1. Introduction
Approximately 2.4 billion years ago, cyanobacteria initiated one of the most significant developments in Earth’s history. By evolving the capability to perform oxygenic photosynthesis, they were the driving force behind the change in the planet's atmosphere, generating oxygen and thereby facilitating aerobic life and enabling the evolution of life as we know it [
1]. Today, owing to their ability to utilize light energy to sequester CO
2, cyanobacteria could play a crucial role in shaping biotechnology as green, sustainable cell factories. As the only photosynthetic bacteria capable of oxygenic photosynthesis [
2], cyanobacteria are promising platforms for sustainable bioproduction. They harness light energy to efficiently convert CO
2 into value-added products such as acetone, ethanol, sucrose, succinate, or the plastic alternative polyhydroxybutyrate (PHB), releasing oxygen as the sole by-product [
3]. However, improving production efficiency and reducing costs requires bioengineering [
4]. A deeper understanding of cyanobacterial carbon flux is fundamental to optimizing these efforts.
One of the most commonly used host strains for pathway engineering studies of cyanobacteria is the model organism
Synechocystis sp. PCC 6803 (
Synechocystis) [
3]. In non-diazotrophic cyanobacteria, such as
Synechocystis, starvation of the essential nutrient nitrogen is a powerful driving force to switch metabolism from biomass growth to desired product formation [
5,
6,
7]. To increase the yield of products such as PHB, lactate, or succinate, carbon needs to be redirected from the glycogen reserve into product synthesis [
5,
8,
9]. As a non-diazotrophic cyanobacterium,
Synechocystis is unable to fix atmospheric nitrogen and therefore requires a combined nitrogen source for growth. Upon encountering nitrogen-limiting conditions,
Synechocystis initiates a survival program termed chlorosis, resulting in cells entering a long-lived dormant state [
10]. During the initial phase of chlorosis,
Synechocystis degrades its light-harvesting complexes, the phycobilisomes, resulting in a color change of the cells from green to yellow and strongly reduced photosynthetic activity [
11,
12,
13,
14]. Concomitantly, the cells accumulate glycogen reserves as carbon and energy storage to survive prolonged nitrogen starvation and support a highly orchestrated resuscitation process once a nitrogen source becomes available again [
10,
15].
Blocking glycogen as an energy and carbon sink in
Synechocystis is most commonly achieved by deleting the
glgC gene, but can also be accomplished by deleting
pgm [
16] or by simultaneous deletion of
glgA1 and
glgA2 [
17]. The phosphoglucomutase (PGM) encoded by
pgm connects glycogen metabolism to the central carbon metabolism by catalyzing the interconversion of glucose-6-phosphate and glucose-1-phosphate. While the glucose-1-phosphate adenylyltransferase (GlgC) encoded by
glgC catalyzes the conversion of glucose-1-phosphate to ADP-glucose, the first committed, irreversible step of glycogen synthesis, the two glycogen synthase isoenzymes GlgA1 and GlgA2 assemble the resulting ADP-glucose monomers into glycogen polymers.
Modifying glycogen metabolism in cyanobacteria strongly influences their carbon metabolism [
18]. Obstructing carbon flow into the glycogen sink results in the redirection of photosynthetically fixed carbon into alternative carbon sinks during nutrient starvation. A
Synechocystis strain lacking
glgC displays growth comparable to the wild type (WT) under continuous illumination and nitrogen-replete conditions [
17,
19]. However, upon encountering nitrogen starvation, unlike the WT, Δ
glgC is unable to accumulate glycogen and therefore is impaired in executing the chlorosis program [
17]. Furthermore, under nitrogen-starved conditions, deletion of
glgC results in the excretion of photosynthetically fixed carbon as organic acids, mainly 2-oxoglutarate (2-OG) and pyruvate, rather than its storage as glycogen. This overflow metabolism suggests that if glycogen synthesis is impaired during nitrogen starvation, the cells are overloaded with carbon influx and excrete products in response [
19].
Overflow metabolism has been extensively studied in heterotrophic bacteria, yeasts, and higher organisms. There, it has been described as a mechanism of energy spilling, which occurs to consume excess energy when catabolism is faster than anabolism [
20]. In heterotrophic organisms, overflow metabolism most commonly occurs under excess glucose conditions, where it is often characterized by excretion of fermentation products like ethanol, acetate, or lactate. Additionally, the excretion of metabolites from glycolysis, the pentose-phosphate pathway, the tricarboxylic acid (TCA) cycle, and free amino acids by bacteria into the culture medium has been reported. This accumulation of central carbon metabolism intermediates was especially pronounced under cultivation with limited nitrogen supply [
21]. For the photoautotrophic
Synechocystis, metabolic overflow into the medium has been predominantly observed as a result of impaired glycogen metabolism during nitrogen depletion [
17], but could also be triggered by cultivation under high-light in nitrogen-repleted conditions [
22]. These findings led to the hypothesis that metabolite overflow in
Synechocystis functions as a mechanism for energy or redox balancing as well as an alternative carbon sink [
17,
22].
Here, we aimed to gain a deeper insight into the carbon flow and metabolite excretion of
Synechocystis by extensively analyzing the changes within intracellular and extracellular metabolites upon nitrogen starvation across various
Synechocystis carbon metabolism mutants. Particular emphasis was placed on investigating a regulatory mutation of the phosphoglycerate mutase (PGAM) reaction. The PGAM reaction directs the first product of CO
2 fixation, 3-phosphoglycerate, towards lower glycolysis to replenish numerous anabolic pathways. During nitrogen starvation, PGAM activity is inhibited by the small protein PirC (P
II-interacting regulator of carbon metabolism), which is under the control of the carbon/nitrogen/energy balance-sensing signaling protein P
II [
23,
24]. A
pirC knock-out mutant (Δ
pirC) lacks this inhibition, maintaining high PGAM activity and increased carbon flow into lower glycolysis during nitrogen-depletion, resulting in reduced glycogen accumulation and overproduction of the biopolymer PHB during chlorosis [
23]. In addition to the Δ
pirC mutant, we analyzed the single knockout strains Δ
glgA1, Δ
glgA2, Δ
glgC, Δ
pgm, and Δ
glnB, as well as a Δ
glgCΔ
pirC double-knockout strain. Deletion of
glgA1,
glgA2,
glgC, or
pgm blocks the glycogen sink to different extents, thereby disrupting the main carbon sink. Since
glgC deletion completely inhibits glycogen synthesis, concomitant deletion of
glgC and
pirC could potentially further amplify carbon flow into lower glycolysis and downstream metabolites. The Δ
glnB strain, lacking P
II, can no longer sense nitrogen stress or properly coordinate the C/N response and can therefore no longer orchestrate the appropriate response [
25].
Using a combination of enzymatic glycogen quantification, high-performance liquid chromatography-mass spectrometry (HPLC-MS), and targeted liquid chromatography-tandem mass spectrometry (LC-MS/MS), we investigate the changes in intracellular and extracellular metabolites between vegetative and nitrogen-depleted carbon flux mutants and WT strains, to reveal which other disruptions of carbon flow result in metabolite overflow and potentially identify the metabolic changes triggering this response.
