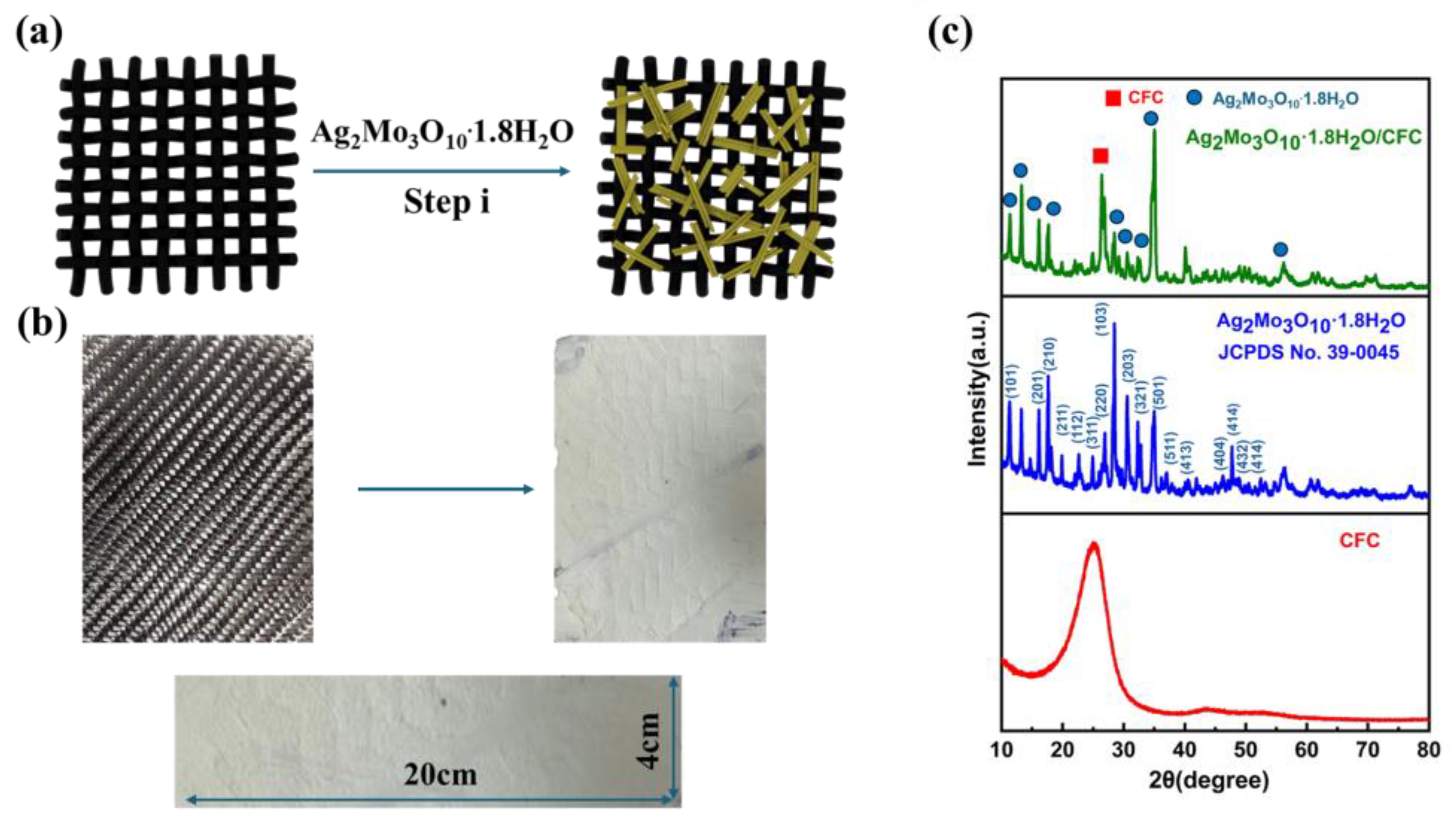
2.2. Preparation of a Ag₂Mo₃O₁₀·1.8H₂O /Carbon Framework
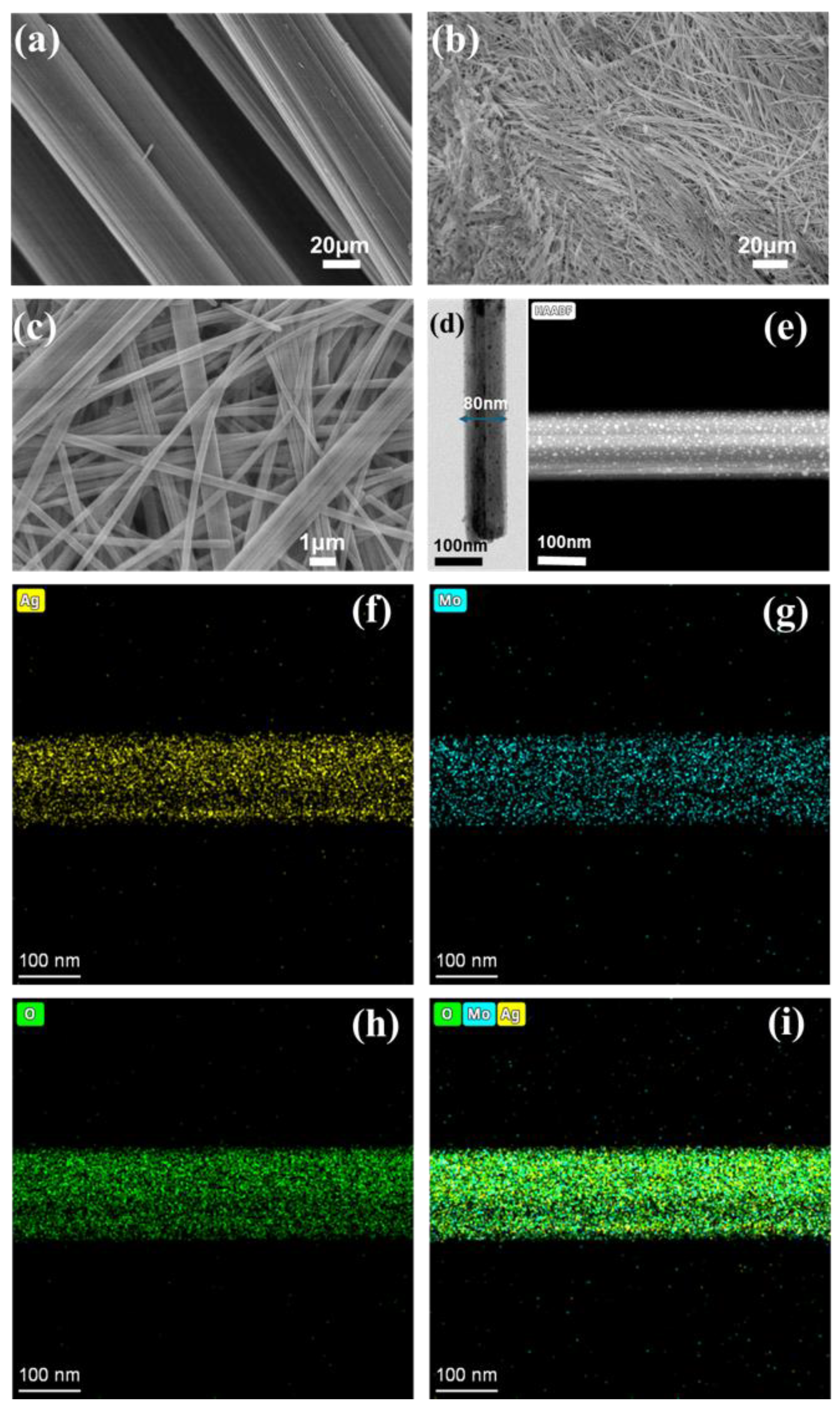
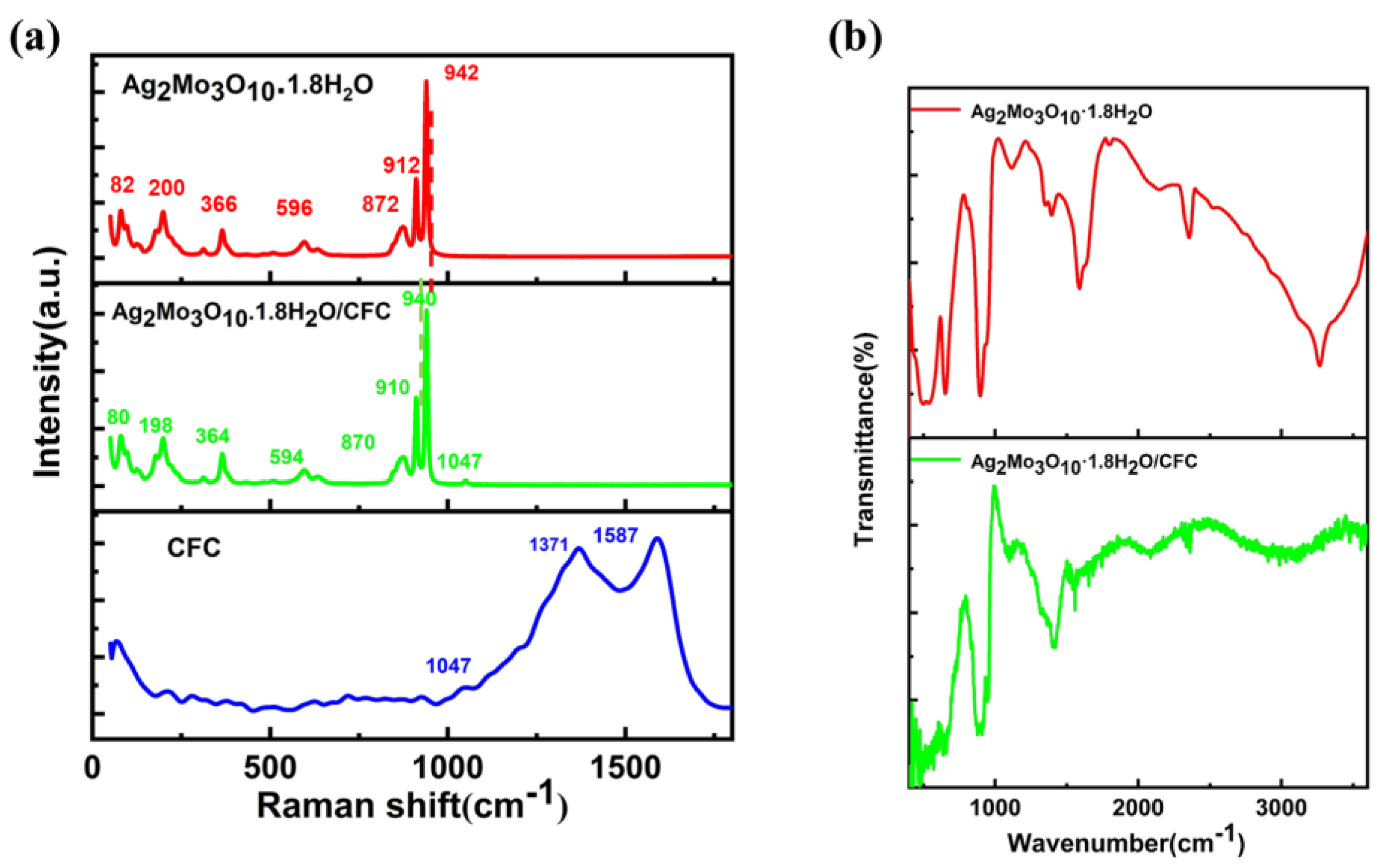
2.3. Characterization
2.4. Water Purification and Resource Utilization Test
3. Results and Discussion
3.1. Formation of the Ag₂Mo₃O₁₀·1.8H₂O /CFC Framework
4. Conclusions
Funding
Author contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Zhang, Z.; et al. Laccase immobilized on nanocomposites for wastewater pollutants degradation: current status and future prospects. Bioprocess and Biosystems Engineering 2023, 46, 1513–1531. [Google Scholar] [CrossRef]
- Chan- Shih, S.; Wu- Jung, H. Improving the Performance of the Reverse Osmosis Process with Fiber filter and Ultrafiltration: Promoting Municipal Sewage Reclamation and reuse for Industrial Processes. Sustainability 2022, 14, 5443–5443. [Google Scholar] [CrossRef]
- Wu-Chun, D.; Zhu-Kai, D.; Hu-Xin, K.; et al. Ozone microbubbles treatment with different gas-liquid mixing conditions and its application on printing and dyeing wastewater and Escherichia coli. Desalination and Water Treatment 2022, 252, 400–407. [Google Scholar]
