Submitted:
05 September 2025
Posted:
01 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Extract Isolation Methods
2.1.1. Ultrasound Assisted Extraction
2.1.2. Extraction in Soxhlet Extractor
2.1.3. Microwave Assisted Extraction
2.2. Characterization of Extracts
2.2.1. Gravimetric Analysis
2.2.2. UV-Vis Spectrometry
2.2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.4. LC-Orbitrap-HRMS
2.2.5. Data Processing in Compound Discoverer
2.3. Emulsion Preparation
| Sample name | Blank | Emulsion A | Emulsion B | Emulsion C | Emulsion D |
| Ingredients (%) | Water phase (75%) | ||||
| Water | 70 | 0 | 35 | 40 | 35 |
| Glycerin | 3,5 | 3,5 | 3,5 | 3,5 | 3,5 |
| Xanthan gum | 1,5 | 1,5 | 1,5 | 1,5 | 1,5 |
| EDTA | 0,5 | 0,5 | 0,5 | 0,5 | 0,5 |
| Reflux 1 | 0 | 70 | 35 | 30 | 0 |
| Reflux 3 | 0 | 0 | 0 | 0 | 35 |
| Oil Phase (25%) | |||||
| Olive oil | 13 | 13 | 13 | 13 | 13 |
| Cetyl alcohol | 2 | 2 | 2 | 2 | 2 |
| Cetearyl alcohol | 2 | 2 | 2 | 2 | 2 |
| Polysorbate 60 | 2 | 2 | 2 | 2 | 2 |
| Shea butter | 2 | 2 | 2 | 2 | 2 |
| Steatic acid | 2 | 2 | 2 | 2 | 2 |
| Beeswax | 2 | 2 | 2 | 2 | 2 |
2.4. Characterization of Emulsions
2.4.1. pH and Viscosity Stability
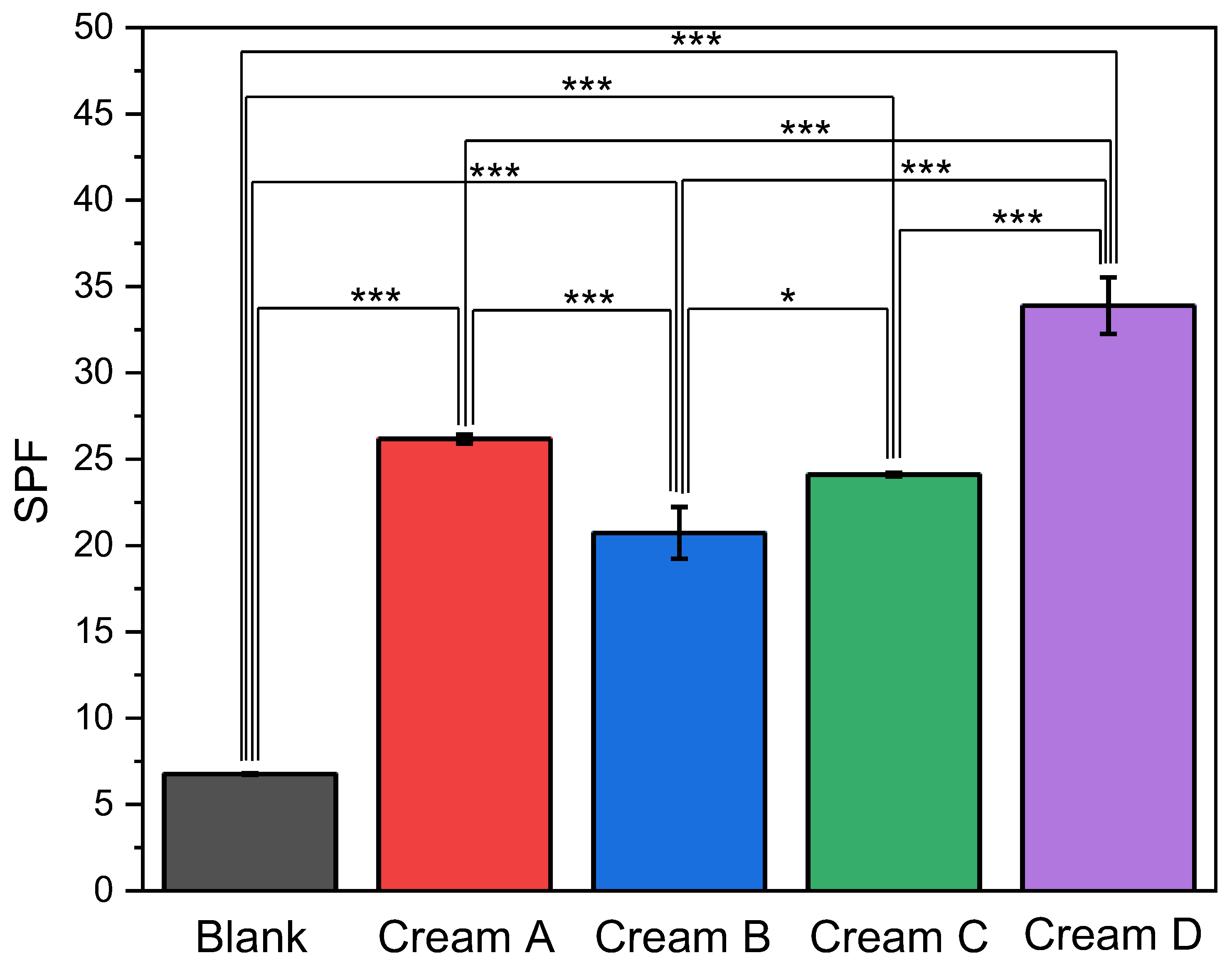
2.4.2. Sun Protection Factor (SPF)
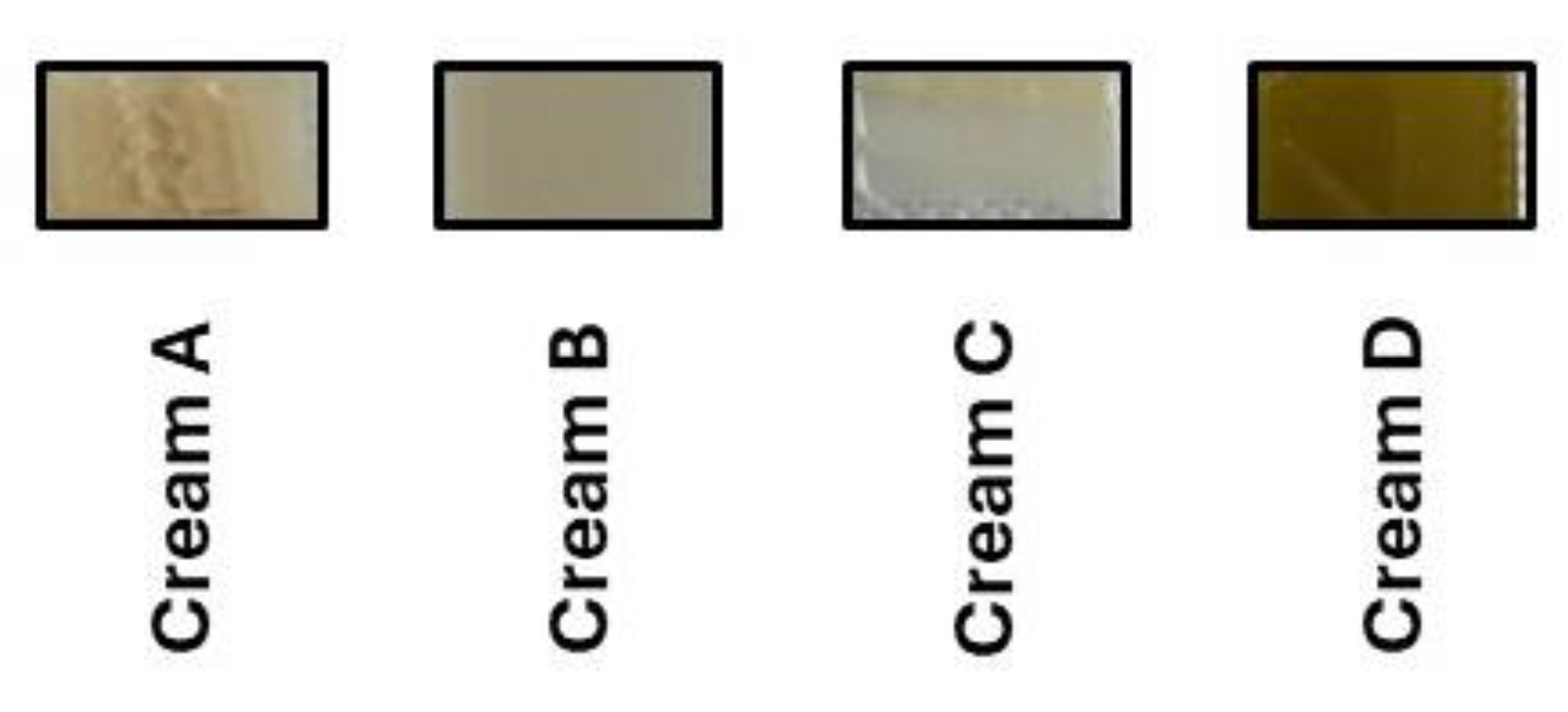
2.4.3. Color Measurement
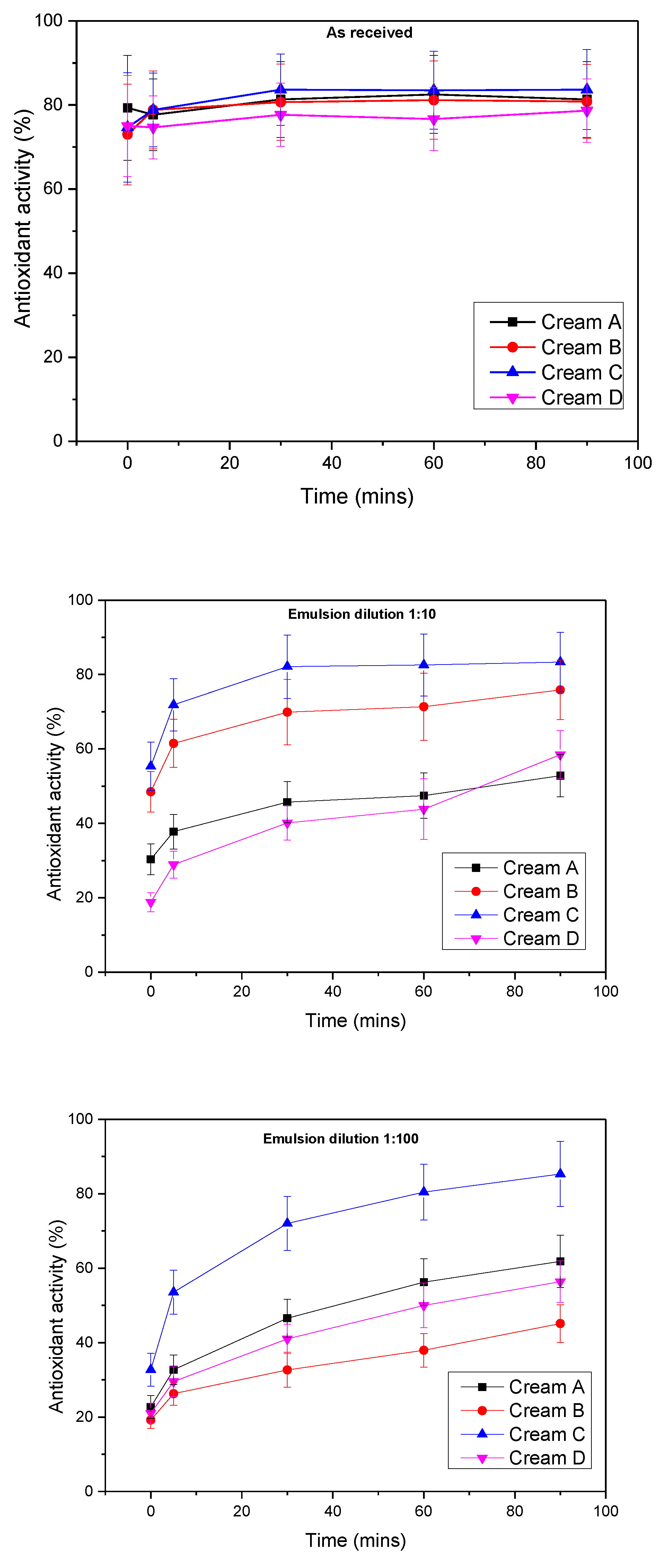
2.4.4. Antioxidant Study
