Submitted:
12 October 2025
Posted:
13 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis and Characterization of S-Mn₂O₃
2.3. Catalytic Degradation and Quenching Experiments
2.3.1. Active Oxidizing Species Detection
2.3.2. Detection of •OH Radicals
2.3.3. Detection of Singlet Oxygen (¹O₂)
2.3.4. Detection of SO₄•⁻ Radicals
2.3.5. Detection of High-Valent Metal Ions
3. Results
3.1. Structure and Chemical Composition of Mn₂O₃ and S-Mn₂O₃
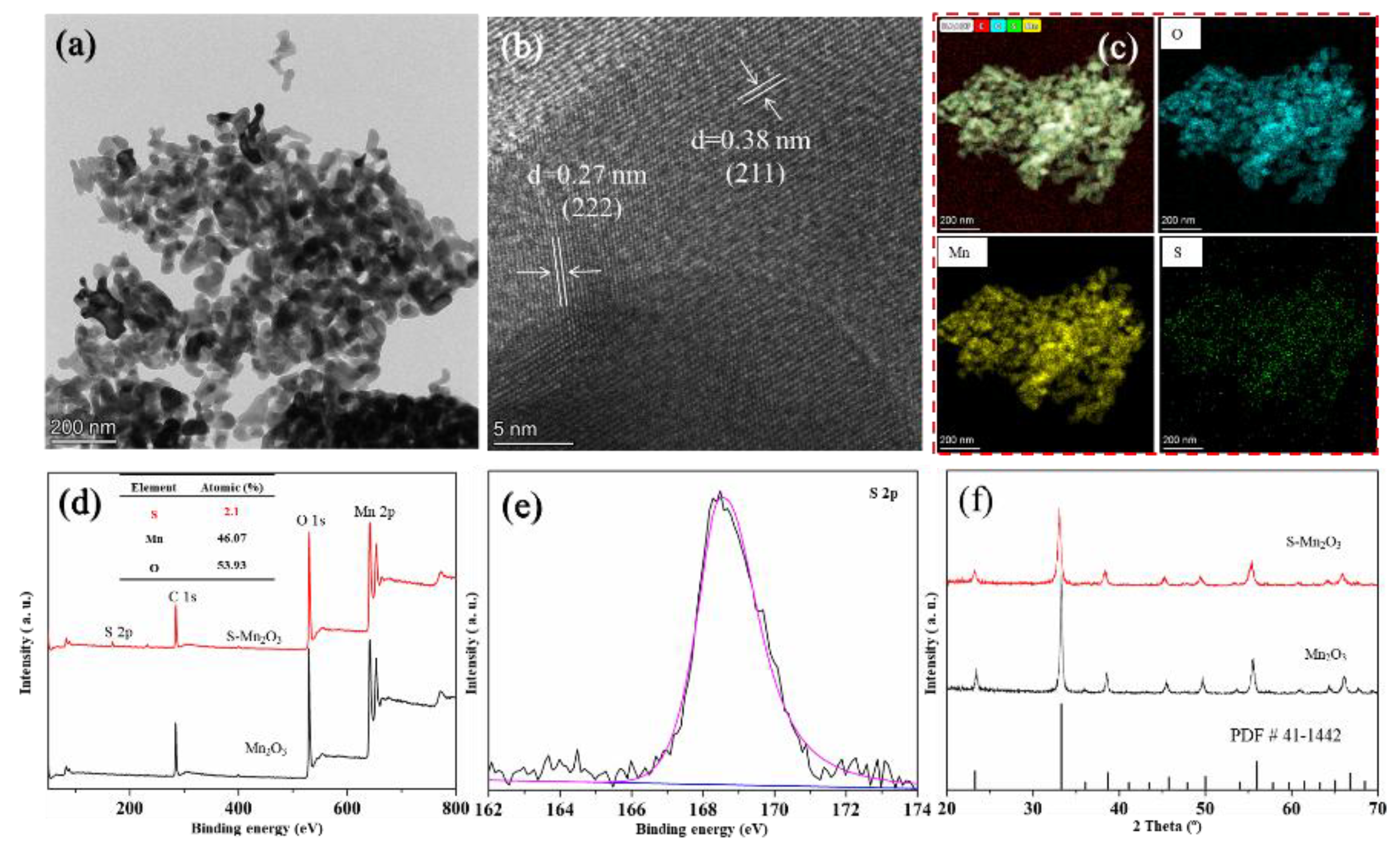
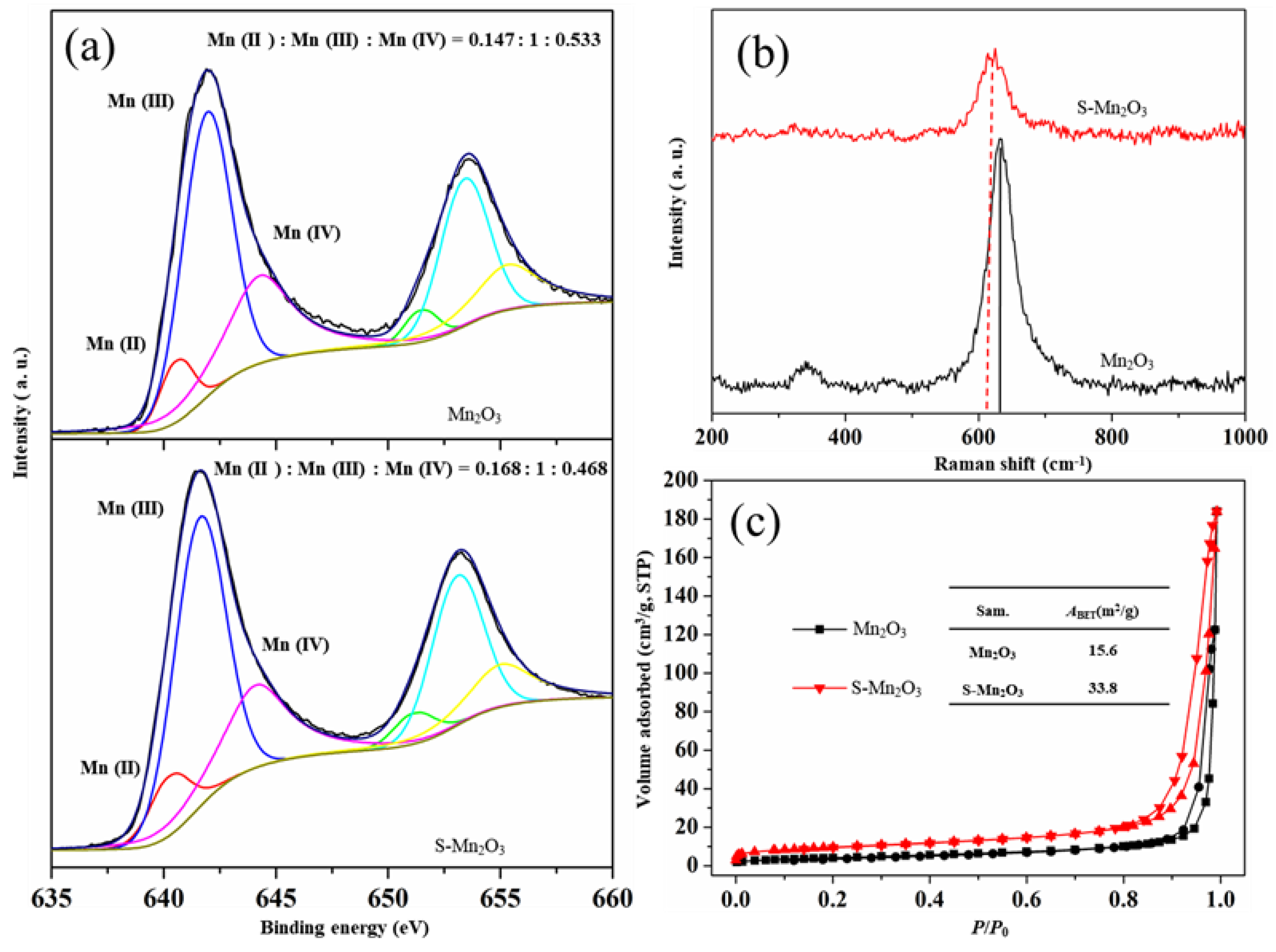
3.2. Catalytic Performance of Mn₂O₃ and S-Mn₂O₃
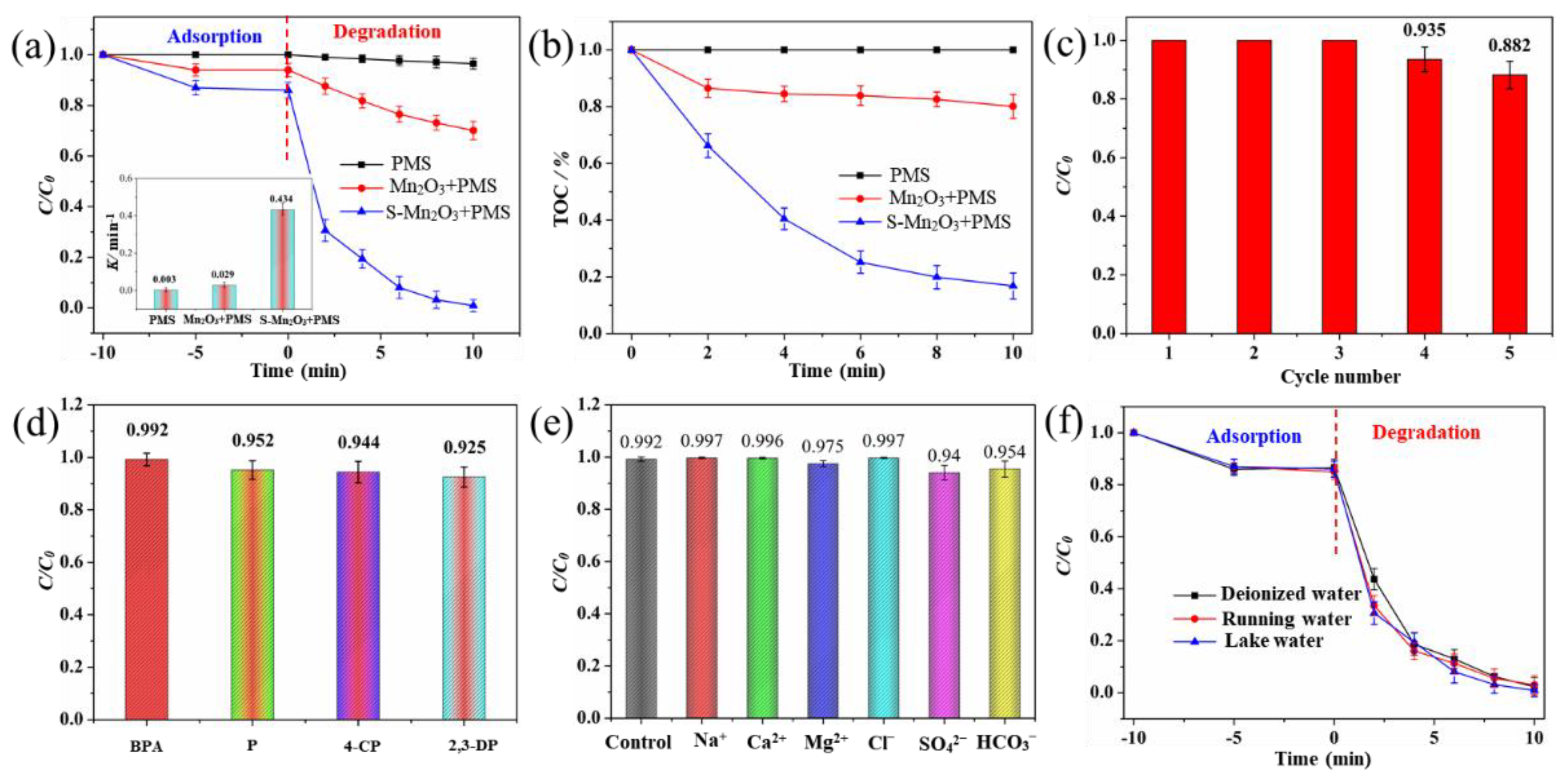
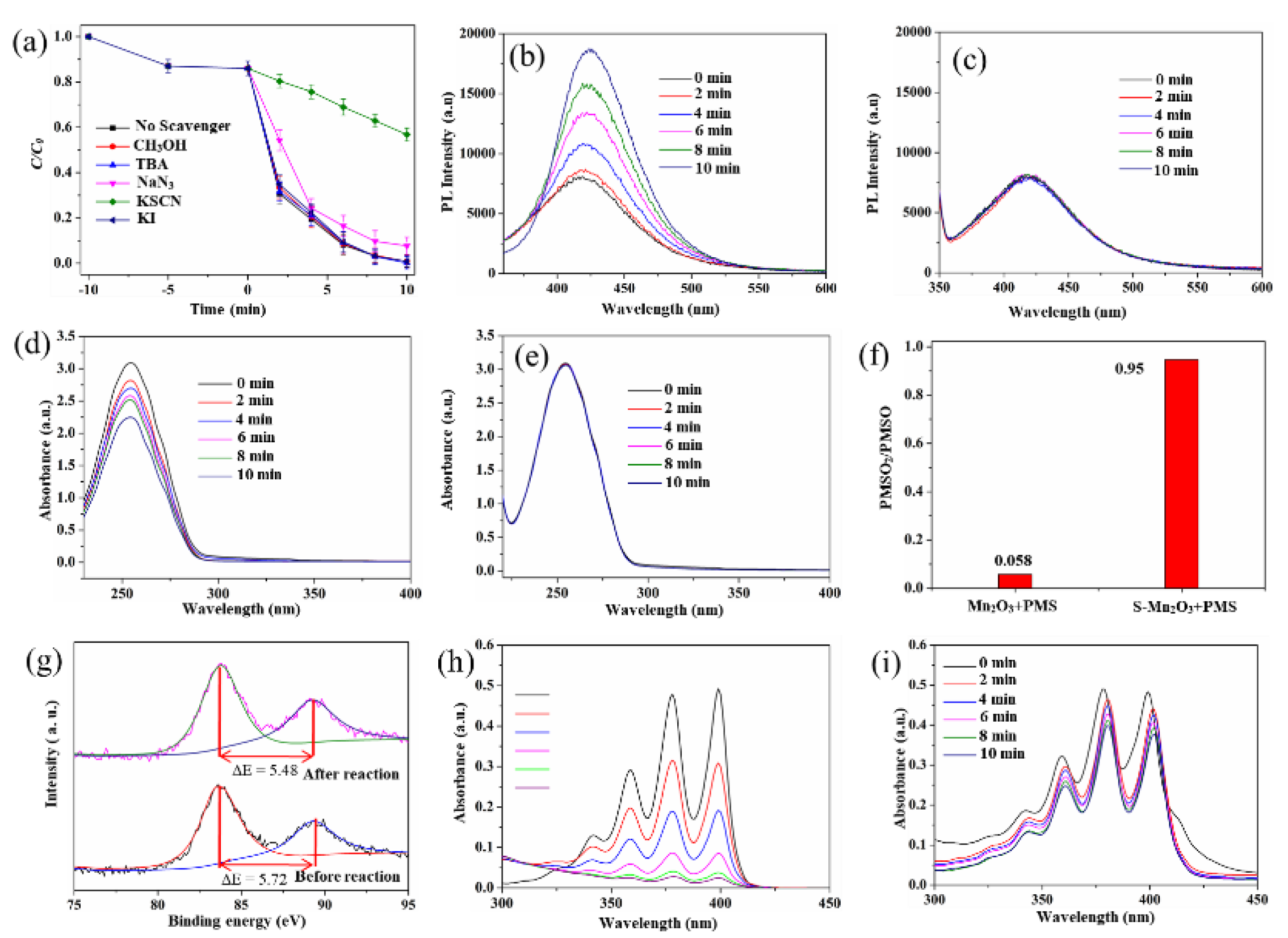
3.3. Catalytic Mechanism of S-Mn₂O₃/PMS System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hodges, B.C.; Cates, E.L.; Kim, J.H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotech. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Cui, B.; Pei, F.; Li, Y.P.; Zhao, R.; Zhang, J.; Wang, F.R.; Gao, Z.X.; Wang, S. Improving peroxymonosulfate activation mediated by oxygen vacancy-abundant BaTiO3/Co3O4 composites: The vital roles of BaTiO3. J. Solid State Chem. 2023, 323, 124049. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.Z.; Feng, J.; Ren, Y.M.; Ma, J. Impact of crystal types of AgFeO2 nanoparticles on the peroxymonosulfate activation in the water. Environ. Sci. Technol. 2019, 53, 4500–4510. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xie, X.; Hu, G.; Prabhakaran, V.; Saha, S.; Gonzalez-Lopez, L.; Phakatkar, A.H.; Hong, M.; Shahbazian-Yassar, R.; Ramani, V.; Al-Sheikhly, M.I.; Jiang, D.