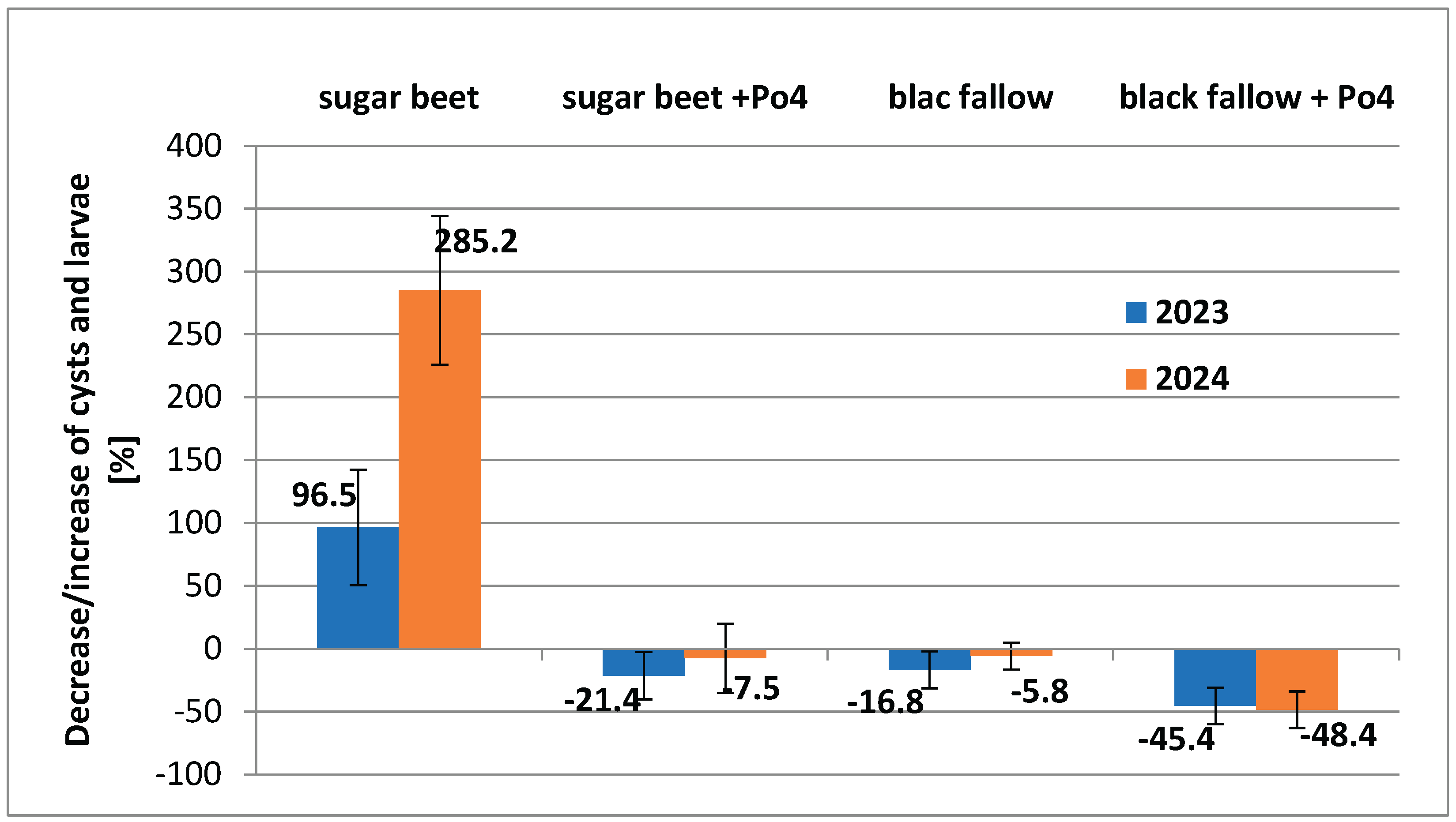1. Introduction
Sugar beet (
Beta vulgaris L.) is one of the most important crops in Europe and the second among the sugar crops in the world [
1]. Its production may be disturbed by several pathogens and pests. Among them,
Heterodera schachtii is one of the most important pests in sugar beet production [
2,
3]. Its control is in many countries, e.g., in Poland, restricted to biological methods only. In Poland, there are only two officially registered chemical substances to protect crops against nematodes, including
H. schachtii on table beet crops, but not in sugar beet cultivation. The most broadly used agricultural practices to control this group of pests belong to the integrated methods of plant protection. Among them, crop rotation, resistant sugar beet varieties against nematodes, and nematocidal plants are the most used [
3]. Thus, searching for a practical and useful method to control sugar beet cyst nematode is crucial and highly demanded [
3].