2.5. Statistical Analysis
3. Results
3.1. Characterization of Extracts
3.2. Characterization of Emulsions
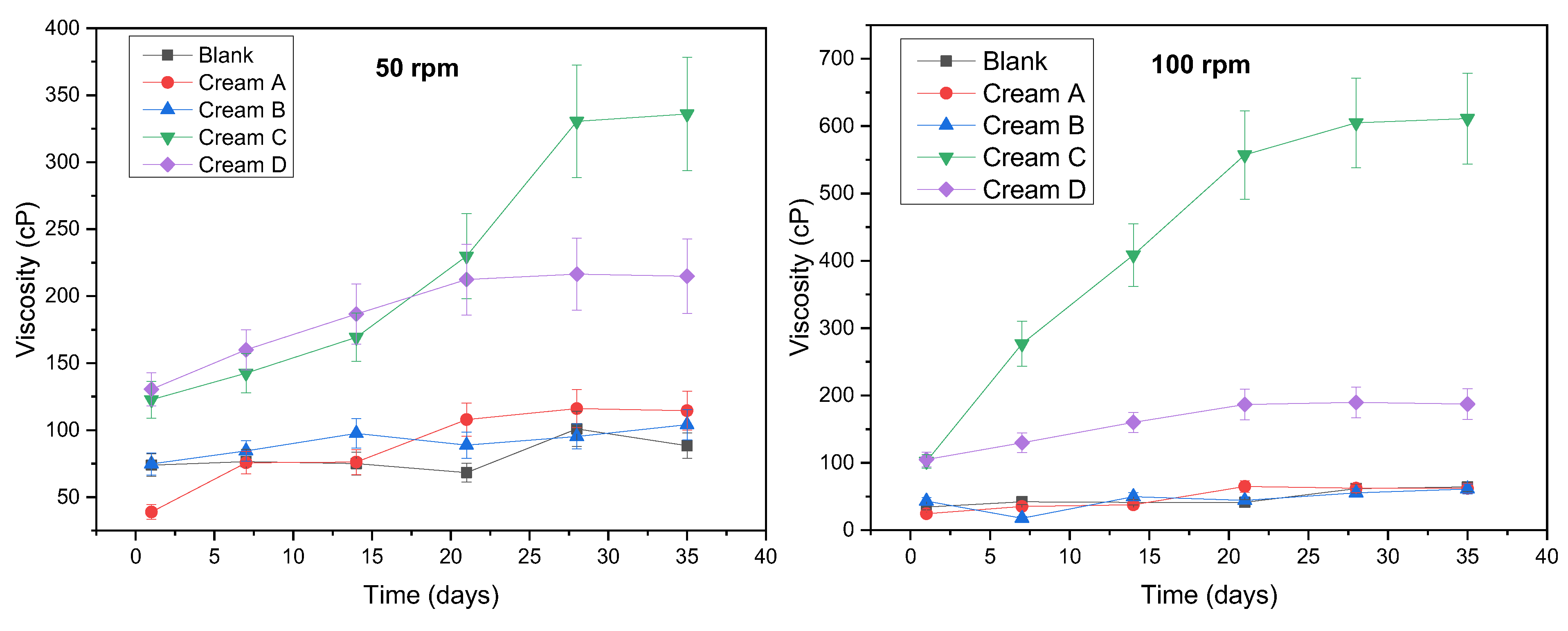
3.2.1. pH and Viscosity Stability
3.2.2. Determination of CIELAB Values
3.2.3. Sun Protection Factor (SPF) Measurements
3.2.4. Antioxidant Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| A. glutinosa | Alnus Glutinosa |
| DPPH | (2,2-diphenyl-1-picryl-hydrazyl-hydrate) |
| SPF | Sun Protection Factor |
| EtOH | Ethanol |
| BS | Blank sample |
References
- V. Goodarzi, H. Zamani, L. Bajuli, and A. Moradshahi, “Evaluation of antioxidant potential and reduction capacity of some plant extracts in silver nanoparticles’ synthesis,” Mol Biol Res Commun, vol. 3, no. 3, pp. 165–174, 2014, [Online]. Available: http://mbrc.shirazu.ac.ir.
- Irshad, R. Jawad, Q. Mushtaq, A. Spalletta, P. Martin, and U. Ishtiaq, “Determination of antibacterial and antioxidant potential of organic crude extracts from Malus domestica, Cinnamomum verum and Trachyspermum ammi,” Sci Rep, vol. 15, no. 1, Dec. 2025. [CrossRef]
- D. M. Kasote, S. S. Katyare, M. V. Hegde, and H. Bae, “Significance of antioxidant potential of plants and its relevance to therapeutic applications,” Jun. 11, 2015, Ivyspring International Publisher. [CrossRef]
- H. T. Hoang, J. Y. Moon, and Y. C. Lee, “Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives,” Dec. 01, 2021, MDPI. [CrossRef]
