1. Introduction
With an estimated population of 1 million in the United States (US), transgender and gender-diverse (TGD) individuals face unique healthcare disparities exacerbated by a lack of TGD-focused medical research [
1,
2]. Healthcare needs of the LGBTQ+ (lesbian, gay, bisexual, transgender, queer, and more) community differ from those of heteronormative populations, and the limited research focusing on this population places their long-term health at risk [
1,
3]. Estimates of TGD adolescents in the US are anywhere between 1 and 6 million children, all of whom have unique healthcare needs [
4,
5]. For these individuals, Gonadotropin-releasing hormone agonist (GnRHa) puberty blockers can aid in treating peripubertal gender dysphoria, alleviating psychosocial stress caused by the incongruity between sex assigned at birth and gender identity. While the psychosocial benefits of GnRHa treatment are well founded, with recent confirmation of GnRHa puberty blocking treating gender dysphoria, the systemic physiological effects of this treatment remain partly a mystery – particularly in organs with high endocrine sensitivity, such as the liver.
Originally synthesized 60 years ago, GnRHa drugs are FDA-approved for treating central precocious puberty (CPP), a condition where puberty starts at a detrimentally young age [
6]. In adults, GnRHa is used to treat sex-specific disorders and cancers, including endometriosis, IVF fertility, and prostate cancer [
7,
8]. Over the past 30 years, these agents have been used as an off-label treatment for adolescent gender dysphoria [
9]. Despite their widespread use, the long-term side effects of GnRHa treatment during adolescence are not fully known. In recent FDA petitions, heart and brain damage have been added to the list of potential side effects from GnRHa use [
10,
11].
GnRHa induces this ‘puberty blocked’ phenotype by interrupting the production and secretion of sex-specific hormones such as estrogen and testosterone at the start of puberty. Disruptions in these hormone levels are often linked to an increased risk of hepatic damage in both males and females, with sex-specific injury patterns Moreover, the liver displays distinct sexual dimorphic variations, including hepatocyte size, vascular architecture, and the abundance of specific cell types within the liver [
12]. Overall, female livers exhibit a greater hepatoprotective capacity and display quicker recovery rates than male livers [
12,
13]. Given the liver's critical role, the disruption in sex-hormone levels induced by GnRHa puberty blocking may cause liver injuries.
Hepatic injury is characterized by oxidative stress, inflammation, and liver fibrosis. Without removing the cause of injury, this can change injury-response cell populations and increase fibrogenesis due to prolonged and amplified inflammatory responses. Liver injury frequently is caused by increased cellular metabolism generating damaging reactive oxygen species (ROS). Thus increased oxidative stress is often the first sign of liver injury [
14,
15]. Increased levels of ROS further induces mitochondrial and DNA oxidative damage, and if not accommodated for, leads to fibrosis and hepatocellular apoptosis [
14,
15,
16]. DNA and mitochondrial oxidative damage can be measured through 8-hydroxy-2'-deoxyguanosine (8-OHdG) and malondialdehyde (MDA), respectively.
Beyond oxidative stress, molecular mechanisms of hepatic inflammation are coordinated through proinflammatory signals, including tissue inhibitor of metalloproteinases (TIMP)1 and transforming growth factor beta (TGFβ)1 [
17,
18]. Liver injury stimuli, such as TIMP1 and TGFβ1, often promote the infiltration of leukocytes (marked CD45+), including Kupffer cells, dendritic cells, neutrophils, and lymphocytes [
18,
19]. Similarly, other hepatic injury-response cells also recruited by TGFβ1 include hepatic stellate cells (HSC) and cholangiocytes (CK19+) [
20,
21]. Intrahepatic cholangiocytes can activate and incite further injury responses, including differentiating into hepatocytes and inciting biliary fibrogenesis through myofibroblast stimulation [
22,
23]. This process is termed ductular reaction (DR) and is characterized by an increased presence of CK19+ cells, as cholangiocytes become a reservoir for hepatocyte replacement during prolonged injury [
23,
24].
Hepatic inflammatory responses, including DR, are often accompanied by fibrosis, which is characterized by increased deposition of collagen fibers and expression of profibrotic biomarkers [
25]. Alpha smooth muscle actin (〈SMA) promotes this fibrotic response, and the simultaneous increased expression of TIMP1 limits the breakdown of collagen fibers [
21,
25]. When the injury stimulus is removed during the final stage of liver injury response, TIMP1 levels decrease, signaling tissue remodeling and replacing excess collagen fibers with healthy hepatocytes [
25].
Within this context, the effect of GnRHa in the liver is largely unknown. This study, therefore, investigates whether GnRHa puberty blocking results in oxidative stress, inflammation, and fibrosis, and whether these effects differ between biological sexes.
2. Materials and Methods
2.1. Animal Trial
An adolescent rodent model was used to mimic the peripubertal timeframe of GnRHa puberty blocking. Rats enter puberty at three to four weeks of age and reach their adult form by eight weeks of age [
26]. The animal trial adhered to Institutional Animal Care and Use Committee-approved protocols under the Animal Welfare Act guidelines (protocol: 2301CD-DH-R-26). Male and female Sprague-Dawley rats at three weeks-of-age were sourced from Envigo (Indianapolis, Indiana). Following a week of acclimation to standard housing conditions, including a 12:12 light/dark cycle and ad libitum access to water and standard rat chow, the trial started with 4-week-old rats, aligning with this species’ pubertal onset [
26]. Animals were randomly assigned to the control group (saline) or the treatment group (GnRHa), with five to six animals of both sexes in each group. Based on a previous study by Roth et al., GnRHa groups received a daily subcutaneous injection of 100 υg triptorelin (Creative Peptides (Shirley, NY USA) Cat #110967) – denoted as GnRHa, while control animals received a daily saline injection [
27]. Animal body weights were monitored weekly. After 4 weeks of daily GnRHa administration (or control), animals were humanely sacrificed using a 50 mg/kg dose of sodium pentobarbital.