Pleurotus ostreatus is a mushroom species well known for its nematocidal properties [
2]. This feature was discovered by Prof. G. Barron (University of Guelph) in the 1980s [
4]. Vegetative mycelium of oyster mushroom produces hyphae knobs, tipped with a drop of a substance that may paralyze nematodes. Since then, several studies have been conducted that have tested the nematocidal potential of
Pleurotus spp. mycelia; however, a complex field application method has not been presented [
2,
5,
6].
P. ostreatus, known as the oyster mushroom, is one of the most popular edible mushrooms in the world, that are industrially cropped. Shortly after the discovery of the nematocidal properties of oyster mushroom vegetative mycelium, the substance responsible for this property was discovered. It was identified as (E)-2-decenedioic acid, a ten-carbon hydrocarbonic substance [
6,
7]. Other studies have shown that this substance is probably not the only one responsible for the nematocidal activity of
Pleurotus sp. [
8,
9]. In 2023, Lee et al. [
10] identified another compound as responsible for the paralysis nematodes by the mycelium. It was 3-octanone, a very popular in the fungal world compound that is one of those responsible for a typical mushroom aroma [
11,
12]. 3-octanone is a liquid substance with a very strong mushroom scent. This discovery explains why, in some research, good results were obtained even when parts of fruiting bodies of oyster mushrooms were used [
8,
13].
In our research, we decided to test the potential of the vegetative mycelium of the oyster mushroom in controlling the cyst nematode
H. schachtii in the field. In the experiment, we compared the activity of the mycelium in the fallowed soil with the activity of the mycelium added to the soil on which sugar beets were sown and cultivated. The two-year experiments were conducted in soil heavily contaminated with this phytopathogenic nematode, providing an opportunity to test the
P. ostreatus mycelium and its application method selected in previous experiments [
14,
15].
2. Materials and Methods
2.1. Organisms
Plants used in the experiment were susceptible to nematodes, sugar beet (Beta vulgaris L. subsp. vulgaris) varieties. In the pot experiment, a variety Fantazja was applied, and in the field experiment - cv. Janetka. Both cultivars were kindly delivered by Kutnowska Hodowla Buraka Cukrowego (Kutnowska Sugar Beet Breeding company, Poland).
Mycelia of Pleurotus ostreatus, a wild strain, designated Po4 and its progeny, homokaryotic (Po4-8, Po4-30) and heterokaryotic (Po4-3dix17, Po4-14x17, Po4-2dix1) crosses, were chosen for laboratory and field experiments. Based on the results of previous tests, Po4 mycelium was selected as a model mycelium for field conditions [
14,
15]. The mycelia used in the laboratory pot experiment were initially grown on PDA medium, and when they had covered the entire surface of the plate, autoclaved barley grains were added to them. The grains were incubated until they were overgrown.
The population of Heterodera schachtii was determined according to the methodology described by Kaczorowski [
16]. Before conducting the basic procedure, soil samples were dried at room temperature and sieved through a 2 mm sieve to remove any straw residues and extracted by Seinhorst apparatus - a method using soil washing with water and separating the organic and inorganic fractions based on their weight and ability to float on the water surface.. Cysts obtained in this procedure were isolated from smaller organic particles by dissecting needle under binocular (ProLab Scientific Motic SMZ160 Binocular) and then transferred to a microscope slides, crushed with a metal spatula in a drop of distilled water to obtain a suspension of nematode eggs and larvae, and counted under the microscope (Nikon Eclipse E200 + Delta Optical 1080 camera; magnification 40x). The same procedure was used to determine the
H. schachtii population at the beginning (Pi) and at the end of the experiments (Pf), both in the pot and field experiments. Based on the obtained results, the percentage of decrease/increase in the number of
H. schachtii eggs and larvae was calculated.
2.2. Laboratory Pot Experiments
Laboratory pot experiments were conducted in a cultivation chamber. Plastic pots with a capacity of 1 dm
3 (dimensions 11 × 11 cm) were filled with an approximately 7 cm layer of turbid soil (Luvisol soil according to FAO classification) (
Table 1). A layer of autoclaved barley straw was introduced on the soil surface in each pot. Then, thirty barley grains, overgrown with appropriate mycelium, were placed on the straw layer in each pot. The straw layers were covered with the remaining turbid soil, filling the pots. In each pot, six seeds of sugar beet were sown. The pots left without plants served as control ones (black fallow). Pots were incubated for 90 days in a growing chamber at 20°C and a relative humidity of approximately 65% under artificial lighting with an intensity of 1450 lx. The experiment was performed in three replicates.
At the end of the experiment, the soil was removed from pots to determine the H. schachtii population (Pf).
