1. Introduction
Cancer and its treatment impact the health-related quality of life (QoL) in many ways. Patients often experience a range of symptoms, including pain, fatigue, dyspnoea, loss of appetite, and others (Tewes et al., 2021). Treatments, such as chemotherapy or radiation, often lead to unwanted side effects, including nausea, vomiting, constipation, alopecia and peripheral neuropathy, which could compromise daily functioning (Pedersen et al., 2013). This high symptom load is also associated with increased levels of emotional suffering, such as anxiety and depression, and poor physical and societal functioning, all of which impact patients’ QoL (Gour and Chaudhary, 2023). Hence, the QoL outcome of patients is a crucial clinical consideration in cancer management (Jitender et al., 2018; Nguyen et al., 2023; Tamburini et al., 2000). Complementary and supportive care alongside primary cancer treatments is needed to enhance overall well-being and improve disease outcomes (Nolazco and Chang, 2023; Olver et al., 2020).
Rice bran arabinoxylan compound (RBAC) is a plant-based food supplement that has exhibited immune restorative function in cancer patients through upregulating natural killer cell activity and enhancing inflammatory and cytotoxic responses (Ooi et al., 2024a). Prior systemic reviews of available research evidence found RBAC to improve the QoL of cancer patients when used as a complementary therapy with conventional cancer treatment (Ooi et al., 2018; Ooi et al., 2024a). A recent double-blind, placebo-controlled pilot trial (RBAC-QoL study) also demonstrated that RBAC improved global QoL scores as well as role and social functioning of cancer patients beyond placebo effects during systemic cancer treatment (Ooi et al., 2025). Patients in the RBAC group also reported lower symptom scores in fatigue, pain, dyspnoea, and appetite loss compared to the placebo group. Although it was suggested that RBAC could improve QoL by enhancing nutritional status and modulating immune function, the biological pathway by which RBAC impacts perceived QoL remained unclear (Ooi et al., 2025).
Tryptophan is an essential amino acid needed for many human biological functions. As a precursor protein, tryptophan is synthesised into many bioactive compounds, including nicotinamide (vitamin B6), serotonin (neurotransmitter), melatonin (hormone), tryptamine, kynurenine, and many others with wide-ranging roles in human health (Friedman, 2018). Diminished tryptophan levels due to chronic inflammation could lower immune responsiveness and influence mood, physical strength, and haematopoiesis, thus affecting the QoL (Fuchs et al., 2010). Specifically, in cancer patients, the inflammation-induced breakdown of tryptophan along the kynurenine pathway was found to relate to fatigue, depression, and anaemia (Lanser et al., 2020). As such, tryptophan metabolism and signalling have garnered considerable attention in cancer research as therapeutic strategies for enhancing patient outcomes (Yan et al., 2024).
The present research is a secondary analysis of the RBAC-QoL data, which aimed to explore the relationship between RBAC supplementation and tryptophan metabolism in cancer patients and validate whether the tryptophan-kynurenine pathway could be a determinant of QoL. The research questions were: (1) In cancer patients under active treatment, could RBAC supplementation affect the levels of tryptophan, kynurenine, and kynurenine to tryptophan ratio (KTR)? (2) Are tryptophan, kynurenine, and KTR related to the reported QoL outcomes of the patients? (3) Are tryptophan, kynurenine, and KTR significant factors in predicting QoL compared to other blood markers for haematological, immune, inflammatory and nutritional markers?
2. Materials and Methods
The RBAC-QoL study was a randomised, placebo-controlled trial conducted from June 2020 to April 2024 in New South Wales, Australia. The specifics of the trial, including the study protocol (Ooi et al., 2020), interim analysis (Ooi et al., 2024b), and the final report (Ooi et al., 2025) have been published for open access. Briefly, the trial recruited adult patients with any solid organ cancer (≥ stage II) who were undergoing outpatient chemotherapy or immunotherapy. Participants were randomly assigned to consume either RBAC or a placebo powder as an oral supplement (3 g/day) for 24 weeks while continuing their oncological treatment. During the trial, the participants attended five study visits (six weeks apart) to complete QoL questionnaires and underwent blood tests. The data collectors and the treating oncologists of the participants were blinded to group assignments. The RBAC-QoL study was approved by the Human Research Ethics Committee (HREC) of Concord Repatriation General Hospital, Sydney Local Health District (Application No. 2019/ ETH00489) and the Charles Sturt University HREC (Protocol No. H19244). All participants provided written extended informed consent, allowing their data and biological samples to be used for future, related or extended projects. For more details, readers are encouraged to refer to the study publications.
2.1. Participant Profile and Blood Markers
The following data from the RBAC-QoL study were used in the current analysis: (1) Participant profile including age, sex, cancer type, stage, and primary treatment, and their group assignment; (2) QoL outcome measures based on the European Organisation for the Research and Treatment of Cancer (EORTC) core 30-item QoL questionnaire (QLQ-C30); (3) Blood test results including the complete blood count, liver function tests, electrolytes, urea, creatinine, C-reactive protein (CRP), and prealbumin; (4) 15 cytokine/chemokine markers, including granulocyte-macrophage colony-stimulating factor, interferon-gamma (IFN-γ), interleukin (IL)-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, monocyte chemoattractant protein-1, and tumour necrosis factor-alpha (TNF-α).
2.2. QoL Measures
QoL measurement was based on the scoring of QLQ-C30, following the procedures stipulated by EORTC (Fayers et al., 2001). This study included the global QoL scale, five functional scales (physical, role, emotional, cognitive, and social), nine symptom-related measures (fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea) and a single financial impact score for analysis. The summary score (SQ), calculated as the mean of the combined 13 QLQ-C30 scale and item scores (excluding the global QoL and financial impact items), was used as the primary indicator of a patient’s overall QoL. SQ is a more reliable and robust QoL measure in oncology research compared to individual scale and item scores (Kasper, 2020).