3. Results
3.1. Extracellular Metabolite Levels Do Not Simply Mirror Intracellular Metabolite Levels
To investigate overflow excretion of metabolites within
Synechocystis, the accumulation of pyruvate, succinate, 2-OG, malate, and glutamate within the medium of
Synechocystis Δ
glgA1, Δ
glgA2, Δ
glgC, Δ
pgm, Δ
pirC, Δ
glnB, and Δ
glgCΔ
pirC strains was measured during vegetative and nitrogen-depleted growth. In addition, the corresponding intracellular dynamics of these metabolites were determined to investigate the relation between intracellular accumulation and excretion of metabolites. As controls, we analyzed metabolite levels in two
Synechocystis WT strains. Most mutants were generated in a glucose-tolerant (GT) WT (WT-GT) background. However, since the Δ
glnB, Δ
glgC, and Δ
glgCΔ
pirC deletions could not be obtained in this background, a glucose-sensitive (GS) WT (WT-GS) was included as an additional control. In addition, because intracellular pyruvate levels could not be captured by our analytical method, the levels of the neighboring metabolites phosphoenolpyruvate (PEP) and acetyl-CoA were used as an approximation for changes in the intracellular pyruvate pool. To compare the metabolic state of the selected strains during vegetative growth and nitrogen starvation, cells from an exponentially growing preculture were used to inoculate nitrogen-deficient and nitrogen-replete cultures, which were sampled after two days of vegetative growth or two days of nitrogen starvation, as cells should have completed their adaptation to nitrogen depletion within 48 h [
13]. Importantly, even glycogen-free strains remain viable after two days of nitrogen starvation, despite their inability to properly adapt, with viability only decreasing after prolonged starvation (Gründel 2012, Doello 2022). Since overflow metabolism in cyanobacteria is triggered by disruption of the glycogen sink [
17,
18,
29], we also measured the amount of glycogen accumulation in the analyzed strains under the different conditions.
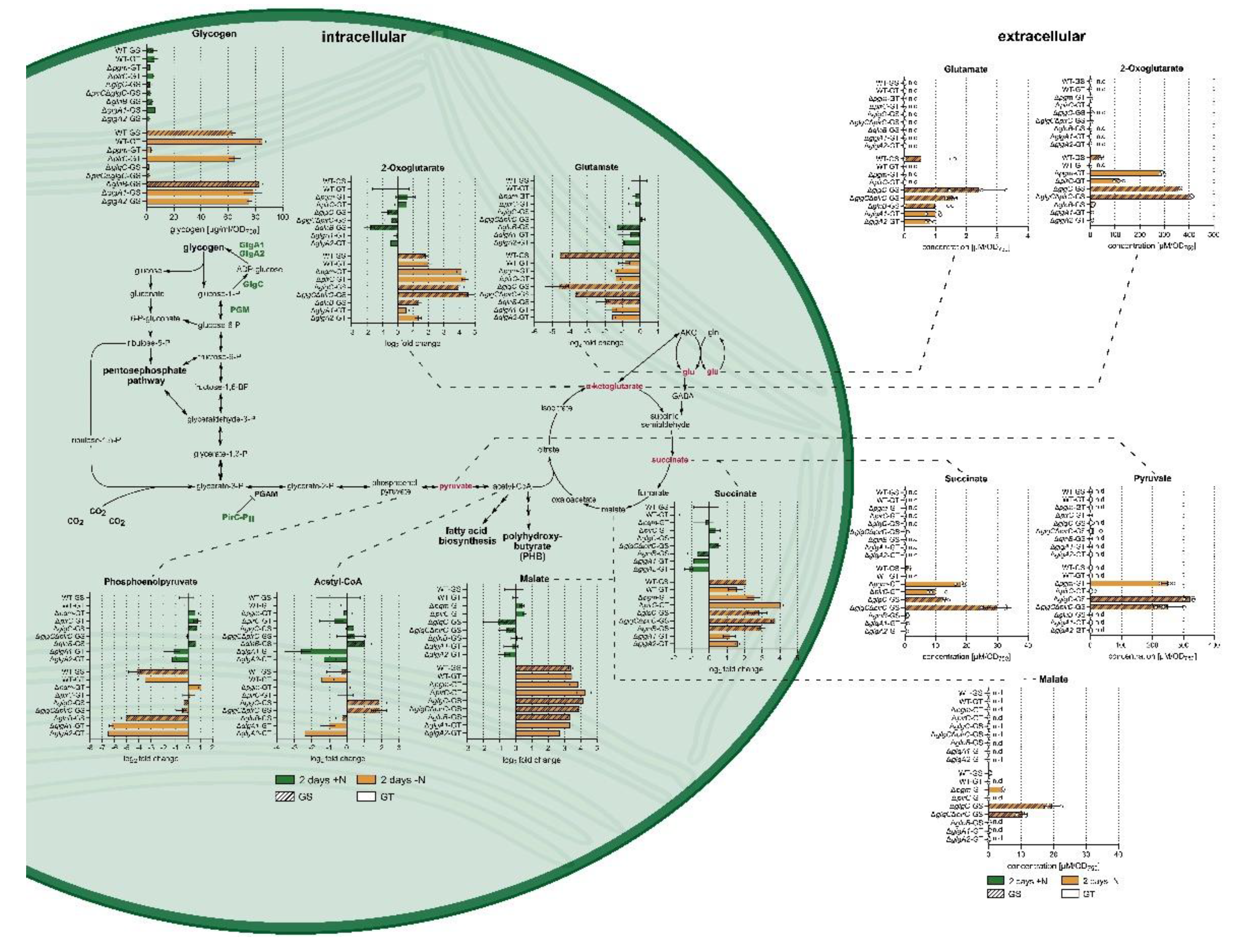
Quantification of glycogen confirmed that deletion of either
pgm or
glgC results in loss of glycogen accumulation during chlorosis, as both strains exhibited only marginal values close to the detection limit of the assay [
28] (
Figure 1). Simultaneously, deletion of
pirC reduced the amount of accumulated glycogen moderately, in agreement with previous publications [
16,
17,
23].
Our results also show that for all strains, metabolite excretion in noteworthy amounts only occurred during nitrogen starvation (
Figure 1). As previously reported, during nitrogen starvation, considerable amounts of pyruvate and 2-OG were excreted by mutants unable to accumulate glycogen due to the deletion of
pgm or
glgC [
17,
29]. Specifically, Δ
pgm released 250 µM/OD
750 pyruvate and 291 µM/OD
750 2-OG, Δ
glgC excreted 322 µM/OD
750 and 364 µM/OD
750, and Δ
glgCΔ
pirC excreted 250 µM/OD
750 and 409 µM/OD
750, respectively. Moreover, our results show that these strains also released small amounts of succinate and malate during nitrogen starvation, which, to our knowledge, has not been reported for
Synechocystis before. In detail, Δ
glgCΔ
pirC released the highest amount of succinate (30 µM/OD
750), followed by Δ
pgm (18 µM/OD
750) and Δ
glgC (13 µM/OD
750). For malate, Δ
glgC showed the highest excretion (19 µM/OD
750), whereas Δ
glgCΔ
pirC (11 µM/OD
750) and Δ
pgm (4 µM/OD
750) released lower amounts. Interestingly, the Δ
pirC strain also exhibited a distinct pattern of metabolite excretion. Despite retaining the ability to synthesize glycogen, albeit to lower levels than the corresponding WT, the Δ
pirC strain excreted 115 µM/OD
750 2-OG and 10 µM/OD
750 succinate, but neither pyruvate nor glutamate. The concurrent deletion of
glgC and
pirC resulted in an additive effect for succinate and 2-OG excretion, while extracellular pyruvate concentrations were comparable for the double-mutant and Δ
glgC (~250 µM/OD
750).
