Submitted:
30 September 2025
Posted:
01 October 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Global Filarial Endemicity
Filariasis: Causative Organisms
Pathophysiology
Clinical Stages
Immunomodulation via Parasite-Derived Molecules
- 1.
- Downregulating the proliferation of CD4+ T cells and conventional B cells, and
- 2.
- Downregulating the production of IL-4 and IFN-γ.
- 3.
- Increasing the production of IL-10 by B1B cells, leading to their enhanced proliferation.
- 4.
- Activating antigen-presenting cells to promote Th2 development and suppress Th1 reactions [64].
Molecular Mimicry
Modulation of TLR and NF-κB Signalling Cascades Induced by Filarial Parasites
Effector T Cell Modulation
Role of Regulatory T Cells in Filarial Evasion and Pathogenesis
Apoptosis Induction in Host Immune Cells
Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of interest
Abbreviations
| APCs | Antigen Presenting Cells |
| TGF-β | Transforming Growth Factor-beta |
| MIF | Macrophage Migration Inhibitory Factor |
| SOCS | Suppressor of Cytokine Signaling |
| AAMs | Alternatively Activated Macrophages |
| CTLA-4 | Cytotoxic T- T-lymphocyte-associated protein 4 |
| PD-1 | Programmed cell death protein 1 |
| ICOS | Inducible Co-stimulator |
| IDO | Indoleamine 2,3-dioxygenase |
| NEDD4 | Neural precursor cell-expressed developmentally downregulated protein 4 |
| Cbl-b | Casitas B-lineage lymphoma proto-oncogene-b |
| c-Cbl | Casitas B-lineage lymphoma |
| TLR | Toll-like receptor |
| NLR | Nod-like receptor |
| RLR | RIG-I-like receptor |
| CLR | C-type lectin receptor |
| ALR | Absent in Melanoma 2-like receptor |
| GATA-3 | binding protein 3 |
| PC | Phosphorylcholine |
| IFN-γ | Interferon-gamma |
| MHC | Major histocompatibility complex |
| RELMα | Resistin-like molecule-α |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| TSLP | Thymic stromal lymphopoietin |
| ILCs | Innate lymphoid cells |
| PKC-γ | Protein kinase C gamma |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PGE2 | Prostaglandin E2 |
| ADCC | Antibody-dependent cell-mediated toxicity |
| NFκB | Nuclear factor kappa B. |
References
- Taylor, M.J., A. Hoerauf, and M. Bockarie, Lymphatic filariasis and onchocerciasis. The Lancet, 2010. 376(9747): p. 1175-1185.
- Rajamanickam, A. and S. Babu, Unraveling the dynamics of human filarial infections: immunological responses, host manifestations, and pathogen biology. Pathogens, 2025. 14(3): p. 223.
- Babu, S. and T.B. Nutman, Immunology of lymphatic filariasis. Parasite immunology, 2014. 36(8): p. 338-346.
- Simonsen, P., et al., Manson's Tropical Diseases, 23rd Edition: The Filariases. 2014. p. 737-765.
- Liatl, L.B., Brugian f~ filariasis, transmitted by.
- Ehrens, A., A. Hoerauf, and M.P. Hübner, Eosinophils in filarial infections: Inducers of protection or pathology? Frontiers in immunology, 2022. 13: p. 983812.
- Bhuvaneswari, A., et al., Mosquitoes, lymphatic filariasis, and public health: A systematic review of Anopheles and Aedes surveillance strategies. Pathogens, 2023. 12(12): p. 1406.
- Nanduri, J. and J.W. Kazura, Clinical and laboratory aspects of filariasis. Clinical microbiology reviews, 1989. 2(1): p. 39-50.
- Murdoch, M., Mapping the burden of onchocercal skin disease. British Journal of Dermatology, 2021. 184(2): p. 199-207.
- Gyasi, M.E., O.N. Okonkwo, and K. Tripathy, Onchocerciasis, in StatPearls [Internet]. 2023, StatPearls Publishing.
- Elouardi, C., et al., Imported loiasis: Diagnostic and therapeutic challenges. Infectious Diseases Now, 2025: p. 105053.
- Downes, B. and K. Jacobsen, A systematic review of the epidemiology of mansonelliasis. African Journal of Infectious Diseases, 2010. 4(1).