2.3. Tryptophan and Kynurenine Assay
Levels of tryptophan and kynurenine (Sigma Aldrich, Castle Hill, NSW, Australia) were analysed using serum samples collected from the RBAC-QoL study, applying a previously validated liquid chromatography (LC) method (Chen et al., 2024) with minor modifications. The LC system consisted of a Shimadzu Nexera XR LC system (Rydalmere, NSW, Australia) with these binary mobile phases: 20 mM ammonium acetate (adjusted to pH 4.5 with acetic acid) mixed with either 5% acetonitrile (A) or 70% acetonitrile (B). The following gradient was applied at a flow rate of 1.2 mL/min: 0-1 min (0-5% B), 1-2 min (5% B), 2-5min (5-50% B), 5-10min (50-90% B), 10-11min (90% B), and 11-12min (90-0% B). The stationary phase was a Waters X-Bridge C18 column (3.5µm, 4.6 x 150mm; Dundas, NSW, Australia), with the column temperature maintained at 35 °C.
Tryptophan and kynurenine were monitored using fluorescence detection (excitation: 297 nm; emission: 347 nm) and ultraviolet detection (360 nm), respectively. Serum samples (50 µL) underwent protein precipitation with the addition of 100 µL methanol. The mixture was vortexed and centrifuged at 14,000 × g for 10 min. The resulting supernatant was transferred to the injection vial, and 10 µL was injected into the HPLC system. The total run time was 15 min, with tryptophan and kynurenine eluting at 3.95 min and 2.8 min, respectively. Quantification was performed using external calibration curves with quantification ranges of 1.91 µM (limit of quantification [LOQ]) to 122.41 µM for tryptophan and 0.94 µM (LOQ) to 60.03 µM for kynurenine. The precisions and accuracies at LOQ levels were within acceptable ranges for both tryptophan (precision: 1.35% coefficient of variation [CV], n = 3; accuracy: < 15.41% of nominal concentration) and kynurenine (precision: 4.82% CV, n = 3; accuracy: < 18.43%), following The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) (2022).
2.4. Statistical Methods
Statistical analysis was performed using RStudio version 2024.12.1 Build 563 (Posit, Boston, MA, USA), running R version 4.4.2. Every data point collected from participants in the trial was used regardless of withdrawal (intention-to-treat principle). Repeated measures analysis of variance (RM-ANOVA) was used to compare between-group differences over multiple time points, with F statistics, degrees of freedom, p-values, and effect sizes reported after sphericity correction. Pairwise comparisons were conducted when statistical significance was observed with the false discovery rates (fdr) applied to adjust the p-values for multiple comparisons. If any significant between-group differences in the baseline characteristics were identified, an analysis of covariance (ANCOVA) was to be performed on the significant outcomes from the primary analysis to account for potential confounding factors.
Spearman’s rank correlation (rs) was used to determine the strength and direction of the relationship between any two variables. The strength of the correlation coefficient was interpreted as negligible (rs < 0.2), weak (0.2 ≤ rs < 0.4), moderate (0.4 ≤ rs < 0.6), strong (0.6 ≤ rs < 0.8), and very strong (rs ≥ 0.8). Similarly, the p-values of the correlation coefficients were adjusted for multiplicity with the fdr method. A linear mixed model was used to fit and analyse the significance of the relationship between the multiple factors that could determine an outcome variable. A stepwise backward reduction approach based on the Akaike information criterion was performed to reduce the fixed and random effects for the mixed model.
Continuous variables were reported as mean ± standard deviation. The difference between any two means was analysed using two-sided Student’s t-statistics. Fisher’s exact test was used to determine if there were nonrandom associations between two categorical variables. Missing data was handled with pairwise deletion. A p-value of less than or equal to 0.05 was considered statistically significant.
3. Results
3.1. Participant Characteristics
Nineteen of the 29 participants in the RBAC-QoL study provided additional serum samples for further analysis. Hence, tryptophan and kynurenine assays were performed on this subgroup, and their baseline characteristics are shown in
Table 1.
The participants were mainly older adults with later-stage cancer (stage III and IV), predominantly male (84.2%), with a mean age of 68.4 ±7.03. Primary cancer sites were mainly skin (melanoma, 31.6%), colorectal (26.3%), and lung (21.0%), with a single count of bladder, oesophagus, kidney, and stomach cancer each. More than half (57.9%) had recurrent cancer. The participants were treated with either chemotherapy (63.2%) or immunotherapy (36.8%). Ten patients were from the placebo group, and nine were from the RBAC group. There were no significant differences between groups in all characteristics except for age. The mean age of the RBAC group was significantly higher than the placebo group (71.9 ± 7.83 vs. 65.2 ± 4.56, p = 0.043).