-E.; Shao, Y.; Hu, L. Ta-TiOx nanoparticles as radical scavengers to improve the durability of Fe-N-C oxygen reduction catalysts. Nat. Energy 2022, 7, 281–289. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Z.; Hou, S.; Wang, A.; Fang, J. Transformation of gemfibrozil by the interaction of chloride with sulfate radicals: Radical chemistry, transient intermediates and pathways. Water Res. 2022, 209, 117944. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Ji, Y.; Kong, D. Transformation of bromide in thermo activated persulfate oxidation processes. Water Res. 2015, 78, 1–8. [Google Scholar] [CrossRef]
- Yun, E.T.; Lee, J.H.; Kim, J.; Park, H.D.; Lee, J. Identifying the nonradical mechanism in the peroxymonosulfate activation process: Singlet oxygenation versus mediated electron transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef]
- Shang, Y.; Xu, X.; Gao, B.; Wang, S.; Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 2021, 50, 5281–5322. [Google Scholar] [CrossRef]
- Miao, J.; Song, J.; Lang, J.; Zhu, Y.; Dai, J.; Wei, Y.; Long, M.; Shao, Z.; Zhou, B.; Alvarez, P.J.J.; Zhang, L. Single-atom MnN5 catalytic sites enable efficient peroxymonosulfate activation by forming highly reactive Mn(IV)-oxo species. Environ. Sci. Technol. 2023, 57, 4266–4275. [Google Scholar] [CrossRef]
- Li, X.; Wen, X.; Lang, J.; Wei, Y.; Miao, J.; Zhang, X.; Zhou, B.; Long, M.; Alvarez, P.J.J.; Zhang, L. CoN1O2 single-atom catalyst for efficient peroxymonosulfate activation and selective cobalt(IV)=O generation. Angew. Chem. Int. Ed. 2023, 62, e202303267. