Submitted:
19 November 2025
Posted:
20 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
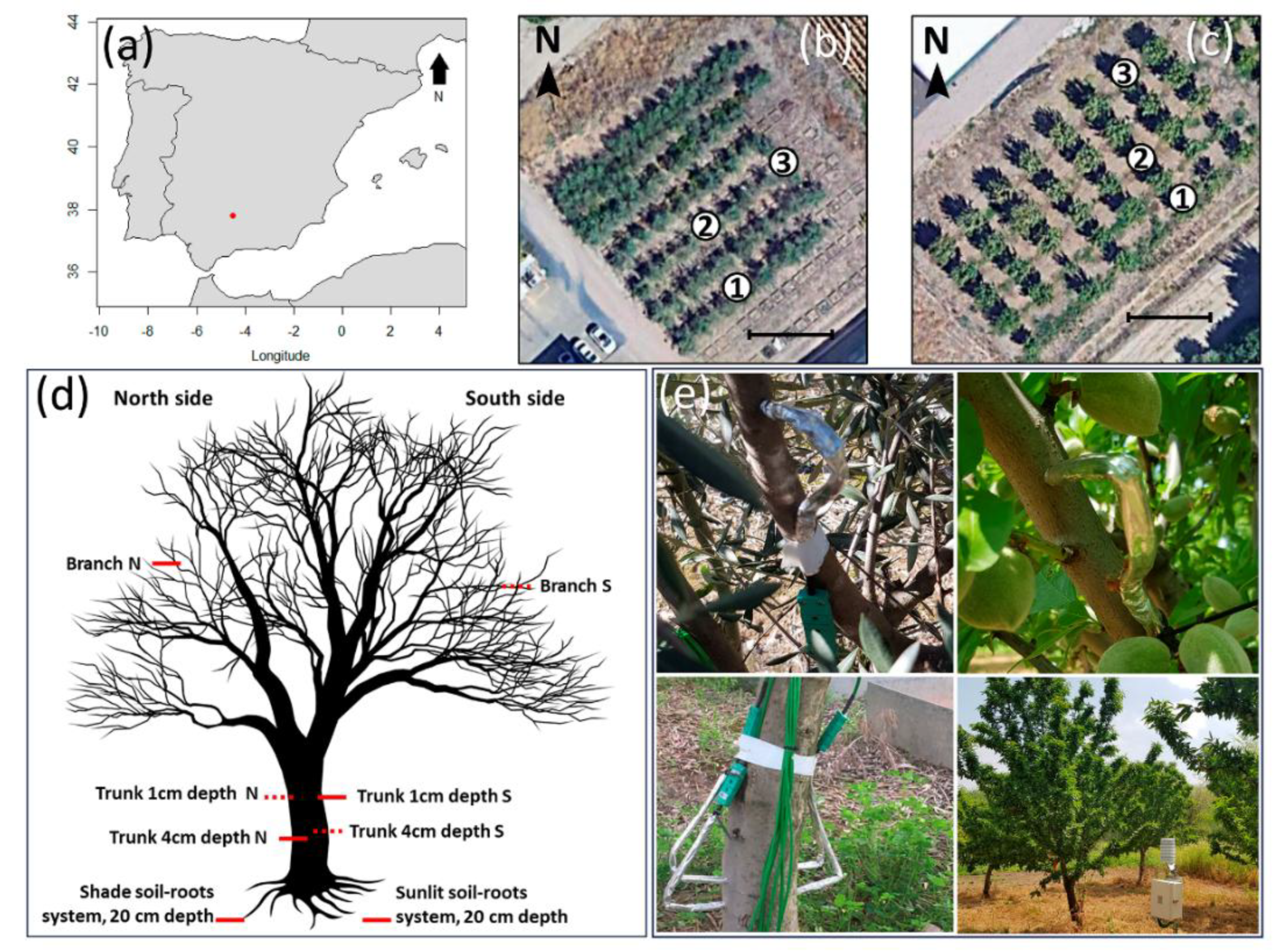
2.1. Study Site
2.2. Temperature Measurements
2.3. Data Analyses
3. Results