The soil used in the pot experiment was characterized by a neutral pH, average nitrogen and potassium content, and high phosphorus content, with a known initial population of beet cyst nematode (Pi) (
Table 2).
2.3. Field Experiments
Field studies were conducted at the Kutnowska Sugar Beet Breeding company (Kutnowska Hodowla Buraka Cukrowego, Poland), on plots organized in tents used for breeding purposes, where the levels of soil nematode contamination had been previously determined (
Table 3).
The experiment consisted of 4 variants (
Table 3) and was conducted on 2 x 2 m plots in partially open cultivation tents, equipped with an irrigation system. The experiment was conducted in four replications. The chemical characteristics of the soil are presented in
Table 4. Soil inoculation with
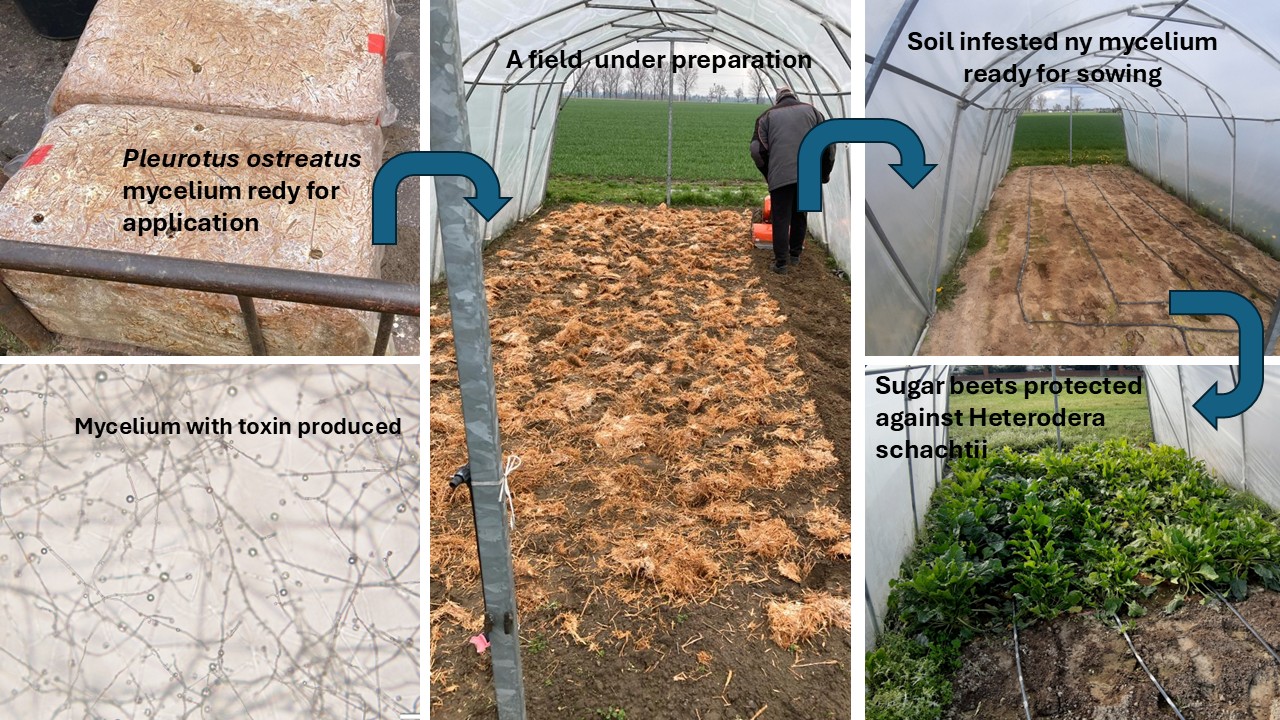
P. ostreatus mycelium (Po4 wild strain) was done a day before sowing (
Table 1).
P. ostreatus Po4 mycelium was introduced as an overgrown straw-composed substrate. The dosage of the straw substrate was established at a dose corresponding to approximately 18 t/ha. The straw-composed substrate bales, weighing approximately 13 kg each, were produced by the Rusieccy farm (
https://rusieccy.pl/), in accordance with the rules used in production of oyster mushroom mycelium. The straw substrate bales containing mycelium were initially crushed by hand and then mixed with soil using a rotary tiller.
The fresh mass yield of leaves and roots grown in the experiment was determined by measuring the biomass collected from 1 m² of each experimental plot. The expected yield of fresh and dry matter of the aboveground parts and roots was determined (using the drying method). The content of sucrose and molasses-forming substances (K, Na, and N-α-NH
2) in the sugar beet pulp was also determined using a technological line with a Venema autoanalyzer. Calculations of technological sugar yield were made using Reinefeld's formula (1) [
17]:
Where: TPC – technological sugar yield [t/ha]; PK – root yield [t/ha]; %ZC – percentage sugar content (polarization); K, Na, N-α-NH2 – content of molasses-forming substances.