- Salmon, V. G., Breen, A. L., Kumar, J., Lara, M. J., Thornton, P. E., Wullschleger, S. D., & Iversen, C. M. (2019). Alder distribution and expansion across a tundra hillslope: implications for local N cycling. Frontiers in plant science, 10, 1099.
- L. Skrypnik, N. Grigorev, D. Michailov, M. Antipina, M. Danilova, and A. Pungin, “Comparative study on radical scavenging activity and phenolic compounds content in water bark extracts of alder (Alnus glutinosa (L.) Gaertn.), oak (Quercus robur L.) and pine (Pinus sylvestris L.),” European Journal of Wood and Wood Products, vol. 77, no. 5, pp. 879–890, Sep. 2019. [CrossRef]
- Ren, X., He, T., Chang, Y., Zhao, Y., Chen, X., Bai, S., Wang, L., Shen, M., & She, G. (2017). The genus Alnus, a comprehensive outline of its chemical constituents and biological activities. Molecules, 22(8), 1383.
- Dinić, J., Novaković, M., Podolski-Renić, A., Stojković, S., Mandić, B., Tešević, V., Vajs, V,. Isaković, A,. & Pešić, M. (2014). Antioxidative activity of diarylheptanoids from the bark of black alder (Alnus glutinosa) and their interaction with anticancer drugs. Planta medica, 80(13), 1088-1096.
- Sukhikh, S., Ivanova, S., Skrypnik, L., Bakhtiyarova, A., Larina, V., Krol, O., Prosekov, A., Frolov, A., Povydysh, M., & Babich, O. (2022). Study of the Antioxidant Properties of Filipendula ulmaria and Alnus glutinosa. Plants, 11(18), 2415.