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.N.; Klu, P.K.; Wang, C.H.; Zhang, W.X.; Luo, R.; Zhang, M.; Qi, J.W.; Sun, X.Y.; Wang, L.J.; Li, J.S. Metal-organic framework-derived hollow Co3O4/carbon as efficient catalyst for peroxymonosulfate activation. Chem. Eng. J. 2019, 363, 234–246. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, X.; Jin, P.; Dzakpasu, M.; Wang, X.C.; Zhang, Q.; Zhang, L.; Yang, L.; Ding, D.; Wang, W.; Wu, K. MOF-templated synthesis of CoFe2O4 nanocrystals and its coupling with peroxymonosulfate for degradation of bisphenol A. Chem. Eng. J. 2018, 353, 329–339. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Cui, B.; Pei, F.; Li, Y.; Zhao, R.; Zhang, J.; Wang, F. Zixuan Gao and Shan Wang. Spontaneous polarization-driven charge migration in BaTiO3/Co3O4/C for enhanced catalytic performance. CrystEngComm 2023, 25, 4219–4230. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Sun; R.; Huang; Z.; Han; X.; Wang; H.; Chen; C.; Liu; Y.; Zheng; X.; Zhang; W.; Hong; X.; Li, W. Crystallinity engineering for overcoming the activity-stability tradeoff of spinel oxide in Fenton-like catalysis. Proc. Natl. Acad. Sci. U.S.A. 2023, 120, e2220608120. [CrossRef]
- Wang, L.; Jiang; J.; Pang; S.; Zhou; Y.; Li; J.; Sun; S.; Gao; Y.; Jiang, C. Oxidation of bisphenol A by nonradical activation of peroxymonosulfate in the presence of amorphous manganese dioxide. Chem. Eng. J. 2018, 352, 1004–1013. [CrossRef]
- Wang, L.; Xu; H.; Jiang; N.; Pang; S.; Jiang; J.; Zhang, T. Effective activation of peroxymonosulfate with natural manganesecontaining minerals through a nonradical pathway and the application for the removal of bisphenols. J. Hazard. Mater. 2021, 417, 126152. [CrossRef]