- Aleksandra, K.; Izabela, K. From Environmental Threat to Control: A Review of Technologies for Removal of Quaternary Ammonium Compounds from Wastewater. Membranes 2025, 16, 1. [Google Scholar] [CrossRef]
- Yang, F.; Wang-Xing, B. Research recap of membrane technology for tannery wastewater treatment: a review. Collagen and Leather 2023, 5. [Google Scholar] [CrossRef]
- Wang, F.; Hou- Xin, Y. Preparation of Cu-MnO2/GO/PVDF Catalytic Membranes via Phase Inversion Method and Application for Separation Removal of Dyes. Membranes 2025, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Xie- Ya, W.; Liu- Ze, W.; et al. Water Purification Efficiency and Membrane Fouling Behavior of Ceramic Membrane-Nanofiltration in Treating Water Treatment Plant Production Wastewater. Membranes 2025, 15, 387. [Google Scholar] [CrossRef]
- Saheli, R. Carbon Nanotube and Graphene Oxide-Based Polymer Nanocomposite Membranes for Oil–Water Separation—Recent Advances and Future Prospects. ChemistrySelect 2025, 10. [Google Scholar]
- Mei, Y.; Su-Yan, H.; Li, Z.; Bai- Shi, Q.; Yuan- Meng, Y.; Li, L.; Yan, Z.; Wu, J.; Zhu- Lian, W. BiOBr nanoplates@TiO2 nanowires/carbon fiber cloth as a functional water transport network for continuous flow water purification. Dalton Transactions 2017, 46, 347. [Google Scholar] [CrossRef]
- Eoin, P.M.; Alexa, E.; et al. Polymer/iron-oxide nanocomposite adsorbents for cycled magnetically-enabled extraction of aqueous micropollutants. RSC Sustainability 2025. [Google Scholar]
- Ahmah, R.; Saeedeh, H.; et al. Retraction notice to "Preparation and characterization of nano composites from metal oxides and activated carbon from banana peel (MO@BPAC, MO=NiO, CuO and ZnO) for 2 nitrophenol removal from aqueous solutions. Heliyon 2025, 11, e41245. [Google Scholar]
- Muni, R.; Sumalatha, B. A prototype integrated approach for sustainable treatment of organic dyes system using ZnO–CuO–AgO heterostructure as photocatalyst. In Scientific Reports; 2025. [Google Scholar]
- Vahid, H.; Gholamreza, K.; Dariush, M. High-efficiency removal of methyl orange from wastewaters using polyimide/chitosan-MoS2-UiO-66 nanofiber adsorbents. In Scientific Reports; 2025. [Google Scholar]