- Ratz-Lyko, J. Arct, and K. Pytkowska, “Methods for evaluation of cosmetic antioxidant capacity,” Skin Research and Technology, vol. 18, no. 4, pp. 421–430, Nov. 2012. [CrossRef]
- T. Dinkova-Kostova, “Phytochemicals as protectors against ultraviolet radiation: Versatility of effects and mechanisms,” Oct. 2008. [CrossRef]
- K. B. Chakraborty and G. Scott, “MECHANISMS OF ANTIOXIDANT ACTION: SYNERGISM BETWEEN ANTIOXIDANTS AND ‘U.V. ABSORBERS,’” Pergamon Press, 1977.
- Schuster, N. Ortmayr, G. J. Oostingh, and B. Stelzhammer, “Compounds Extracted from Larch, Birch bark, Douglas Fir, and Alder Woods with Four Different Solvents: Effects on Five Skin-related Microbes.”.
- N. Tsouka, D. Lazari, N. Nikolaidis, K. Dimitriadis, E. Vouvoudi, and K. Theodoropoulos, “Dyeing of Cotton and Wool Fibers with the Aqueous Extract of Alnus glutinosa: Evaluation of Their Ultraviolet Protection Factor, Their Color fastness and the Antioxidant Activity of the Aqueous Extract,” Fibers and Polymers, vol. 25, no. 5, pp. 1825–1833, May 2024. [CrossRef]
- S. Dahija, S. Haverić, J. Čakar, and A. Parić, “Antimicrobial and cytotoxic activity of Alnus glutinosa (L.) Gaertn., A. incana (L.) Moench, and A. viridis (Chaix) DC. extracts,” Journal of Health Sciences, vol. 6, no. 2, pp. 100–104, Jul. 2016. [CrossRef]
- X. Pan, G. Niu, and H. Liu, “Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves.” [Online]. Available: www.elsevier.com/locate/cep.
- Schymanski, E. L., Jeon, J., Gulde, R., Fenner, K., Ruff, M., Singer, H. P., & Hollender, J. (2014). Identifying small molecules via high resolution mass spectrometry: communicating confidence.
- Bikiaris, N. D., Michailidou, G., Lazaridou, M., Christodoulou, E., Gounari, E., Ofrydopoulou, A., Lambropoulou, A., Vergkizi-Nikolakaki, S., Lykidou, S., & Nikolaidis, N. (2020). Innovative skin product emulsions with enhanced antioxidant, antimicrobial and UV protection properties containing nanoparticles of pure and modified chitosan with encapsulated fresh pomegranate juice. Polymers, 12(7), 1542.
- E. Dalla, I. Koumentakou, N. Bikiaris, E. Balla, S. Lykidou, and N. Nikolaidis, “Formulation, Characterization and Evaluation of Innovative O/W Emulsions Containing Curcumin Derivatives with Enhanced Antioxidant Properties,” Antioxidants, vol. 11, no. 11, Nov. 2022. [CrossRef]
- N. D. Bikiaris, I. Koumentakou, S. Lykidou, and N. Nikolaidis, “Innovative Skin Product O/W Emulsions Containing Lignin, Multiwall Carbon Nanotubes and Graphene Oxide Nanoadditives with Enhanced Sun Protection Factor and UV Stability Properties,” Applied Nano, vol. 3, no. 1, pp. 1–15, Jan. 2022. [CrossRef]
- Dutra, E. A., Oliveira, D. A. G. D. C., Kedor-Hackmann, E. R. M., & Santoro, M. I. R. M. (2004). Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Revista Brasileira de Ciências Farmacêuticas, 40, 381-385.
- R. M. Sayre, P. P. Agin, G. J. LeVee, and E. Marlowe, “A COMPARISON OF IN VIVO AND IN VITRO TESTING OF SUNSCREENING FORMULAS,” Photochem Photobiol, vol. 29, no. 3, pp. 559–566, 1979. [CrossRef]
- M. Šicklep and M. Čandek-Potokar, “Pork color measurement as affected by bloom time and measurement location,” Journal of Muscle Foods, vol. 18, no. 1, pp. 78–87, Jan. 2007. [CrossRef]
- H. Nawaz, M. A. Shad, N. Rehman, H. Andaleeb, and N. Ullah, “Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds,” Brazilian Journal of Pharmaceutical Sciences, vol. 56, 2020. [CrossRef]
- Ghasemzadeh, H. Z. E. Jaafar, and A. Rahmat, “Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (Zingiber officinale Roscoe) extracts,” Journal of Medicinal Plants Research, vol. 5, no. 7, pp. 1147–1154, 2011, [Online]. Available: http://www.academicjournals.org/JMPR.
- N. Ghaffar and A. Perveen, “Solvent polarity effects on extraction yield, phenolic content, and antioxidant properties of Malvaceae family seeds: a comparative study,” N Z J Bot, 2024. [CrossRef]
- E. H. Anouar, J. Gierschner, J. L. Duroux, and P. Trouillas, “UV/Visible spectra of natural polyphenols: A time-dependent density functional theory study,” Food Chem, vol. 131, no. 1, pp. 79–89, Mar. 2012. [CrossRef]
- Felföldi-Gáva, S. Szarka, B. Simándi, B. Blazics, B. Simon, and Á. Kéry, “Supercritical fluid extraction of Alnus glutinosa (L.) Gaertn.,” Journal of Supercritical Fluids, vol. 61, pp. 55–61, Jan. 2012. [CrossRef]
- Daneshfar, H. S. Ghaziaskar, and N. Homayoun, “Solubility of gallic acid in methanol, ethanol, water, and ethyl acetate,” J Chem Eng Data, vol. 53, no. 3, pp. 776–778, Mar. 2008. [CrossRef]
- M. Fedor and M. J. Toda, “Investigating Hydrogen Bonding in Phenol Using Infrared Spectroscopy and Computational Chemistry,” J Chem Educ, vol. 91, no. 12, pp. 2191–2194, Dec. 2014. [CrossRef]
- Kledecka, A., Siejak, P., Pratap-Singh, A., Kowalczewski, P. Ł., Fathordoobady, F., Jarzębski, M., & Smułek, W. (2022). Extracts from Frangula alnus Mill. and Their Effects on Environmental and Probiotic Bacteria. Plants, 11(20), 2719.
