1. Introduction
Plant-based ingredients are the main components of modern swine diets, and these materials contain substantial amounts of non-starch polysaccharides such as phytic acid, β-mannan, xylan, and β-glucan. These compounds are largely indigestible for pigs and can increase the viscosity of intestinal contents, thereby impairing the activity of endogenous digestive enzymes and limiting nutrient release [
1]. Increased digesta viscosity is also associated with enhanced fermentation by undesirable microorganisms, which may negatively influence gut health and reduce growth performance [
2]. As feed cost remains the major expense in pig production, improving nutrient utilization has become an important goal for enhancing both productivity and sustainability.
The use of exogenous enzymes has been widely explored as a strategy to improve the breakdown of fiber-rich feed components. Enzymes such as β-mannanase, xylanase, β-glucanase, and cellulase have been reported to improve nutrient digestibility, modulate microbial populations in the large intestine, and enhance growth performance in pigs [
3,
4,
5,
6]. Some studies have demonstrated marked improvements, including increases of approximately 15% in daily weight gain when specific non-starch polysaccharide–degrading enzymes were added to wheat- or barley-based diets [
7,
8]. However, responses to enzyme supplementation vary considerably depending on diet composition, enzyme type, animal age, and gut conditions, and inconsistent results continue to be reported across studies [
9]. These discrepancies highlight the need for further evaluation using both controlled laboratory techniques and animal-based assessments.
In this context, the present study aimed to investigate the effects of an exogenous enzyme mixture on the digestibility of various swine diets using in vitro assays and to verify these findings in an in vivo nursery pig model. By integrating laboratory-based digestion assessments with physiological measurements in pigs, this work seeks to provide a clearer understanding of when and how non-starch polysaccharide–degrading enzymes contribute to improved feed utilization.
2. Materials and Methods
2.1. Diets and Exogenous NSP Enzyme
Seven commercial swine diets were evaluated: creep, nursery, starter, grower, finisher, gestating, and lactating diets. Ingredient composition and chemical analysis of all diets are shown in
Table 1. The diets were divided into two groups: a control group without enzyme supplementation and a treatment group supplemented with 100 g/ton of Hostazym
® X (Huva Phama, Bulgaria), containing cellulase, α-amylase, protease, and hemicellulose from
Trichoderma longibrachiatum.
2.2. In Vitro Digestibility
Five hundred grams of each diet were analyzed for dry matter (DM) digestibility at 0, 12, and 24 hours using a Daisy Incubator (ANKOM Technology, USA). Starch digestibility was assessed via the Boisen and Fernandez method [
10], simulating gastric (pH 3.0) and intestinal (pH 6.8) conditions using pepsin and pancreatin. Digesta residues were dried (130 °C) and ashed (500 °C) for nutrient composition analysis following AOAC (1980) methods [
11].
2.3. In Vivo Digestibility in Nursery Pigs
The study was conducted under a completely randomized design and approved by the Ethics Committee of the Faculty of Veterinary Medicine, Chiang Mai University (SO24/2018). Ten male starter pigs (Large White × Landrace × Duroc) with an initial body weight of 16.71 ± 0.36 kg were housed individually and assigned to control or enzyme-supplemented diets. Feed and water were provided ad libitum for 30 days. Growth performance was assessed based on initial weight, final weight, average daily gain (ADG), and feed conversion ratio (FCR).
Before the experiment ended, 0.5% chromic oxide was added to the feed to trace fecal passage. Fecal samples (100 g/pig) were collected and stored at -20 °C for digestibility analysis. Pigs were euthanized with thiopental sodium IV, and samples from the duodenum, jejunum, and ileum were collected for histological analysis.
2.4. Laboratory Methods
Digesta viscosity was measured using a Brookfield Digital Viscometer. Crude protein was analyzed by the Kjeldahl method (AOAC 2001.11), crude fiber by AOAC 962.09, crude fat by AOAC 920.39, and ash/dry matter by NFTA 2.1.4. Digestibility was assessed via spectrophotometry with chromic oxide as the marker, calculated using Fenton and Fenton’s (1979) formula [
12].
2.5. Histological Analysis
Intestinal samples were fixed in glutaraldehyde and paraformaldehyde, embedded in paraplast, and sectioned at 5 μm. Sections were stained with hematoxylin and eosin, and villus height was measured from 40 villi per intestinal segment. Epithelial cell density was determined by counting nuclei per unit area. Crypt mitosis was assessed in randomly selected crypts, with measurements performed using an Olympus Imaging Software system.
2.6. Microbiota and Volatile Fatty Acid Analysis
Digesta samples (1 g) from the ileum, cecum, and colon were diluted and cultured for
Enterobacter spp.,
Escherichia coli, and
Lactobacillus spp. using the spread plate method [
13]. Volatile fatty acid profiles were analyzed by gas-liquid chromatography [
14].
