Introduction
BC is the most common cancer among women globally and in Bangladesh and is the leading cause of cancer-related deaths in women [
1]. There were approximately 2.3 million BC cases recorded worldwide and 670,000 BC related deaths in 2022, making it the most common cancer among women and the second most common cancer overall [
2]. BC affects one in seven women worldwide, with male BC accounting for less than 1% of cases [
3,
4]. BC remains a growing global challenge, with the health burden shifting towards low- and middle-income countries such as Bangladesh. In Bangladesh, BC affects 22.5/100,000 females, primarily those aged 15–44 years, with high morbidity and mortality largely due to inadequate screening programs [
5]. If the current trend continues, BC cases are expected to rise to 3.2 million annually, with 1.1 million deaths affecting countries with lower Human Development Index (HDI) [
6].
Genetic polymorphisms are the most common type of human genetic variation, differing from DNA mutations in their lower frequency and population-specific presence [
7]. These polymorphisms influence cancer susceptibility and have predictive value, particularly for BC development [
8]. While genetic predisposition is a known risk factor, the precise molecular mechanisms of BC etiology remain unclear, further complicating its pathogenesis and management [
9,
10,
11]. The
MDM family, comprising
MDM2 and
MDM4 proteins, is crucial for genomic stability and stress response regulation via the tumor suppressor gene p53. Elevated levels of
MDM2 and
MDM4, which are prevalent in BC, disrupt p53 function via degradation and transcriptional repression increasing cancer risk [
11].
MDM4 plays a critical role in BC proliferation by inhibiting the transcriptional activity of wild type p53 [
12]. Although
MDM4 lacks E3 ligase activity, its heterodimerization with
MDM2 enhances
MDM2’s enzymatic activity, efficiently inactivating p53 [
13]. Overexpression of
MDM4, common in malignancies such as BC, reduces p53 activity and contributes to oncogenesis [
11,
14]. The p53 tumor suppressor protein is crucial for cellular responses to stress, such as DNA damage and hypoxia, by promoting DNA repair, cell cycle arrest, and apoptosis [
15,
16].
Genetic variants of
MDM4, particularly rs4245739 and rs1380576, have been extensively studied for their association with cancer susceptibility. SNP rs4245739 in the 3′ untranslated region (UTR) of
MDM4 decreases its expression by forming a microRNA-191 target site, impacting p53 regulation [
15]. Genome-wide association studies (GWAS) have identified rs4245739 as a BC risk factor, whereas rs1380576 is linked to multiple cancers, including BC and gastric cancer [
17]. A meta-analysis conducted in 2021 found rs4245739 to be inversely associated with BC risk where a protective effect was observed in the Asian population [
18]. Conversely, multiple studies that include a 2018 case-control study in a Southeast Iranian population reported that the rs1380576 polymorphism is associated with reduced risk of BC in some populations [
15]. However, the results were not universally conclusive and varied across ethnicities therefore extensive research is needed to validate the findings. These polymorphisms highlight the role of
MDM4 in cancer pathogenesis and its effect on DNA repair mechanisms [
15]. Targeting
MDM4, including strategies to block its expression or activity, is a proposed therapeutic approach for BC that aims to restore p53 functionality and improve treatment outcomes [
11].
Genetic susceptibility to diseases like BC often vary significantly across various ethnicities due to differences in allele frequency, gene-environment interactions and lifestyle factors. Therefore, it is necessary to evaluate specific single nucleotide polymorphisms (SNPs) within diverse populations to understand their relevance in distinct genetic backgrounds. To address this gap, our study aimed to examine the association of two polymorphisms in the MDM4 Gene (rs4245739 and rs1380576) with BC risk in the Bangladeshi population, where genetic data on BC susceptibility remain limited. To the best of our knowledge, this is the first study investigating these SNPs in relation to BC in this population. We believe our findings will provide valuable population specific insights and contribute to a broader understanding of genetic risk factors for BC across various ethnic groups.
Materials and Methods
Materials
TaKaRa Taq DNA polymerase master mix (PMM), restriction enzymes, and DNA ladders were supplied by TAKARA BIO Inc. (Japan). SeaKem LE Agarose was procured from Lonza (USA) and Tris-borate-EDTA (TBE) buffer was sourced from Solarbio Life Sciences (China). The DNA extraction kit was provided by Favorgen (USA), and Midori Green was purchased from Nippon Genetics (Europe). Primers were obtained from Macrogen (Korea) and restriction enzymes were purchased from New England Biolabs (USA).