- D. Sethi, N. Jada, A. Tiwari, S. Ramasamy, T. Dash, and S. Pandey, “Photocatalytic destruction of Escherichia coli in water by V2O5/TiO2,” J Photochem Photobiol B, vol. 144, pp. 68–74, 2015. [CrossRef]
- G. Telysheva, T. Dizhbite, O. Bikovens, J. Ponomarenko, S. Janceva, and J. Krasilnikova, “Structure and antioxidant activity of diarylheptanoids extracted from bark of grey alder (Alnus incana) and potential of biorefinery-based bark processing of European trees,” in Holzforschung, Jun. 2011, pp. 623–629. [CrossRef]
- F. Rippke, E. Berardesca, and T. M. Weber, “PH and Microbial Infections,” Current Problems in Dermatology (Switzerland), vol. 54, pp. 87–94, 2018. [CrossRef]
- M. Uyama, K. Ikuta, T. Teshigawara, K. Watanabe, and R. Miyahara, “The viscosity stability of O/W emulsion containing α-gel through an ionic-complex system,” 2013. [Online]. Available: http://www.jstage.jst.go.jp/browse/jos/http://mc.manusriptcentral.com/jjocs.
- Ali, N. Akhtar, and H. M. S. Khan, “Assessment of physical stability and antioxidant activity of polysiloxane polyalkyl polyether copolymer-based creams,” J Chem, 2013. [CrossRef]
- V. M. Di Mambro and M. J. V. Fonseca, “Assays of physical stability and antioxidant activity of a topical formulation added with different plant extracts,” J Pharm Biomed Anal, vol. 37, no. 2, pp. 287–295, Feb. 2005. [CrossRef]
- N. Wathoni, A. Haerani, N. Yuniarsih, and R. Haryanti, “A review on herbal cosmetics in Indonesia,” 2018, Innovare Academics Sciences Pvt. Ltd. [CrossRef]
- J. D’Orazio, S. Jarrett, A. Amaro-Ortiz, and T. Scott, “UV radiation and the skin,” 2013, MDPI AG. [CrossRef]
- L. Li, L. Chong, T. Huang, Y. Ma, Y. Li, and H. Ding, “Natural products and extracts from plants as natural UV filters for sunscreens: A review,” Jun. 01, 2023, John Wiley and Sons Inc. [CrossRef]
- E. M. Hussen and S. A. Endalew, “In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina,” BMC Complement Med Ther, vol. 23, no. 1, Dec. 2023. [CrossRef]
- Rosado, C., Tokunaga, V. K., Sauce, R., De Oliveira, C. A., Sarruf, F. D., Parise-Filho, R., Elisabete, M., De Almeida, T.S., Robles Velasco, M, V,. & Baby, A. R. (2019). Another reason for using caffeine in dermocosmetics: Sunscreen adjuvant. Frontiers in physiology, 10, 519.
- S. Bhattacharya and A. P. Sherje, “Development of resveratrol and green tea sunscreen formulation for combined photoprotective and antioxidant properties,” J Drug Deliv Sci Technol, vol. 60, Dec. 2020. [CrossRef]
- M. Y. Park, H. J. Kwon, and M. K. Sung, “Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model,” Life Sci, vol. 88, no. 11–12, pp. 486–492, Mar. 2011. [CrossRef]
- Ayaz, M., Junaid, M., Ahmed, J., Ullah, F., Sadiq, A., Ahmad, S., & Imran, M. (2014). Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BMC complementary and alternative medicine, 14(1), 145.
- Baral, M., Biswas, S., Chakraborty, S., Ghosh, A. K., da Silva, J. A. T., Panda, S., & Chakraborty, P. (2010). In vitro antioxidant activity of the whole plant of Amaranthus spinosus Linn. International Journal of Biomedical and Pharmaceutical Sciences, 5(1), 75-78.
- Imamović, I. Ivazović, A. Alispahić, E. Bečić, M. Dedić, and A. Dacić, “Assessment of the suitability of methods for testing the antioxidant activity of anti-aging creams,” Applied Sciences (Switzerland), vol. 11, no. 4, pp. 1–14, Feb. 2021. [CrossRef]
- Ali, N. Akhtar, M. S. Khan, M. T. Khan, A. Ullah, and M. I. Shah, “Effect of Moringa oleifera on undesireble skin sebum secretions of sebaceous glands observed during winter season in humans,” 2013.
- Smeriglio, A., D’Angelo, V., Cacciola, A., Ingegneri, M., Raimondo, F. M., Trombetta, D., & Germanò, M. P. (2022). New insights on phytochemical features and biological properties of Alnus glutinosa stem bark. Plants, 11(19), 2499.