- Tu, C.; Le, T. g-C3N4 Nanosheet/ZnO Nanosponge Heterojunctions for Efficient Visible-Light Photocatalytic Degradation of Methylene Blue. ChemNanoMat 2025. [Google Scholar]
- Tu-Wen, J.; Cai-Wei, Q. Selective adsorption of hazardous substances from wastewater by hierarchical oxide composites: A review. Toxics 2024, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Xu-Ke, Y.; Wang, L.; Zhang-Yu, X.; et al. Graphene oxide enabled self-assembly of silver trimolybdate nanowires into robust membranes for nanosolid capture and molecular separation. Nanoscale 2023, 15, 6607–6618. [Google Scholar] [CrossRef]
- Jing, Z.; Victoria, S.C. Adsorption of octahedral mono-molybdate and poly-molybdate onto hematite: A multi-technique approach. Journal of Hazardous Materials 2022, 431, 128564. [Google Scholar]
- Yang, L.; Hong, Z.H.; Wu, J.; Singh, H.K.; Yeole, K.V.; Mhaske, S.T.; et al. RSC Advances 2014, 4, 25556. H.K. Singh, K.V. Yeole, S.T. Mhaske. Synthesis and characterization of layer-by-layer assembled magnesium zinc molybdate nanocontainer for anticorrosive application. Chemical Engineering Journal. 2016,7: 414-426.