Study Subjects
Participants aged 18 to 50 years with a BMI of 18.5-24.9 kg/m2 who had significant physical or neurological illnesses, identified through comprehensive physical, neurological, and laboratory assessments, were excluded from the study. Histopathologically confirmed BC patients (cases) and age-matched healthy individuals (controls) of Bangladeshi ethnicity were recruited randomly. A total of 112 BC patients and 124 age- and sex-matched HCs were enrolled between April 1, 2023, and June 30, 2023. We recruited over 95% female and around 5% male volunteers to minimize gender bias. All enrolled participants completed the study, with no cases of attrition. As this was a pilot study, formal power calculation was not necessary. Patients with BC were recruited from Dhaka Cancer and General Hospital, located on Sat Masjid Road, Dhaka. The study used well-prepared inpatient and outpatient facilities for participant evaluation. A qualified oncologist diagnosed BC patients based on established diagnostic criteria, while also evaluating HCs. Demographic and clinical data were collected, and genetic analysis was performed on blood samples. Randomized selection ensured unbiased sampling, while laboratory personnel were blinded to case-control status to minimize bias. Genotyping analysis was conducted at the Rufaida BioMeds Laboratory in Dhaka, Bangladesh. To ensure participant confidentiality, the authors had no access to personally identifiable information during or after the data collection.
DNA Extraction and Genotyping
Blood samples were collected from all participants in sterile ethylenediaminetetraacetic acid-containing tubes. Genomic DNA was extracted for a single nucleotide polymorphism (SNP) study using a commercial genomic DNA extraction kit (Favorgen, USA), following the manufacturer’s protocol. Briefly, the collected blood samples were lysed, and the lysate was carefully transferred into binding column tubes. The tube was washed with supplied ethanol containing two different types of washing buffers and DNA was eluted using nuclease-free water. The isolated DNA was stored at -20 °C.
The purity of the extracted DNA was evaluated using 1% agarose gel electrophoresis and quantification was performed at 260 nm using a UV-VIS spectrophotometer (Shimadzu Corporation, Japan). A thermal cycler (miniPCR, USA) was used to perform the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay, which was employed to detect SNPs at specific genomic sites. Table 1 summarizes the thermal cycling conditions and primers used to amplify the target regions. The PCR reaction [PCR 2X master mix (10 µL), DNA (2 µL of 100ngm/ µL), forward primer (2 µL of 5 pmol/µL), reverse primer (2 µL of 5 pmol/µL), and ddH2O (4 µL)] was performed using a TaKaRa Taq DNA polymerase master mix (PMM), and successful amplification was confirmed by 1% agarose gel electrophoresis.
The PCR products were digested using MspI (134 bp, 110 bp and 24 bp) and BsrI (195 bp, 172 bp and 23 bp) restriction enzymes at 60 °C for 1 h each [PCR-amplified product (8 µL), 10X QuickCut buffer (1 µL), and enzyme (1µL)]. These enzymes were used to analyze the SNPs at rs4245739 and rs1380576. The digested products were visualized on 3% agarose gels stained with Midori Green. All gels were independently reviewed by two analysts; discrepancies were resolved by repeat amplification and digestion. To ensure reliability of the results, 20% of the samples were subjected to sanger sequencing through a commercial service provider, concordance with PCR-RFLP was 100%.
Statistical Analyses
Statistical analysis was performed using R software (version 4.4.1; R Foundation for Statistical Computing, Vienna, Austria;
https://www.r-project.org/). Hardy-Weinberg equilibrium was tested using the ‘HardyWeinberg’ principle. Pearson’s Chi-square tests were used to check the association between categorical variables across different genotype groups. Adjusted Odds ratios (aORs) with 95% confidence intervals (CIs) were calculated using multivariate logistic regression models, incorporating age and BMI as covariates.
Ethical Consideration
The study was approved by the Institutional Review Board (IRB) of BRAC University (Approval No. BRACUIRB120220005). Informed written consent was obtained from all participants, who were assured of their right to withdraw from the study at any time without penalty. All the procedures were conducted in accordance with the guidelines and regulations outlined in the approved research protocol.
Results
Association of MDM4 (rs4245739) Polymorphism with BC
The genotype and allele frequencies of MDM4 (rs4245739) polymorphism were assessed among BC cases and controls (Table 2). The HWE analysis showed that it adhered to Hardy-Weinberg equilibrium (p > 0.05).