2.4. Data Analyses
Statistical analysis was performed using Microsoft Excel 2010. Mean values and standard deviations (SDs) were calculated at a 95% confidence level, and results were expressed as mean ± SD.
3. Results
3.1. Laboratory Experiments
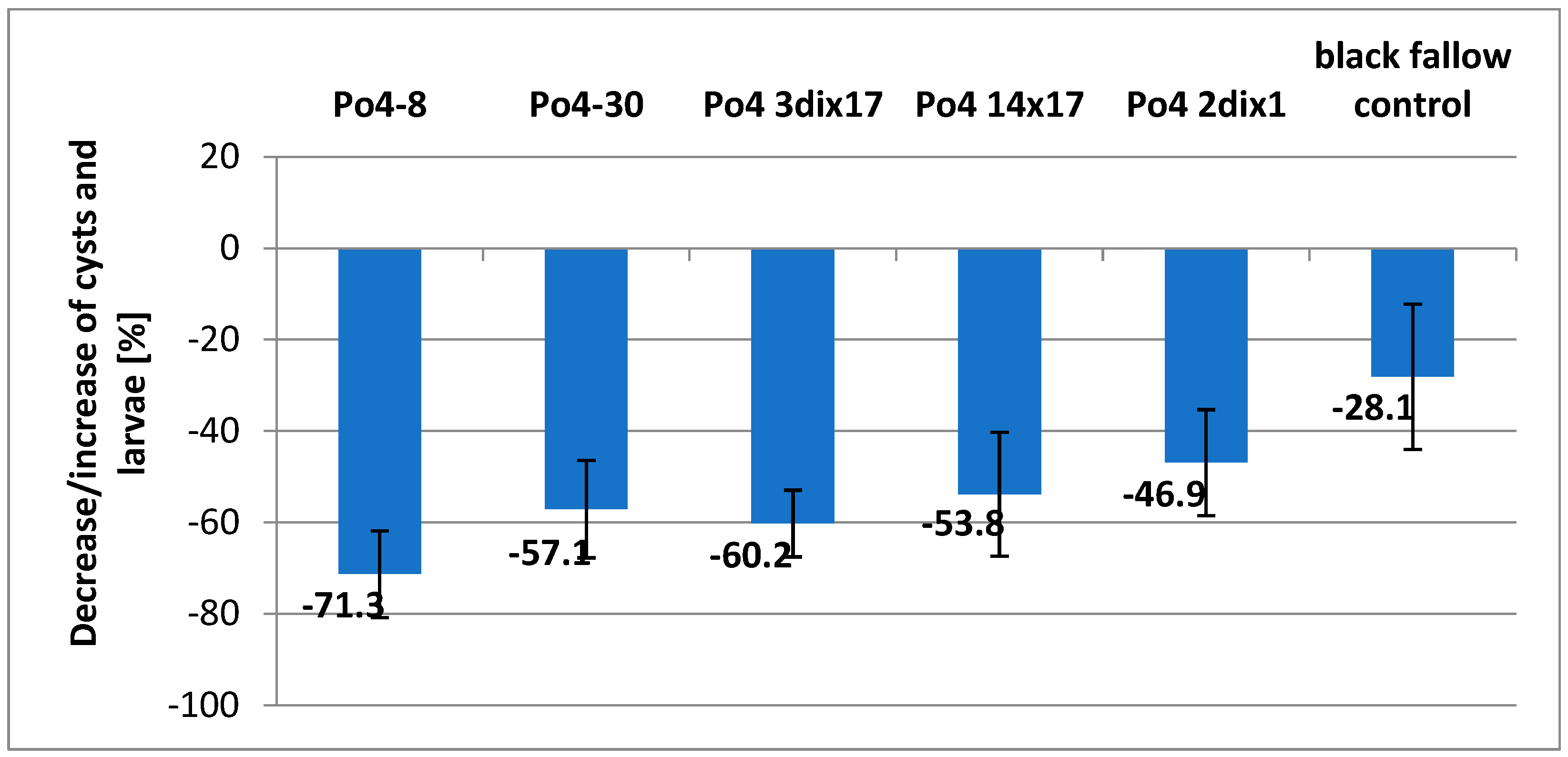
The laboratory experiments have shown that
P. ostreatus mycelia can significantly reduce the
H. schachtii population under the black fallow conditions (without plants sown). In just 90 days of the experiment, population reductions ranging from 71.3 to 46.9% were obtained, based on the counting of cysts and larvae in 100 g samples (
Figure 1). The experiment was relatively short. This effect was partially due to the fallowing of the soil, which is one of the known methods for reducing the potential for
H. schachtii occurrence. (
Figure 1).