2.2. Tissue Processing
Following the end of the animal trial, liver tissues were collected and processed in two ways: 1) mounting in optimal cutting temperature (OCT) media, snap freezing in liquid nitrogen, and storing at -80ºC; 2) fixing in neutral buffered formalin for 24 hours, followed by incubating in 70% ethanol for 24 hours, and then the tissues are sent to iHisto company (Salem, MA) for mounting in paraffin blocks.
2.3. Oxidative Damage
Low levels of ROS, the source of oxidative damage, are expected and accommodated within healthy cells [
14]. During cellular stress, ROS levels increase above the level of endogenous antioxidants, and the resulting mitochondrial, lipid, and DNA oxidative damage causes cellular dysfunction [
15]. Thus, the expression of 8-OHdG (dilution 1:50; Santa Cruz Biotechnology Cat# sc-66036, RRID:AB_832272), a sign of DNA oxidative damage was evaluated via immunofluorescence (IF) performed using 8 μm thick OCT sectioned liver slides according to the following simplified steps: 1) fix slides in 10% neutral buffered formalin for 10 minutes; 2) rinse in cold phosphate-buffered saline (PBS); 3) incubate in permeabilization solution (0.1% Triton X-100 (intracellular target) or 1% Tween 20 (extracellular) in PBS); 4) rinse in PBS; 5) incubate in 1% goat serum blocking buffer for 20 minutes; 6) incubate in primary antibody overnight in 4ºC; 7) rinse slides in PBS; 8) incubate in permeabilization solution for 10 minutes; 9) incubate in secondary antibody for 60 minutes; 10) Rinse in PBS; 11) Mount in fluorescent DAPI media and seal sides with nail polish. Slides were stored in the dark at -20ºC until imaged using a Zeiss 700 confocal microscope. Further, the level of mitochondrial oxidative stress in liver tissues was measured through a thiobarbituric acid-reactive substance (TBARS) MDA assay using homogenized snap-frozen liver tissues (catalog #10009055, Caymen Chemical, Michigan, USA).
2.4. Inflammatory Response
To measure the injury response of leukocyte infiltration caused by peripubertal GnRHa administration was measured via IHC specific for CD45+ cells (dilution 1:50; Santa Cruz Biotechnology Cat# sc-1178, RRID:AB_627074) according to the following simplified protocol: 1) deparaffinize; 2) incubate in 6% H202 and methanol to block exogenous peroxides for 5 minutes; 3) rinse in water; 4) pressure cook in citrate buffer for 30 minutes; 5) rinse in water; 6) wash in tris-buffered saline with 0.1% Tween 20 (TBST) for 5 minutes; 7) incubate in horse serum blocking buffer for 30 minutes; 8) incubate in primary antibody overnight in 4ºC fridge; 9) wash slides in phosphate-buffered saline with 0.1% Tween 20 (PBST); 10) incubate in secondary antibody for 30 minutes; 11) wash in PBS; 12) incubate in VECTASTAIN ABC reagent for 30 minutes; 13) wash in PBS; 14) incubate in DAB peroxidase substrate solution for 2 to 5 minutes; 15) counterstain in hematoxylin for 2 minutes; 16) rinse in water; 17) dehydrate and mount. Furthermore, the cholangiocyte injury response to peripubertal GnRHa administration was assessed by staining CK19+ cells via IHC (dilution 1:50; Abcam Cat# ab220193, RRID: AB_2814863) using the previously described IHC protocol.
2.5. Evaluation of Fibrotic Changes
Picrosirius red (PSR) staining highlights the presence of collagen I and III fibrosis. PSR staining was performed on 5 μm thick paraffin liver sections following these steps: 1) deparaffinize slides using xylene and 100-70% ethanol; 2) stain in hematoxylin for 1 min; 3) rinse in water; 4) stain with Picrosirius red for 1 hour; 5) rinse in acidified water (5% HCL); and 6) dehydrate and mount. Besides detecting collagen fibers, fibrosis was also measured by the expression of profibrotic 〈SMA (dilution 1:50; Thermo Fisher Scientific Cat# 14-9760-82, RRID:AB_2572996) and TIMP1 (dilution 1:50; Santa Cruz Biotechnology Cat# sc-21734, RRID:AB_628359) via IF performed according to the above-described protocol.
2.6. Tissue Morphology
Hematoxylin and eosin (H&E) staining elucidates tissue morphology changes, parenchymal apoptosis/necrosis, and inflammatory cell loci. Using 5 μm thick sections, H&E staining was performed following these simplified steps: 1) deparaffinize slides using xylene and 100-70% ethanol, 2) stain slides in hematoxylin for 3 minutes, 3) rinse in water and moderate in 95% ethanol, 4) stain in eosin for 1 minute, and 5) dehydrate and mount. Histotechnologist evaluation of H&E slides was performed by Patrick Wilson. H&E slides were given numerical ratings based on a standardized scoring system for metabolic-dysfunction-associated fatty liver disease, assessing lobular inflammation and cell structure changes induced by peripubertal GnRHa administration [
28].
2.6. Quantification and Analysis of Data
The above-detailed cellular and molecular signs of hepatic injury were imaged across multiple lobes of the liver. These images were then quantified and analyzed using ImageJ and R software, respectively. All experiments were performed using 3 or 4 animals per group. Shapiro-Wilk tests for normality were complete, and for the results that adhered to a normal distribution, a two-way ANOVA and post hoc Tukey tests were used. Non-normal distributions found by the Shapiro-Wilk tests for normality were treated as nonparametric. This data was then evaluated through Kruskal-Wallis tests, followed by Dunn’s test for multiple comparisons, to identify significant differences between control and treatment groups, with an alpha level of 0.05.