Remarkably, intracellular and extracellular metabolite dynamics did not always mirror each other. 2-OG was mainly excreted by the strains strongly accumulating 2-OG intracellularly, namely Δ
pgm, Δ
pirC, Δ
glgC, and Δ
glgCΔ
pirC, and all strains excreting pyruvate had higher intracellular PEP and acetyl-CoA levels compared to the WT (
Figure 1). However, not all strains with increased intracellular PEP and acetyl-CoA levels excreted pyruvate into the medium. This could be observed for the Δ
glgA1, Δ
glnB, and Δ
pirC strains. Furthermore, succinate accumulated intracellularly in a similar way within the Δ
pirC and the Δ
glgCΔ
pirC strain, but the latter excreted approximately three times more succinate into the medium. Malate was only excreted by the nitrogen-starved Δ
pgm, Δ
glgC, and Δ
glgCΔ
pirC strains, despite similar intracellular malate levels across all strains.
Intriguingly, glutamate was excreted even though intracellular levels declined under nitrogen starvation (
Figure 1). Small amounts were excreted by one WT-GS replicate and by the corresponding Δ
glnB mutant. The Δ
glgC strain excreted the highest amount of glutamate, even though its intracellular glutamate levels dropped as strongly as in the cognate WT-GS. The Δ
glgCΔ
pirC strain showed a slightly smaller decrease in intracellular glutamate but nevertheless excreted similar amounts to the Δ
glgC strain. Among the glucose-tolerant strains, intracellular glutamate decreased less than in the GS-background during nitrogen depletion. However, the Δ
glgA1 and Δ
glgA2 mutants excreted small amounts of glutamate, whereas Δ
pgm and Δ
pirC did not excrete any.
Altogether, these results show that metabolites were not simply excreted when they accumulated intracellularly, and metabolite excretion was not exclusively triggered by the blocking of the glycogen sink. Interestingly, metabolite excretion also did not categorically result in intracellular balancing of metabolite levels. Most strains that excreted metabolites did not exhibit intracellular metabolite levels comparable to those within the WT. Thus, metabolite excretion did not result in restoring intracellular metabolite levels to WT metabolite levels.
3.2. Intracellular Central Carbon and Nitrogen Metabolism
To further investigate which underlying carbon fluxes contribute to metabolite excretion, we analyzed the intracellular levels of 124 metabolites across all strains under both vegetative (
Figure S1) and nitrogen-starved conditions (
Figure S2). We focused on metabolites from central carbon and nitrogen metabolism captured by our measurement method, specifically comparing intermediates of glycolysis starting from glycogen, the TCA cycle, and the glutamine synthetase/glutamate synthase cycle (GS/GOGAT) during nitrogen starvation to the respective WT metabolite levels during vegetative growth.
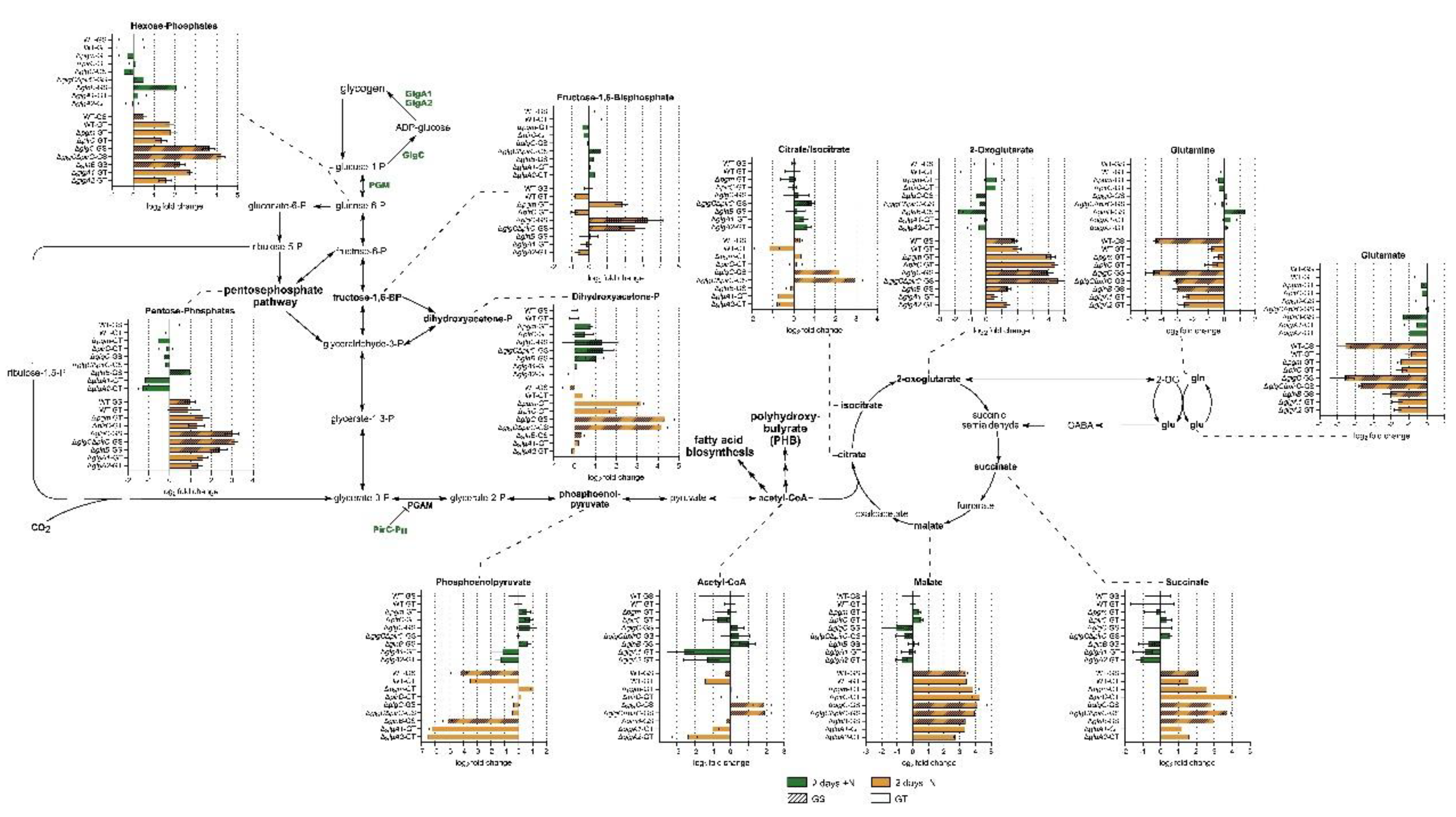
The results show that nitrogen depletion resulted in a buildup of hexose-phosphates and pentose-phosphates within all strains compared to their respective WT under vegetative growth conditions (
Figure 2).
Interestingly, sugar-phosphate accumulation was more pronounced within strains lacking glgC as well as in the ΔglnB strain. Notably, ΔglnB exhibited higher intracellular levels of hexose-phosphates and pentose-phosphates than its cognate WT even during vegetative growth.
All strains displaying pronounced overflow metabolism, namely Δ
pgm, Δ
glgC, Δ
pirC, and Δ
glgCΔ
pirC, exhibited higher intracellular dihydroxyacetone-phosphate (DHAP) levels than their respective WT during nitrogen starvation (
Figure 2). Similarly, fructose-1,6-bisphosphate accumulated within all these strains, except Δ
pirC, during nitrogen depletion.