3.1. Temporal Dynamics of Sensor Monthly Average Temperature and Its Relationship with Air Temperature
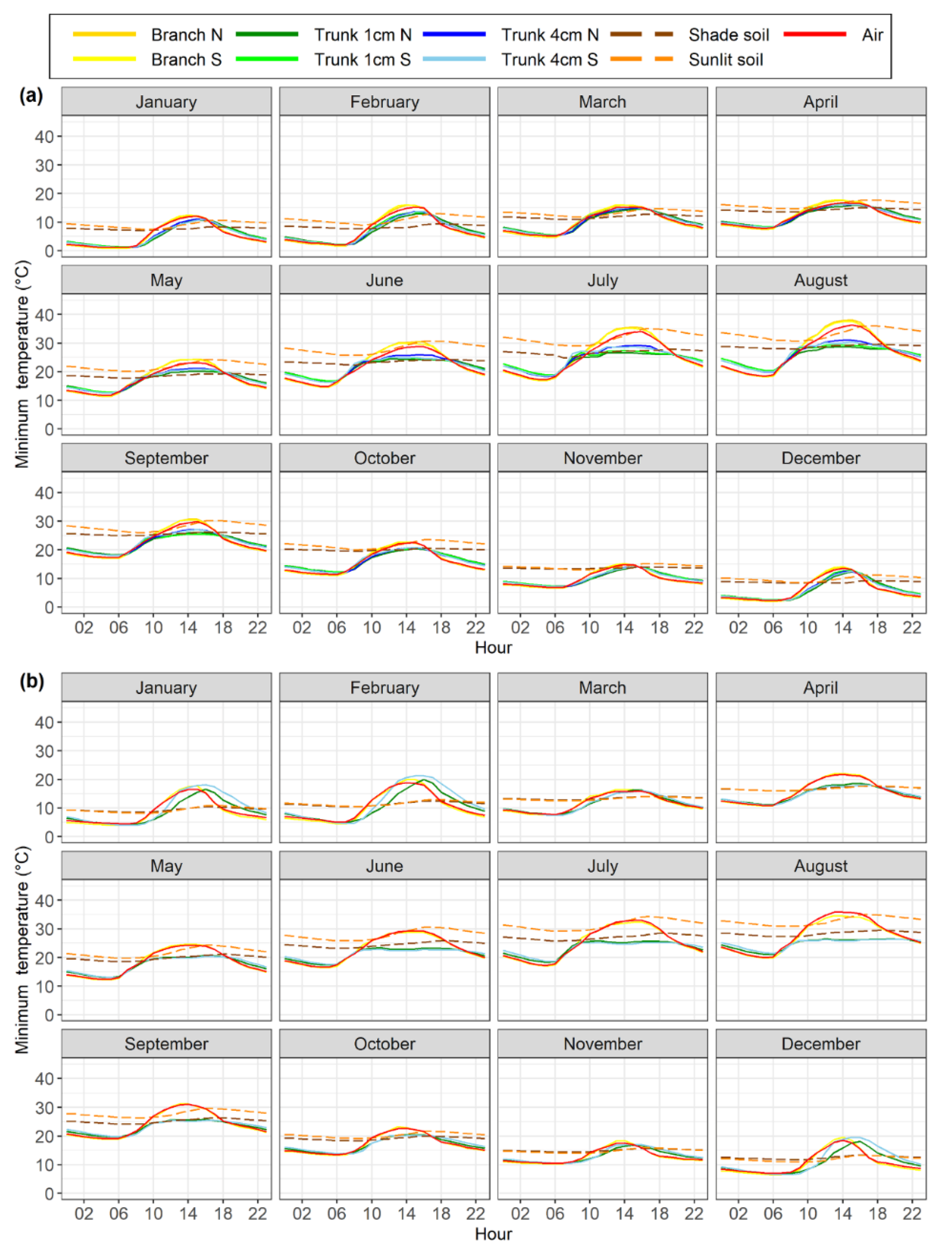
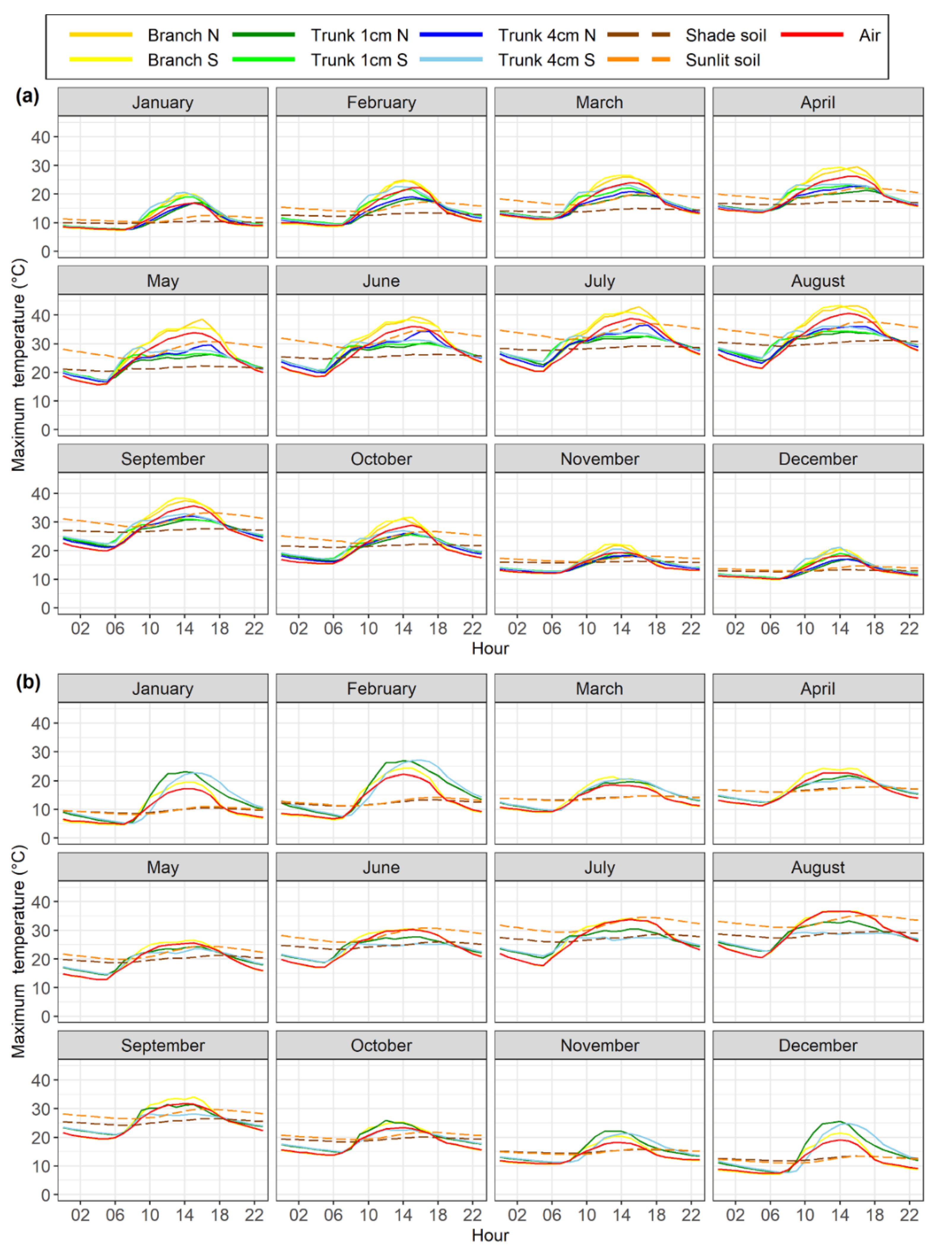
3.1.1. Air Temperatures
3.1.2. Xylem Temperatures
3.1.3. Soil Temperature