3. Results
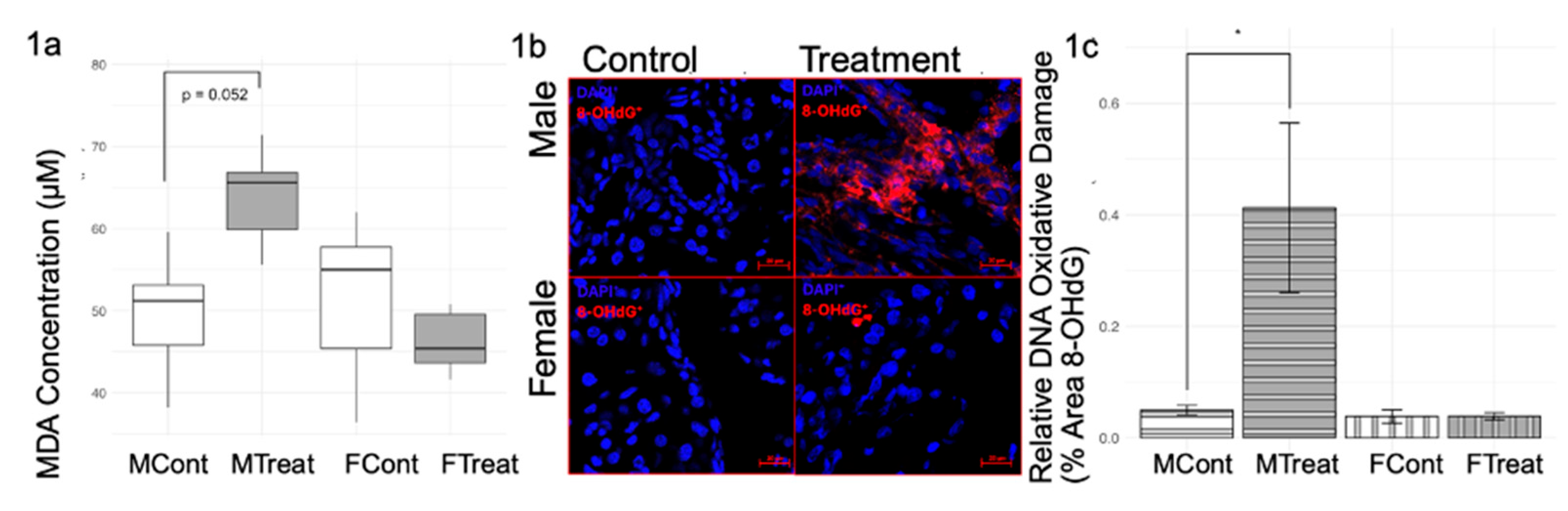
3.1. GnRHa Administration Leads to Sex-Specific Increased Oxidative Stress and Inflammatory Responses
Drug-induced hepatic injury often includes oxidative stress, leukocyte infiltration, and cholangiocyte proliferation [
29]. Further, sex steroids normally regulate hepatic redox balance; thus, we first evaluated oxidative stress signs as early indicators of hepatic injury (
Figure 1). TBARS assay for MDA revealed a trend towards an increase (p= 0.052) in lipid oxidative stress in GnRHa males but not in GnRHa females (
Figure 1a). This same trend was noted in the IF staining for 8-OHdG for DNA oxidative damage (
Figure 1b,c).
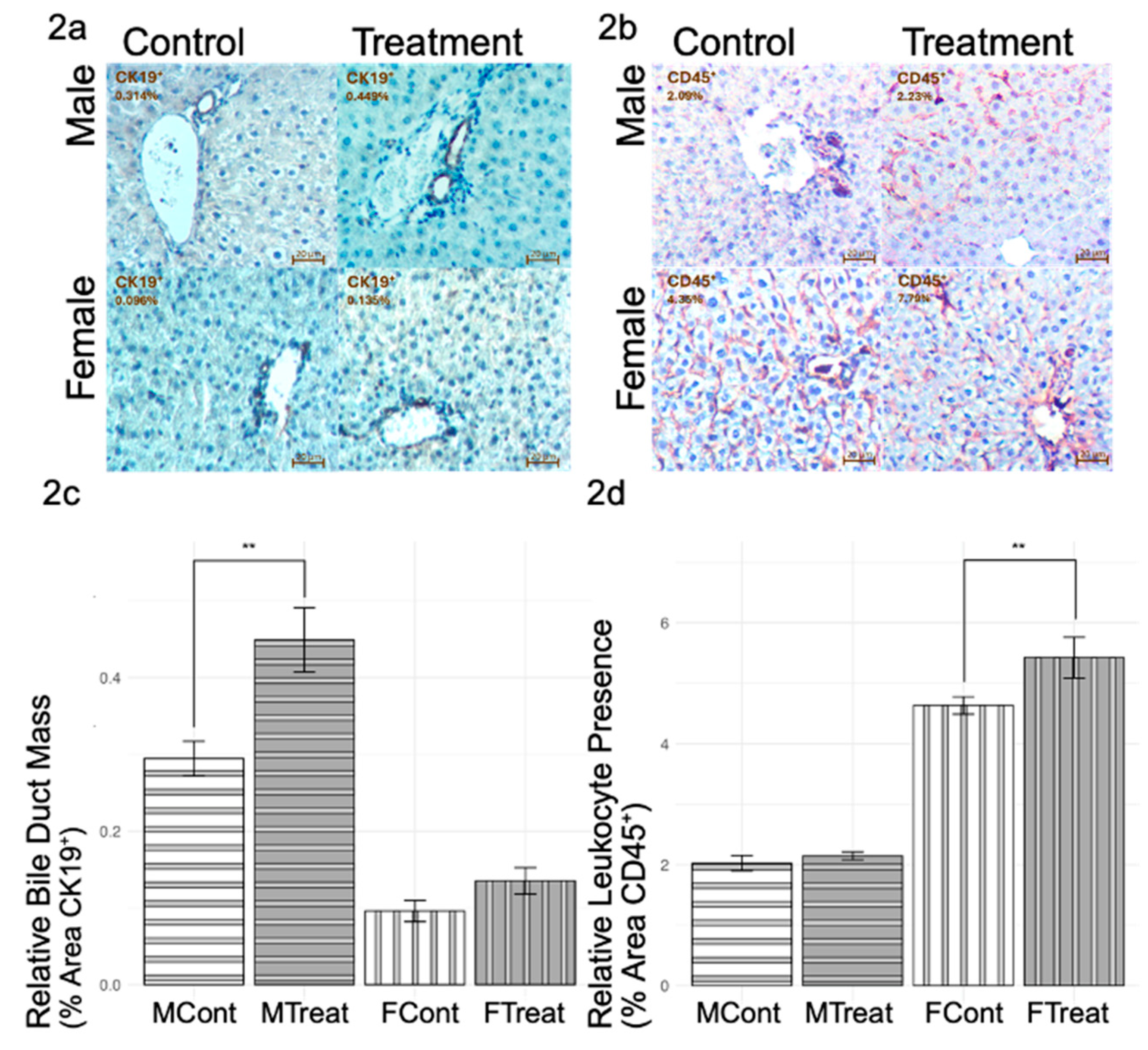
Beyond oxidative damage, further inflammatory responses exhibit sex-specific trends that are induced during GnRHa administration (
Figure 2). IHC staining for CK19 revealed increased bile duct mass in GnRHa-treated males but not in GnRHa-treated females (
Figure 2a,c). Conversely, IHC staining for CD45 highlighted a significant increase in leukocyte presence in GnRHa-treated females but not in GnRHa-treated males, as shown in