In WT cells, PEP levels strongly decreased upon N-starvation (
Figure 2). The same was observed for the Δ
glnB and the Δ
glgA mutants. Interestingly, those strains that export 2-OG (Δ
pgm, Δ
pirC, Δ
glgC, and Δ
glgCΔ
pirC) maintained similar intracellular PEP levels during nitrogen depletion as during vegetative cultivation, or in the case of Δ
pgm, even slightly increased PEP levels during nitrogen depletion.
Figure 2 also shows that all strains, including the WT strains, accumulated TCA cycle intermediates upon nitrogen starvation. However, while both WT accumulated predominantly malate, succinate, and 2-OG, but no or only slight amounts of citrate/isocitrate, especially ΔglgC and ΔglgCΔpirC accumulated increased intracellular citrate/isocitrate levels during nitrogen depletion. The four strains excreting the most metabolites, Δpgm, ΔglgC, ΔpirC, and ΔglgCΔpirC, also displayed increased intracellular 2-OG levels during nitrogen-depleted growth, while the ΔglgA1 and ΔglgA2 strains had noticeably lower intracellular 2-OG levels during vegetative growth and nitrogen-depleted growth compared to the respective WT. During vegetative growth, lower intracellular 2-OG levels could also be observed for the ΔglnB strain.
While intracellular malate levels were comparable within all strains during nitrogen-limited cultivation, the accumulation of succinate was more pronounced in the ΔglnB, ΔpirC, and ΔglgCΔpirC.
Within the main nitrogen assimilation pathway, the GS/GOGAT, both WT variants decreased their intracellular glutamine and glutamate levels during nitrogen starvation. However, there was a striking difference in the extent of this decrease between the WT-GT and WT-GS. While the WT-GS strongly decreased both metabolites during chlorosis, there was only a slight decrease in glutamine and glutamate levels within the WT-GT.
Within most mutant strains, glutamate dynamics upon nitrogen depletion were similar to those of their cognate WT. Noticeable exceptions were the ΔglgCΔpirC and especially the ΔglnB strain, which decreased their intracellular glutamate level during nitrogen depletion not as strongly as the corresponding WT. This could also be observed for the glutamine dynamics within those strains. In contrast, the glutamine levels within the ΔglgA mutants decreased stronger than in their respective WT, while the glutamine dynamics of Δpgm, ΔpirC, and ΔglgC during nitrogen depletion were comparable to those of their respective WT.
Collectively, these results suggest that metabolite excretion correlates with intracellular accumulation of key intermediates from glycolysis and the TCA cycle under nitrogen-starved conditions.
3.3. Intracellular Energy and Redox Balance
To determine whether our data supports the theory that overflow metabolism in
Synechocystis is a mechanism for maintaining energy and redox balance [
17,
22,
32], we examined the intracellular dynamics of AMP/ADP/ATP, NAD/NADH, NADP/NADPH, and reduced/oxidized glutathione in the various carbon metabolism mutants.
During nitrogen starvation, ATP, ADP, and AMP levels decreased in both WT strains (
Table 1).
In contrast, all carbon metabolism mutants, except Δ
pirC, showed a considerable increase in their intracellular AMP levels upon nitrogen shift (
Table 1). This increase was strongest for the Δ
pgm strain, which increased its AMP levels sixfold upon nitrogen starvation. Interestingly, all carbon metabolism mutants maintained similar or merely slightly altered ADP levels to those during vegetative growth under nitrogen-depleted conditions. These AMP and ADP dynamics were also reflected in the adenylate energy charge, calculated as (ATP + 0.5 ADP)/(ATP+ADP+AMP), of the various strains (
Figure S3). Except for Δ
pirC, the energy charge decreased in all strains during nitrogen starvation (
Figure S3). Interestingly, the energy charge decrease was significantly more pronounced than in the respective WT for Δ
pgm and Δ
glgA2.
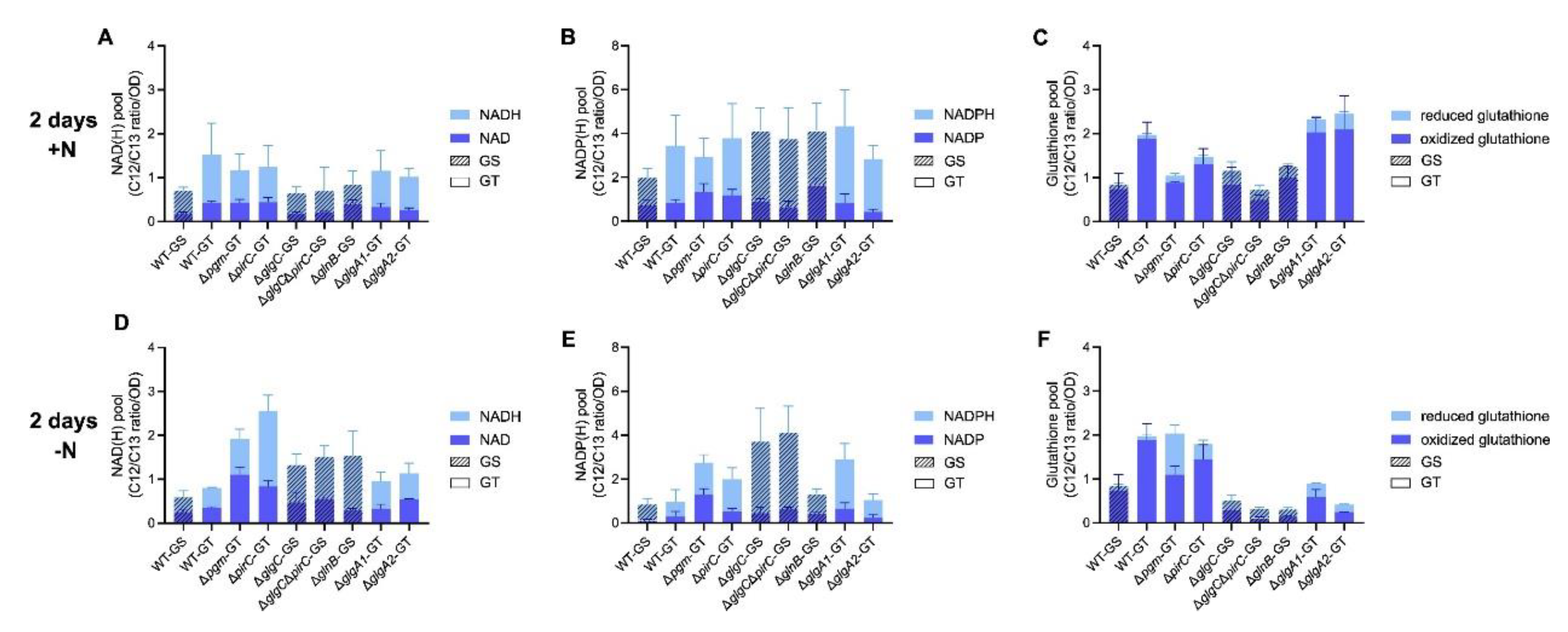
Investigation of the total NAD(H) pool revealed that total NAD(H) levels slightly decreased in both WT backgrounds upon nitrogen starvation. In contrast, Δ
pgm, Δ
pirC, Δ
glgC, Δ
glgCΔ
pirC, and Δ
glnB maintained high NAD(H) levels or even increased them (
Figure 3A and Figure 3D).