3.2. Between-Group Analysis
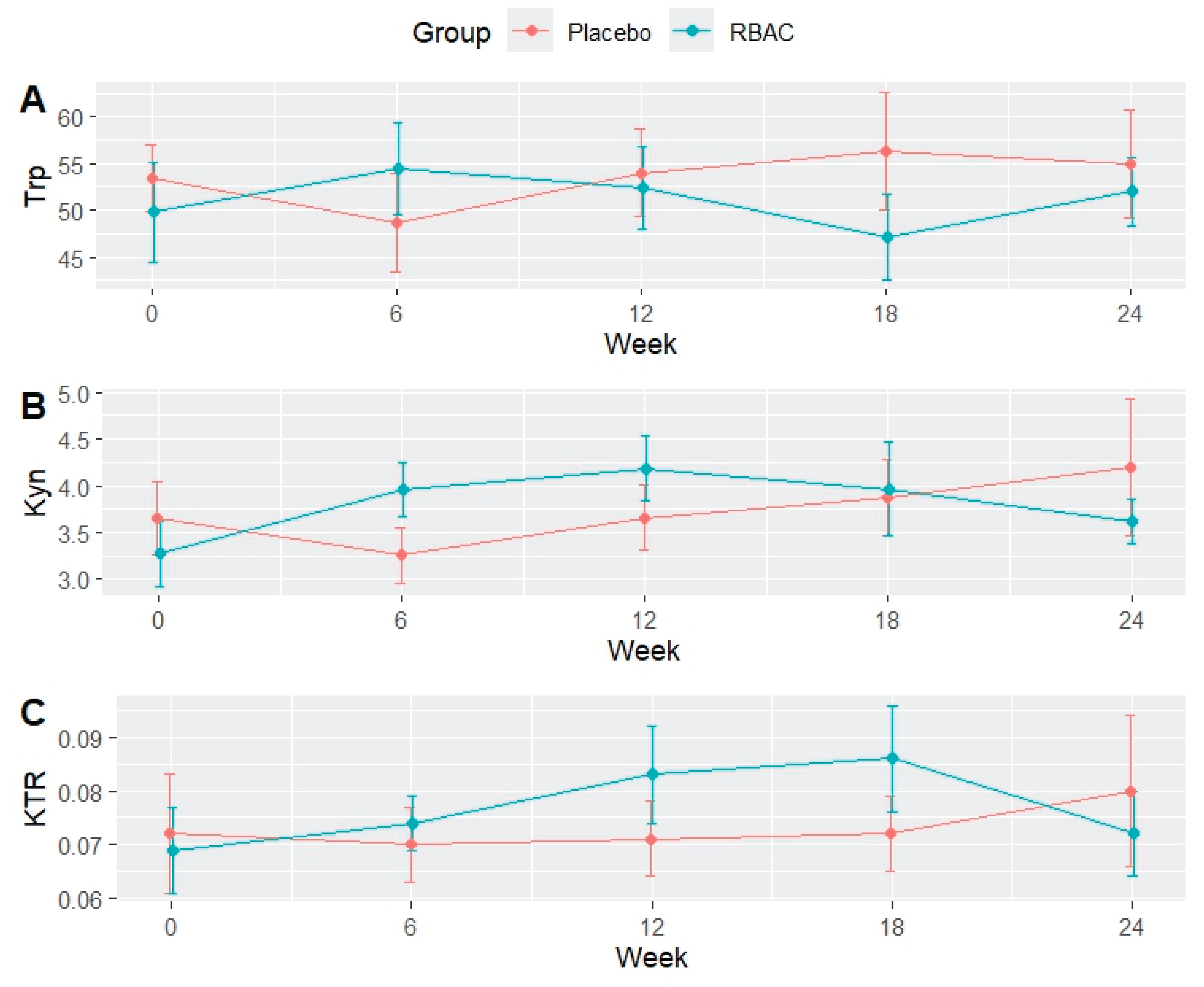
The levels of tryptophan, kynurenine, and KTR were analysed with RM-ANOVA to determine the differences between groups. No significant difference was detected for tryptophan and kynurenine. However, analysis of KTR detected a marginally significant difference over time (F[4,48] = 2.864, p = 0.052, eta2[g] = 0.074) but not between the groups or the interaction of group and time. Pairwise analysis revealed no significant differences, which may be attributed to the small effect size.
Figure 1 shows the between-group differences of tryptophan, kynurenine, and KTR over time. The levels of tryptophan, kynurenine, and KTR fluctuated, and no clear trend was detected. Adjusted analysis was performed with age as a covariate to account for the significant between-group differences in age. The results of the ANCOVA analysis revealed no significant differences between groups for all three parameters. The details of the data analysis are presented in the Supplementary Material.
3.3. Tryptophan and QoL Outcomes
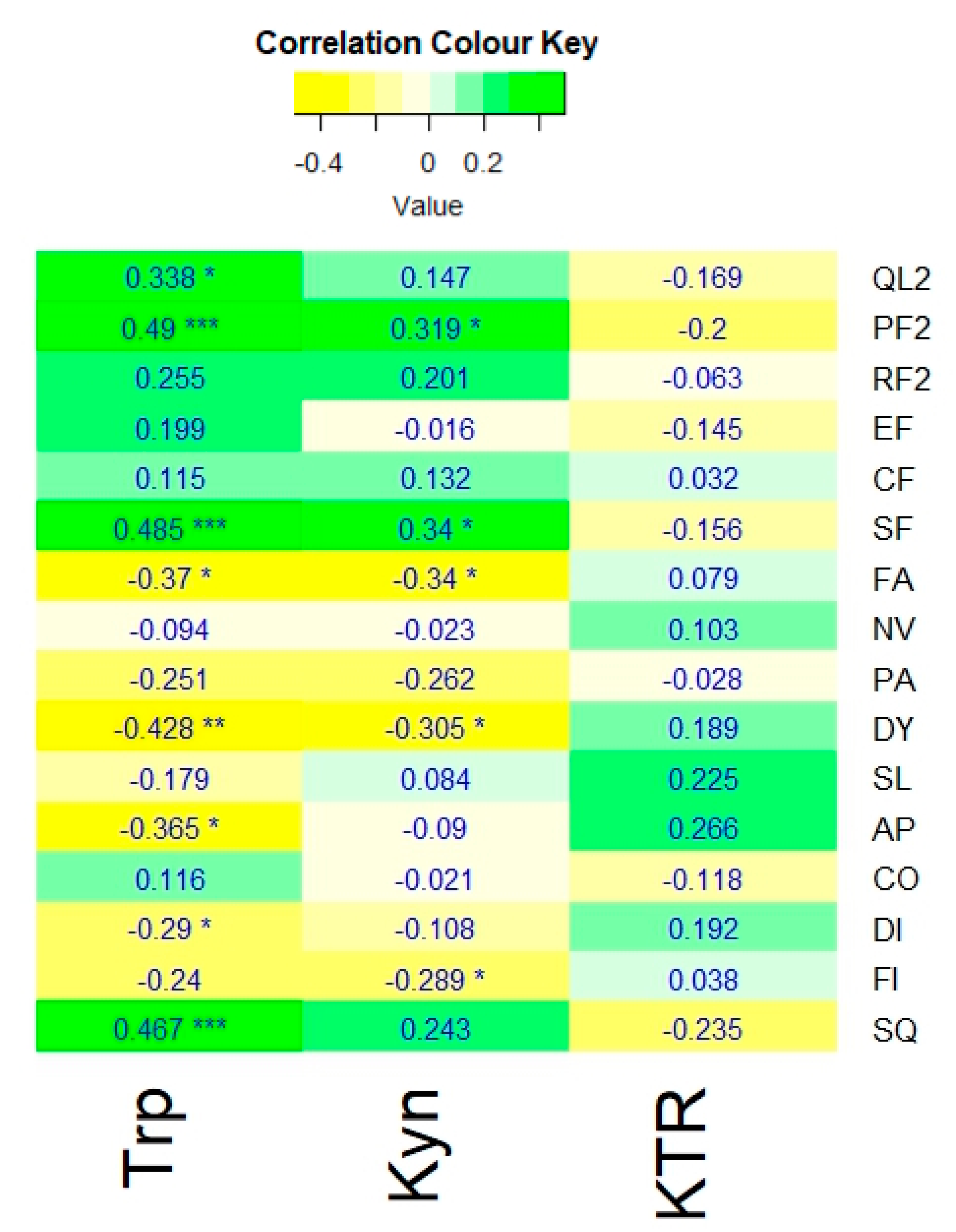
Figure 1 shows the Spearman’s coefficients of tryptophan, kynurenine, and KTR with the QoL outcome measures based on the QLQ-C30.
Tryptophan levels of the participants showed significant positive correlations with global QoL (rs = 0.338, p ≤ 0.05), physical functioning (rs = 0.49, p ≤ 0.001), social functioning (rs = 0.485, p ≤ 0.001), and overall QoL measured with SQ, the summary score of QLQ-C30 (rs = 0.467, p ≤ 0.001). Significant negative correlations were also detected between tryptophan and the symptom scores of fatigue (rs = -0.37, p ≤ 0.05), dyspnoea (rs = -0.428, p ≤ 0.001), appetite loss (rs = -0.365, p ≤ 0.05), and diarrhoea (rs = -0.29, p ≤ 0.05).