Figure 2b,d. Overall, GnRHa-treated males appeared to show a more advanced injury response (indicated by higher DNA oxidative stress levels and higher bile duct mass) than GnRHa-treated females (indicated by the lack of MDA oxidative stress or bile duct mass change). These results put together suggest that while GnRHa-treated males exhibit greater oxidative stress and biliary remodeling, females display a robust immune response, indicating distinct pathways of hepatic injury between biological sexes.
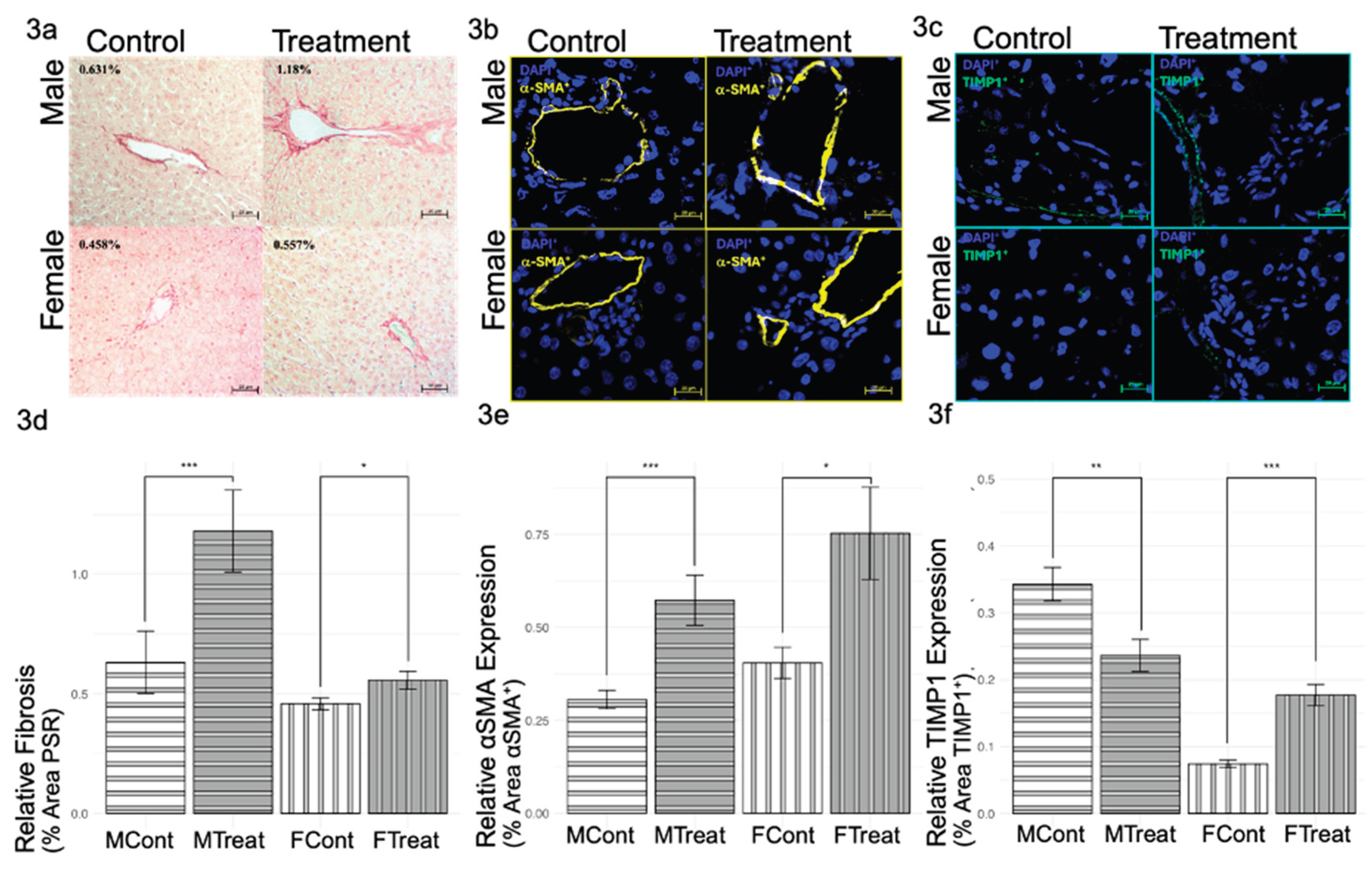
3.2. GnRHa Administration Leads to Increased Fibrosis
Oxidative stress and inflammatory signaling pathways in hepatic injury often include profibrotic signals and increased collagen deposition. Therefore, fibrosis investigations included evaluating the level of collagen fibers and the expression levels of profibrotic biomarkers 〈SMA and TIMP1. PSR Staining for collagen fibers (types I and III) revealed significantly increased fibrosis in both sexes, with males showing a greater fibrotic response than females (
Figure 3a,d). Similarly, the expression of profibrotic biomarker 〈SMA showed a significant increase in both sexes (
Figure 3b,e). Notably, profibrotic TIMP1 levels in GnRHa males were significantly lowered, while GnRHa-treated females showed increased TIMP1 (
Figure 3c,f). Overall, increased fibrosis was induced by GnRHa in both sexes, though TIMP1 levels suggest a difference in mechanistic pathways that lead to the same fibrotic end.