2.7. Statistical Analysis
All measured data on viscosity value, percent digestibility, villous morphology, microbial population, volatile fatty acid, and growth performance was statistically analyzed using the two independent samples t-test to identify significant differences between the results of the treatments. Analysis was done with Duncan’s multiple range test using the R program (Version 4.4.1). P<0.05 was set as the level of statistical significance.
3. Results
3.1. In Vitro Digestibility
The results of percent digestibility of dry matter (DM), crude protein (DP), crude fat (EE), and crude fiber (DF) of creep, nursery, starter, grower, fattening, gestating, and lactating diets is shown in
Table 2 shows that the percent digestibility of the creep diet was not significantly different between the groups for all parameters (P>0.05).
Table 2 presents the percent digestibility of various nutrients across different diet types. In the control group, crude fat digestibility was lower than in the treatment group, although the difference was not statistically significant (P>0.05). Similarly, the digestibility of crude protein and crude fiber in the starter diet was lower in the control group, while dry matter, crude fat, and ash digestibility showed no significant differences. For the grower diet, the control group exhibited lower digestibility values for dry matter, crude fat, crude fiber, and ash compared to the treatment group, with no significant difference observed in crude protein digestibility. In the finisher diet, all measured parameters showed lower digestibility in the control group (P>0.05). In the gestating diet, crude protein, crude fiber, and ash digestibility were lower in the control group, while dry matter and crude fat digestibility remained comparable between groups. Similarly, in the lactating diet, dry matter, crude protein, and ash digestibility were lower in the control group, whereas crude fat and crude fiber digestibility showed no significant differences.
In vivo digestibility in nursery pigs:
Table 3 shows the growth performance of nursery pigs in the control group and the treatment group (NSP-enzyme supplement) were not significantly different for all parameters. However, the final weight, ADG, and FCR of the treatment group trended to be higher than the control group (P=0.07)
Table 4 shows the volatile fatty acid concentrations at the cecum of nursery pigs in the control group and the treatment group (NSP-enzyme supplement) were not significantly different for all parameters. Moreover, the treatment group had trended of the volatile fatty acid concentrations to higher than control group (P=0.01).
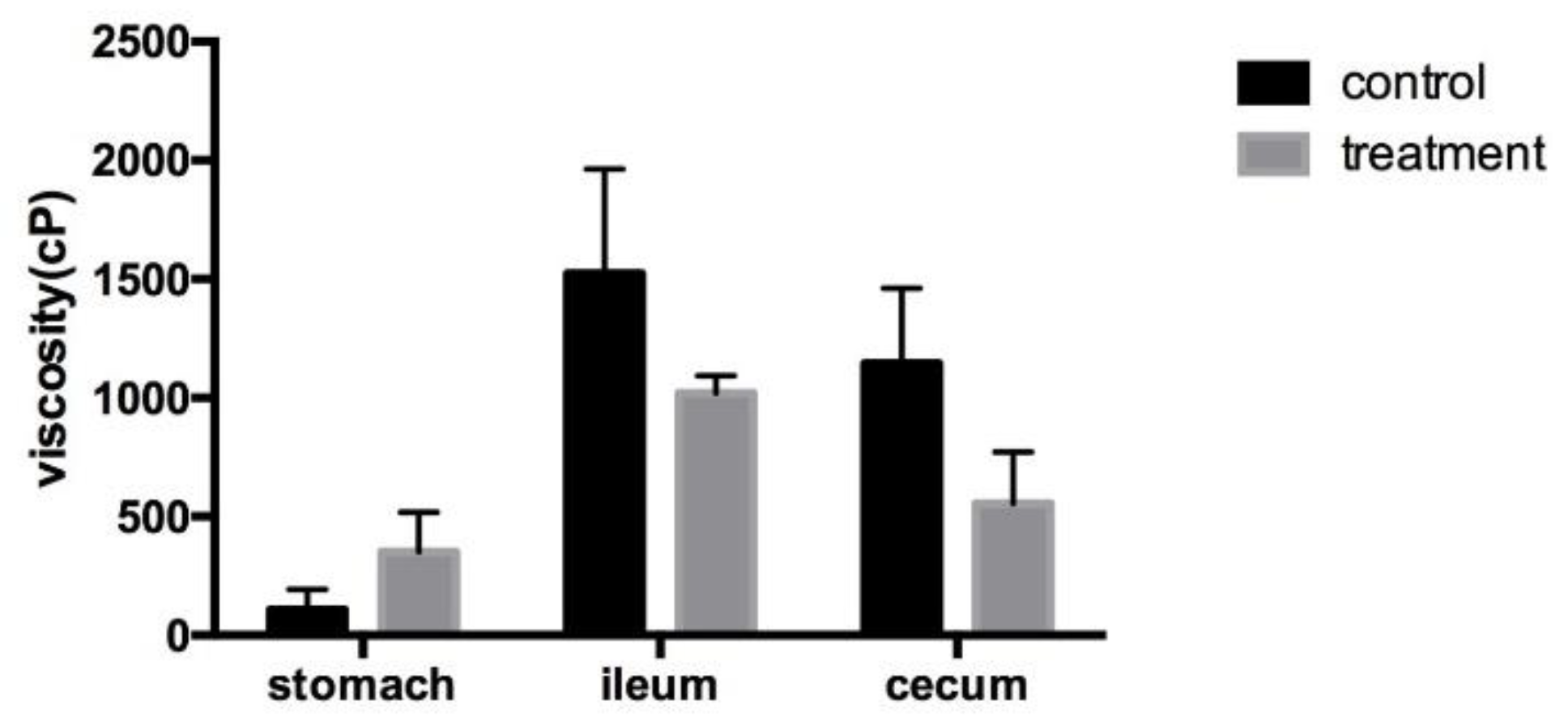
Figure 1 shows the viscosity level in the control and treatment groups. The viscosity levels were not significantly different. However, the viscosity of the treatment group was less than control group, especially the viscosity of ileum and cecum.
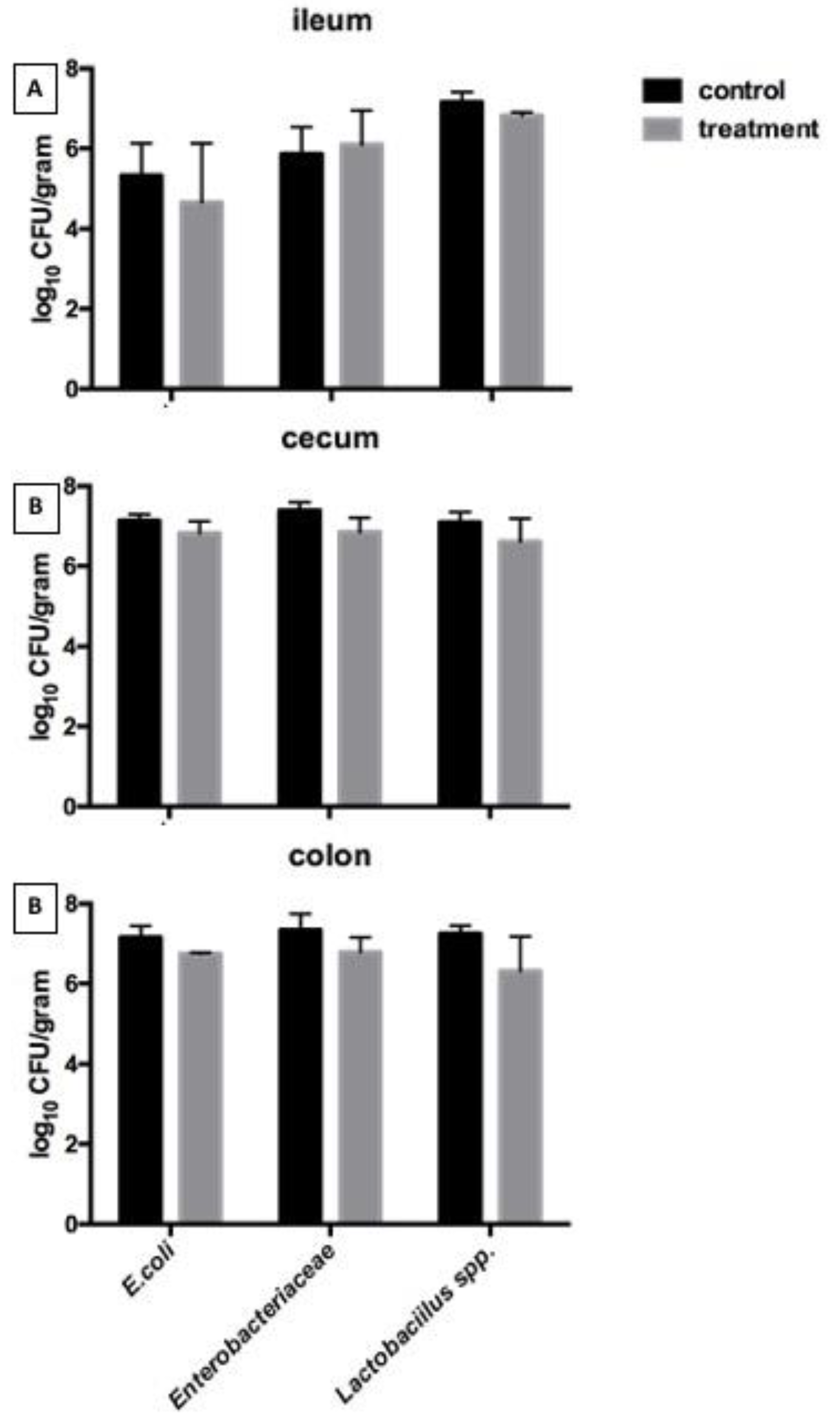
Figure 2 shows the microbial populations in the digestive system including
Escherichia coli,
Enterobactericeae, and
Lactobacillus spp. in the ileum, cecum, and colon. None of the parameters were significantly different between the two groups.