- Simonetti, O., et al., Dracunculiasis over the centuries: the history of a parasite unfamiliar to the West. Le Infezioni in Medicina, 2023. 31(2): p. 257.
- Risch, F., et al., Human filariasis—contributions of the Litomosoides sigmodontis and Acanthocheilonema viteae animal model. Parasitology research, 2021. 120(12): p. 4125-4143.
- Bakowski, M.A. and C.W. McNamara, Advances in antiwolbachial drug discovery for treatment of parasitic filarial worm infections. Tropical Medicine and Infectious Disease, 2019. 4(3): p. 108.
- Sangshetti, J.N., et al., Two decades of antifilarial drug discovery: A review. RSC advances, 2017. 7(33): p. 20628-20666.
- Nutman, T.B., Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphatic research and biology, 2013. 11(3): p. 144-148.
- Chulanetra, M. and W. Chaicumpa, Revisiting the mechanisms of immune evasion employed by human parasites. Frontiers in cellular and infection microbiology, 2021. 11: p. 702125.
- Chakraborty, S., et al., Lymphatic filariasis: perspectives on lymphatic remodeling and contractile dysfunction in filarial disease pathogenesis. Microcirculation, 2013. 20(5): p. 349-364.
- Medeiros, Z.M., et al., Lymphatic filariasis: a systematic review on morbidity and its repercussions in countries in the Americas. International journal of environmental research and public health, 2021. 19(1): p. 316.
- Azhar, S., et al., Basic insights into lymphatic filariasis. Zoonosis, Unique Scientific Publishers, Faisalabad, Pakistan, 2023. 2: p. 73-88.
- Jungmann, P., J. Figueredo-Silva, and G. Dreyer, Bancroftian lymphadenopathy: a histopathologic study of fifty-eight cases from northeastern Brazil. The American journal of tropical medicine and hygiene, 1991. 45(3): p. 325-331.
- Kumaraswami, V., The clinical manifestations of lymphatic filariasis. 2000.
- Bennuru, S. and T.B. Nutman, Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLoS pathogens, 2009. 5(12): p. e1000688.
- Babu, S. and T.B. Nutman. Immunopathogenesis of lymphatic filarial disease. in Seminars in immunopathology. 2012. Springer.
- DeVries, C.R., Basic science of lymphatic filariasis. Indian Journal of Urology, 2005. 21(1): p. 5-8.
- Mues, K.E., et al., Changes in antibody levels during and following an episode of acute adenolymphangitis (ADL) among lymphedema patients in Léogâne, Haiti. PLoS One, 2015. 10(10): p. e0141047.
- Babu, S. and T.B. Nutman, Vascular Responses in Human Lymphatic Filariasis, in Vascular Responses to Pathogens. 2016, Elsevier. p. 209-220.
- Dierks, C., et al., Plasma Proteomics Reveals Distinct Signatures in Occult and Microfilaremic Loa loa Infections. The Journal of Infectious Diseases, 2025: p. jiaf344.
- Maizels, R.M., et al., Immune evasion genes from filarial nematodes. International journal for parasitology, 2001. 31(9): p. 889-898.
- Allen, J.E. and P.N. Loke, Divergent roles for macrophages in lymphatic filariasis. Parasite Immunology, 2001. 23(7): p. 345-352.
- Moreno, Y. and T.G. Geary, Stage-and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS neglected tropical diseases, 2008. 2(10): p. e326.
- Allen, J.E. and R.M. Maizels, Diversity and dialogue in immunity to helminths. Nature Reviews Immunology, 2011. 11(6): p. 375-388.
- Jafari, N. and S. Abediankenari, Role of microRNAs in immunoregulatory functions of epithelial cells. BMC immunology, 2024. 25(1): p. 84.
- Miller, J.F., The function of the thymus and its impact on modern medicine. Science, 2020. 369(6503): p. eaba2429.
- Maizels, R.M. and H.J. McSorley, Regulation of the host immune system by helminth parasites. Journal of Allergy and Clinical Immunology, 2016. 138(3): p. 666-675.
- Ajendra, J. and J.E. Allen, Neutrophils: Friend or foe in Filariasis? Parasite Immunology, 2022. 44(6): p. e12918.