Kynurenine also demonstrated significant positive correlations with physical (rs = 0.319, p ≤ 0.05) and social (rs = 0.34, p ≤ 0.05) functioning and negative correlations with fatigue (rs = -0.34, p ≤ 0.05) and dyspnoea (rs = -0.305, p ≤ 0.05); albeit weaker compared to those of tryptophan. Additionally, kynurenine levels were negatively correlated with financial impact (rs = -0.289, p ≤ 0.05). In contrast, the KTR exhibited no significant correlations with any of the QoL outcome measures.
Figure 1.
A heatmap showing the Spearman’s correlation coefficients of tryptophan (Trp), kynurenine (Kyn), and kynurenine to tryptophan ratio (KTR) with the QLQ-C30 measures: global QoL scale (QL2), physical [PF2], role [RF2], emotional [EF], cognitive [CF], and social [SF] functions, as well as fatigue (FA), nausea & vomiting (NV), pain (PA), dyspnoea (DY), insomnia (SL), appetite loss (AP), constipation (CO), diarrhoea (DI), financial impact (FI). And the Summary score (SQ). Significance: adjusted p-value ≤ 0.05 *, ≤ 0.01 **, ≤ 0.001 ***.
Figure 1.
A heatmap showing the Spearman’s correlation coefficients of tryptophan (Trp), kynurenine (Kyn), and kynurenine to tryptophan ratio (KTR) with the QLQ-C30 measures: global QoL scale (QL2), physical [PF2], role [RF2], emotional [EF], cognitive [CF], and social [SF] functions, as well as fatigue (FA), nausea & vomiting (NV), pain (PA), dyspnoea (DY), insomnia (SL), appetite loss (AP), constipation (CO), diarrhoea (DI), financial impact (FI). And the Summary score (SQ). Significance: adjusted p-value ≤ 0.05 *, ≤ 0.01 **, ≤ 0.001 ***.
3.4. Significant Factors Influencing QoL Outcomes
Correlation analysis was conducted to compare all blood tests and cytokine markers with SQ, assessing how tryptophan and kynurenine, relative to other factors, influence QoL outcomes.