For the individual metabolites, NADH decreased slightly in both WT, while NAD remained mostly stable, whereas Δ
pgm, Δ
pirC, Δ
glgCΔ
pirC, and Δ
glgA2 exhibited increased NAD under nitrogen starvation. NADH levels were stable in Δ
pgm and Δ
glgA2, slightly increased in Δ
glgCΔ
pirC and Δ
glnB, and noticeably increased in Δ
pirC. Similarly, the total NADP(H) pool was comparable across strains during vegetative growth, except for a slightly elevated NADP level in Δ
glnB (
Figure 3B and Figure 3E). Upon nitrogen depletion, total NADP(H) decreased in the WT strains, as well as in Δ
pirC, Δ
glnB, and Δ
glgA2. In contrast, Δ
pgm, Δ
glgC, Δ
glgCΔ
pirC, and Δ
glgA1 maintained NADP(H) levels similar to those during vegetative growth. Simultaneously, the individual NADP and NADPH levels decreased in both WT and Δ
glgA2, but remained stable in Δ
pgm, Δ
glgC, Δ
glgCΔ
pirC, Δ
pirC, and Δ
glgA1. Although NAD(H) and NADP(H) pools as well as individual cofactor levels varied across strains, the NAD/NADH and NADP/NADPH ratios were generally stable, with significant changes observed only for nitrogen-starved Δ
pgm and Δ
glnB (
Figure S4). This suggests that most strains maintain their NAD(H)/NADP(H) redox balance despite altered cofactor pools.
We also investigated the redox state of glutathione within the different strains, as glutathione is a key molecule in the antioxidative defense system in cyanobacteria. It helps maintain protein cysteine residues in their reduced state and detoxifies reactive oxygen species, thereby protecting cells from oxidative damage. Glutathione is synthesized from glutamate, cysteine, and glycine, linking its function to glutamate metabolism [
33,
34,
35].
In both WT strains and in ΔpirC, the total glutathione pool as well as its composition remained largely unchanged between vegetative growth and nitrogen starvation (Figure 3C and 3F). In contrast, ΔglgC, ΔglgCΔpirC, ΔglnB, ΔglgA1, and ΔglgA2 showed a decrease in total glutathione levels, whereas Δpgm exhibited an increase in total glutathione. The drop of the glutathione pool in ΔglnB, ΔglgA1, and ΔglgA2 was mainly driven by a strong decrease in oxidized glutathione, while in ΔglgC and ΔglgCΔpirC, only a slight decrease in the oxidized fraction was observed. The increase in the glutathione pool in Δpgm, by comparison, resulted from the accumulation of reduced glutathione under nitrogen starvation. So, in contrast to the largely stable NAD(H) and NADP(H) ratios, analysis of the glutathione redox balance indicates a strain-specific imbalance, with ΔglnB, ΔglgA1, and ΔglgA2 displaying a more pronounced decrease in oxidized glutathione, while Δpgm accumulated reduced glutathione.
Overall, these results show that most strains largely preserve energy and redox homeostasis during nitrogen depletion, whereas ΔglnB, ΔglgA1, and ΔglgA2 show moderate imbalances, and Δpgm displays the most pronounced disruption, combining reduced energy charge from AMP accumulation with a distinct shift toward reduced glutathione.
3.4. Metabolite Excretion Represents a Major Alternative Carbon Sink
Gründel et al. previously proposed that overflow excretion provides an alternative carbon sink in the absence of glycogen storage [
17]. To assess how fixed carbon was partitioned between glycogen storage and metabolite excretion within the investigated strains, we converted measured glycogen amounts into intracellular glucose equivalents (
Equation S1) and, analogously, calculated the intracellular metabolite concentrations removed from the cell via excretion from the extracellularly detected amounts of metabolites (
Equation S2).
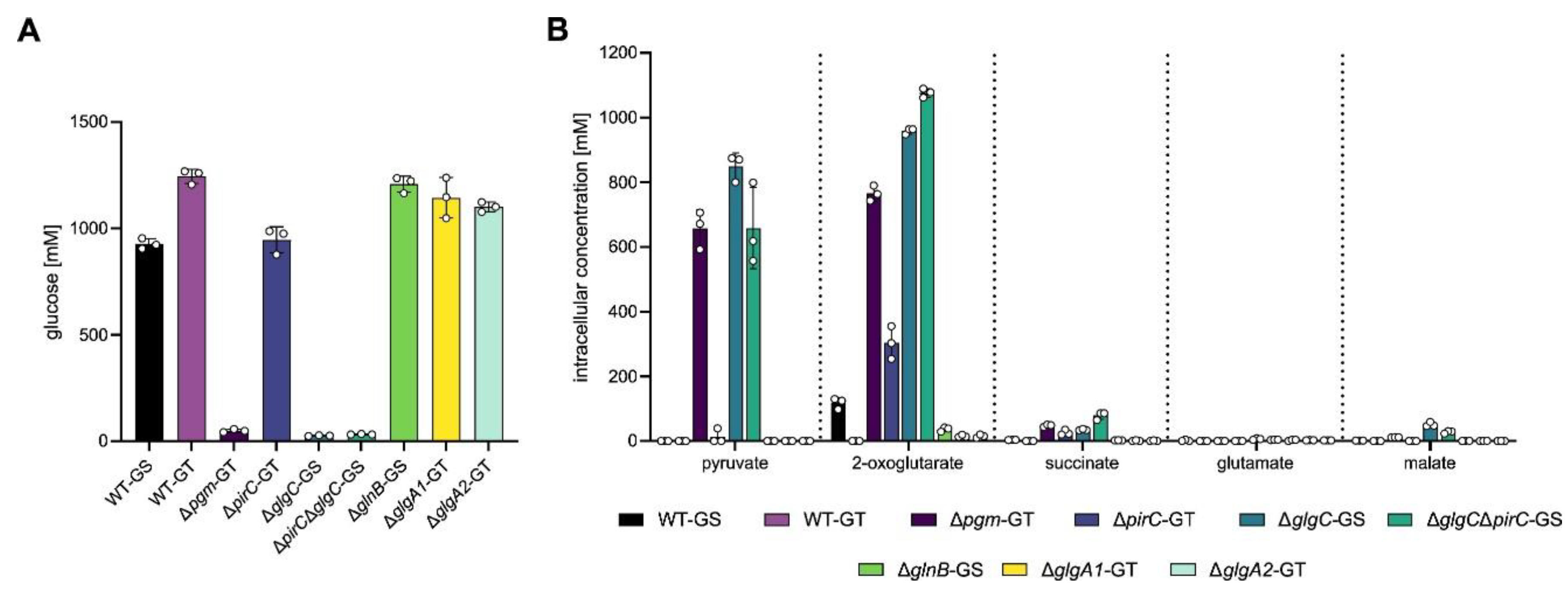
As shown in
Figure 4A, WT-GS, WT-GT, Δ
pirC, Δ
glnB, Δ
glgA1, and Δ
glgA2 accumulated glycogen corresponding to 0.93–1.25 M intracellular glucose under nitrogen starvation. While cells of the Δ
pgm mutant failed to accumulate noteworthy amounts of glycogen, they instead excreted organic acids corresponding to an intercellular equivalent of ca. 657 mM pyruvate, 765 mM 2-OG, 48 mM succinate, and 12 mM malate (
Figure 4B). Similarly, Δ
glgC excreted an equivalent of ca. 848 mM pyruvate, 958 mM 2-OG, 35 mM succinate, and 51 mM malate, while Δ
glgCΔ
pirC released an equivalent of ca. 658 mM pyruvate, 1076 mM 2-OG, 79 mM succinate, and 28 mM malate.