3.3. Gross & Tissue Morphology Unaltered by GnRHa Puberty Blocking
Weekly body weights for male and female samples were not significantly different between the control and treatment groups (Supplemental Figures S1a,b). Further, histological examination of tissue morphology via H&E staining showed no significant GnRHa-induced changes (Supplemental
Figure S1). Both treatment and control groups showed minimal but healthy levels of parenchymal cell death, with no signs of pathological steatosis. The absence of gross or histological abnormalities suggests that any GnRHa-induced hepatic alterations occur at the molecular and cellular level.
4. Discussion
This study shows that GnRHa administration induces sex-specific fibrotic liver injury responses, with male rats showing increased DNA and lipid oxidative stress in liver tissue and females not reflecting this pattern. This increased oxidative stress aligns with higher collagen deposition in male, as endogenous ROS accumulation is a strong driver of fibrogenesis. 8-OHdG and MDA are signs of ROS-induced damage, a common theme of hepatic injury and linked with the expression of profibrotic biomarkers, including 〈SMA [
15,
30]. A recent study found that CCl4 hepatic injury increases in both 〈SMA and MDA levels, further highlighting the link between oxidative damage and active fibrosis [
31]. GnRHa-treated male rats reflected these hepatic injury trends of increased 8-OHdG and lipid oxidative stress in conjunction with increased 〈SMA. Interestingly, female rats did not replicate this pattern, highlighting the incredible sexual dimorphism of the liver and the sex-hormone-linked molecular mechanisms present during the peripubertal period [
12].
Estrogen and testosterone are the main sex-specific hormones driving puberty. The lack of testosterone in GnRHa males likely contributes to the increased oxidative stress shown in this study. Previous studies have shown that testosterone deprivation is linked to increased liver fibrosis in males. In castrated male rats, Boukari et al. found that MDA levels rise in the brain, prostate, and seminal vesicles and return to normal when supplemented with testosterone at low levels, suggesting that testosterone acts as a potent antioxidant [
32]. Jin et al. conclude that adolescent males reflect the same hepatic sex-specific response. With peripubertal GnRHa treatment, male rats appeared to have an oxidative stress-driven fibrotic injury response. Recent research has shown that endothelial cells respond to GnRHa hormonal withdrawal with reduced capacity to accommodate endogenous ROS [
32]. In females, increased estrogen expression has been linked to biliary injury, often accompanied by significantly increased oxidative stress, as explored by Zu et al. [
33]. Interestingly, the lack of this oxidative response in GnRHa female rats is inconsistent with the link between estrogen deficiency and hepatic oxidative damage found in prior research. This suggests female resistance to oxidative stress may be estrogen independent.
Liver injury responses can include the activation of inflammatory cells, including Kupffer cells, HSCs, cholangiocytes, and other infiltrating leukocytes. While cholangiocyte injury response shares many functions with that of hepatocytes, including coordinating further injury responses through TGFβ1 signaling, cholangiocytes have not been found to utilize TIMP1 signaling to promote fibrogenesis [
22,
34]. Thus, it is unsurprising that male rats showed increased cholangiocytes in the absence of increased TIMP1 expression upon GnRHa administration. Defamie et al. also recently found that TIMP1-deficient progenitor cells preferentially differentiate into cholangiocytes over hepatocytes [
35]. The positive relationship between oxidative damage and the cholangiocyte population in both sexes suggests that oxidative stress is a key contributor of DR during hepatic injury response.
Unlike male rats, puberty blocked females showed increased immune cell infiltration, not oxidative damage or DR. The increased presence of CD45+ immune cells with simultaneous increased TIMP1 expression supports a more leukocyte-mediated injury response pattern in females. Sharing a common progenitor cell, CD45+ inflammatory cells that respond to hepatic injury include liver-resident Kupffer macrophages, as well as circulating T-cells, B-cells, granulocytes, and macrophages [
36]. These leukocytes have been the highlight of many studies, documenting the complex and cooperative way inflammatory responses are coordinated during liver injury. Similarly, the evidence that estrogen has a protective impact on the liver and can modulate inflammatory responses is well documented [
12]. Thus, disruptions in estrogen levels may instigate altered injury response mechanisms in females. Increased leukocyte presence in female rats during peripubertal GnRHa administration in conjunction with increased expression of TIMP1 and 〈SMA indicates active inflammation and the recruitment of circulating inflammatory responsive cells.