- Huang, J.; Dai; Y.; Singewald; K.; Liu; C.; Saxena; S.; Zhang, H. Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions. Chem. Eng. J. 2019, 370, 906–915. [CrossRef]
- Li, H.; Yuan, N.; Qian, J. Bingcai Pan. Mn2O3 as an Electron Shuttle between Peroxymonosulfate and Organic Pollutants: The Dominant Role of Surface Reactive Mn(IV) Species. Environ. Sci. Technol. 2022, 56, 7–4498. [Google Scholar]
- Huang, Q.; Zhang; W.; Li; F.; Zhang; M.; Li; Q.; Yang, J. Highly Efficient Peroxymonosulfate Activation by Molten Salt-Assisted Synthesis of Magnetic Mn-Fe3O4 Supported Mesoporous Biochar Composites for SDz Degradation. ACS EST Water 2024, 4, 4591–4603. [CrossRef]
- Y.; Li; H.; Yu; W.; Pan; Y.; Li; L.; Wang; Y.; Pu; L.; Ding; J.; Gao; G.; Pan, B.Peroxydisulfate Activation and Singlet Oxygen Generation by Oxygen Vacancy for Degradation of Contaminants. Environ. Sci. Technol. 2021, 55, 2110–2120. [CrossRef]
- Yang, Y.; Zhang; P.; Hu; K.; Duan; X.; Ren; Y.; Sun; H.; Wang, S. Sustainable redox processes induced by peroxymonosulfate and metal doping on amorphous manganese dioxide for nonradical degradation of water contaminants. Appl. Catal. B 2021, 286, 119903. [CrossRef]
- Huang, K.Z.; Zhang, H. Direct electron-transfer-based peroxymonosulfate activation by iron-doped manganese oxide (δ-MnO2) and the development of galvanic oxidation processes (GOPs). Environ. Sci. Technol. 2019, 53, 12610–12620. [CrossRef]
- M.-K.; Huang; G.; Mei; S.; Wang; Z.; Zhang; Y.; Hua; T.; Zheng; L.; Yu, H. Interface-Promoted Direct Oxidation of p-Arsanilic Acid and Removal of Total Arsenic by the Coupling of Peroxymonosulfate and Mn-Fe-Mixed Oxide. Environ. Sci. Technol. 2021, 55, 7063–7071. [CrossRef] [PubMed]