The homozygous AA genotype was more prevalent in BC cases (65.18%) compared to controls (53.23%). The heterozygous CA genotype was found in 3.57% of cases and 4.03% of controls. Additionally, the homozygous CC genotype was present in cases at a higher frequency (31.25%) compared to controls (42.74%).
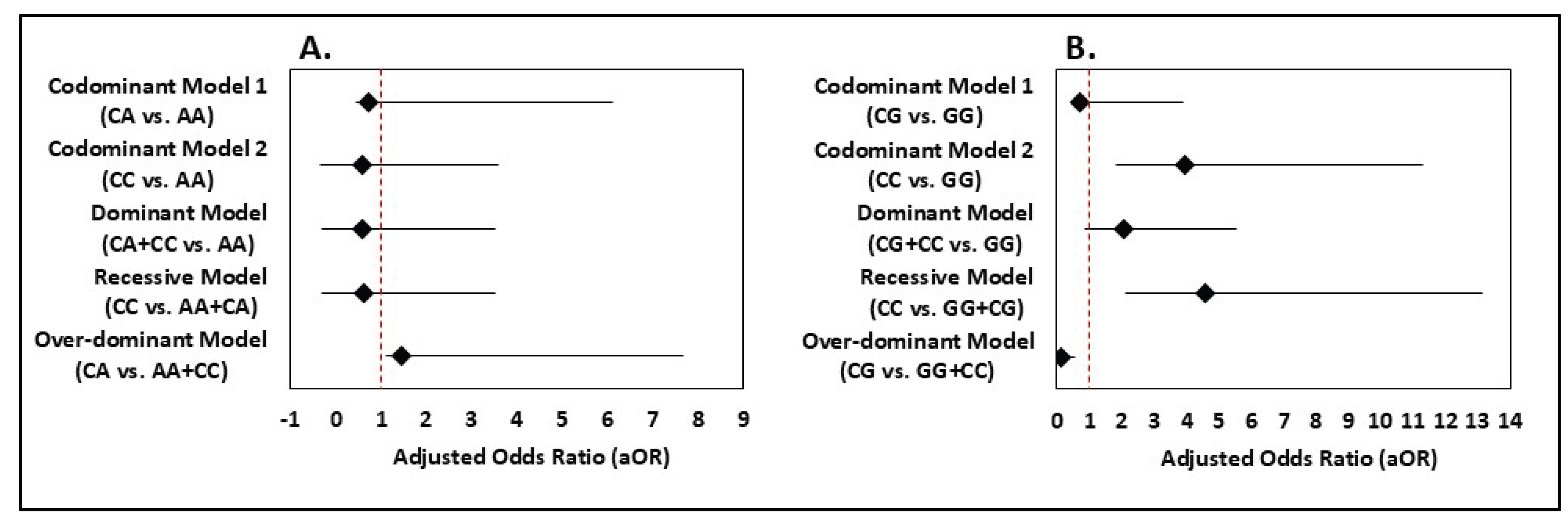
In the codominant model 1 (CA vs. AA), the adjusted odds ratio (aOR) was 0.712 (95% CI: 0.271-5.420) indicating no significant association (p = 0.802) (
Figure 1). Similarly, codominant model 2 (CC vs. AA) did not show a significant association (aOR = 0.588, 95% CI: 0.932-3.010, p = 0.085), although a protective trend was noted. The dominant model (CA + CC vs. AA) yielded an adjusted OR of 0.599 (95% CI: 0.920-2.930, p = 0.093), indicating a potential protective effect, though not statistically significant (
Figure 1). The recessive model (CC vs. CA + AA) also showed a non-significant association (aOR = 0.609, 95% CI: 0.931-2.900, p = 0.086) (
Figure 1). In the over-dominant model (CA vs. CC + AA), the association was not significant (aOR = 1.461, 95% CI: 0.345-6.197, p = 0.607) (
Figure 1). Overall, no statistically significant association was found between
MDM4 rs4245739 polymorphism and BC risk (
Figure 1).
Association of MDM4 (rs1380576) Polymorphism with BC
Table 3 shows genotype and allele frequencies of MDM4 rs1380576 in cases and controls. Both groups were in HWE (p > 0.05). The normal homozygous GG genotype was more frequent in controls (70.97%) than in cases (54.46%). The rare homozygous CC genotype occurred far more often in cases (43.75%) than in controls (14.52%).