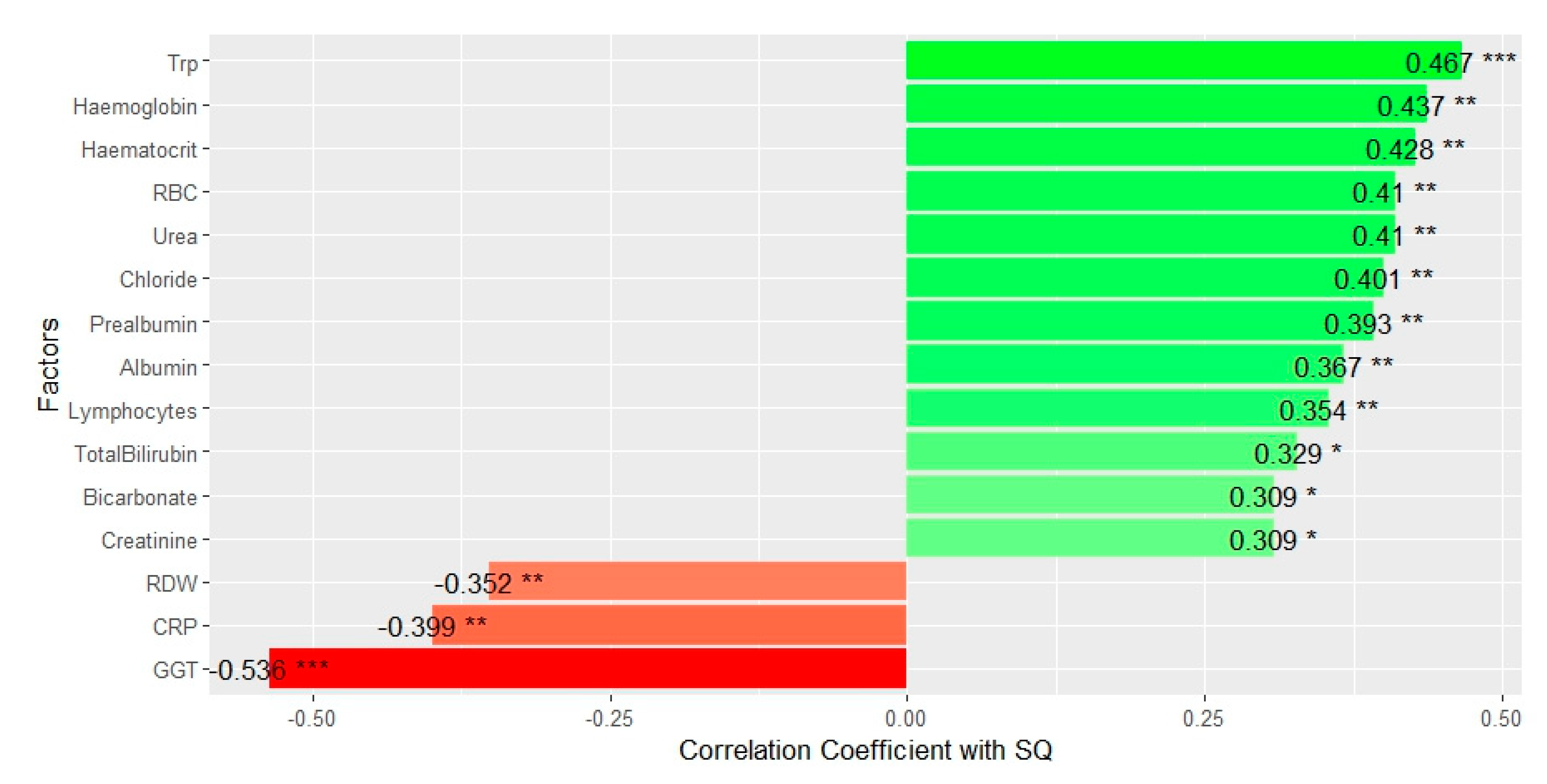
Figure 2 illustrates the list of significant factors for SQ, arranged in order of decreasing correlation coefficients.
Many blood markers appeared to have a positive correlation with SQ, including haematological (red blood cell count [RBC], haemoglobin, haematocrit), electrolytes (chloride, bilirubin, bicarbonate), liver function (urea), and immune and nutritional markers (lymphocyte, albumin, prealbumin). Tryptophan was featured in the chart as the most prominent positive factor. Factors negatively correlated with SQ were the inflammatory marker, CRP, red cell distribution width (RDW) and gamma-glutamyl transferase (GGT). Serum cytokine levels, however, did not exhibit any significant correlations with SQ (see the Supplementary Material for the Spearman’s correlation test results for all factors).
3.5. Linear Mixed Model Analysis
A linear mixed model, which accounted for group-specific effects and a random slope for weeks, was used to fit the data with SQ as the dependent outcome measure and all significant blood markers as fixed effects of the model. The model formula is shown below:
The details of the model fitting are provided in the Supplementary Material. Among the factors, only tryptophan (p < 0.001), GGT (p = 0.006), lymphocytes (p = 0.011), and total bilirubin (p = 0.045) were found to be significant predictors of SQ, with notable differences between groups.
Stepwise reduction of the full model above yielded a simplified model with only two independent predictors for the SQ score between groups after eliminating total bilirubin as the least significant predictor and the effect of weeks on the random slope. The simplified formula is shown below:
The fixed effect coefficient of tryptophan is 6.35 (p < 0.001) and GGT is -10,450.60 (p < 0.001) in this simplified model. The results of the model fitting are presented in the Supplementary Material.