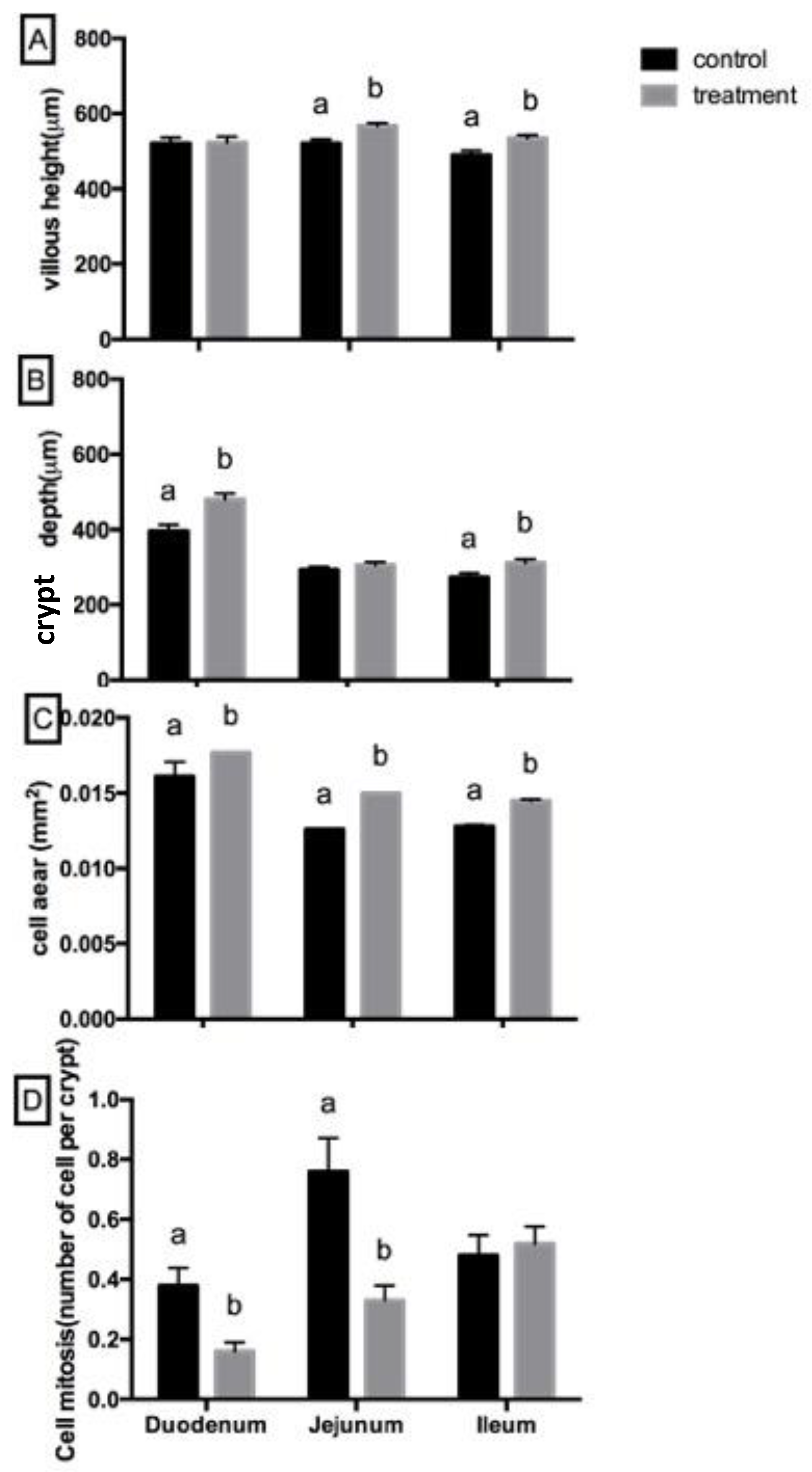
Figure 3 illustrates the villous morphology, including villous height, crypt depth, cell area, and cell mitosis in the duodenum, jejunum, and ileum of starter pigs. Villous height was higher in the treatment group, particularly in the jejunum and ileum, while crypt depth was greater in the duodenum and ileum (P>0.05). The treatment group also exhibited a larger cell area across all intestinal segments. Conversely, cell mitosis was lower in the duodenum and jejunum of the treatment group but showed no significant difference in the ileum (P>0.05).