- Du, J.; Bao, J.; Fu, X.; Lu, C.; Kim, S.H. Mesoporous sulfur-modified iron oxide as an effective Fenton-like catalyst for degradation of bisphenol A. Appl. Catal. B: Environ. 2016, 184, 132–141. [Google Scholar] [CrossRef]
- Guo, L.; Chen, F.; Fan, X.; Cai, W.; Zhang, J. S-doped α-Fe2O3 as a highly active heterogeneous Fenton-like catalyst towards the degradation of acid orange 7 and phenol. Appl. Catal. B: Environ. 2010, 96, 162–168. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, W.H.; Liu, J.W.; Lin, P.; Zhang, J.F.; Huang, T.L.; Liu, K.Q. Mesoporous sulfur-doped CoFe2O4 as a new Fenton catalyst for the highly efficient pollutants removal. Appl. Catal. B: Environ. 2021, 295, 120273. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, G.; Wang, L.; Huang, T.; Qin, L. Highly active S-modified ZnFe2O4 heterogeneous catalyst and its photo-Fenton behavior under UV–visible irradiation. Ind. Eng. Chem. Res. 2011, 50, 7219–7227. [Google Scholar] [CrossRef]
- Han, G.; Zhang; X.; Liu; W.; Zhang; Q.; Wang; Z.; Cheng; J.; Yao; T.; Gu; L.; Du; C.; Gao; Y.; Yin, G. Substrate Strain Tunes Operando Geometric Distortion and Oxygen Reduction Activity of CuN2C2 Single-Atom Sites. Nat. Commun. 2021, 12, 6335. [CrossRef]
- Tani, Y.; Miyata; N.; Ohashi; M.; Ohnuki; T.; Seyama; H.; Iwahori; K.; Soma, M. Interaction of Inorganic Arsenic with Biogenic Manganese Oxide Produced by a Mn-Oxidizing Fungus, Strain KR21-2. Environ. Sci. Technol. 2004, 38, 6618–6624. [CrossRef]