Beyond oxidative stress and changes in injury response cell populations, this study shows that GnRHa administration induces liver fibrosis in both sexes. Increased collagen deposition was accompanied by increased expression of 〈SMA in both sexes, while TIMP1 expression was decreased in males but increased in females, highlighting TIMP1-independent fibrosis. TIMP1 levels increase during fibrogenesis to promote collagen extracellular matrix (ECM) production in early-stage injury and decrease in late-stage injury to favor MMP degradation of excess collagen ECM formed in earlier stages [
25]. The sex-specific differential expression of TIMP1 does not fully explain the fibrotic response seen in this study; indeed, as Thiele et al. recently demonstrated, TIMP1 is not essential for liver fibrogenesis [
37]. During GnRHa administration, male rats appeared to have a more advanced fibrotic response than females. This suggests that the DR and oxidative stress seen in males are critical for the progression of fibrosis. Interestingly, the sex-specific expression of TIMP1 is consistent with the differential levels of leucocyte infiltration. Studies showed that TIMP1 promotes the expression of monocyte chemoattractant protein (MCP)-1 to recruit CD45+ macrophages to the liver [
17]. Our data suggest that the increased leukocytes in females following GnRHa administration may be attributed to a higher level of TIMP1 expression. Both male and female rats showed increased 〈SMA expression, further highlighting active fibrogenesis during GnRHa administration, suggesting that at least some injury responses are similar between the sexes.
5. Conclusions
Overall, our study highlights the sex-specific nature of GnRHa-induced hepatic injury responses. While both GnRHa-treated males and females showed increased fibrosis, the male rats indicated a later injury stage with a more locally controlled damage response, evidenced by higher collagen levels, higher 〈SMA levels, lower TIMP1 levels, and DR (CK19+). Females show a delayed injury response, potentially mediated by leukocytes, evidenced by lower collagen levels, higher αSMA levels, higher TIMP1 levels, and higher CD45+ levels.
Our research underscores the importance of considering sex-specific responses in the development and administration of treatments, suggesting that further studies are needed to fully understand the implications of GnRHa therapy on liver health and to optimize treatment protocols for all patients, including sex-specific co-treatments to supplement liver health. Additionally, dosage trials are needed to evaluate if altered GnRHa-induced hepatic damage is dose-dependent. GnRHa has a long history of use in managing central precocious puberty and other conditions, with minimal and manageable side effects [
38,
39]. While recent studies have raised concerns about potential risks, including increased heart [
10] and brain damage [
11], the therapeutic benefits of GnRHa for treating serious psychological disorders should continue to be evaluated within the context of individual patient needs and risks.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplemental Figure S1: GnRHa administration does not induce significant changes in body weight or obvious tissue morphology changes.
Author Contributions
Conceptualization, K.J.K. and Y.H.; methodology, K.J.K., Y.H., and D.H.; validation, Y.H., R.J., P.W., and K.J.K.; formal analysis, K.J.K.; investigation, M.Y., M.P., K.J.K., R.,J., P.W., and Y.H..; resources, Y.H., D.H., and K.J.K.; data curation, K.J.K.; writing—original draft preparation, K.J.K.; writing—review and editing, Y.H. and K.J.K.; visualization, K.J.K.; supervision, Y.H.; project administration, K.J.K.; funding acquisition, Y.H. and K.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Care and Use Committee and the protocol approved under the Animal Welfare Act guidelines (protocol: 2301CD-DH-R-26, May 4, 2023).
Data Availability Statement
Data for this project is available upon request (corresponding author: Yuyan Han – yuyan.han@unco.edu). This is an ongoing project, so the full data set is not published for public access at this time.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 8-OHdG |
8-hydroxy-2'-deoxyguanosine |
| aSMA |
alpha smooth muscle actin |
| CPP |
central precocious puberty |
| DR |
ductular reaction |
| GnRH |
gonadotropin-releasing hormone |
| GnRHa |
gonadotropin-releasing hormone agonist |
| H&E |
hematoxylin and eosin |
| HSC |
hepatic stellate cells |
| IF |
immunofluorescence |
| IHC |
immunohistochemistry |
| LGBTQ+ |
lesbian, gay, bisexual, transgender, queer, and more |
| MCP-1 |
monocyte chemoattractant protein 1 |
| MDA |
malondialdehyde |
| OCT |
optimal cutting temperature |
| PBS |
phosphate-buffered saline |
| PBST |
PBS with 0.1% Tween 20 |
| PSR |
Picro-Sirius Red |
| ROS |
reactive oxygen species |
| TBARS |
thiobarbituric acid-reactive substance |
| TBST |