- L. Angeli, S. Imperiale, Y. Ding, M. Scampicchio, and K. Morozova, “A novel stoichio-kinetic model for the dpph• assay: The importance of the side reaction and application to complex mixtures,” Antioxidants, vol. 10, no. 7, Jul. 2021. [CrossRef]
- K. Mishra, H. Ojha, and N. K. Chaudhury, “Estimation of antiradical properties of antioxidants using DPPH- assay: A critical review and results,” Food Chem, vol. 130, no. 4, pp. 1036–1043, Feb. 2012. [CrossRef]
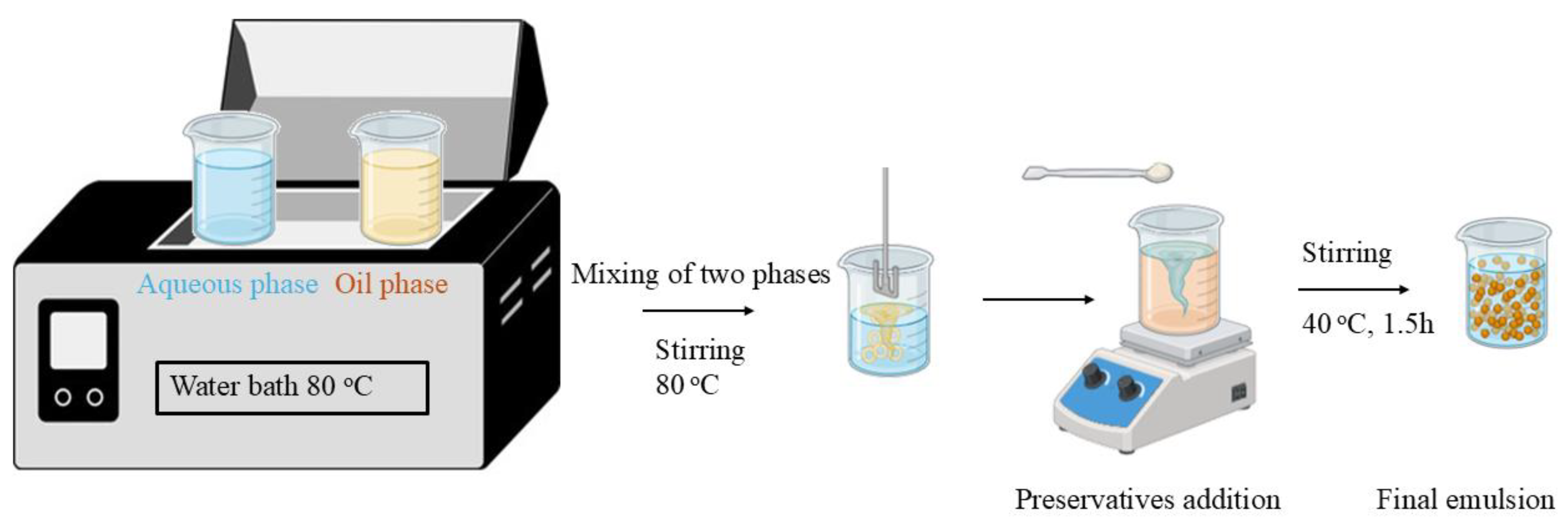
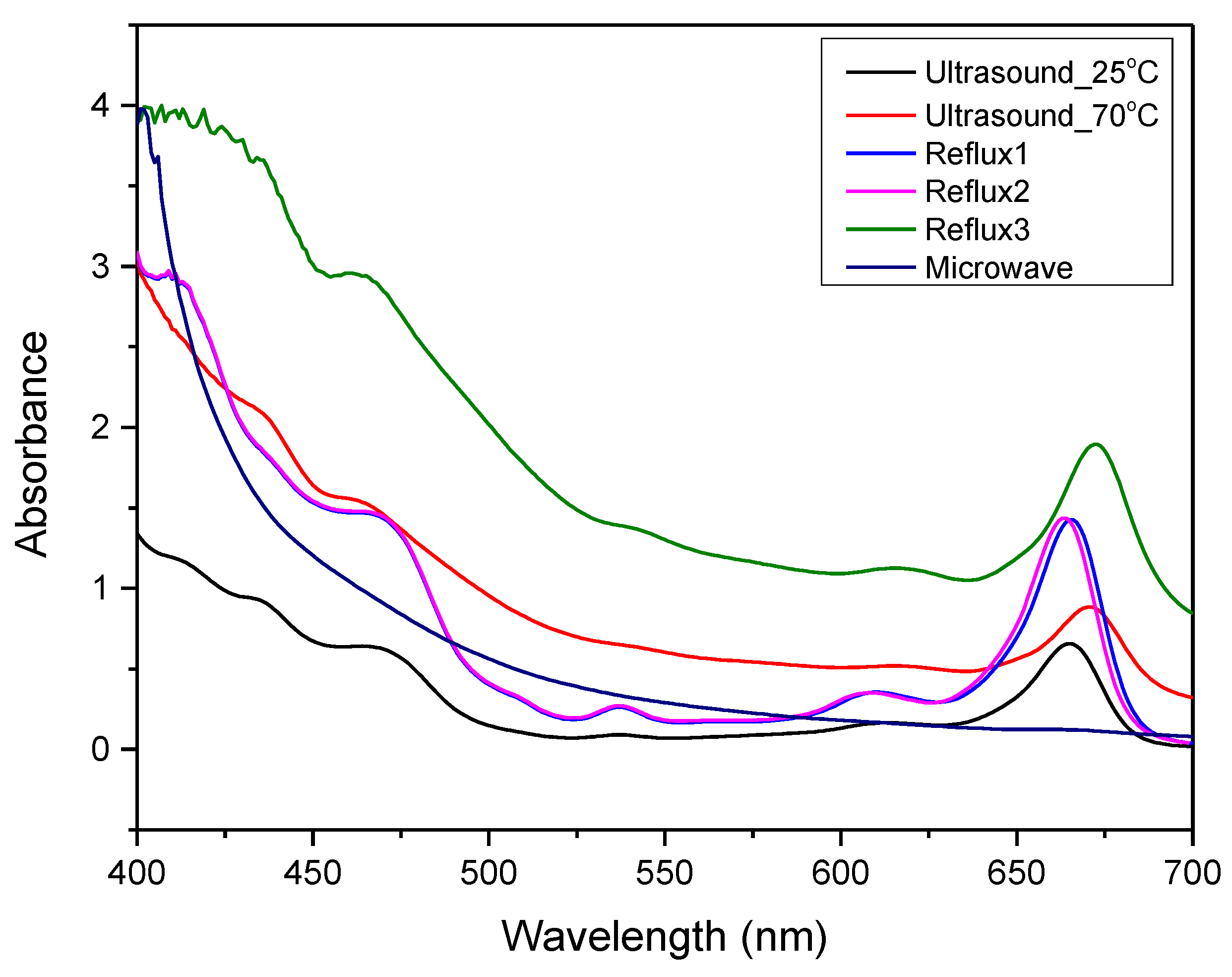
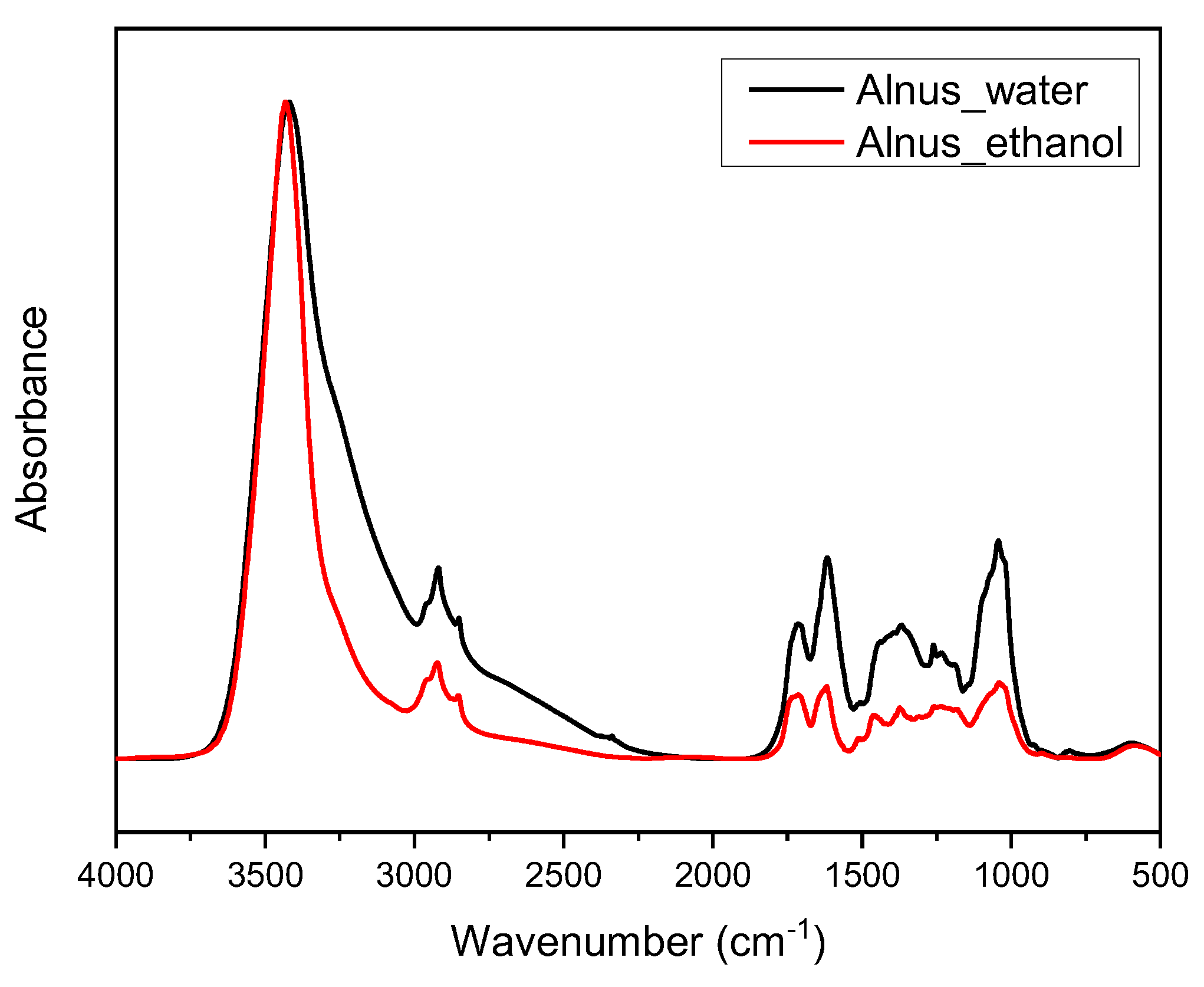
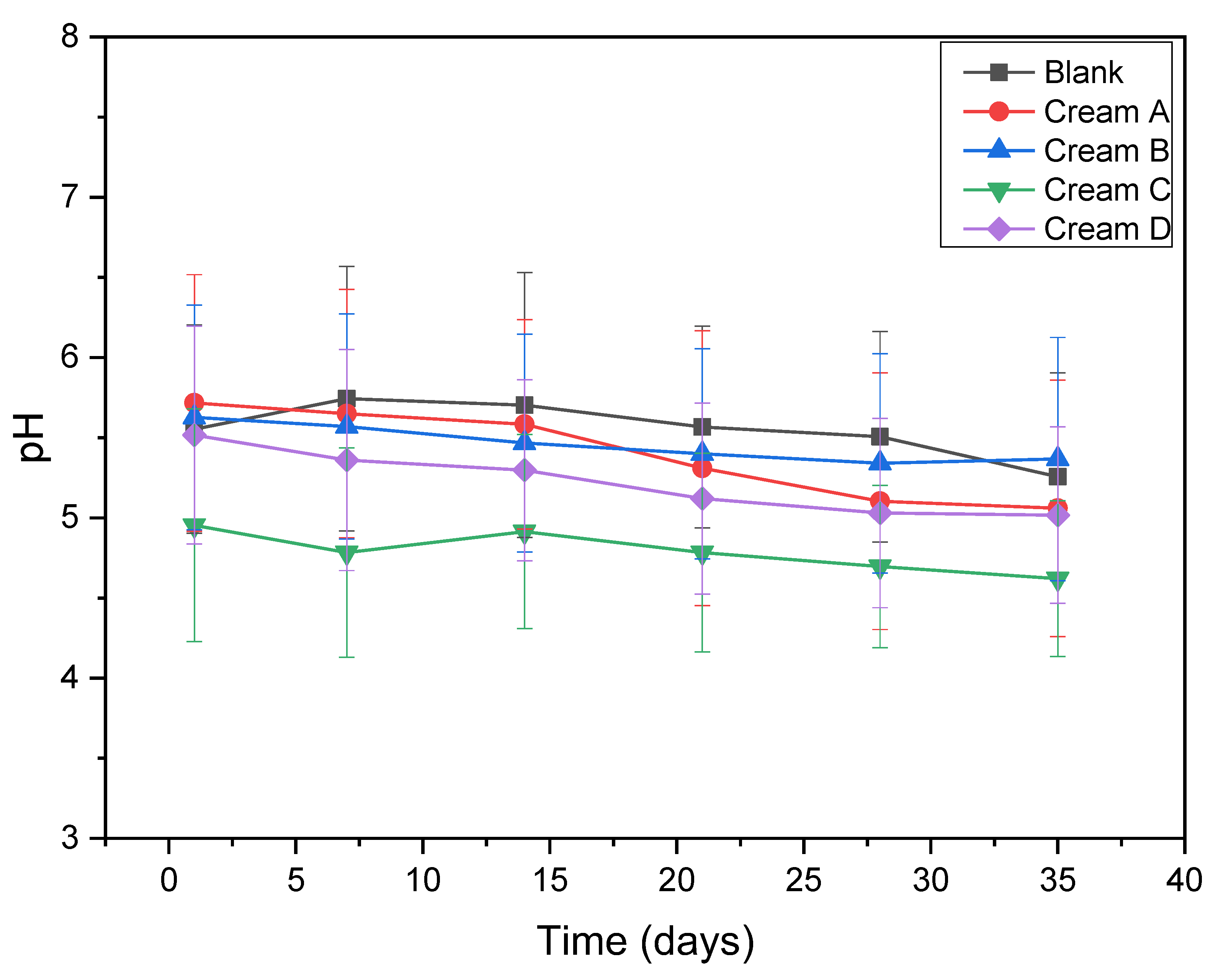
| Process | Temperature (oC) | Time (hours) | Solvent |
| Soxhlet 1 | 70 | 3 | Water |
| Soxhlet 2 | 90 | 3 | Water |
| Soxhlet 3 | 70 | 1 | Ethanol |
| Extract | Extractive yield (%) |
| Ultrasound_25oC | 8,20 ± 0,35 |
| Ultrasound_70oC | 14,60 ± 0,50 |
| Soxhlet 1 | 18,40 ± 0,70 |
| Soxhlet 2 | 24,90 ± 0,60 |
| Soxhlet 3 | 42,50 ± 1,10 |
| Microwave | 4,80 ± 0,20 |
| Compound | Formula | Calc. MW | m/z | RT [min] |
| (1E)-1,7-bis(4-hydroxyphenyl)hept-1-en-3-one | C19H20O3 | 296,14198 | 295,13471 | 7,996 |
| 12-oxo Phytodienoic Acid | C18H28O3 | 292,20499 | 291,19752 | 11,331 |
| 15-Deoxy-Δ12,14-prostaglandin J2-2-glycerol ester | C23H34O5 | 390,23984 | 391,24712 | 10,836 |
| 3-(4-{[1,3-Dihydroxy-1-(4-hydroxy-3-methoxyphenyl)-2-propanyl]oxy}-3-methoxyphenyl)propyl 6-deoxy-alpha-L-mannopyranoside | C26H36O11 | 524,22731 | 523,22004 | 5,701 |