- Hao, Y.; Sun; S.; Du; X.; Qu; J.; Li; L.; Yu; X.; Zhang; X.; Yang; X.; Zheng; R.; Cairney; J. M.; Lu, Z. Boosting Oxygen Reduction Activity of Manganese Oxide Through Strain Effect Caused By Ion Insertion. Small 2022, 18, 2105201. [CrossRef]
- Zhang, D.; Li; Y.; Wang; P.; Qu; J.; Li; Y.; Zhan, S. Dynamic Active-Site Induced by Host-Guest Interactions Boost the Fenton Like Reaction for Organic Wastewater Treatment. Nat. Commun. 2023, 14, 3538. [CrossRef]
- Weng, Z.; Lin; Y.; Guo; S.; Zhang; X.; Guo; Q.; Luo; Y.; Ou; X.; Ma; J.; Zhou; Y.; Jiang; J.; Han, B. Site Engineering of Covalent Organic Frameworks for Regulating Peroxymonosulfate Activation to Generate Singlet Oxygen with 100% Selectivity. Angew. Chem. Int. Ed. 2023, 62, e202310934. [CrossRef]
- Lu, M.; Kang, G. , Yajuan Deng. Construction of mesoporous S-doped Co3O4 with abundant oxygen vacancies as an efficient activator of PMS for organic dye degradation. CrystEngComm 2023, 25, 2767–2777. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, K.K.; Zhao, W.X.; Jiang, H.B.; Liu, L.L.; Hu, D.W.; Cui, B.; Sun, Y. Novel nanoparticle-assembled tetrakaidekahedron Bi25FeO40 as efficient photo-Fenton catalysts for Rhodamine B degradation. Adv. Powder Technol. 2022, 33, 103579. [Google Scholar] [CrossRef]
- Rong, S.; Zhang, P.; Yang, Y.; Zhu, L.; Wang, J.; Liu, F. MnO2 framework forinstantaneous mineralization of carcinogenic airborne formaldehyde at roomtemperature. ACS Catal. 2017, 7, 1057–1067. [Google Scholar] [CrossRef]