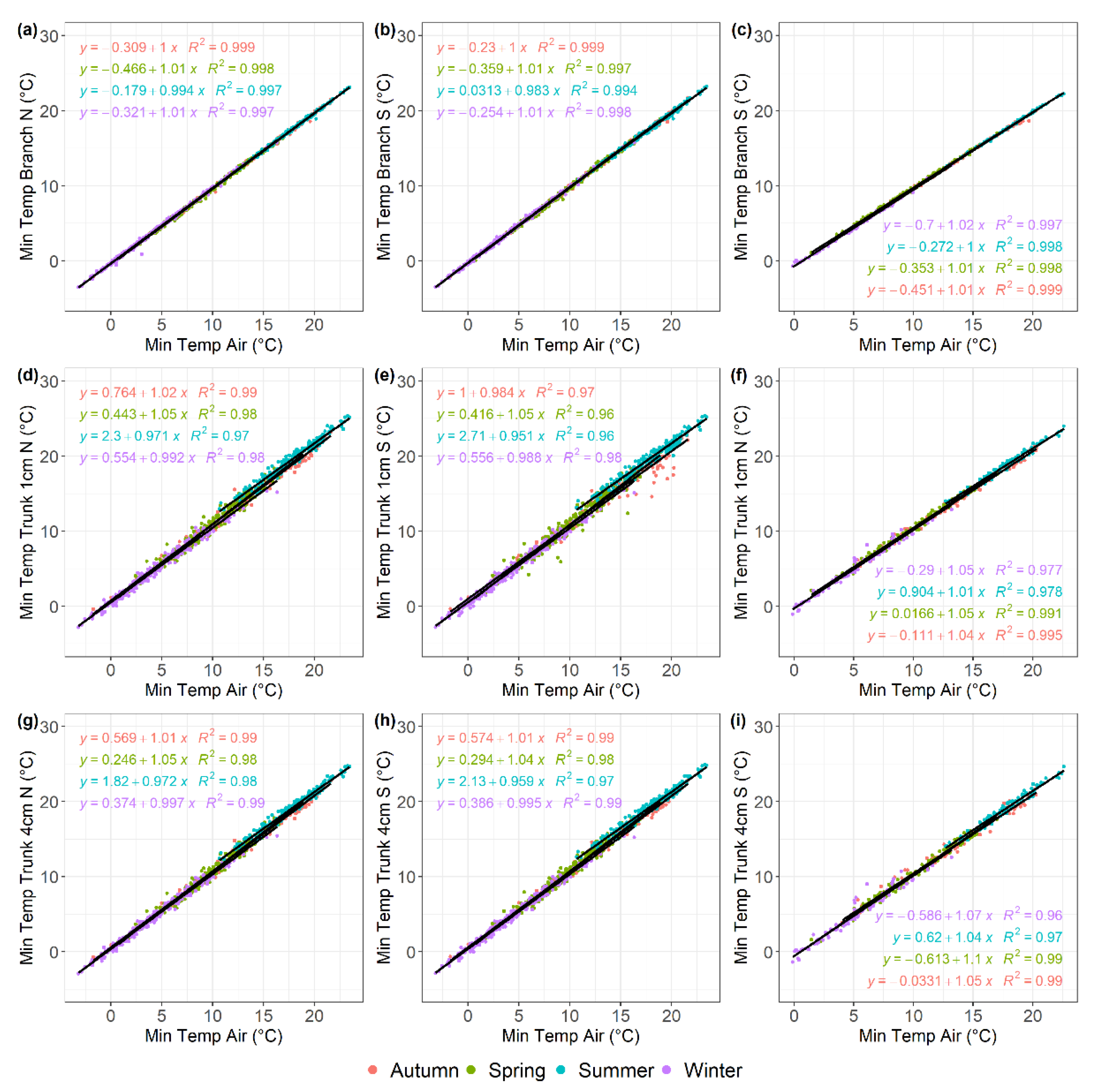
3.2. Seasonal Relationships Between Air and Sensor Temperatures
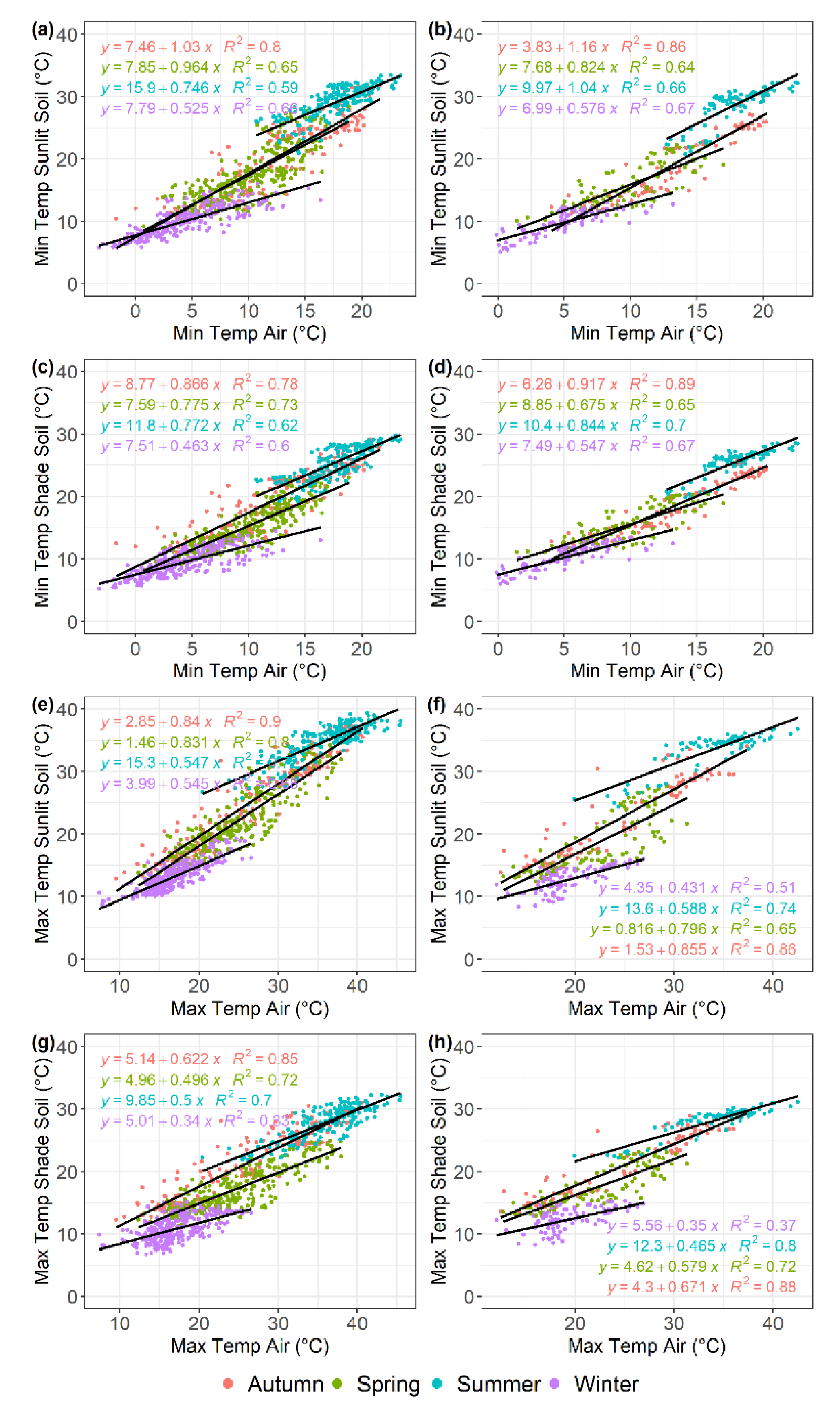
3.2.1. Minimum Temperatures
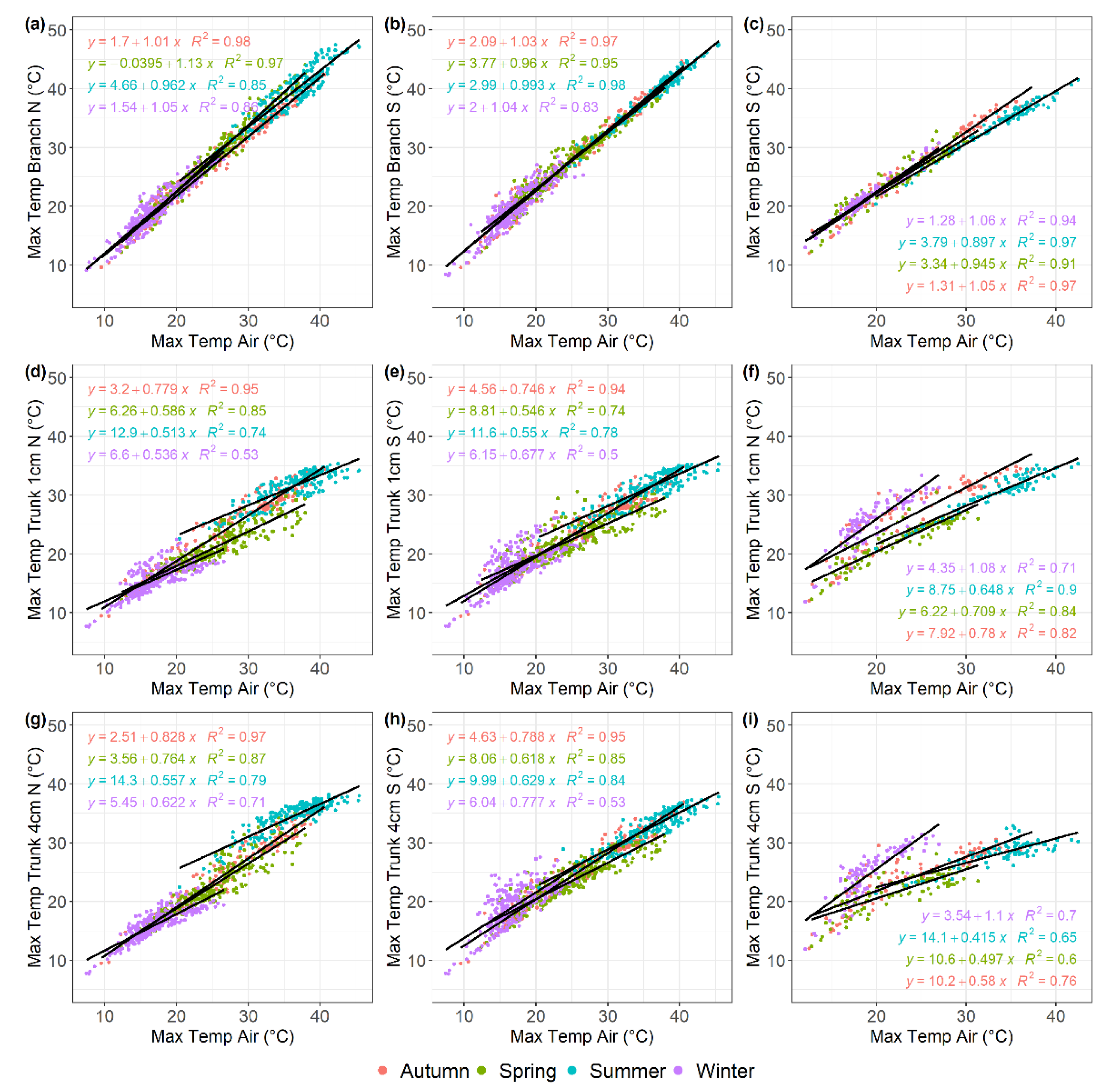
3.2.2. Maximum Temperatures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and agriculture data: Crops and livestock products. Food and Agriculture Organization of the United Nations. 2022. Available online: http://www.fao.org/faostat/en/#data/QCL.