Considering the carbon content of the excreted metabolites, Δpgm, ΔglgC, and ΔglgCΔpirC excreted approximately 1–1.3 M glucose equivalents, representing ca. 81 %, 138 %, and 140 %, respectively, of the carbon that would have been stored as glycogen by the respective WT under the same conditions. This indicates that the fixed carbon normally directed into glycogen storage is redirected into overflow excretion when glycogen synthesis is impaired. Notably, among the three mutants, Δpgm excreted the least carbon despite its equal inability to store glycogen, and deletion of pirC in the ΔglgC background did not lead to a substantial increase in carbon excretion.
4. Discussion
Cyanobacteria, like the model organism Synechocystis, hold great potential as a chassis for sustainable biotechnology. However, efficient bioengineering for increased efficiency and reduced costs requires a deeper understanding of their carbon partitioning. Here, we analyzed the carbon flow and overflow excretion of various carbon flux mutants to increase our understanding of carbon flux and overflow metabolism within Synechocystis.
It has been previously shown that disrupting the glycogen sink within
Synechocystis by deleting Δ
glgC results in excretion of 2-OG and pyruvate, and that drastically reducing glycogen accumulation by deleting Δ
pgm has a similar effect [
17,
29]. Our data not only confirms this but also reveals that overflow metabolism within these strains is not limited to the excretion of 2-OG and pyruvate, as small amounts of malate, succinate, and, in the case of Δ
glgC, also glutamate were additionally excreted. Nevertheless, pyruvate and 2-OG remain the most prominently excreted metabolites (
Figure 1). This aligns with findings by Kato et al., who reported that
Synechococcus elongatus PCC 7942 excretes several metabolites in addition to 2-OG and pyruvate when glycogen synthesis is blocked during nitrogen starvation. Additionally, Kato et al. showed that, in the presence of a nitrogen source, a
glgC-deficient mutant of
Synechococcus elongatus PCC 7942 primarily excreted glutamate as an alternative carbon sink to glycogen [
18]. Here we show that the Δ
glgC mutant in
Synechocystis excretes small amounts of glutamate even during nitrogen starvation, when intracellular glutamate levels decrease considerably. This is a clear indication against excretion as a result of passive metabolic overflow and suggests the contribution of an active transport mechanism that is triggered under specific metabolic conditions.
Our results also suggest that, while metabolite excretion in
Synechocystis is primarily triggered by blocking the glycogen sink, it can be further modulated by other changes in the carbon flow. Despite accumulating WT levels of glycogen under nitrogen starvation, Δ
glgA1 and Δ
glgA2 excreted small amounts of glutamate (
Figure 1), while displaying considerably lower intracellular 2-OG, glutamate, and glutamine levels compared to the WT (
Figure 2). This suggests that deletion of either homolog subtly alters carbon flux partitioning and slightly increases residual GS/GOGAT activity compared to the WT, diverting more 2-OG into glutamate synthesis and excretion. Such reallocation of carbon flow could also explain the significant reduction in energy charge compared to the WT during chlorosis observed in the
glgA2 mutant. To date, no regulatory feedback of GlgA activity influencing nitrogen assimilation within
Synechocystis has been described. However, our data suggests a potential connection between the glycogen synthases and the GS/GOGAT reaction, with glycogen synthase activity potentially influencing nitrogen assimilation.
We also observed that increasing carbon flux toward the TCA cycle via increased PGAM reaction through deletion of
pirC [
23] favored excretion of succinate and 2-OG at the expense of pyruvate excretion, as Δ
pirC exclusively excreted 2-OG and succinate (
Figure 1). This effect of
pirC deletion could also be observed for the Δ
glgCΔ
pirC strain. Concomitant deletion of
pirC and
glgC only subtly altered metabolite excretion, with the double mutant excreting slightly more 2-OG and succinate than the Δ
glgC single mutant. This was unexpected, as previous work had shown that Δ
pirC accumulates substantially more pyruvate than succinate intracellularly after 48 h of nitrogen starvation [
23]. However, we did not detect any extracellular pyruvate in the Δ
pirC strain after two days of nitrogen depletion. Thus, Δ
pirC did not excrete all metabolites that accumulated intracellularly, suggesting that metabolites are probably not simply excreted by passive diffusion but via a selective transport mechanism. This agrees with the work of Benson et al., suggesting that an unknown transporter facilitates overflow excretion of pyruvate in
Synechococcus PCC7942 [
36] and the hypothesis that overflow excretion is facilitated by mechanosensitive channels in glycogen-deficient cyanobacteria [
18].
We also observed that strains accumulating metabolites to similar intracellular levels varied in the extent of metabolite excretion. The amount of 2-OG excreted by Δ
pgm and especially Δ
pirC was comparably small, despite Δ
pirC, Δ
pgm, Δ
glgC, and Δ
glgCΔ
pirC displaying equally increased intracellular 2-OG levels upon nitrogen starvation. Similarly, while Δ
pirC and Δ
glgCΔ
pirC had comparable intracellular succinate levels during nitrogen depletion, Δ
glgCΔ
pirC excreted considerably more succinate. Malate followed the same pattern, being excreted exclusively by Δ
pgm, Δ
glgC, and Δ
glgCΔ
pirC, even though intracellular malate levels were comparable across strains (
Figure 1). Together, these findings suggest that excretion is not a simple function of intracellular metabolite abundance but may require metabolite levels to surpass a strain-specific threshold before being released.
This hypothesis is also supported by our observations concerning TCA cycle intermediates. Both WT predominantly accumulated downstream TCA intermediates such as succinate and malate upon nitrogen starvation. In accordance with the work of Kato et al. [
18], disturbance of the glycogen sink within the metabolite-excreting mutants Δ
pirC, Δ
pgm, Δ
glgC, and Δ
glgCΔ
pirC resulted in intracellular accumulation of TCA cycle intermediates during nitrogen starvation. Interestingly, this accumulation within the mutants was more pronounced within citrate/isocitrate and 2-OG, leading to stronger differences in early TCA cycle intermediate levels between the mutants and their respective WT. Simultaneously, only slight differences in succinate and almost no differences in the change of the malate levels could be observed (
Figure 2). These observations could be explained by a threshold-dependent release of TCA intermediates, specifically 2-OG. The reaction generating 2-OG is a well-described bottleneck of the TCA cycle during nitrogen starvation. During nitrogen starvation, the GS/GOGAT comes to a halt, and 2-OG is no longer consumed for nitrogen assimilation, resulting in its buildup [
25]. Threshold-dependent excretion of this accumulated 2-OG could provide a means of buffering the TCA cycle, thereby helping to maintain balanced levels of downstream TCA intermediates. Thus, downstream TCA intermediates only accumulate more pronouncedly than in the WT once overflow excretion is not sufficient to buffer increased carbon flow into the TCA. This was mainly observed in strains in which the carbon flow towards lower glycolysis and the TCA cycle was increased due to the deletion of
pirC.
Remarkably, if metabolite excretion is triggered in a threshold-dependent manner, this threshold apparently lies above normal WT metabolite levels, as excretion did not consistently restore intracellular concentrations to WT values (
Figure 1).
Metabolite excretion also did not result in global balancing of intracellular metabolite levels. We observed stronger metabolic differences between strains excreting large amounts of metabolites and their respective WT than between strains with little or no excretion (
Figure S2). Across most of the 124 measured intracellular metabolites, strains that excrete substantial amounts of metabolites (Δ
pgm, Δ
glgC, Δ
pirC, and Δ
glgCΔ
pirC) showed a more pronounced increase in intracellular metabolites during nitrogen starvation compared to their respective WT. This was not observed for strains with minimal or no excretion (
Figure S2). This indicates that while metabolic overflow is the result of a severely unbalanced metabolism, it is apparently not sufficient to restore WT metabolite balance. Nonetheless, excretion may act as a regulatory mechanism to prevent excessive accumulation and maintain metabolite levels within a defined physiological range.