- Chen, B.; Wu, B.; Yu, L.; Crocker, M.; Shi, C. Investigation into the catalytic roles of various oxygen species over different crystal phases of MnO2 for C6H6 and HCHO oxidation. ACS Catal. 2020, 10, 6176–6187. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, M.; Li, H.; Gao, J.; Li, W.; Chen, L.; Zhang, S. Sujing Li。Phosphate-induced electronic tuning of MnO2: Unlocking enhancedactivation and complete oxidation of propane. Applied Catalysis B: Environment and Energy 2025, 372, 125291. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, D.; Xu, X.; Sheng, Y.; Xu, J.; Han, Y.-F. Application of operandospectroscopy on catalytic reactions. Curr. Opin. Chem. Eng. 2016, 12, 1–7. [Google Scholar] [CrossRef]
- Pei, W.; Ma, X.; Wu, Y.; Wang, Y.; Zhou, L.; Lei, J.; Yamashita, H.; Zhang, J. A-site defect regulates d-band center in perovskite-type catalysts enhancing photo-assisted peroxymonosulfate activation for levofloxacin removal via high-valent iron-oxo species. Applied Catalysis B: Environment and Energy 2025, 371, 125273. [Google Scholar] [CrossRef]
- Chen, Y.L.; Bai, X.; Ji, Y.T.; Shen, T. Reduced graphene oxide-supported hollow Co3O4@N-doped porous carbon as peroxymonosulfate activator for sulfamethoxazole degradation. Chem. Eng. J. 2022, 430, 132951. [Google Scholar] [CrossRef]
- Zheng, P.; Pan, Z.; Zhang, J. Synergistic enhancement in catalytic performance of superparamagnetic Fe3O4@bacilus subtilis as recyclable fenton-like catalyst. Catalysts 2017, 7, 349–359. [Google Scholar] [CrossRef]
- Yang, Z.S.W.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. ,Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation oxygen vacanciesand reaction intermediates. Appl. Catal. B: Environ. 2020, 260, 118150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