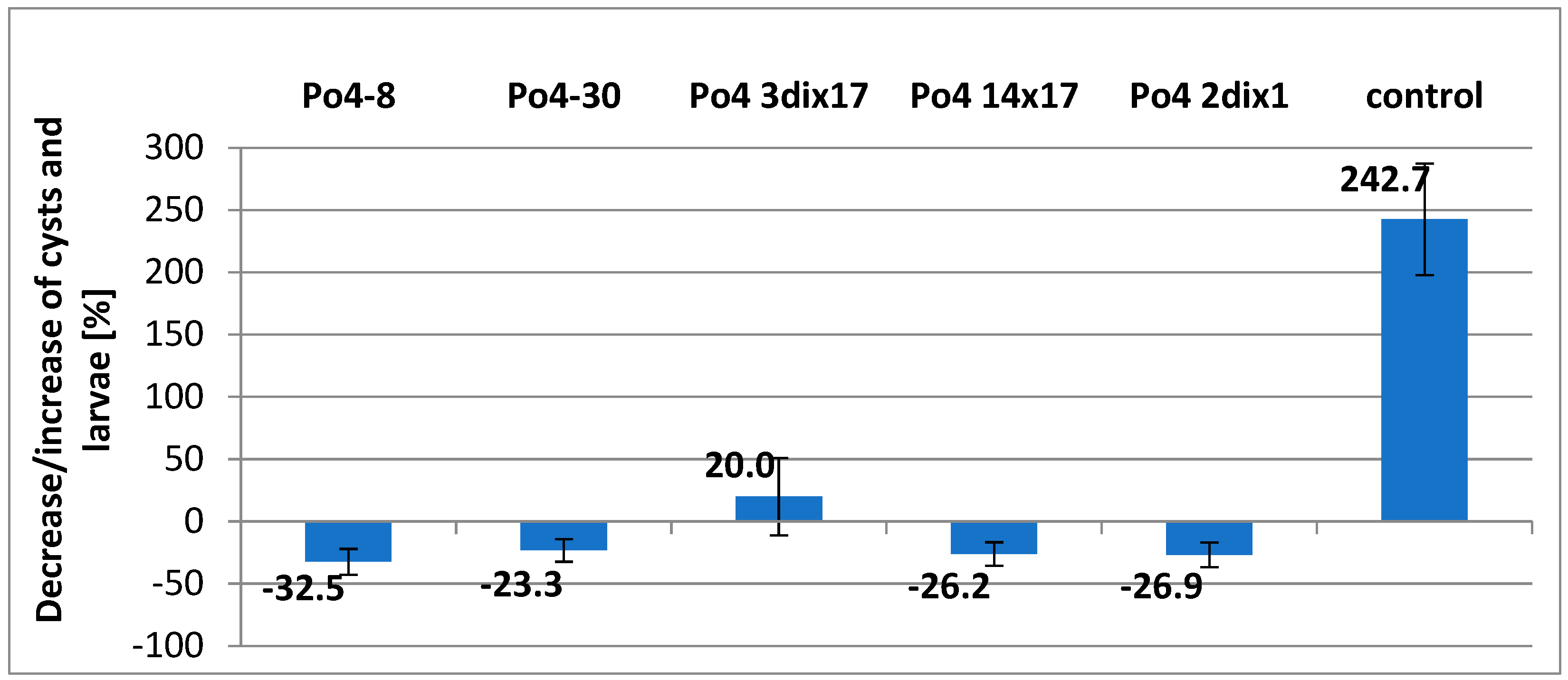
The variants of the experiment with growing sugar beet plants also gave good results. However, the reduction was not as good as in black fallows, but this part of the test was conducted under conditions favorable to
H. schachtii. The reductions of
H. schachtii achieved 32.5 for the homokaryotic mycelium Po4-8 to 23.3% - for another homokaryon Po4-30. Two heterokaryotic mycelia (Po4-14x17, Po4-2dix1) resulted in very similar reduction, which was on the level of 26.0%. Heterokaryotic mycelium Po4-3di x17 did not give good results in the variant with sugar beet plants. The result was not significant, and in this case, we observed a 20% increase in the nematode population. Although in contrast with the control variant in which sugar beets were grown (242.7% increase), this result is still good. The population of the nematode didn’t increase much (
Figure 2). Comparing this fungus activity in the black fallow, it can be seen that the result was the second highest (60.2% of reduction) (
Figure 1 and
Figure 2).