Logistic regression revealed a robust association of the CC genotype with increased BC risk in the codominant model (CC vs. GG: aOR = 3.93; 95% CI = 2.09-7.38; p < 0.001). The dominant model (CG+CC vs. GG) also showed significant risk (aOR = 2.04; 95% CI = 1.19-3.50; p = 0.009). The recessive model (CC vs. GG+CG) yielded an aOR of 4.58 (95% CI = 2.46-8.55), indicating strong risk elevation. In the over-dominant model (CG vs. GG+CC), CG carriers appeared protected (aOR = 0.11; 95% CI = 0.02-0.47; p = 0.003).
Together, these data highlight the C allele’s risk-enhancing effect and the rarity but potential protective nature of the CG heterozygote in this population.
Comparative Analysis of MDM4 (rs4245739) and (rs1380576) Polymorphism
A side-by-side evaluation of both SNPs highlights their markedly different associations with BC susceptibility in the Bangladeshi cohort. The MDM4 rs4245739 A>C polymorphism showed no statistically significant association with BC under any genetic model, with all odds ratios hovering around 0.6 and p-values > 0.05.
In contrast, the rs1380576 C>G polymorphism displayed consistently strong associations with increased BC risk. Individuals carrying the CC genotype had nearly a four-fold higher risk compared with GG carriers in the codominant model. The dominant model also indicated significantly increased risk. The recessive model again confirmed the elevated risk for CC carriers. Interestingly, the over-dominant model showed a strong protective effect for the heterozygous genotype, reflecting its low frequency among cases.
Taken together, these findings show that while rs4245739 appears neutral in this population, rs1380576 exerts a pronounced effect on BC susceptibility, with the CC genotype conferring the highest risk and the CG genotype possibly conferring protection
Socio-Demographic History
The demographic characteristics of BC patients (n=112) and healthy controls (n=124) used in our research are presented in Table 4. The mean age of BC patients was 46.9 ± 12.5 years, while that of the control group was 47.8 ± 13.1 years with no statistically significant observed between them (p = 0.58). Moreover, the mean BMI was also comparable between the two groups (25.7 ± 4.9 kg/m2 for cases and 25.2 ± 5.1 kg/m2 for controls).
In terms of occupation, the distribution was comparable across both groups. Among cases, 33.9% were housewives, 60.7% were working women, and 5.4% were students. In the control group, 35.5% were housewives, 58.9% were working women, and 5.6% were students. The differences in occupational status between cases and controls were not statistically significant (p = 0.46). The mean number of children was similar between groups, with cases reporting 2.13 ± 0.91 and controls 2.09 ± 0.87 (p = 0.45).
Further socio-demographic analysis revealed that education level (p = 0.71), socio-economic status (p = 0.29), residential status (p = 1.0), and menopausal status (p = 0.881) also showed no significant differences between cases and controls. This indicates that these demographic factors were evenly distributed across both groups and are unlikely to have influenced BC susceptibility in this study population.
Histopathological Characteristics of BC Patients
The clinical and histopathological features of BC cases are summarized in Table 5. All patients presented with a breast lump (100%), while 40.19% reported weight loss, 39.29% presented with peau d’orange, and 18.75% experienced nipple discharge. Histological analysis revealed that the majority of tumors were invasive ductal carcinoma (75%), followed by ductal carcinoma in situ (14.29%) and invasive lobular carcinoma (10.71%). Regarding clinical staging, 46.43% of patients were in Grade I, 37.5% in Grade II, and 16.07% in Grade III.
Hormone receptor analysis showed ER positivity in 65.19% of cases, PR positivity in 71.43%, and HER2 positivity in 28.57%. Tumor size ranged from <2 cm in 5.36% of cases to >5.0 cm in 39.29% of cases, with the majority (55.36%) between 2.1–5.0 cm. Lymph node involvement was observed in 26.79% of cases.
Discussion
This study examines the role of MDM4 rs4245739 and rs1380576 polymorphisms on BC risk in 112 BC patients and 124 healthy controls. Based on an assumed minor allele frequency of approximately 0.30 for the investigated SNPs in South Asian populations and an anticipated odds ratio of 2.0 for the association with BC, a power calculation (α=0.05, two-sided) yielded an estimated power of ~77% to detect such an effect under a dominant model. This indicates that the present sample size is sufficient to detect moderate-to-large effects of the studied polymorphisms in this population. Genotype comparisons and logistic regression analyses were used to calculate the odds ratios (ORs), p-values, and 95% confidence intervals (CIs) using various genetic models. We investigated the association of MDM4 rs4245739 and rs1380576 polymorphisms with BC risk using various genetic models. Although rs4245739 did not show significant associations in any model, rs1380576 displayed strong and statistically significant links with BC risk, particularly for the CC genotype.