- De Aranzabal, I.; Schmitz, M.F.; Aguilera, P.; Pineda, F.D. Modelling of landscape changes derived from the dynamics of socio-ecological systems: A case of study in a semiarid Mediterranean landscape. Ecol. Indic. 2008, 8, 672–685. [Google Scholar] [CrossRef]
- Kochhar, S.L.; Gujral, S.K. Plant Physiology: Theory and Applications; Cambridge University Press: Cambridge, UK, 2020; ISBN 978-1-108-96347-3. [Google Scholar]
- Principles of Agronomy for Sustainable Agriculture; Villalobos, F.J.; Fereres, E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-46115-1.
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Mahan, J.R.; McMichael, B.L.; Wanjura, D.F. Methods for reducing the adverse effects of temperature stress on plants: A review. Environ. Exp. Bot. 1995, 35, 251–258. [Google Scholar] [CrossRef]
- Stockfors, J. Temperature variations and distribution of living cells within tree stems: implications for stem respiration modeling and scale-up. Tree Physiol. 2000, 20, 1057–1062. [Google Scholar] [CrossRef]
- Derby, R.W.; Gates, D.M. The temperature of tree trunks-calculated and observed. Am. J. Bot. 1966, 53, 580–587. [Google Scholar] [CrossRef]
- Larcher, W. Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst. - Int. J. Deal. Asp. Plant Biol. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Prudencio, Á.S.; Martínez-Gómez, P.; Dicenta, F. Analysis of the modulation of dormancy release in almond (Prunus dulcis) in relation to the flowering and ripening dates and production under controlled temperature conditions. Agronomy 2020, 10, 277. [Google Scholar] [CrossRef]
- Orlandi, F.; Rojo, J.; Picornell, A.; Oteros, J.; Pérez-Badia, R.; Fornaciari, M. Impact of climate change on olive crop production in Italy. Atmosphere 2020, 11, 595. [Google Scholar] [CrossRef]
- Montanaro, G.; Doupis, G.; Kourgialas, N.; Markakis, E.; Kavroulakis, N.; Psarras, G.; Koubouris, G.; Dichio, B.; Nuzzo, V. Management options influence seasonal CO2 soil emissions in Mediterranean olive ecosystems. Eur. J. Agron. 2023, 146, 126815. [Google Scholar] [CrossRef]
- Atkinson, D.; Porter, J.R. Temperature, plant development and crop yields. Trends Plant Sci. 1996, 1, 119–124. [Google Scholar] [CrossRef]
- Ritchie, J.T.; Nesmith, D.S. Temperature and crop development. In Modeling Plant and Soil Systems; John Wiley & Sons, Ltd., 1991; pp. 5–29 ISBN 978-0-89118-223-8.
- Peña Quiñones, A.J.; Hoogenboom, G.; Salazar Gutiérrez, M.R.; Stöckle, C.; Keller, M. Comparison of air temperature measured in a vineyard canopy and at a standard weather station. PLOS ONE 2020, 15, e0234436. [Google Scholar] [CrossRef]
- Edwards, N.T.; Hanson, P.J. Stem respiration in a closed-canopy upland oak forest. Tree Physiol. 1996, 16, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, V. The bark of trees: thermal properties, microclimate and fauna. Oecologia 1986, 69, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.E.; Andresen, J.A. A finite-difference model of temperatures and heat flow within a tree stem. Can. J. For. Res. 2002, 32, 548–555. [Google Scholar] [CrossRef]
- Aubrecht, D.M.; Helliker, B.R.; Goulden, M.L.; Roberts, D.A.; Still, C.J.; Richardson, A.D. Continuous, long-term, high-frequency thermal imaging of vegetation: Uncertainties and recommended best practices. Agric. For. Meteorol. 2016. [Google Scholar] [CrossRef]
- Cannon, J.; Warren, L.; Ohlson, G.; Hiers, J.; Shrestha, M.; Mitra, C.; Hill, E.; Bradfield, S.; Ocheltree, T. Applications of low-cost environmental monitoring systems for fine-scale abiotic measurements in forest ecology. Agric. For. Meteorol. 2022, 321, 108973. [Google Scholar] [CrossRef]
- Mayr, S.; Wieser, G.; Bauer, H. Xylem temperatures during winter in conifers at the alpine timberline. Agric. For. Meteorol. 2006, 137, 81–88. [Google Scholar] [CrossRef]
- Vermunt, B.; Cuddington, K.; Sobek-Swant, S.; Crosthwaite, J.C.; Barry Lyons, D.; Sinclair, B.J. Temperatures experienced by wood-boring beetles in the under-bark microclimate. For. Ecol. Manag. 2012, 269, 149–157. [Google Scholar] [CrossRef]
- Bär, A.; Mayr, S. Bark insulation: Ten Central Alpine tree species compared. For. Ecol. Manag. 2020, 474, 118361. [Google Scholar] [CrossRef]
- Peña Quiñones, A.J.; Keller, M.; Salazar Gutierrez, M.R.; Khot, L.; Hoogenboom, G. Comparison between grapevine tissue temperature and air temperature. Sci. Hortic. 2019, 247, 407–420. [Google Scholar] [CrossRef]
- Järvan, M.; Edesi, L.; Adamson, A.; Võsa, T. Soil microbial communities and dehydrogenase activity depending on farming systems. Plant Soil Environ. 2014, 60, 459–463. [Google Scholar] [CrossRef]
- Calderón, R.; Lucena, C.; Trapero-Casas, J.L.; Zarco-Tejada, P.J.; Navas-Cortés, J.A. Soil temperature determines the reaction of olive cultivars to Verticillium dahliae pathotypes. PLOS ONE 2014, 9, e110664. [Google Scholar] [CrossRef] [PubMed]
- Hunjan, M.S.; Lore, J.S. Climate change: impact on plant pathogens, diseases, and their management. In Crop Protection Under Changing Climate; Jabran, K., Florentine, S., Chauhan, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-46111-9. [Google Scholar]
- Katan, J. Soil temperature interactions with the biotic components of vascular wilt diseases. In Vascular Wilt Diseases of Plants: Basic Studies and Control; Tjamos, E.C., Beckman, C.H., Eds.; Springer: Berlin, Heidelberg, Germany, 1989; ISBN 978-3-642-73168-6. [Google Scholar]
- Feil, H.; Purcell, A.H. Temperature-dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Dis. 2001, 85, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K. Interactive effects of broccoli residue and temperature on Verticillium dahliae microsclerotia in soil and on wilt in cauliflower. Phytopathology 1996, 86, 1303. [Google Scholar] [CrossRef]
- Henneberger, T.S.M.; Stevenson, K.L.; Britton, K.O.; Chang, C.J. Distribution of Xylella fastidiosa in sycamore associated with low temperature and host resistance. Plant Dis. 2004, 88, 951–958. [Google Scholar] [CrossRef]
- Testi, L.; Villalobos, F.J.; Orgaz, F. Evapotranspiration of a young irrigated olive orchard in southern Spain. Agric. For. Meteorol. 2004, 121, 1–18. [Google Scholar] [CrossRef]
- Trenberth, K.E. What are the seasons? Bull. Am. Meteorol. Soc. 1983, 64, 1276–1282. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.0.3; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/.