3.2. Field Experiments
In the field experiment, in which Po4 mycelium served as a model one, we observed good reduction results in the case of black fallow. The reduction of
H. schachtii population reached 45.4% in 2023 and 48.4% in 2024. These results were significantly higher than those obtained for black fallow without mycelium and for the variant with sugar beet cultivation. Unlike in the sugar beet plots, reduction was achieved even when sugar beet was grown simultaneously with mycelium applied to the soil. In this case, the reduction could be around 20% or unchanged (
Figure 3). This experiment showed that even under favorable conditions for the nematode pest, its population can remain at a non-increasing level.
The expected sugar beet root yield per 1 ha of area treated with
P. ostreatus was at the same level as for standardly grown sugar beets in 2023, and higher in 2024 (
Table 5). A similar result was obtained for leaf yield and
Foliage index, which represents the ratio of leaf to root yield (
Table 5).
Other parameters characterizing the yield quality of the crop harvested from plots treated with
P. ostreatus mycelium did not exceed those obtained for plots without mycelium. The exception were potassium and sodium contents in 2024, which were higher and influenced sugar production. In 2024, potassium and sodium levels were lower in the mycelium-treated variant (16.62%) than in the control variant (17.13%), but the
Biological sugar yield was higher in this case and was expected to reach 17.1 t·ha
-1 (
Table 5).
4. Discussion
The laboratory and field experiment revealed that
P. ostreatus may be useful under natural field conditions by reducing the
H. schachtii population or inhibiting its development. To our knowledge, it is the first time when that mycelium was introduced into agricultural soil against
H. schachtii. Previously, similar experiments have been conducted in the laboratory to control
H. schachtii or to protect vegetables against other plant pathogenic nematodes [
2,
18,
19].
Laboratory-tested mycelia showed varying levels of nematocidal activity, consistent with our previous studies [
14,
15] and expectations. Pineda-Alegría et al. [
20] reported similar observations for
Pleurotus djamor against
Haemonchus contortus. In this study, one of the tested strains showed lower anthelminthic activity than others. This research also demonstrated that
Pleurotus sp. mushrooms may produce a wide range of active compounds that may have nematocidal activity and that are contained in their fruiting bodies. Therefore, the nematocidal activity does not derive solely from the 3-octanone, as suggested by the study of Lee et al. [
10]. The results obtained in the laboratory are very promising for future research and field applications. However, we expected similarly high results in the field as in the laboratory, but the results obtained in the field were less effective.
Our expectations were also suggested by the results of Palizi et al. [
2]. This difference may be due to numerous factors affecting the mycelium under natural conditions. In particular, lower soil water content, higher temperatures, wind, the influence of other microorganisms or plants in the soil, etc., can significantly alter the field effects observed previously in the laboratory. Another important factor is the content of organic matter, such as cellulose and lignocellulose (plant residues). Agricultural soils treated by mineral fertilizers usually do not contain sufficiently high levels of organic matter [
21]. However, the addition of organic matter, such as spent mushroom substrate, can affect soil structure by increasing porosity in the topsoil and subsoil [
22] and by improving mineral content [
23]. Undecomposed organic matter reach in complex compounds like lignin and cellulose, provides a natural habitat for the
P. ostreatus mycelium, and there, the nematodes bodies serve as a source of supplemental nitrogen for the mycelium [
24].
The expected yield quality was comparable to that obtained with standard sugar beet cultivation. This strongly suggests that the straw with oyster mushroom mycelium can be considered a good biological nematicide without negatively impacting yield.
Similar results, such as increased biomass yield, were obtained by Tazuba et al. [
25] for banana plants (
Musa sp.) and Nyangwire et al. [
19] for eggplant (
Solanum melongena). However, in both studies, only pot tests were conducted, in which spent mushroom substrates of
P. ostreatus were used to control
Radopholus similis or root-knot nematodes (
Meloidogyne spp.). Good results of a field experiment using spent mushroom substrate were obtained for tomatoes [
26]. In field conditions, when two types of spent compost (on rice or wheat straws) of
Pleurotus sajor-caju were used to protect plants against the root-knot nematode (
Meloidogyne incognita), the nematode reduction efficiency reached 80.2-85.5% at a dose of 1200 g/m
2 of spent mushroom substrate. Furthermore, a significant increase in fruit yield and its parameters was observed. The authors recommended treatment with spent mushroom compost based on wheat straw. Furthermore, greenhouse experiment on control
Meloidogyne javanica with
Pleurotus djamor spent mushroom substrate in lettuce cultivation showed a 98.68% reduction in nematode reproduction and a 99.75% reduction in population density [
27]. However, due to higher concentrations of spent mushroom substrate, some phytotoxic effects were observed in lettuce, such as a decrease in vegetative parameters, chlorophyll content, and nitrogen balance in the leaves, but an increase in anthocyanin content. On the other hand, the results suggest the induction of lettuce resistance to
M. javanica.
5. Conclusions
The two-year field experiments have shown that, under natural conditions in a cultivated field infested with Heterodera schachtii, it is possible to reduce its population using a natural method involving vegetative mycelium of Pleurotus ostreatus (oyster mushroom). This method produces good results, which may provide an alternative to chemical treatments. However, we tested only one strain of mycelium in the field; the laboratory experiments have shown that individual mycelia may vary in activity against H. schachtii. Therefore, it is worthwhile to develop this method and search for effective mycelium.