- Young-Jung, H.; Mira, P.; Woo-Seok, K.; Kyong-Yop, R.; Soo-Jin, P. Preparation and characterization of carbon black/pitch-based carbon fiber paper composites for gas diffusion layers. Composites Part B 2019, 159, 362–368. [Google Scholar]
- Huang- Shan, C.; Ye, J.; Su- Mi, M.; Zhang, Y.; Meng, Y.; Meng, X.; Xia- Xin, X. Efficient preparation and characterization of carbon fiber paper using phenolic resin in-pulp addition method. International Journal of Hydrogen Energy 2024, 71, 506–514. [Google Scholar] [CrossRef]
- Vipin, K.; Sebastian, M.; et al. Design of Mixed-Metal Silver Decamolybdate Nanostructures for High Specific Energies at High Power Density. Advanced Materials 2016, 8, 6966–6975. [Google Scholar]
- Pennycook, SJ; Nellist, P. D. Scanning transmission electron microscopy: imaging and analysis; Springer: Berlin, 2011. [Google Scholar]
- Yang- Xiang, L.; Wang, Y.; Xu, X.; Qu, Y.; Ding, X.; Chen, H. Surface plasmon resonance-induced visible-light photocatalytic performance of silver/silver molybdate composites. Chinese Journal of Catalysis 2017, 38, 260–269. [Google Scholar] [CrossRef]
- Moura, JVB; Ferreira, W.C.; da Silva-Filho, J.G.; Alabarse, F.G.; Freire, P.T.C.; Luz-Lima, C. Ag2Mo3O10 nanorods under high pressure: In situ Raman spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2023, 299, 122871. [Google Scholar] [CrossRef]
- Nagaraju, G; Chandrappa, G.T.; Livage, J. Synthesis and characterization of silver molybdate nanowires, nanorods and multipods. Bulletin of Materials Science 2008, 31, 367–371. [Google Scholar] [CrossRef]
- Jiang- De, M.; Chen- Hui, N.; Xie, H.; Cheng, K.; Li, L.; Xie, K.; Wang- Yu, Q. Fe, N, S co-doped cellulose paper carbon fibers as an air-cathode catalyst for microbial fuel cells. Environmental Research 2023, 221, 115308. [Google Scholar] [CrossRef]
- Joel- Marcos, S.; Assad, U. K.; Liu- Tian, Y; Xu, Z; Alan, R. E.; Liu- Guo, L. Capacitive Organic Dye Removal by Block Copolymer Based Porous Carbon Fibers. In Advanced Materials Interfaces.; 2020. [Google Scholar]
- Zhang- Shou, W.; Yang- Hong, C.; Huang- Hui, Y.; Gao- Hui, H.; Wang- Xiang, X.; Cao- Ru, Y.; Li- Jia, X.; Xu- Xi, J.; Wang- Xiang, K. Unexpected ultrafast and high adsorption capacity of oxygen vacancy-rich WOx/C nanowire networks for aqueous Pb2+ and methylene blue removal Journal of Materials. Chemistry A 2017, 1–11. [Google Scholar]
- Qu- Tong, Q.; Huang- Xi, Y.; Wang, B. Effects of the Surface Structure on the Water Transport Behavior in PEMFC Carbon Fiber Papers. ACS Omega 2022, 7, 5992−5997. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, ANC; Ferreira, W.C.; Duarte, A.V. In situ high-temperature Raman scattering study of monoclinic Ag2Mo2O7 microrods. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2023, 295, 122632. [Google Scholar] [CrossRef] [PubMed]
- Maria Kdo Nascimento- Silva, L.; João, V.B.M. Silver Trimolybdate (Ag2Mo3O10.2H2O) Nanorods: Synthesis, Characterization, and Photo-Induced Antibacterial Activity under Visible-Light Irradiation. Bioinorganic Chemistry and Applications 2024. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