Comparing the amount of carbon excreted as metabolites with that stored as glycogen revealed how excess carbon, which cannot be stored as glycogen, is redirected into the excretion of metabolites such as succinate, malate, pyruvate, and 2-OG (
Figure 4). These findings highlight that metabolic overflow is important for intracellular homeostasis, as excess carbon and metabolites appear to be excreted using the medium as an alternative sink. Although overflow excretion alone is insufficient to maintain balanced metabolite levels, it might keep intracellular metabolite concentrations within a specific range that allows key regulatory and signaling processes to function. Previous publications indicate that metabolite-level regulation governs key processes. Doello et al. described that the recovery from chlorosis and the enzymes required to return to vegetative growth are regulated by metabolite levels [
30]. Additionally, Carrieri et al. suggested that overflow excretion is regulated at the metabolite level, by a difference in metabolic status, not a difference in sensing and responding to stress [
37]. Maintaining particularly hub metabolites, such as pyruvate and 2-OG, below critical levels may help cells maintain metabolic stability. 2-OG occupies a central role at the intersection of carbon and nitrogen metabolism and serves as an important regulatory signal [
25,
38], while pyruvate links glycolysis, fermentation, and amino acid synthesis [
39,
40], underscoring its central role in coordinating multiple metabolic pathways. Thus, accumulation of these hub metabolites exceeding specific critical levels may initiate excretion, acting as a buffer to protect central metabolism and enabling cells to respond efficiently to and during nitrogen starvation.
Another metabolite potentially influencing carbon partitioning and metabolic overflow is DHAP. We observed a correlation between high intracellular DHAP levels and metabolite excretion during nitrogen starvation for Δ
pgm, Δ
glgC, Δ
pirC, and Δ
glgCΔ
pirC (
Figure 2). DHAP is the precursor of methylglyoxal (MG), a toxic metabolic byproduct that is detoxified via conjugation of MG with reduced glutathione [
41]. If DHAP accumulation in turn resulted in increased MG synthesis, it could trigger stress responses, such as rerouting the carbon flow into alternative carbon sinks, resulting in overflow excretion. However, we did not observe any indicative differences within the reduced glutathione pools of Δ
pgm, Δ
glgC, Δ
pirC, and Δ
glgCΔ
pirC compared to their respective WT, which would suggest increased detoxification within these strains during nitrogen starvation (
Figure 3C and Figure 3F). Alternatively, changes in DHAP levels could also be part of transient changes within sugar-phosphate intermediates, which have been observed in
Synechocystis during early chlorosis and are likely due to a shift in carbon partitioning [
42,
43]. As Δ
pgm, Δ
glgC, Δ
pirC, and Δ
glgCΔ
pirC have a modified carbon flux due to their respective gene deletions, these changes in carbon partitioning might be simply more pronounced in these strains. This agrees with our results, which show an increase in intracellular hexose-P and pentose-P within all strains, with this increase being most pronounced in strains lacking
glgC and therefore their glycogen sink.
Previous publications concluded that the intracellular redox and energy balance of
Synechocystis plays an important role in the excretion of organic acids [
22,
32]. Here, we did not observe strong differences in redox or energy homeostasis during nitrogen starvation across strains, with the notable exception of the Δ
pgm strain. However, our results suggest that the differences we observed within the adenylate pool as well as the NAD(H) and NADP(H) pools were not caused by differences within the redox or energy balancing of the various strains. Alternatively, the observed changes could be connected to changes in the purine metabolism. In
Synechocystis, purine metabolism intermediates are substrates for nicotinamide nucleotide synthesis [
33,
44]. Interestingly, it has been shown that these intermediates additionally act as regulators of NAD synthesis in other organisms [
45]. Thus, changes in purine metabolism could impact NAD(H) and NADP(H) dynamics in
Synechocystis. We observed that purine metabolism is strongly downregulated in WT cells under nitrogen starvation, as seen by decreases in intermediates such as 5-amino-1-(5-phospho-D-ribosyl)imidazole and 5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamide (AICAR), with similar trends in the Δ
glnB and Δ
glgA strains (
Figure S5). In contrast, Δ
pgm, Δ
pirC, Δ
glgC, and Δ
glgCΔ
pirC mutants maintained considerably higher levels of downstream intermediates, including 5-carboxyamino-1-(5-phospho-D-ribosyl)imidazole, AICAR, adenylosuccinate, and, particularly in Δ
pgm, elevated ADP and AMP levels. These differences in purine nucleotide pools could provide the basis for the altered NAD(H) and NADP(H) dynamics observed in the respective mutants. The observation that Δ
pgm redirected fewer glucose equivalents into overflow excretion than the other glycogen-deficient mutants would also fit this theory. Instead of reallocating carbon flow into overflow excretion, Δ
pgm might partially divert carbon flow into purine metabolism, resulting in greater differences within the Δ
pgm adenylate, NAD(H), and NADP(H) pools. Additionally, the increased AMP accumulation accounts for the significantly reduced energy charge evident in Δ
pgm during nitrogen depletion.
Interestingly, we also observed some differences between the glucose-sensitive and glucose-tolerant WT. During nitrogen chlorosis, the WT-GT did not decrease its intracellular glutamine and glutamate levels as strongly as the WT-GS (
Figure 2). Additionally, despite differences in intracellular glutamine and glutamate levels, glutamate excretion was observed only in one WT-GS replicate. The WT-GT consistently showed no glutamate excretion, suggesting no reproducible differences in glutamate excretion between the two WT strains. Instead, small extracellular amounts of malate, succinate, and 2-OG were detected across all replicates of the nitrogen-starved WT-GS (
Figure 1). These results suggest minor differences in carbon partitioning between glucose-sensitive and glucose-tolerant strains, particularly within the GS-GOGAT pathway. Furthermore, the overflow excretion of metabolites by WT-GS suggests that
Synechocystis not only releases metabolites due to artificial changes in its carbon flux but also naturally excretes small amounts of specific metabolites during nitrogen starvation. It has been demonstrated that cyanobacteria growing in soil crusts release organic carbon to establish a resource trading relationship with heterotrophic nitrogen-fixing bacteria, exchanging carbon for combined nitrogen sources [
46]. Furthermore, cyanobacteria within these communities utilize the excreted metabolites glutamate and mainly GABA as an interspecies signal for spatial organization [
47]. Thus, excretion of specific metabolites by the WT might be part of the normal nitrogen starvation response as a mechanism to communicate with their environment or to attract other beneficial bacteria to help them cope with this shortage of nutrients. Further research is required to elucidate whether the excretion of specific metabolites by
Synechocystis plays a role as an environmental signal.
Figure 1.
Comparison of intracellular changes of selected metabolite levels with the amounts of excreted metabolites. The intracellular values represent log2-fold changes in metabolites for WT-GS, WT-GT, Δpgm-GT, ΔpirC-GT, ΔglgC-GS, ΔglgCΔpirC-GS, ΔglnB-GS, ΔglgA1-GT, and ΔglgA2-GT after 2 days of vegetative growth (green) or 2 days of nitrogen starvation (orange), relative to the metabolite levels of the corresponding WT during vegetative growth. Each bar represents the mean log2-fold change of a triplicate, including the negative and positive standard deviation (SD). The extracellular values represent the mean metabolite concentration calculated from triplicates including the SD, the individual values of the biological replicates are depicted as dots.