Author Contributions
R.N.— methodology, formal analysis, investigation, data curation, visualization, writing—original draft preparation; M.Nb.— conceptualization, investigation, data curation, supervision, writing— editing; M.No.— conceptualization, investigation, supervision, writing—review; E.M.—conceptualization, methodology, software, formal analysis, resources, visualization, data curation, writing—original draft preparation, writing—review & editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Agriculture and Rural Development grant number under the program “Biological Progress”, the project for the years 2021–2026, task 22 “Influence of environmental parameters and biological variability of Pleurotus ostreatus in terms of. nematicidal activity on Heterodera schachtii”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request..
Acknowledgments
This work was carried out at MCBR UO (International Research and Development Center of the University of Opole), which was established as part of a project co-financed by the European Union under the European Regional Development Fund, RPO WO 2014-2020, Action 1.2 Infra-structure for R&D. Agreement No. RPOP.01.02.00-16-0001/17-00 dated January 31, 2018.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Abbreviations
The following abbreviations are used in this manuscript:
References
- Mwangi, N.G.; Stevens, M.D.; Wright, A.J. J.; Watts, W.D.; Edwards, S.G.; Hare, M.C.; Back, M.A. Population dynamics of stubby root nematodes (Trichodorus and Paratrichodorus spp.) associated with ‘Docking disorder’ of sugar beet (Beta vulgaris L.), in field rotations with cover crops in East England. Annals of Applied Biology 2025, 187(2), 177-191. [CrossRef]
- Palizi, P.; Goltapeh, E.M.; Pourjam, E.; Safaie, N. Potential of oyster mushrooms for the biocontrol of sugar beet nematode (Heterodera schachtii). Journal of Plant Protection Research 2009, 49(1), 27-33. [CrossRef]
- Hauer, M.; Koch, H.-J.; Krüssel, S.; Mittler, S.; Märländer, B. Integrated control of Heterodera schachtii Schmidt in Central Europe by trap crop cultivation, sugar beet variety choice and nematicide application. Applied Soil Ecology 2016, 99, 62-69. [CrossRef]
- Barron, G.L.; Thorn, R.G. Destruction of nematodes by species of Pleurotus. Can. J. Bot. 1987, 65, 774-778.
- Castro, L.R.I.; Delmastro, S.; Curvetto N.R. Spent oyster mushroom substrate in a mix with organic soil for plant pot cultivation. Micologia Aplicada International 2008, 20(1), 17-26.
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as alternatives for biological control. Part II: metabolites from nematophagous basidiomycetes and non-nematophagous fungi. Appl Microbiol Biotechnol 2016, 100, 3813–3824. [CrossRef]
- Kwok, O.C.H.; Plattner, R.; Weisleder, D.; Wicklow, D.T. A nematicidal toxin from Pleurotus ostreatus NRRL 3526. Journal of Chemical Ecology 1992, 18(2), 127-36. [CrossRef]
- Landi, N.; Ragucci, S.; Russo, R.; Valletta, M.; Pizzo, E.; Ferreras, J.M.; Di Maro, A. The ribotoxin-like protein Ostreatin from Pleurotus ostreatus fruiting bodies: Confirmation of a novel ribonuclease family expressed in basidiomycetes. Int. J. Biol. Macromol. 2020, 161, 1329–1336.
- Žužek, M.C.; Maček, P.; Sepčić, K.; Cestnik, V.; Frangež, R. Toxic and lethal effects of ostreolysin, a cytolytic protein from edible oyster mushroom (Pleurotus ostreatus), in rodents. Toxicon 2006, 48, 264–271.
- Lee C.-H. et al. A carnivorous mushroom paralyzes and kills nematodes via a volatile ketone. Sci. Adv. 2023, 9, eade4809. [CrossRef]
- Moliszewska, E. Mushroom flavour. Acta Universitatis Lodziensis. Folia Biologica et Oecologica, 2014, 10, 80–88. [CrossRef]
- Moliszewska, E.; Nabrdalik, M.; Dickenson, J. Mushrooms as Sources of Flavours and Scents, ss. 252-285. (in:) Sridhar K. R. and S. K. Deshmukh (ed.), Advances in Macrofungi: Pharmaceuticals and Cosmeceuticals, 2021, CRC Press, Taylor & Francis Group, USA, ISBN: 103204277X, 9781032042770, ss. 328. [CrossRef]
- Khan, A.; Saifullah, M.I.,; Hussain, S. Organic control of phytonematodes with Pleurotus species, Pakistan Journal of Nematology, 2014, 32(2), 155-161. https://www.cabidigitallibrary.org/doi/pdf/10.5555/20143309504.