The
MDM4 is a critical regulator of the p53 tumor suppressor pathway, which plays a central role in controlling cell cycle progression, DNA repair, apoptosis and cellular response to stress and DNA damage. Under normal conditions,
MDM4 binds directly to wild type p53 and inhibits its transcriptional activity which prevents p53 from activating genes involved in cell cycle arrest and apoptosis [
14]. However, when
MDM4 is overexpressed as observed in most cancer cells, it excessively accumulates p53 thus allowing abnormal cells to survive and proliferate. Normal breast epithelial cells exhibit 20% and 10% levels of
MDM2 and
MDM4, respectively, but abnormal elevations are strongly linked to BC progression and prognosis [
13]. Overexpression or amplification of
MDM4 is a mechanism by which tumors with wild type p53 inactivate its function, contributing to cancer development. Mouse models overexpressing
MDM4 develop spontaneous tumors, demonstrating that
MDM4 acts as an oncogene in vivo [
19]. Additionally, the
MDM4 forms a heterodimer complex with
MDM2 that enhances
MDM2’a E3 ubiquitin ligase activity thus initiating p53 degradation and further reducing the tumor suppressor function of p53 [
20]. Moreover, recent evidence suggests that
MDM4 can promote tumorigenesis in cells that lack functional p53, through p53-independent oncogenic mechanisms, making it a critical target in cancers regardless of p53 mutation status [
21]. Therefore, the
MDM4 gene has drawn attention because of its dual function in controlling the tumor suppressor p53 and its possible involvement in oncogenesis via p53-independent pathways. These properties make
MDM4 a key candidate for the study of BC susceptibility and progression.
MDM4, particularly the rs4245739 and rs1380576 polymorphisms, has been studied for its expression in various BC subtypes and its role in tumorigenesis [
22]. The rs4245739 A>C polymorphism, located in the 3 untranslated regions (UTR) of
MDM4, is believed to affect mRNA stability and protein expression levels. These changes have been proposed to modulate tumorigenesis by altering the p53-dependent pathways. The
MDM4 rs4245739 polymorphism creates a novel target site for microRNA-191 in the 3’-untranslated region [
23]. Therefore, miR-191 can bind to the
MDM4-C allele mRNA selectively, resulting in decreased
MDM4 protein expression that in turn reduces the inhibition of p53 activities, leading to decreased cancer risk. In contrast,
MDM4-A allele lacks the miR-191 binding site so
MDM4 mRNA transcripts with the A allele are not suppressed by miR-191 [
23]. This leads to higher expression of
MDM4 mRNA and protein in individuals with the A allele, as observed in cancers such as ovarian cancer and retinoblastoma [
24]. Our study found no significant association between the rs4245739 polymorphism and BC susceptibility in the Bangladeshi population. Similarly, a study conducted in the Iranian-Azeri population also reported no significant association between this SNP and BC risk or clinicopathological association that further reinforces the results of Gansmo et al. on lung, colon and prostate cancer [
25,
26]. According to a meta-analysis conducted in 2021 reported that rs4245739 is significantly associated with reduced overall cancer risk in the Asian population and no significant association was observed in the Caucasian population [
14]. However, three GWAS studies reported that this SNP increased the risk of ER-negative or triple negative BC (defined by the absence of ER, progesterone receptor and human epidermal growth factor receptor-2) in the Caucasian population [
27,
28,
29]. According to Garcia-Closaset, the rs4245739 polymorphism is located in an ER-negative specific BC risk locus therefore it was assumed it increases the susceptibility of ER-negative specific BC [
27]. However, there is a large difference in the distribution of the rs4245739 polymorphism on the Asian and Caucasian population therefore large cohort studies are needed to validate the findings [
14].
Although previous studies in some Asian populations reported a protective effect of the G allele, our data show a clear risk associated with the C allele and CC genotype in Bangladeshi women. This ethnic divergence underscores the importance of population-specific studies. The functional role of rs1380576 is still unclear but may involve regulation of MDM4 splicing or expression, thereby altering p53 activity. Future functional assays could clarify whether the observed heterozygote protection reflects distinct molecular mechanisms.