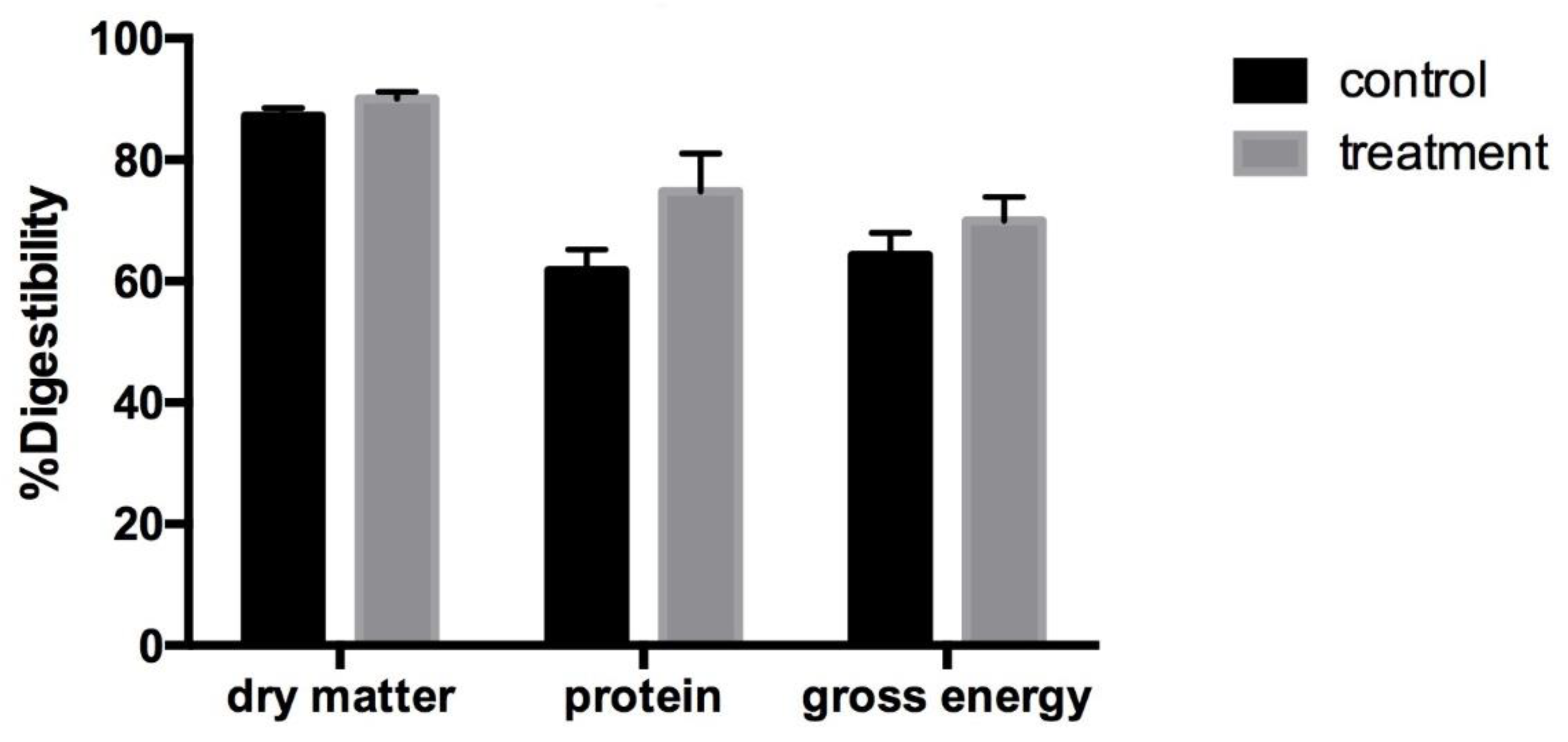
Figure 4 shows the percent digestibility of nutrients in the feces of nursery pigs in the control group and the treatment group. There was no significant difference in this parameter between the groups. However, both percent digestibility of protein and gross energy in the treatment group were higher than the control group after the first and second week.