- de Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research, Version 1.3-7; R Package; 2023. Available online: https://CRAN.R-project.org/package=agricolae.
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means, Version 1.8. R Package, 2024. Available online: https://doi.org/10.32614/CRAN.package.emmeans. [CrossRef]
- Gansert, D.; Burgdorf, M.; Lösch, R. A novel approach to the in situ measurement of oxygen concentrations in the sapwood of woody plants. Plant Cell Environ. 2001, 24, 1055–1064. [Google Scholar] [CrossRef]
- Gartner, B.L. Patterns of xylem variation within a tree and their hydraulic and mechanical consequences. In Plant Stems; Gartner, B.L., Ed.; Physiological Ecology; Academic Press: San Diego, CA, USA, 1995; ISBN 978-0-12-276460-8. [Google Scholar]
- Quick, D.D. Continuous measurements of water status in deeply rooted Southern California chaparral shrub species; M.S. thesis, California State University, Fullerton, USA, 2016; ISBN 978-1-339-75307-2; Available online: https://scholarworks.calstate.
- Lindroth, A.; Mölder, M.; Lagergren, F. Heat Heat storage in forest biomass improves energy balance closure. Biogeosciences 2010, 7, 301–313. [Google Scholar] [CrossRef]
- Trcala, M.; Čermák, J. Nonlinear finite element analysis of thermal inertia in heat-balance sap flow measurement. Int. J. Therm. Sci. 2014, 76, 200–207. [Google Scholar] [CrossRef]
- Kunert, N.; Mercado Cárdenas, A. Effects of xylem water transport on CO2 efflux of woody tissue in a tropical tree, Amazonas State, Brazil. Hoehnea 2012, 39, 139–144. [Google Scholar] [CrossRef]
- López-Bernal, Á.; Alcántara, E.; Testi, L.; Villalobos, F.J. Spatial sap flow and xylem anatomical characteristics in olive trees under different irrigation regimes. Tree Physiol. 2010, 30, 1536–1544. [Google Scholar] [CrossRef]
- Cohen, Y.; Cohen, S.; Cantuarias-Aviles, T.; Schiller, G. Variations in the radial gradient of sap velocity in trunks of forest and fruit trees. Plant Soil 2008, 305, 49–59. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Nadezhdin, V.; Ferreira, M.I.; Pitacco, A. Variability with xylem depth in sap flow in trunks and branches of mature olive trees. Tree Physiol. 2007, 27, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, M.; Acosta, M.; Marek, M.; Kutsch, W.L.; Janous, D. Dependence of the Q10 values on the depth of the soil temperature measuring point. Plant Soil 2007, 292, 171–179. [Google Scholar] [CrossRef]
- Tang, M.; Gao, X.; Zhang, C.; Zhao, X.; Wu, P. Sloping land use affects soil moisture and temperature in the Loess Hilly region of China. Agronomy 2020, 10, 774. [Google Scholar] [CrossRef]
- Bertrand, C.; González Sotelino, L.; Journée, M. Quality control of 10-min soil temperatures data at RMI. Adv. Sci. Res. 2015, 12, 23–30. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; King, J.S.; Burton, A.J.; Brown, S.E. Responses of tree fine roots to temperature. New Phytol. 2000, 147, 105–115. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
- Bejarano-AIcazar, J. Etiology, Importance, and distribution of Verticillium wilt of cotton in Southern Spain. Plant Dis. 1996, 80, 1233. [Google Scholar] [CrossRef]
- Román Ecija, M.; Landa, B.B.; Testi, L.; Navas Cortés, J.A. Extreme temperature differentially affects growth and survival of Xylella fastidiosa strains. In Proceedings of the 3rd European Conference on Xylella fastidiosa; 2021. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Trapero, A. Enhanced production of microsclerotia in recalcitrant Verticillium dahliae isolates and its use for inoculation of olive plants. J. Appl. Microbiol. 2016, 121, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef]
- Janse, J.D.; Obradovic, A. Xylella Fastidiosa: Its biology, diagnosis, control and risks. J. Plant Pathol. 2010, 92, S35–S48. [Google Scholar]
- Amanifar, N.; Taghavi, M.; Salehi, M. Xylella fastidiosa from almond in Iran: overwinter recovery and effects of antibiotics. Phytopathol. Mediterr. 2016, 55, 337–345. [Google Scholar]