| 3,4,5-trihydroxycyclohex-1-ene-1-carboxylic acid | C7H10O5 | 174,0525 | 173,04517 | 0,843 |
| 3-Methoxy-5,7,3’,4’-tetrahydroxy-flavone | C16H12O7 | 316,0593 | 317,06685 | 7,304 |
| Adenosine | C10H13N5O4 | 267,0977 | 268,10498 | 0,814 |
| Asiatic acid | C30H48O5 | 488,35155 | 487,34427 | 12,551 |
| Azelaic acid | C9H16O4 | 188,10472 | 187,09744 | 6,111 |
| Cafestol | C20H28O3 | 316,20256 | 317,20984 | 11,684 |
| Caffeic acid | C9H8O4 | 180,04196 | 179,03468 | 4,541 |
| Citric acid | C6H8O7 | 192,02685 | 191,01957 | 0,825 |
| Corchorifatty acid F | C18H32O5 | 328,22593 | 327,21865 | 7,641 |
| D-(-)-Fructose | C6H12O6 | 180,0631 | 179,05575 | 0,769 |
| D-(-)-Quinic acid | C7H12O6 | 192,06315 | 191,05587 | 0,819 |
| D(+)-Phenyllactic acid | C9H10O3 | 166,0626 | 165,05533 | 4,999 |
| Docosahexaenoic acid ethyl ester | C24H36O2 | 356,27268 | 357,27996 | 12,17 |
| Gallic acid | C7H6O5 | 170,02114 | 169,01386 | 0,816 |
| Genistein | C15H10O5 | 270,05373 | 269,04629 | 7,428 |
| Gentisic acid | C7H6O4 | 154,02613 | 153,01878 | 0,858 |
| L-Phenylalanine | C9H11NO2 | 165,07974 | 166,08702 | 0,857 |
| L-Tyrosine | C9H11NO3 | 181,07474 | 182,08203 | 0,862 |
| Luteolin | C15H10O6 | 286,0486 | 285,04132 | 7,271 |
| Miquelianin | C21H18O13 | 478,07619 | 477,06891 | 5,675 |
| N-[4-cyano-1-(4-fluorophenyl)-1H-pyrazol-5-yl]cyclohexanecarboxamide | C17H17FN4 O | 312,13715 | 311,12988 | 6,301 |