4. Discussion
Non-starch polysaccharides are known to exert variable effects on digestive processes depending on the botanical origin and characteristics of the feed ingredients, particularly cereals such as wheat and rye [
15]. Their capacity to increase digesta viscosity can reduce nutrient accessibility and impair enzymatic activity within the gastrointestinal tract [
1]. As pigs lack endogenous enzymes capable of degrading these complex polysaccharides [
16], the use of exogenous NSP-degrading enzymes has been proposed as a strategy to overcome these limitations. In agreement with this concept, the present in vitro findings demonstrated improved digestibility in several diet types, especially those containing higher NSP levels such as starter, grower, finisher, gestating, and lactating diets. This trend is consistent with earlier reports suggesting that enzyme efficacy is closely linked to the amount and type of NSP present in the diet [
9,
17]. However, the absence of improvement in creep and nursery diets indicates that enzyme effects may be limited when NSP content is inherently low, a concept also reflected in previous observations.
The in vivo results showed that although the differences in growth performance were not statistically significant, pigs receiving the enzyme-supplemented diet tended to have higher final body weight, improved average daily gain, and lower feed conversion ratio. These tendencies are in line with earlier studies demonstrating positive growth responses to NSP enzyme supplementation in various production stages [
6,
18,
19]. Nonetheless, reductions in ileal digestibility were observed, which may be related to accelerated digesta transit time, as previously suggested by Chen
et al. [
20]. Similar inconsistencies have been reported in pigs fed corn–soybean or wheat-based diets, where enzyme supplementation sometimes yielded limited improvements in digestibility or growth [
21,
22]. These divergent findings highlight the multifactorial nature of enzyme responses, which are influenced by dietary formulation, enzyme characteristics, gut environment, and animal developmental stage. Conversely, studies such as Barrera
et al. [
4] and Emiola
et al. [
8] have shown sizable performance improvements in finishing pigs when NSP enzymes were added to fiber-rich diets, suggesting that diet composition remains a key determinant of enzyme efficacy.
Although viscosity and microbial populations did not differ significantly between treatments in the present study, a tendency toward lower viscosity in the ileum and cecum was noted. Given that arabinoxylans are major contributors to viscosity [
23], and that xylanase supplementation can reduce viscosity by hydrolyzing arabinoxylan structures [
22], the observed trend may still be biologically relevant. The absence of marked changes in microbial populations may reflect the influence of multiple interacting factors, including feed matrix, animal health status, and baseline microbiota composition [
24]. Further studies incorporating advanced microbiome profiling may help clarify the relationship between NSP degradation, gut microbial communities, and fermentation end products such as volatile fatty acids [
25,
26].
The morphological evaluation of the small intestine showed tendencies toward increased villous height, deeper crypts, and larger absorptive cell area in the enzyme-supplemented group, particularly in the jejunum and ileum. These structural improvements may support enhanced nutrient absorption capacity. Previous research has indicated that NSPs influence epithelial development [
27], while enzyme supplementation may alleviate NSP-induced disturbances [
28]. Reduced cell mitosis observed in the duodenum and jejunum of pigs receiving enzymes could suggest more stable epithelial turnover and reduced cellular stress, a pattern consistent with earlier findings on improved intestinal health associated with enzyme use [
16,
29,
30]. These physiological responses help explain the favorable growth trends observed, despite the lack of statistical significance.
Overall, the findings of this study support the hypothesis that exogenous NSP enzymes can enhance nutrient utilization and influence gastrointestinal physiology in pigs, particularly when diets contain substantial NSP levels. However, the variability in responses underscores the need for further work to better define the conditions under which enzyme supplementation is most effective. Future research should consider incorporating detailed chemical characterization of NSP fractions, evaluating enzyme–substrate interactions under different dietary matrices, and employing high-resolution microbial and metabolite analyses to elucidate underlying mechanisms. Such information will help refine feeding strategies and improve the practical application of NSP enzymes in swine production.
5. Conclusions
The present study demonstrates that non-starch polysaccharide enzyme supplementation can improve nutrient digestibility in several types of swine diets, particularly those used during the finishing period and in breeding herds where fiber-rich ingredients are common. In vivo responses showed favorable tendencies in growth performance and intestinal morphology, suggesting that enzyme supplementation may support more efficient nutrient utilization when diet composition and physiological conditions are appropriate. However, the effectiveness of enzyme supplementation is influenced by multiple factors, including the inherent non-starch polysaccharide content of the diet, the health and developmental stage of the pigs, and the suitability of the gut environment for enzyme activity. Further research should focus on identifying diet–enzyme interactions, evaluating the use of alternative raw materials, and refining feeding strategies to improve the consistency of responses at the herd level. Continued investigation into digestive mechanisms and gut microbial fermentation may help optimize the practical use of non-starch polysaccharide enzymes in modern swine production systems.