Figure 1.
Comparison of intracellular changes of selected metabolite levels with the amounts of excreted metabolites. The intracellular values represent log2-fold changes in metabolites for WT-GS, WT-GT, Δpgm-GT, ΔpirC-GT, ΔglgC-GS, ΔglgCΔpirC-GS, ΔglnB-GS, ΔglgA1-GT, and ΔglgA2-GT after 2 days of vegetative growth (green) or 2 days of nitrogen starvation (orange), relative to the metabolite levels of the corresponding WT during vegetative growth. Each bar represents the mean log2-fold change of a triplicate, including the negative and positive standard deviation (SD). The extracellular values represent the mean metabolite concentration calculated from triplicates including the SD, the individual values of the biological replicates are depicted as dots.
Figure 2.
Carbon and nitrogen metabolism. Log2-fold changes in metabolites for WT-GS, WT-GT, Δpgm-GT, ΔpirC-GT, ΔglgC-GS, ΔglgCΔpirC-GS, ΔglnB-GS, ΔglgA1-GT, and ΔglgA2-GT after 2 days of vegetative growth (green) or 2 days of nitrogen starvation (orange), normalized to the metabolite levels of the corresponding WT during vegetative growth. Each bar represents the mean log2-fold change of a triplicate, including the negative and positive SD.
Figure 2.
Carbon and nitrogen metabolism. Log2-fold changes in metabolites for WT-GS, WT-GT, Δpgm-GT, ΔpirC-GT, ΔglgC-GS, ΔglgCΔpirC-GS, ΔglnB-GS, ΔglgA1-GT, and ΔglgA2-GT after 2 days of vegetative growth (green) or 2 days of nitrogen starvation (orange), normalized to the metabolite levels of the corresponding WT during vegetative growth. Each bar represents the mean log2-fold change of a triplicate, including the negative and positive SD.
Figure 3.
NAD(H) (A and D), NADP(H) (B and E), and reduced/oxidized glutathione pool (C and F) within WT-GS, WT-GT, Δpgm-GT, ΔpirC-GT, ΔglgC-GS, ΔglgCΔpirC-GS, ΔglnB-GS, ΔglgA1-GT, and ΔglgA2-GT during vegetative (A–C) and nitrogen-depleted growth (D–F). The bars represent the total amount of NAD(H), NADP(H), and glutathione, composed of the stacked values of the respective metabolite in their reduced (light blue) and oxidized (dark blue) form. Each bar represents the mean of a triplicate, including the SD.
Figure 3.
NAD(H) (A and D), NADP(H) (B and E), and reduced/oxidized glutathione pool (C and F) within WT-GS, WT-GT, Δpgm-GT, ΔpirC-GT, ΔglgC-GS, ΔglgCΔpirC-GS, ΔglnB-GS, ΔglgA1-GT, and ΔglgA2-GT during vegetative (A–C) and nitrogen-depleted growth (D–F). The bars represent the total amount of NAD(H), NADP(H), and glutathione, composed of the stacked values of the respective metabolite in their reduced (light blue) and oxidized (dark blue) form. Each bar represents the mean of a triplicate, including the SD.
Figure 4.
Conversion of measured glycogen amounts into intracellular glucose equivalents stored in form of glycogen per cell (A) and corresponding intracellular metabolite concentrations removed from each cell via excretion calculated from the extracellularly detected amounts of pyruvate, 2-OG, succinate, glutamate, and malate (B) for WT-GS (black), WT-GT (light purple), Δpgm-GT (dark purple), ΔpirC-GT (blue), ΔglgC-GS (teal), ΔglgCΔpirC-GS (green), ΔglnB-GS (bright green), ΔglgA1-GT (yellow), ΔglgA2-GT (turquoise). Each bar represents the mean of a triplicate, including the SD. The individual values for each biological replicate are depicted as dots.
Figure 4.
Conversion of measured glycogen amounts into intracellular glucose equivalents stored in form of glycogen per cell (A) and corresponding intracellular metabolite concentrations removed from each cell via excretion calculated from the extracellularly detected amounts of pyruvate, 2-OG, succinate, glutamate, and malate (B) for WT-GS (black), WT-GT (light purple), Δpgm-GT (dark purple), ΔpirC-GT (blue), ΔglgC-GS (teal), ΔglgCΔpirC-GS (green), ΔglnB-GS (bright green), ΔglgA1-GT (yellow), ΔglgA2-GT (turquoise). Each bar represents the mean of a triplicate, including the SD. The individual values for each biological replicate are depicted as dots.
Table 1.
ATP, ADP, and AMP C12/C13 ratios normalized to the OD750 of the respective cultures at the time of sampling. The values represent intracellular metabolite levels of all strains after two days of vegetative growth (+N) and after two days of nitrogen-depleted growth (-N). Each value represents the mean of a triplicate, including the SD.
Table 1.
ATP, ADP, and AMP C12/C13 ratios normalized to the OD750 of the respective cultures at the time of sampling. The values represent intracellular metabolite levels of all strains after two days of vegetative growth (+N) and after two days of nitrogen-depleted growth (-N). Each value represents the mean of a triplicate, including the SD.
| |
ATP |
ADP |
AMP |
| |
+N |
-N |
+N |
-N |
+N |
-N |
| WT-GS |
0.31 |
± 0.15 |
0.06 |
± 0.03 |
3.53 |
± 1.68 |
1.84 |
± 0.71 |
1.37 |
± 0.39 |
1.15 |
± 0.28 |
| WT-GT |
0.39 |
± 0.10 |
0.15 |
± 0.07 |
3.44 |
± 0.90 |
1.18 |
± 0.94 |
0.74 |
± 0.06 |
0.50 |
± 0.29 |
| Δpgm-GT |
0.44 |
± 0.19 |
0.38 |
± 0.05 |
4.09 |
± 0.73 |
5.63 |
± 0.94 |
1.24 |
± 0.08 |
7.35 |
± 0.60 |
| ΔpirC-GT |
0.45 |
± 0.21 |
0.51 |
± 0.20 |
3.66 |
± 1.53 |
2.77 |
± 1.12 |
1.03 |
± 0.18 |
0.78 |
± 0.08 |
| ΔglgC-GS |
0.47 |
± 0.12 |
0.13 |
± 0.10 |
3.81 |
± 0.65 |
2.66 |
± 1.18 |
0.60 |
± 0.28 |
2.15 |
± 0.88 |
| ΔglgCΔpirC-GS |
0.25 |
± 0.02 |
0.28 |
± 0.07 |
3.29 |
± 1.66 |
6.14 |
± 2.15 |
1.10 |
± 0.64 |
3.32 |
± 0.50 |
| ΔglnB-GS |
0.99 |
± 0.12 |
0.14 |
± 0.02 |
6.04 |
± 1.15 |
4.58 |
± 0.53 |
0.88 |
± 0.24 |
2.90 |
± 0.22 |
| ΔglgA1-GT |
0.29 |
± 0.21 |
0.16 |
± 0.07 |
3.18 |
± 1.22 |
4.72 |
± 1.71 |
1.14 |
± 0.42 |
2.92 |
± 0.85 |
| ΔglgA2-GT |
0.19 |
± 0.05 |
0.05 |
± 0.02 |
2.18 |
± 0.21 |
1.59 |
± 0.95 |
0.77 |
± 0.29 |
2.60 |
± 0.65 |