tris-buffered saline with 0.1% Tween 20 |
| TGD |
transgender and gender-diverse |
| TGFβ1 |
transforming growth factor beta 1 |
| TIMP1 |
tissue inhibitor of metalloproteinases |
| US |
United States |
References
- Call, D.C.; Challa, M.; Telingator, C.J. Providing Affirmative Care to Transgender and Gender Diverse Youth: Disparities, Interventions, and Outcomes. Curr Psychiatry Rep 2021, 23, 33. [Google Scholar] [CrossRef]
- Knudson, G.; Winter, S.; Baral, S.; Reisner, S.; Wylie, K. An Introduction to Gender Diversity. In Comprehensive Care of the Transgender Patient; Elsevier, 2020; pp. 1–7. ISBN 978-0-323-49642-1. [Google Scholar]
- Telfer, M.M.; Pang, K.; Pace, C.; Tollit, M. The Creation of the Australian Standards of Care and Treatment Guidelines for Trans and Gender Diverse Children and Adolescents. Journal of Adolescent Health 2018, 62, S49–S50. [Google Scholar] [CrossRef]
- Meerwijk, E.L.; Sevelius, J.M. Transgender Population Size in the United States: A Meta-Regression of Population-Based Probability Samples. Am J Public Health 2017, 107, e1–e8. [Google Scholar] [CrossRef]
- Weixel, T.; Wildman, B. Geographic Distribution of Clinical Care for Transgender and Gender-Diverse Youth. Pediatrics 2022, 150, e2022057054. [Google Scholar] [CrossRef]
- Popovic, J.; Geffner, M.E.; Rogol, A.D.; Silverman, L.A.; Kaplowitz, P.B.; Mauras, N.; Zeitler, P.; Eugster, E.A.; Klein, K.O. Gonadotropin-Releasing Hormone Analog Therapies for Children with Central Precocious Puberty in the United States. Front. Pediatr. 2022, 10, 968485. [Google Scholar] [CrossRef]
- Huang, C.; Shen, X.; Mei, J.; Sun, Y.; Sun, H.; Xing, J. Effect of Recombinant LH Supplementation Timing on Clinical Pregnancy Outcome in Long-Acting GnRHa Downregulated Cycles. BMC Pregnancy Childbirth 2022, 22, 632. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Endometriosis. In Endocrinology: Adult and Pediatric; Elsevier, 2016; pp. 2242–2254.e5. ISBN 978-0-323-18907-1. [Google Scholar]
- Fisher, A.D.; Ristori, J.; Romani, A.; Cassioli, E.; Mazzoli, F.; Cocchetti, C.; Pierdominici, M.; Marconi, M.; Ricca, V.; Maggi, M.; et al. Back to the Future: Is GnRHa Treatment in Transgender and Gender Diverse Adolescents Only an Extended Evaluation Phase? The Journal of Clinical Endocrinology & Metabolism 2024, 109, 1565–1579. [Google Scholar] [CrossRef]
- Cicione, A.; Nacchia, A.; Guercio, A.; Gravina, C.; Franco, A.; Grimaldi, M.C.; Tema, G.; Lombardo, R.; Tubaro, A.; De Nunzio, C. Cardiovascular Adverse Events-Related to GnRH Agonists and GnRH Antagonists: Analysis of Real-Life Data from Eudra-Vigilance and Food and Drug Administration Databases Entries. Prostate Cancer Prostatic Dis 2023, 26, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Karamanis, G.; Frisell, T.; Holmberg, M.; Halldin, M.; Sylvén, S.; Skalkidou, A.; Papadopoulos, F.C. Incidence of Idiopathic Intracranial Hypertension in Individuals With Gonadotropin-Releasing Hormone Analogue Treatment for Gender Dysphoria in Sweden. JAMA Pediatr 2023, 177, 726. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yuan, Y.; Che, Z.; Tan, X.; Wu, B.; Wang, C.; Xu, C.; Xiao, J. The Hepatoprotective and Hepatotoxic Roles of Sex and Sex-Related Hormones. Front. Immunol. 2022, 13, 939631. [Google Scholar] [CrossRef]
- Della Torre, S. Non-Alcoholic Fatty Liver Disease as a Canonical Example of Metabolic Inflammatory-Based Liver Disease Showing a Sex-Specific Prevalence: Relevance of Estrogen Signaling. Front. Endocrinol. 2020, 11, 572490. [Google Scholar] [CrossRef]
- Conde De La Rosa, L.; Goicoechea, L.; Torres, S.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Role of Oxidative Stress in Liver Disorders. Livers 2022, 2, 283–314. [Google Scholar] [CrossRef]
- Mooli, R.G.R.; Mukhi, D.; Ramakrishnan, S.K. Oxidative Stress and Redox Signaling in the Pathophysiology of Liver Diseases. Comprehensive Physiology 2022, 12, 3167. [Google Scholar] [CrossRef]
- Karihtala, P.; Kauppila, S.; Puistola, U.; Jukkola-Vuorinen, A. Divergent Behaviour of Oxidative Stress Markers 8-Hydroxydeoxyguanosine (8-OHdG) and 4-Hydroxy-2-Nonenal (HNE) in Breast Carcinogenesis: 8-OHdG and HNE in Breast Carcinogenesis. Histopathology 2011, 58, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, X.; Wang, Y.; Shen, S.; Chen, W.; Liu, T.; Wang, P.; Fan, X.; Liu, L.; Jia, J.; et al. TIMP-1 Promotes Expression of MCP-1 and Macrophage Migration by Inducing Fli-1 in Experimental Liver Fibrosis. J Clin Transl Hepatol 2024, 000, 000–000. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P. the IT-LIVER Consortium TGF-β Signalling and Liver Disease. FEBS J 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Justo, B.L.; Jasiulionis, M.G. Characteristics of TIMP1, CD63, and Β1-Integrin and the Functional Impact of Their Interaction in Cancer. IJMS 2021, 22, 9319. [Google Scholar] [CrossRef]