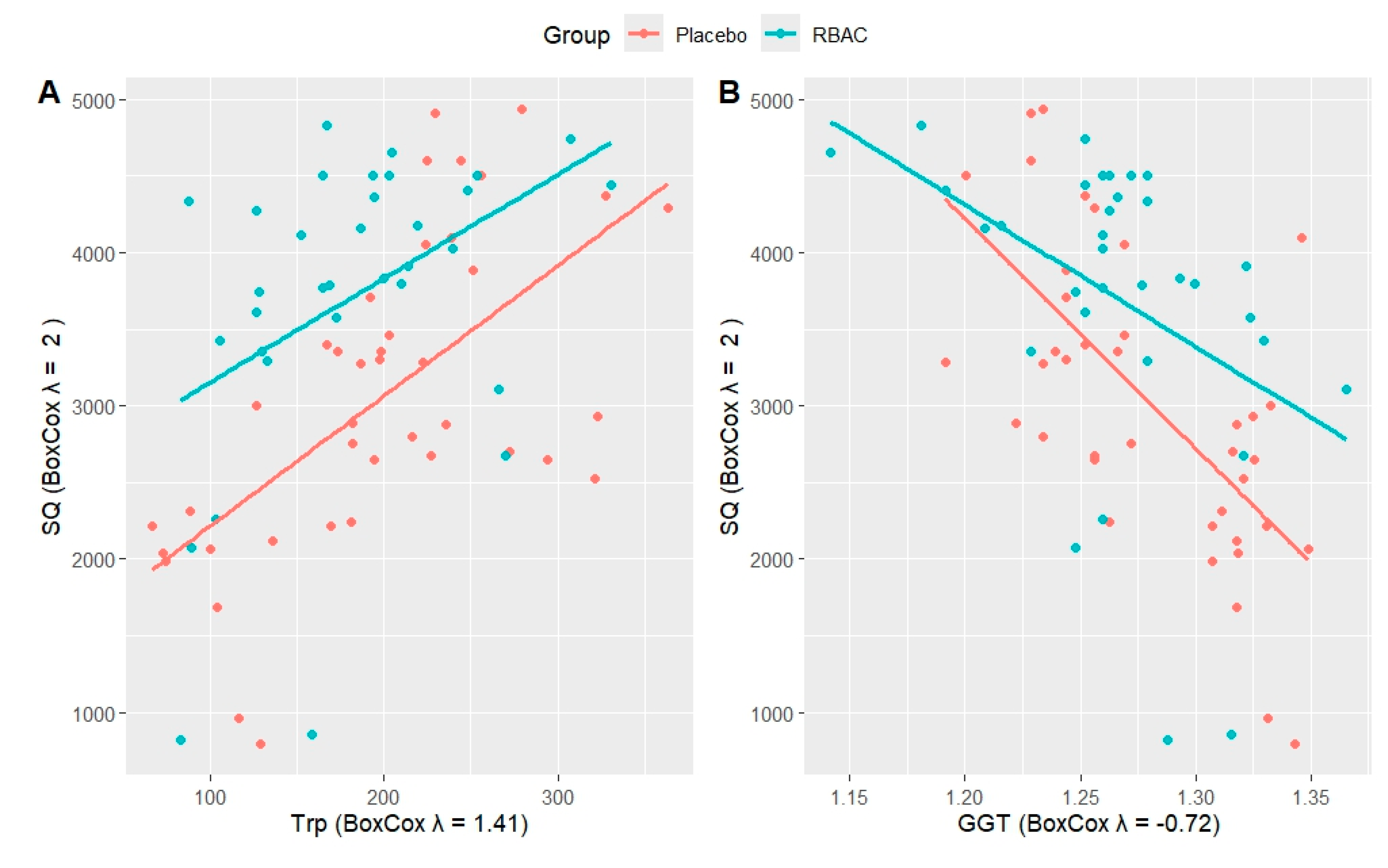
Figure 3 presents a two-dimensional visual representation of how tryptophan and GGT could predict SQ, comparing the RBAC and placebo groups. The increase in tryptophan was accompanied by the rise in SQ for both groups at a similar rate. Notwithstanding, the RBAC group had a consistently higher SQ than the placebo group at any level of tryptophan. Hence, there appeared to be an additive effect on SQ with RBAC and serum tryptophan level.
Higher GGT levels were associated with lower SQ, with both the RBAC and placebo groups exhibiting similar SQ levels at low GGT levels. However, the rate of deterioration between groups differed, with the drop in SQ being much steeper in the placebo group. Hence, in the case of liver function decline, indicated by elevated serum GGT, RBAC was more effective than placebo in preserving SQ.
4. Discussion
This study explored the potential relationship between RBAC supplementation, tryptophan, and the associated kynurenine metabolite in cancer patients’ QoL. Tryptophan revealed significant correlations with several components of QoL, including a positive correlation with global QoL, physical and social functioning and a negative correlation with fatigue, dyspnoea, appetite loss and diarrhoea (see
Figure 1). Moreover, serum tryptophan levels correlated significantly with the SQ, the summary score for QoL (
p < 0.001); however, the correlation between kynurenine and KTR with SQ was not significant.
Several blood markers were also found to correlate with SQ, including haematological, renal, liver, immune, and nutritional markers. RBC, haemoglobin, haematocrit, RDW, lymphocytes, chloride, bicarbonate, urea, creatinine, total bilirubin, CRP, prealbumin, albumin, and GGT exhibited significant correlations with SQ scores. However, from the linear mixed model analysis with stepwise reduction, tryptophan remained one of two prominent independent factors that could predict SQ, with the other being GGT, a liver function marker.
These findings are consistent with previous reports in the literature. Schroecksnadel et al. (2007) demonstrated that cancer patients with progressive disease had significantly lower tryptophan levels than those with stable/remitting disease (p < 0.01), and the decrease in tryptophan concentration was related to decreased QoL (rs = 0.256, p < 0.01) and increased fatigue (rs = -0.179; p < 0.05). An earlier study among colorectal cancer patients with liver metastases also reported that reduced serum tryptophan was significantly associated with scores of physical symptoms (r = -0.51, p = 0.01) and sickness impact profile (r = -0.42, p = 0.01) that undermined QoL (Huang et al., 2002). Similarly, serum tryptophan was found to be a significant independent predictor of both physical symptoms (p < 0.01) and sickness impact (p < 0.04) using stepwise regression analysis (Huang et al., 2002).
In another study by Kurz et al. (2012), tryptophan breakdown was also found to correlate significantly with fatigue (rs = 0.376, p = 0.007), anaemia (rs = 0.409, p = 0.003), and overall QoL scores (rs = 0.382, p = 0.006) of lung cancer patients measured with the FACT (Functional Assessment of Cancer Therapy) questionnaires. It is worth noting that none of the earlier studies used the QLQ-C30 as the QoL questionnaire. Hence, the results from the present study validated the earlier findings with an internationally accepted QLQ-C30 measuring instrument, confirming the observation that higher serum tryptophan levels in cancer patients could mean higher overall QoL.
In the earlier reported results of the RBAC-QoL study, RBAC supplementation was shown to improve the QoL of cancer patients beyond placebo (Ooi et al., 2025). The present analysis found little evidence suggesting that RBAC supplementation directly affected tryptophan and kynurenine levels and their ratio (KTR) over time. Hence, the positive impact of RBAC on QoL was not likely to result from preventing tryptophan degradation. Nonetheless, RBAC supplementation appeared to exhibit QoL-enhancing effects on the patients at the same tryptophan level.