- Kudrys, P.; Nabrdalik, M.; Hendel, P.; Kolasa-Więcek, A.; Moliszewska, E. Trait Variation between Two Wild Specimens of Pleurotus ostreatus and Their Progeny in the Context of Usefulness in Nematode Control, Agriculture 2022, 12(11), 1-17. https://www.mdpi.com/2077-0472/12/11/1819/pdf.
- Nelke, R.; Nabrdalik, M.; Żurek, M.; Kudrys, P.; Hendel, P.; Nowakowski, M.; Moliszewska, E.B. Nematicidal Properties of Wild Strains of Pleurotus ostreatus Progeny Derived from Buller Phenomenon Crosses. Appl. Sci. 2024, 14(17), 7980. [CrossRef]
- Kaczorowski, G. Wpływ chwastów na populację Heterodera schachtii Schmidt na polach gospodarstw buraczanych. Doctoral thesis in Polish [The influence of weeds on the population of Heterodera schachtii Schmidt in the fields of sugar beet farms.]. Akademia Techniczno-Rolnicza, Bydgoszcz 1992, pp. 63.
- Reinefeld, E.; Emmerich, A.; Baumgarten, G.; Winner, C.; Beiß, U. Zur Voraussage des Melassezuckers aus Rübenanalysen. Zucker 1974, 27, 2–15.
- Heydari, R.; E. Pourjam, E.; Goltapeh, M. Antagonistic Effect of Some Species of Pleurotus on the Root-knot Nematode, Meloidogyne javanica in vitro. Plant Pathology Journal 2006, 5, 173-177. [CrossRef]
- Nyangwire, B.; Ocimati, W.; Tazuba, A. F.; Blomme, G.; Alumai, A.; Onyilo, F. Pleurotus ostreatus is a potential biological control agent of root-knot nematodes in eggplant (Solanum melongena). Frontiers in Agronomy 2024, 6, 1464111. [CrossRef]
- Pineda-Alegría, J.A.; Sánchez-Vázquez, J.E.; González-Cortazar, M.; Zamilpa, A.; López-Arellano, M.E.; Cuevas-Padilla, E.J.; Mendoza-de-Gives, P.; Aguilar-Marcelino, L. The Edible Mushroom Pleurotus djamor Produces Metabolites with Lethal Activity Against the Parasitic Nematode Haemonchus contortus. J Med Food. 2017, 20(12), 1184-1192. Epub 2017 Aug 2. [CrossRef] [PubMed]
- Allam, M.; Radicetti, E.; Quintarelli, V.; Petroselli, V.; Marinari, S.; Mancinelli, R. Influence of organic and mineral fertilizers on soil organic carbon and crop productivity under different tillage systems: A meta-analysis. Agriculture 2022, 12(4), 464.
- Nakatsuka, H.; Oda, M.; Hayashi, E.; Tamura, K. Effects of fresh spent mushroom substrate of Pleurotus ostreatus on soil micromorphology in Brazil, Geoderma 2016, 269, 54-60. [CrossRef]
- Castro L.R.I.; Delmastro, S.; Curvetto N.R. Spent oyster mushroom substrate in a mix with organic soil for plant pot cultivation. Micologia Aplicada International 2008, 20(1), 17-26.
- Truong, BN.; Okazaki, K.; Fukiharu, T. et al. Characterization of the nematicidal toxocyst in Pleurotus subgen. Coremiopleurotus . Mycoscience 2007, 48, 222–230. [CrossRef]
- Tazuba, A.F.; Ocimati, W.; Ogwal, G.; Nyangwire, B.; Onyilo, F.; Blomme, G. Spent Pleurotus ostreatus Substrate Has Potential for Controlling the Plant-Parasitic Nematode, Radopholus similis in Bananas. Agronomy 2025, 15, 1040. [CrossRef]
- Mostafa, D.M.; Awd Allah, S.F.A.; Awad-Allah, E.F.A. Potential of Pleurotus sajor-caju compost for controlling Meloidogyne incognita and improve nutritional status of tomato plants. J Plant Sci Phytopathol. 2019, 3, 118-127.
- Lopes, A.D.; de Melo Santana Gomes, S.; Schwengber, R.P. et al. Control of Meloidogyne javanica with Pleurotus djamor spent mushroom substrate. Chem. Biol. Technol. Agric. 10, 13 (2023). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).