- Aseem, S.O.; Jalan-Sakrikar, N.; Chi, C.; Navarro-Corcuera, A.; De Assuncao, T.M.; Hamdan, F.H.; Chowdhury, S.; Banales, J.M.; Johnsen, S.A.; Shah, V.H.; et al. Epigenomic Evaluation of Cholangiocyte Transforming Growth Factor-β Signaling Identifies a Selective Role for Histone 3 Lysine 9 Acetylation in Biliary Fibrosis. Gastroenterology 2021, 160, 889–905.e10. [Google Scholar] [CrossRef]
- Yang, F.; Li, H.; Li, Y.; Hao, Y.; Wang, C.; Jia, P.; Chen, X.; Ma, S.; Xiao, Z. Crosstalk between Hepatic Stellate Cells and Surrounding Cells in Hepatic Fibrosis. International Immunopharmacology 2021, 99, 108051. [Google Scholar] [CrossRef]
- Loarca, L.; Pisarello, M.J.L.; Morton, L.; Huang, B.Q.; O’Hara, S.; Splinter, P.; LaRusso, N. Cholangiocyte Biology. In Primary Sclerosing Cholangitis; Forman, L.M., Ed.; Springer International Publishing: Cham, 2017; pp. 83–97. ISBN 978-3-319-40906-1. [Google Scholar]
- Monga, S.P.; Nejak-Bowen, K. Ductular Reaction and Liver Regeneration: Fulfilling the Prophecy of Prometheus! Cellular and Molecular Gastroenterology and Hepatology 2023, 15, 806–808. [Google Scholar] [CrossRef]
- Ceci, L.; Han, Y.; Krutsinger, K.; Baiocchi, L.; Wu, N.; Kundu, D.; Kyritsi, K.; Zhou, T.; Gaudio, E.; Francis, H.; et al. Gallstone and Gallbladder Disease: Biliary Tract and Cholangiopathies. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley, 2023; pp. 4909–4943. ISBN 978-0-470-65071-4. [Google Scholar]
- Kisseleva, T.; Brenner, D. Molecular and Cellular Mechanisms of Liver Fibrosis and Its Regression. Nat Rev Gastroenterol Hepatol 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Kwekel, J.C.; Desai, V.G.; Moland, C.L.; Branham, W.S.; Fuscoe, J.C. Age and Sex Dependent Changes in Liver Gene Expression during the Life Cycle of the Rat. BMC Genomics 2010, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Leonhardt, S.; Seidel, C.; Luft, H.; Wuttke, W.; Jarry, H. Comparative Analysis of Different Puberty Inhibiting Mechanisms of Two GnRH Agonists and the GnRH Antagonist Cetrorelix Using a Female Rat Model. Pediatr Res 2000, 48, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Rusyn, I.; Arzuaga, X.; Cattley, R.C.; Corton, J.C.; Ferguson, S.S.; Godoy, P.; Guyton, K.Z.; Kaplowitz, N.; Khetani, S.R.; Roberts, R.A.; et al. Key Characteristics of Human Hepatotoxicants as a Basis for Identification and Characterization of the Causes of Liver Toxicity. Hepatology 2021, 74, 3486–3496. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujita, N.; Sugimoto, R.; Urawa, N.; Horiike, S.; Kobayashi, Y.; Iwasa, M.; Ma, N.; Kawanishi, S.; Watanabe, S.; et al. Hepatic Oxidative DNA Damage Is Associated with Increased Risk for Hepatocellular Carcinoma in Chronic Hepatitis C. Br J Cancer 2008, 98, 580–586. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Kadhim, H.M. The Hepatoprotective Effects of the Polyphenol-Enriched n-Butanol Fraction of Cnicus Benedictus against Carbon Tetrachloride-Induced Liver Fibrosis in Rats: In Vivo Study. Toxicology Reports 2025, 14, 101850. [Google Scholar] [CrossRef]
- Boukari, O.; Khemissi, W.; Ghodhbane, S.; Lahbib, A.; Tebourbi, O.; Rhouma, K.B.; Sakly, M.; Hallegue, D. Effects of Testosterone Replacement on Lipid Profile, Hepatotoxicity, Oxidative Stress, and Cognitive Performance in Castrated Wistar Rats. Arch Ital Urol Androl 2023. [Google Scholar] [CrossRef]
- Zu, Y.; Yang, J.; Zhang, C.; Liu, D. The Pathological Mechanisms of Estrogen-Induced Cholestasis: Current Perspectives. Front. Pharmacol. 2021, 12, 761255. [Google Scholar] [CrossRef]
- Milani, S.; Schuppan, D.; Surrenti, C. Transforming Growth Factors Beta1 and Beta2 Are Differentially Expressed in Fibrotic Liver Disease. 1991, 139. [Google Scholar]
- Defamie, V.; Aliar, K.; Sarkar, S.; Vyas, F.; Shetty, R.; Reddy Narala, S.; Fang, H.; Saw, S.; Tharmapalan, P.; Sanchez, O.; et al. Metalloproteinase Inhibitors Regulate Biliary Progenitor Cells through sDLK1 in Organoid Models of Liver Injury. Journal of Clinical Investigation 2025, 135, e164997. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-M.; Hsiao, C.-C.; Lin, C.-W.; Lee, P.-H. Complex Cell Type-Specific Roles of Autophagy in Liver Fibrosis and Cirrhosis. Pathogens 2020, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Thiele, N.D.; Wirth, J.W.; Steins, D.; Koop, A.C.; Ittrich, H.; Lohse, A.W.; Kluwe, J. TIMP-1 Is Upregulated, but Not Essential in Hepatic Fibrogenesis and Carcinogenesis in Mice. Sci Rep 2017, 7, 714. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.G.; Krishna, K.B.; Lee, P.A. Use of Gonadotropin-Releasing Hormone Analogs in Children. Current Opinion in Pediatrics 2021, 33, 442–448. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Di Maio, S.; Soliman, N.; Elsedfy, H. Long-Term Effects and Significant Adverse Drug Reactions (ADRs) Associated with the Use of Gonadotropin-Releasing Hormone Analogs (GnRHa) for Central Precocious Puberty: A Brief Review of Literature. Acta Bio Medica Atenei Parmensis 2019, 90, 345–359. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).