Author Contributions
Conceptualization, P.Y. (Panuwat Yamsakul); methodology, P.Y., T.Y. (Panuwat Yamsakul and Terdsuk Yano); validation, P.Y., T.Y. and T.E. (Panuwat Yamsakul, Terdsuk Yano and Thanaporn Eiamsam-ang); formal analysis, P.Y., T.Y. (Panuwat Yamsakul and Terdsuk Yano); writing—original draft preparation, P.Y. (Panuwat Yamsakul); writing—review and editing, P.Y. (Panuwat Yamsakul). All authors have read and agreed to the published version of the manuscript. Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by the IRTC, the National Science and Technology Development Agency, and Huvepharma Thailand Ltd.
Institutional Review Board Statement
The study was conducted in accordance with the Ethics Committee of the Faculty of Veterinary Medicine, Chiang Mai University (SO24/2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the authors upon request and were used with permission from Chiang Mai university, Thailand
Acknowledgments
The authors would like to express our thanks to the IRTC, the National Science and Technology Development Agency, and Huvepharma Thailand Ltd. for financial support. We would also like to extend thanks to Miss Saowaratcharee Rinut, Scientist of the Food Analysis Laboratory, Faculty of Veterinary Medicine, Chiang Mai University for the laboratory analyses. Finally, we would like to thank the Faculty of Veterinary Medicine for providing access to the infrastructure which made this study possible.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| NSPs |
Non-Starch Polysaccharides |
| ADG |
Average Daily Gain |
| FCR |
Feed Conversion Ratio |
| DM |
Dry Matter |
| CP |
Crud Protein |
| Fat |
Crud Fat |
| CF |
Crud Fiber |
References
- Smith, J.; Brown, K.; Taylor, R.; Johnson, L.; Williams, M.; Anderson, P. The role of non-starch polysaccharides in pig nutrition: A review. J. Anim. Sci. Biotechnol. 2021, 2, 45–56. [Google Scholar]
- Chen, L.; Jiang, T.; Li, X.; Wang, Y.; Zhang, J.; Liu, H.; Li, X. Immunomodulatory activity of β-glucan and mannan-oligosaccharides from Saccharomyces cerevisiae on broiler chickens challenged with feed-borne Aspergillus fumigatus. Pak. Vet. J. 2022, 42, 297–301. [Google Scholar]
- Garcia, A.; Martinez, S.; Rodriguez, M.; Gonzalez, P.; Fernandez, L.; Hernandez, J.; Lopez, M. Impact of xylanase supplementation on nutrient digestibility and growth performance in pigs fed high-fiber diets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1234–1242. [Google Scholar]
- Lee, S.; Kim, D.; Park, J.; Lee, H.; Kim, Y.; Cho, K.; Kim, B. The effects of mannan-oligosaccharides on immune response and gut microbiota in growing pigs. Vet. Immunol. Immunopathol. 2021, 231, 110165. [Google Scholar]
- Alpine, P.M.; O’Shea, C.; Varley, P.; O’Doherty, J. The effect of protease and xylanase enzymes on growth performance and nutrient digestibility in finisher pigs. J. Anim. Sci. 2012, 90, 375–377. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Wang, F.; Liu, X.; Yang, H.; Zhang, J.; Chen, L. Effects of non-starch polysaccharide-degrading enzymes on growth performance, nutrient digestibility, and gut health in growing pigs. J. Anim. Sci. 2023, 101, 1–12. [Google Scholar]
- Barrera, M.; Cervantes, M.; Sauer, W.; Araiza, A.; Torrentera, N. Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. J. Anim. Sci. 2004, 82, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Emiola, I.; Opapeju, F.; Slominski, B.; Nyachoti, C. Growth performance and nutrient digestibility in pigs fed wheat distillers dried grains with solubles-based diets supplemented with a multi-carbohydrase. J. Anim. Sci. 2009, 87, 2315–2322. [Google Scholar] [CrossRef]
- McDonough, J.; Pedersen, C.; Eggum, B.O.; Kay, R.M.; Nielsen, H.J. Critical evaluation of in vitro methods for estimating digestibility in simple-stomach animals. Nutr. Res. Rev. 2020, 4, 141–162. [Google Scholar]
- Boisen, S.; Fernandez, J.A. A Model for the Determination of Starch Digestibility in the Stomach-Small Intestine of Pigs. J. Sci. Food Agric. 1993, 61, 309–313. [Google Scholar]
- AOAC. Official Methods of Analysis, 13th ed.; Official Anal. Chem: Washington, DC, USA, 1980. [Google Scholar]
- Fenton, T.; Fenton, M. An improved procedure for the determination of chromic oxide in feed and feces. J. Anim. Sci. 1979, 59, 631–634. [Google Scholar] [CrossRef]