Conversely, various potential pathogenic mechanisms may elucidate the connection among rs1380576 and BC. Our updated analysis strengthens the evidence that the rs1380576 polymorphism of
MDM4 is linked to BC susceptibility. The CC genotype confers nearly a four-fold higher risk compared with GG carriers and remains significant under both dominant and recessive models. Interestingly, the CG heterozygote appears underrepresented in cases and shows a strong protective effect in the over-dominant model, suggesting a possible heterozygote advantage or gene–environment interaction that merits further exploration. The functional implications of rs1380576 remain unclear, although it has been suggested that it may indirectly modulate the expression or activity of
MDM4 in cancer pathways. According to a case-control study in a Southeast Iranian population, the rs1380576 G allele was significantly associated with a reduced risk of BC under multiple genetic models [
15]. Although rs1380576 is located within the
MDM4 gene, it is not in a known microRNA binding site like rs4245739. It may influence
MDM4 gene regulation, splicing, or mRNA stability and hence modulating the level or activity of
MDM4 protein. Reduced
MDM4 activity would lead to less inhibition of p53, enhancing tumor suppressor activity and lowering cancer risk [
15,
18]. Additionally, a 2021 meta-analysis reported that the rs1380576 polymorphism is negatively associated with cancer in Asians, indicating a protective effect although the association was absent in other ethnicities [
18]. Another Polish study showed that rs1380576 and rs4245739 polymorphisms in
MDM4 were significantly associated with estrogen and progesterone receptor status in early-stage BC, implying a potential role in tumor characteristics and progression [
11]. Moreover, they reported that patients with the CC genotype were susceptible to ER and PR which indicates that the CC genotype might increase the risk of developing BC [
11]. According to a retinoblastoma study in the Chinese population, it was seen that the G allele of rs1380576 appeared to be protective against both the development and aggressiveness of retinoblastoma that was not in line with our findings as our results regarding patients with the G allele were not statistically significant [
17]. However, there is currently limited evidence explaining implications of the rs1380576 polymorphism in BC therefore further investigation across multiple ethnicities is required.
The demographic data of our research showed no significant differences between BC patients and healthy controls in terms of age, BMI, occupation or number of children. These findings suggested that the case and control group were well matched, reducing the chance of confounding due to these factors. These lack of significant differences in BMI and the susceptibility of BC aligned with the findings from previous studies that was done to assess the effect of BMI on BC risk in the Bangladesh population [
30]. However, other studies have reported that higher BMI is a risk factor for BC especially in postmenopausal women therefore further research is needed for assessment [
31]. Furthermore, the similarity in the number of children between the case and control suggests that reproductive history does not have any significant effect on BC risk. Moreover, the demographic analysis also revealed no significant differences between cases and controls with respect to education level, socio-economic class, residential status, or menopausal status, suggesting these factors did not contribute to BC risk in the studied population. This strengthens the reliability of the genetic association results by reducing the likelihood of demographic confounding.
In our study, tissue analysis of BC patients closely reflected global patterns, with invasive ductal carcinoma emerging as the most common type. A large proportion of the tumors were hormone receptor–positive (ER and PR), which is an encouraging finding since these cancers often respond well to hormone-based therapies and are usually linked to better outcomes. About 29% of patients were HER2-positive, a rate similar to what has been reported in other Asian populations. This indicates that a meaningful subset of patients could potentially benefit from HER2-targeted treatments. Additionally, most tumors were identified at medium sizes (2.1–5.0 cm), though nearly 40% were already larger than 5 cm at diagnosis. This points to the urgent need for earlier detection and better screening practices. Lymph node involvement was seen in roughly one in four patients. While not the majority, this remains an important factor, as the presence of cancer in lymph nodes plays a key role in disease staging, treatment decisions, and predicting outcomes.
This study has several limitations that should be acknowledged, including the relatively small sample size of 112 BC patients and 124 healthy controls. While larger cohorts are always preferable, this sample size is comparable to many studies published in high-impact journals that have provided meaningful and widely accepted insights. Additionally, data on various clinical features of BC patients and key risk factors, such as detailed reproductive history, smoking status, breastfeeding practices, and use of oral contraceptives, were not included. Finally, because the study was conducted in a single center with Bangladeshi participants, the findings may not apply to other populations. Larger, multi-center studies with more diverse groups are needed to confirm these results.