A recent study demonstrated that administering L-tryptophan as a dietary supplement at 3g/day significantly increased serum tryptophan levels (p < 0.001) in prostate, breast, or uterine cervical cancer and decreased symptom scores for hot flushes, asthenia, and insomnia, thus leading to overall QoL improvement (p < 0.001) (Peña Vivas et al., 2021). A systematic review of 11 randomised controlled trials also found L-tryptophan supplementation effective in improving the mood of healthy individuals (Kikuchi et al., 2020). Therefore, future research should investigate the combined impact of RBAC and L-tryptophan supplementation to determine whether any synergistic effects exist.
The liver enzyme GGT appeared to work independently from tryptophan, with rising serum GGT counteracting the tryptophan effect and lowering QoL. Not surprisingly, GGT is an extracellular glutathione catabolism enzyme, a powerful antioxidant that helps prevent cellular oxidative stress. Raising serum GGT is an indication of liver injury or oxidative stress and is also associated with higher cancer risk and poorer prognosis (Fentiman, 2012; Kunutsor, 2016). While no study thus far explores the direct relationship between serum GGT and QoL of cancer patients, the liver function and QoL of cancer patients are known to be highly correlated (Li et al., 2019).
The differing rates of degradation in QoL due to increasing GGT between RBAC and placebo groups in the current study, as shown in
Figure 3B, could likely be due to the hepatoprotective effects of RBAC, as demonstrated in several experimental and clinical studies (Zheng et al., 2012a; Zheng et al., 2012b; Lewis et al., 2020a; Lewis et al., 2020b; Salama et al., 2016). Thus, another area of future research could be the effect of RBAC on improving the QoL of cancer patients through the hepatoprotective pathway.
The current study was a secondary analysis of existing data. While the results offered insights into how RBAC supplementation and serum tryptophan could affect the QoL of cancer patients, given its retrospective nature, such a study had inherent limitations. The study design of a parallel trial with repeated measures was not ideal for correlation analysis and predictive modelling. Hence, the observations derived in this study should not be used to infer causality in any way (Trinh, 2018). Additionally, the small sample size available for analysis could lower statistical power and produce unreliable results (Cao et al., 2024). Thus, the present results may not be generalisable to the broader population of cancer patients. Notwithstanding, this secondary analysis could provide supporting data to inform future research on RBAC and tryptophan.
5. Conclusion
This secondary analysis of the RBAC-Qol study found that RBAC supplementation did not affect the tryptophan metabolism among the participants. However, serum tryptophan levels demonstrated significant correlations with many aspects of QoL, including global QoL, physical and social functioning, and symptoms of fatigue, dyspnoea, appetite loss and diarrhoea. Furthermore, serum tryptophan was shown to be a prominent predictor of SQ, which is the summary score of QLQ-C30. RBAC supplementation and serum tryptophan appeared to have an additive effect on the QoL over placebo. Such findings provided supporting data to inform future research.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
SLO: Conceptualisation, Methodology, Software, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Visualisation. BK: Methodology, Formal analysis, Investigation, Validation, Writing - Review & Editing. PSM: Conceptualisation, Methodology, Validation, Writing - Review & Editing. BSP: Conceptualisation, Methodology, Investigation, Validation, Resources, Writing - Review & Editing. SCP: Conceptualisation, Methodology, Validation, Resources, Writing - Review & Editing, Supervision, Project administration.
Ethical considerations
This RBAC-QoL study was approved by the Human Research Ethics Committee (HREC) of Concord Repatriation General Hospital, Sydney Local Health District (Application No. 2019/ ETH00489) and Charles Sturt University HREC (Protocol No. H19244) and was conducted in accordance with the Declaration of Helsinki.
Consent to participate
Extended informed consent was obtained from all individuals participating in the RBAC-QoL study, allowing their data and samples to be used for future, related or extended projects.
Consent for publication
Not applicable.
Declaration of conflicting interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding statement
This RBAC-QoL was funded by Daiwa Pharmaceutical Co., Ltd. (Japan) and BioMedica Nutraceuticals Pty Ltd. (Australia). No additional funding was received for this secondary analysis.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to commercial funding agreement but are available from the corresponding author for non-commercial research use on reasonable request.
References
- Cao Y, Chen RC and Katz AJ (2024) Why is a small sample size not enough? The Oncologist 29(9): 761-763.
- Chen C-J, Kimble B, Van Aggelen A, et al. (2024) Preliminary analyses of tryptophan, kynurenine, and the kynurenine: Tryptophan ratio in plasma, as potential biomarkers for systemic chlamydial infections in koalas. PLOS ONE 19(12): e0314945.
- Fayers PM, Aaronson NK, Bjordal K, et al. (2001) EORTC QLQ-C30 scoring manual. Reportno. Report Number|, Date. Place Published|: Institution|.
- Fentiman IS (2012) Gamma-glutamyl transferase: risk and prognosis of cancer. British Journal of Cancer 106(9): 1467-1468.
- Friedman M (2018) Analysis, nutrition, and health benefits of tryptophan. International Journal of Tryptophan Research 11: 1178646918802282.
- Fuchs D, Schroecksnadel K, Neurauter G, et al. (2010) Quality of life and tryptophan degradation. In: Preedy VR and Watson RR (eds) Handbook of disease burdens and quality of life measures. New York, NY: Springer New York, pp.2027-2045.
- Gour N and Chaudhary M (2023) The quality of life in cancer patients. In: Hassan BAR and Mohammed A (eds) Supportive and palliative care and quality of life in oncology. Rijeka: IntechOpen.
- Huang A, Fuchs D, Widner B, et al. (2002) Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. British Journal of Cancer 86(11): 1691-1696.