- Pierce, J.L.; Lonergan, S.M.; Hutchison, C.L.; Kessler, K.L.; Stahl, C.A.; Wiegand, B.R.; Honeyman, M.S. A comparison of two plating methods for counting Lactobacillus spp. in swine feces. J. Anim. Sci. 2005, 83, 733–736. [Google Scholar]
- Franklin, M.; Mathew, A.; Vickers, J.; Clift, R. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J. Anim. Sci. 2002, 80, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Courtin, C.; Delcour, J. Arabinoxylans and Endoxylanases in Wheat Flour Bread-making. J. Cereal Sci. 2002, 35, 225–243. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Wang, Z.; Zhang, L.; Li, M.; Wang, J.; Liu, H. Effects of multi-carbohydrase supplementation on nutrient digestibility and gut health in pigs fed diets with high non-starch polysaccharides. Anim. Feed Sci. Technol. 2020, 268, 114533. [Google Scholar]
- Hu, J.; Shibata, Y.; Voss, C.; Shemesh, T.; Li, Z.; Coughlin, M.; Kozlov, M.M.; Rapoport, T.R.; Prinz, W.A. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 2008, 319, 1247–1250. [Google Scholar] [CrossRef]
- Fang, Z.; Peng, J.; Liu, Z.; Liu, Y. Responses of non-starch polysaccharide-degrading enzymes on digestibility and performance of growing pigs fed a diet based on corn, soya bean meal and Chinese double-low rapeseed meal. J. Anim. Physiol. Anim. Nutr. 2007, 91, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Kiarie, E.; Nyachoti, C.; Slominski, B.; Blank, G. Growth performance, gastrointestinal microbial activity, and nutrient digestibility in early-weaned pigs fed diets containing flaxseed and carbohydrase enzyme. J. Anim. Sci. 2007, 85, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, W.-w.; Li, G.-h.; Zhang, Y.-y.; Liu, J.-j.; Chen, L.-l.; Jiang, H.-q.; Wang, X.-m. Compound non-starch polysaccharide enzymes improve growth performance, slaughter performance, immune function, and nutrient utilization in broilers. Front. Vet. Sci. 2023, 10, 1162811. [Google Scholar] [CrossRef]
- Passos, A.; Park, I.; Ferket, P.; Heimendahl, E.V.; Kim, S. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim. Nutr. 2015, 1, 19–23. [Google Scholar] [CrossRef]
- Woyengo, B.; Slominski, R.; Jones, R. Growth performance and nutrient utilization of broiler chickens fed diets supplemented with phytase alone or in combination with citric acid and multicarbohydrase. Poult. Sci. 2010, 89, 2221–2229. [Google Scholar] [CrossRef]
- Masey O’Neill, H.V.; Smith, J.A.; Bedford, M.R. Multicarbohydrase applications for corn-soybean meal-based broiler diets. Poult. Sci. 2019, 98, 1–10. [Google Scholar]
- Chen, Q.; Li, M.; Wang, X. Enzymology properties of two different xylanases and their impacts on growth performance and intestinal microflora of weaned piglets. Anim. Nutr. 2016, 2, 18–23. [Google Scholar] [CrossRef]
- Rivière, A.; Ganza, M.; Perpetuini, G.; Pham, H.T.; Taminiau, B.; Daube, G.; Mozzi, F.; De Vuyst, L.; Miescher, S.S. The Ability of Bifidobacteria To Degrade Arabinoxylan Oligosaccharide Constituents and Derived Oligosaccharides Is Strain Dependent. Appl. Environ. Microbiol. 2014, 80, 204–217. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, L.; Sun, X.; Liu, M.; Wang, Y.; Yang, B.; Ai, H.; Chen, C.; Huang, L. Assessing the relationship between the gut microbiota and growth traits in Chinese indigenous pig breeds. BMC Vet. Res. 2025, 21, 284. [Google Scholar] [CrossRef] [PubMed]
- Hedemann, M.; Eskildsen, S.; Lærke, M.; Pedersen, H.N.; Lindberg, J.E.; Laurinen, P.; Knudsen, K.E. Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting fiber concentrations and fiber properties. J. Anim. Sci. 2006, 84, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A review of interactions between dietary fiber and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Kiarie, E.; Romero, L.F.; Mills, A. Role of feed processing on gut health and function in pigs and poultry: Conundrum of optimal particle size and hydrothermal regimens. Front. Vet. Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.R.; Cowieson, A.J. Matrix effects of feed ingredients and exogenous enzymes. Anim. Feed Sci. Technol. 2020, 267, 1–12. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).