| Naringenin | C15H12O5 | 272,06939 | 271,0621 | 6,599 |
| Neochlorogenic acid | C16H18O9 | 354,09627 | 353,08899 | 1,889 |
| Oleanolic acid | C30H48O3 | 456,36174 | 455,35446 | 13,408 |
| Pinolenic acid | C18 H30O2 | 278,22554 | 301,21483 | 12,729 |
| Quercetin-3β-D-glucoside | C21H20O12 | 464,09733 | 463,08966 | 5,794 |
| α-Linolenic acid | C18H30 O2 | 278,22546 | 279,23273 | 11,736 |
| (1E)-1,7-bis(4-hydroxyphenyl)hept-1-en-3-one | C19H20O3 | 296,14198 | 295,13471 | 7,996 |
| 12-oxo Phytodienoic Acid | C18H28O3 | 292,20499 | 291,19752 | 11,331 |
| 15-Deoxy-Δ12,14-prostaglandin J2-2-glycerol ester | C23H34O5 | 390,23984 | 391,24712 | 10,836 |
| 3-(4-{[1,3-Dihydroxy-1-(4-hydroxy-3-methoxyphenyl)-2-propanyl]oxy}-3-methoxyphenyl)propyl 6-deoxy-alpha-L-mannopyranoside | C26H36O11 | 524,22731 | 523,22004 | 5,701 |
| Sample | L* (Lightness) | a* (Red-Green) | b* (Yellow-Blue) | C (Chroma) | h° (Hue Angle) | R% (Reflectance) | K/S (Color Strength) |
| Cream A | 56,04 ± 0,23 | -4,14 ± 0,44 | 14,40 ± 0,30 | 14,88 ± 0,22 | 108,12° | 7,44 (400 nm) | 5,76 |
| Cream B | 48,03 ± 1,46 | -1,46 ± 1,68 | 11,91 ± 0,33 | 11,98 ± 0,32 | 96,13° | 10,84 (482 nm) | 2,05 |
| Cream C | 48,64 ± 1,28 | -0,28 ± 0,99 | 9,68 ± 1,11 | 11,72 ± 0,58 | 93,45° | 5,73 (409 nm) | 1,29 |
| Cream D | 48,63 ± 0,56 | 6,08 ± 0,17 | 17,67 ± 0,83 | 18,80 ± 0,74 | 71,46° | 4,27 (410 nm) | 10,73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