- Jitender S, Mahajan R, Rathore V, et al. (2018) Quality of life of cancer patients. Journal of Experimental Therapeutics and Oncology 12(3): 217-221.
- Kasper B (2020) The eortc qlq-c30 summary score as a prognostic factor for survival of patients with cancer: A commentary. The Oncologist 25(4): e610-e611.
- Kikuchi AM, Aya T and and Iwahori Y (2020) A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. Journal of Dietary Supplements 18(3): 316–333.
- Kunutsor SK (2016) Gamma-glutamyltransferase—friend or foe within? Liver International 36(12): 1723-1734.
- Kurz K, Fiegl M, Holzner B, et al. (2012) Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLOS ONE 7(5): e36956.
- Lanser L, Kink P, Egger EM, et al. (2020) Inflammation-induced tryptophan breakdown is related with anemia, fatigue, and depression in cancer. Frontiers in Immunology 11: 249.
- Lewis JE, Atlas SE, Abbas MH, et al. (2020a) The novel effects of a hydrolyzed polysaccharide dietary supplement on immune, hepatic, and renal function in adults with HIV in a randomized, double-blind, placebo-control trial. Journal of Dietary Supplements 17(4): 429-441.
- Lewis JE, Atlas SE, Higuera OL, et al. (2020b) Corrigendum to “The Effect of a Hydrolyzed Polysaccharide Dietary Supplement on Biomarkers in Adults with Nonalcoholic Fatty Liver Disease”. Evidence-Based Complementary and Alternative Medicine 2020: 10.
- Li L, Mo F, Hui EP, et al. (2019) The association of liver function and quality of life of patients with liver cancer. BMC Gastroenterology 19(1): 66.
- Nguyen LB, Vu LG, Le TT, et al. (2023) Impact of interventions on the quality of life of cancer patients: a systematic review and meta-analysis of longitudinal research. Health and Quality of Life Outcomes 21(1): 112.
- Nolazco JI and Chang SL (2023) The role of health-related quality of life in improving cancer outcomes. Journal of Clinical and Translational Research 9(2): 110-114.
- Olver I, Keefe D, Herrstedt J, et al. (2020) Supportive care in cancer—a MASCC perspective. Supportive Care in Cancer 28(8): 3467-3475.
- Ooi SL, McMullen D, Golombick T, et al. (2018) Evidence-based review of BioBran/MGN-3 arabinoxylan compound as a complementary therapy for conventional cancer treatment. Integrative Cancer Therapies 17(2): 165-178.
- Ooi SL, Micalos PS, Kim J, et al. (2024a) Rice bran arabinoxylan compound as a natural product for cancer treatment – an evidence-based assessment of the effects and mechanisms. Pharmaceutical Biology 62(1): 367-393.
- Ooi SL, Micalos PS, Zielinski R, et al. (2025) Effects of rice bran arabinoxylan compound on quality of life of cancer patients during active treatment: A randomised placebo-controlled pilot trial. Research Square. DOI: 10.21203/rs.3.rs-5837950/v1.
- Ooi SL, Micalos PS, Zielinski R, et al. (2024b) Rice bran arabinoxylan compound and quality of life (RBAC-QoL) of cancer patients: An interim analysis of the RBAC-QoL Study. Cureus 16(1): e53188.
- Ooi SL, Pak SC, Micalos PS, et al. (2020) Rice bran arabinoxylan compound and quality of life of cancer patients (RBAC-QoL): Study protocol for a randomized pilot feasibility trial. Contemporary Clinical Trials Communications 19: 100580.
- Pedersen B, Koktved DP and Nielsen LL (2013) Living with side effects from cancer treatment – a challenge to target information. Scandinavian Journal of Caring Sciences 27(3): 715-723.
- Peña Vivas J, Alonso Garcia A, Fernández Rivero G, et al. (2021) [L-tryptophan as dietetic supplement and treatment for hot flashes, astenia, and insomnia in cancer patients]. Nutrición Hospitalaria 38(3): 568-574.
- Salama H, Medhat E, Shaheen M, et al. (2016) Arabinoxylan rice bran (Biobran) suppresses the viremia level in patients with chronic HCV infection: A randomized trial. International Journal of Immunopathology and Pharmacology 29(4): 647-653.
- Schroecksnadel K, Fiegl M, Prassl K, et al. (2007) Diminished quality of life in patients with cancer correlates with tryptophan degradation. Journal of Cancer Research and Clinical Oncology 133(7): 477-485.
- Tamburini M, Casali PG and Miccinesi G (2000) Outcome assessment in cancer management. Surgical Clinics of North America 80(2): 471-486.
- Tewes M, Baumann F, Teufel M, et al. (2021) Symptoms during outpatient cancer treatment and options for their management. Dtsch Arztebl International 118(17): 291-297.
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) (2022) Bioanalytical method validation and study sample analysis M10. Reportno. Report Number|, Date. Place Published|: Institution|.
- Trinh Q-D (2018) Understanding the impact and challenges of secondary data analysis. Urologic Oncology: Seminars and Original Investigations 36(4): 163-164.
- Yan J, Chen D, Ye Z, et al. (2024) Molecular mechanisms and therapeutic significance of Tryptophan Metabolism and signaling in cancer. Molecular Cancer 23(1): 241.
- Zheng S, Sanada H, Dohi H, et al. (2012a) Suppressive effect of modified arabinoxylan from rice bran (MGN-3) on D-galactosamine-induced IL-18 expression and hepatitis in rats. Bioscience, Biotechnology, and Biochemistry 76(5): 942-946.
- Zheng S, Sugita S, Hirai S, et al. (2012b) Protective effect of low molecular fraction of MGN-3, a modified arabinoxylan from rice bran, on acute liver injury by inhibition of NF-[kappa]B and JNK/MAPK expression. International Immunopharmacology 14(4): 764-769.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).