- Saponari, M.; Boscia, D.; Altamura, G.; Loconsole, G.; Zicca, S.; D’Attoma, G.; Morelli, M.; Palmisano, F.; Saponari, A.; Tavano, D.; et al. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Sci. Rep. 2017, 7, 17723. [Google Scholar] [CrossRef]
- Purcell, A.H. Environmental therapy for Pierce’s disease of grapevines. Plant Dis. 1980, 64, 388–390. [Google Scholar] [CrossRef]
| T/ Crop/Month |
T air | T soil sunlit |
T soil shade |
T branch N |
T branch S |
T trunk 1cm N |
T trunk 1cm S | T trunk 4cm N |
T trunk 4cm S | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum temperatures | ||||||||||||||||||
| Olive | ||||||||||||||||||
| January | 3.24 | b | 8.72 | a | 8.36 | a | 2.97 | b | 3.05 | b | 3.72 | b | 3.73 | b | 3.57 | b | 3.58 | b |
| February | 4.26 | b | 10.88 | a | 9.69 | a | 3.94 | b | 4.04 | b | 4.84 | b | 4.80 | b | 4.65 | b | 4.67 | b |
| March | 7.01 | cd | 13.84 | a | 12.21 | b | 6.62 | d | 6.75 | cd | 7.72 | c | 7.58 | cd | 7.50 | cd | 7.51 | cd |
| April | 9.80 | cd | 16.17 | a | 14.74 | b | 9.41 | d | 9.53 | d | 10.64 | c | 10.59 | c | 10.42 | cd | 10.41 | cd |
| May | 13.20 | d | 22.43 | a | 19.06 | b | 12.88 | d | 13.04 | d | 14.51 | c | 14.45 | c | 14.21 | c | 14.23 | c |
| June | 15.84 | d | 26.72 | a | 22.96 | b | 15.56 | d | 15.62 | d | 17.57 | c | 17.66 | c | 17.16 | c | 17.21 | c |
| July | 18.37 | d | 30.05 | a | 26.08 | b | 18.09 | d | 18.04 | d | 20.17 | c | 20.24 | c | 19.71 | c | 19.79 | c |
| August | 19.58 | d | 31.16 | a | 28.15 | b | 19.26 | d | 19.31 | d | 21.42 | c | 21.43 | c | 20.92 | c | 20.96 | c |
| September | 18.05 | de | 26.81 | a | 25.38 | b | 17.74 | e | 17.79 | e | 19.16 | c | 18.76 | cd | 18.85 | cd | 18.83 | cd |
| October | 12.35 | bc | 20.89 | a | 20.06 | a | 12.08 | c | 12.14 | c | 13.45 | b | 13.38 | b | 13.16 | bc | 13.11 | bc |
| November | 8.24 | b | 14.27 | a | 14.32 | a | 7.95 | b | 8.04 | b | 8.98 | b | 8.87 | b | 8.77 | b | 8.79 | b |
| December | 5.11 | b | 10.47 | a | 10.36 | a | 4.82 | b | 4.90 | b | 5.62 | b | 5.60 | b | 5.48 | b | 5.47 | b |
| Almond | ||||||||||||||||||
| January | 3.80 | b | 8.15 | a | 8.52 | a | 3.15 | b | 3.76 | b | 3.67 | b | ||||||
| February | 5.28 | b | 10.80 | a | 10.74 | a | 4.70 | b | 5.27 | b | 5.01 | b | ||||||
| March | 7.74 | b | 12.71 | a | 12.90 | a | 7.47 | b | 7.93 | b | 7.74 | b | ||||||
| April | 10.48 | b | 15.95 | a | 15.90 | a | 10.32 | b | 11.01 | b | 10.79 | b | ||||||
| May | 12.15 | d | 19.74 | a | 18.55 | b | 11.90 | d | 12.92 | c | 12.94 | c | ||||||
| June | 16.51 | d | 25.78 | a | 23.26 | b | 16.31 | d | 17.33 | c | 17.56 | c | ||||||
| July | 17.19 | d | 29.22 | a | 25.79 | b | 16.99 | d | 18.32 | c | 18.62 | c | ||||||
| August | 19.94 | d | 30.92 | a | 27.26 | b | 19.75 | d | 21.05 | c | 21.45 | c | ||||||
| September | 18.76 | d | 26.31 | a | 24.06 | b | 18.48 | d | 19.40 | c | 19.60 | c | ||||||
| October | 12.92 | cd | 18.97 | a | 18.15 | b | 12.63 | d | 13.28 | cd | 13.45 | c | ||||||
| November | 9.50 | bc | 13.86 | a | 14.35 | a | 9.16 | c | 9.78 | bc | 9.93 | b | ||||||
| December | 6.52 | cd | 10.84 | b | 11.68 | a | 5.92 | d | 6.41 | c | 6.29 | cd | ||||||
| Maximum temperatures | ||||||||||||||||||
| Olive | ||||||||||||||||||
| January | 15.11 | c | 11.76 | d | 9.59 | e | 17.62 | a | 17.83 | a | 14.58 | c | 16.29 | b | 14.77 | c | 17.80 | a |
| February | 19.52 | c | 14.93 | e | 11.30 | f | 22.07 | ab | 22.60 | a | 16.84 | d | 19.40 | c | 17.53 | d | 20.98 | b |
| March | 20.99 | b | 17.82 | d | 14.05 | e | 23.21 | a | 23.96 | a | 18.16 | cd | 20.38 | b | 19.04 | c | 21.28 | b |
| April | 22.90 | b | 20.06 | de | 16.49 | f | 25.92 | a | 25.96 | a | 19.38 | e | 20.81 | cd | 20.69 | cde | 21.70 | bc |
| May | 29.99 | c | 27.84 | d | 21.01 | g | 34.18 | a | 32.33 | b | 24.51 | f | 25.60 | ef | 27.37 | d | 26.87 | de |
| June | 32.74 | b | 32.44 | b | 25.16 | d | 36.17 | a | 35.26 | a | 28.79 | c | 28.77 | c | 32.20 | b | 29.77 | c |
| July | 37.01 | b | 36.14 | bc | 28.61 | f | 40.23 | a | 39.76 | a | 32.22 | e | 32.17 | e | 35.93 | c | 33.35 | d |
| August | 39.15 | b | 36.92 | c | 30.48 | f | 42.29 | a | 42.16 | a | 33.71 | e | 34.08 | e | 35.60 | d | 35.55 | d |
| September | 33.56 | b | 31.84 | c | 27.07 | e | 35.32 | a | 36.51 | a | 29.77 | d | 30.10 | d | 30.68 | cd | 31.44 | c |
| October | 26.73 | b | 25.36 | bc | 21.58 | d | 28.54 | a | 29.40 | a | 24.00 | c | 24.28 | c | 24.53 | c | 25.34 | bc |
| November | 17.67 | cd | 16.82 | d | 15.29 | e | 19.69 | ab | 20.55 | a | 16.60 | de | 17.44 | cd | 16.86 | d | 18.56 | bc |
| December | 16.41 | bc | 13.14 | d | 11.50 | e | 18.35 | a | 18.72 | a | 15.72 | c | 17.30 | b | 15.78 | c | 18.96 | a |
| Almond | ||||||||||||||||||
| January | 17.44 | c | 11.01 | d | 10.52 | d | 20.03 | b | 23.55 | a | 22.89 | a | ||||||
| February | 21.28 | c | 13.59 | d | 13.00 | d | 23.59 | b | 26.30 | a | 26.55 | a | ||||||
| March | 18.71 | b | 14.55 | c | 14.49 | c | 21.55 | a | 20.15 | ab | 20.71 | a | ||||||
| April | 23.27 | b | 17.85 | d | 17.79 | d | 25.39 | a | 21.92 | c | 20.95 | c | ||||||
| May | 26.14 | b | 24.56 | c | 21.25 | e | 27.29 | a | 24.72 | c | 23.75 | d | ||||||
| June | 30.73 | a | 30.84 | a | 26.03 | c | 31.00 | a | 28.03 | b | 26.15 | c | ||||||
| July | 33.86 | a | 34.52 | a | 28.62 | c | 34.24 | a | 30.63 | b | 28.75 | c | ||||||
| August | 37.05 | a | 35.17 | b | 29.58 | d | 37.26 | a | 33.48 | c | 29.70 | d | ||||||
| September | 31.91 | b | 29.89 | c | 26.54 | e | 34.57 | a | 32.00 | b | 28.64 | d | ||||||
| October | 23.78 | b | 22.05 | c | 20.28 | d | 26.05 | a | 26.64 | a | 23.36 | bc | ||||||
| November | 18.41 | c | 16.04 | d | 15.76 | d | 20.78 | b | 22.94 | a | 21.62 | ab | ||||||
| December | 19.16 | c | 13.40 | d | 13.43 | d | 21.71 | b | 25.90 | a | 24.90 | a | ||||||
| Temperature Crop/Sensor | Season | |||||||
|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | |||||
| Minimum temperatures | ||||||||
| Olive orchard | ||||||||
| Branch N | 9.97 | C d | 10.17 | AB d | 10.60 | A e | 10.38 | BC d |
| Branch S | 10.06 | C d | 10.30 | AB d | 10.63 | A e | 10.45 | BC d |
| Trunk 1 cm N | 10.78 | C c | 11.49 | B c | 12.69 | A c | 11.65 | B c |
| Trunk 1 cm S | 10.77 | C c | 11.41 | B c | 12.74 | A c | 11.46 | B c |
| Trunk 4 cm N | 10.63 | C c | 11.24 | B c | 12.23 | A d | 11.38 | B c |
| Trunk 4 cm S | 10.63 | C c | 11.25 | B c | 12.29 | A d | 11.37 | B c |
| Shade soil | 15.53 | D b | 15.87 | C b | 18.66 | A b | 17.72 | B b |
| Sunlit soil | 16.06 | D a | 18.02 | C a | 22.25 | A a | 18.46 | B a |
| Almond orchard | ||||||||
| Branch N | 10.71 | B c | 11.24 | A c | 11.54 | A d | 11.29 | A d |
| Trunk 1 cm N | 11.27 | C b | 11.94 | B b | 12.76 | A c | 12.02 | B c |
| Trunk 4 cm S | 11.11 | C bc | 11.81 | B b | 13.07 | A c | 12.20 | B c |
| Shade soil | 16.46 | C a | 17.10 | B a | 19.30 | A b | 16.72 | BC b |
| Sunlit soil | 16.10 | C a | 17.41 | B a | 22.51 | A a | 17.59 | B a |
| Maximum temperatures | ||||||||
| Olive orchard | ||||||||
| Branch N | 25.51 | D a | 28.16 | B a | 31.02 | A a | 27.20 | C b |
| Branch S | 25.88 | C a | 27.80 | B a | 30.49 | A a | 28.17 | B a |
| Trunk 1 cm N | 21.92 | B c | 21.07 | C d | 23.07 | A d | 22.81 | A d |
| Trunk 1 cm S | 23.85 | A b | 22.65 | C c | 23.16 | B d | 23.29 | B d |
| Trunk 4 cm N | 22.22 | D c | 22.76 | C c | 26.10 | A b | 23.37 | B d |
| Trunk 4 cm S | 25.43 | A a | 23.67 | C b | 24.37 | B c | 24.46 | B c |
| Shade soil | 17.01 | D e | 17.56 | C e | 19.57 | B e | 20.66 | A e |
| Sunlit soil | 19.47 | D d | 22.30 | C c | 26.67 | A b | 24.03 | B c |
| Almond orchard | ||||||||
| Branch N | 25.94 | B b | 26.36 | C a | 27.61 | A a | 27.31 | A a |
| Trunk 1 cm N | 29.37 | B a | 23.97 | B b | 24.15 | A b | 27.37 | A a |
| Trunk 4 cm S | 28.94 | A a | 23.60 | C b | 21.64 | C c | 24.71 | B b |
| Shade soil | 16.41 | C c | 19.45 | B d | 21.51 | A c | 21.04 | A d |
| Sunlit soil | 16.78 | D c | 20.52 | C c | 26.95 | A a | 22.84 | B c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