- Joardar, N. and S.P.S. Babu, A review on the druggability of a thiol-based enzymatic antioxidant thioredoxin reductase for treating filariasis and other parasitic infections. International journal of biological macromolecules, 2020. 142: p. 125-141.
- Kwarteng, A., et al., Highlighting the relevance of CD8+ T cells in filarial infections. Frontiers in Immunology, 2021. 12: p. 714052.
- Lie Kian Joe, L.K.J., Occult filariasis: its relationship with tropical pulmonary eosinophilia. 1962.
- Beaver, P.C., Filariasis without microfilaremia. Am J Trop Med Hyg, 1970. 19(2): p. 181-9.
- UDWADIA, F.E., Tropical eosinophilia—a correlation of clinical, histopathologic and lung function studies. Diseases of the Chest, 1967. 52(4): p. 531-538.
- Ismail, M. and N. Nagaratnam, Arthritis, possibly due to filariasis. 1973.
- Chaturvedi, P., A. Gawdi, and S. Dey, Occult filarial infections. Natl Med J India, 1990. 3: p. 7-9.
- Chandrasoma, P. and K.N. Mendis, Filarial infection of the breast. The American journal of tropical medicine and hygiene, 1978. 27(4): p. 770-773.
- Maizels, R.M. and M. Yazdanbakhsh, Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature Reviews Immunology, 2003. 3(9): p. 733-744.
- Hewitson, J.P., J.R. Grainger, and R.M. Maizels, Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Molecular and biochemical parasitology, 2009. 167(1): p. 1-11.
- Ghosh, I., et al., Thioredoxin peroxidases from Brugia malayi. Molecular and Biochemical Parasitology, 1998. 91(2): p. 207-220.
- Gnanasekar, M., et al., Molecular characterization of a calcium binding translationally controlled tumor protein homologue from the filarial parasites Brugia malayi and Wuchereria bancrofti. Molecular and Biochemical Parasitology, 2002. 121(1): p. 107-118.
- Yadav, S., et al., In silico and in vitro studies on the protein-protein interactions between Brugia malayi immunomodulatory protein calreticulin and human C1q. PloS one, 2014. 9(9): p. e106413.
- Joardar, N., et al., Filarial thioredoxin reductase exerts anti-inflammatory effects upon lipopolysaccharide induced inflammation in macrophages. International Journal of Biological Macromolecules, 2021. 193: p. 1379-1390.
- Desjardins, C.A., et al., Genomics of Loa loa, a Wolbachia-free filarial parasite of humans. Nature genetics, 2013. 45(5): p. 495-500.
- Gomez-Escobar, N., et al., Heterologous expression of the filarial nematode alt gene products reveals their potential to inhibit immune function. BMC biology, 2005. 3(1): p. 8.
- Chhabra, S., et al., Kv1. 3 channel-blocking immunomodulatory peptides from parasitic worms: implications for autoimmune diseases. The FASEB Journal, 2014. 28(9): p. 3952.
- Harnett, W. and M. Harnett, Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. Journal of immunology (Baltimore, Md.: 1950), 1993. 151(9): p. 4829-4837.
- Hewitson, J.P., et al., The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory–secretory products. Molecular and biochemical parasitology, 2008. 160(1): p. 8-21.
- Adjobimey, T. and A. Hoerauf, Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Annals of Tropical Medicine & Parasitology, 2010. 104(6): p. 455-464.
- Khatri, V., N. Chauhan, and R. Kalyanasundaram, Parasite cystatin: immunomodulatory molecule with therapeutic activity against immune mediated disorders. Pathogens, 2020. 9(6): p. 431.
- Manoury, B., et al., Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Current Biology, 2001. 11(6): p. 447-451.
- Mucida, D., et al., Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science, 2007. 317(5835): p. 256-260.
- Bunte, M.J., et al., Helminth glycans at the host-parasite interface and their potential for developing novel therapeutics. Frontiers in Molecular Biosciences, 2022. 8: p. 1358.
- Melendez, A.J., et al., Inhibition of FcεRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nature Medicine, 2007. 13(11): p. 1375-1381.
- Harnett, W., I.B. McInnes, and M.M. Harnett, ES-62, a filarial nematode-derived immunomodulator with anti-inflammatory potential. Immunology letters, 2004. 94(1-2): p. 27-33.
- Harnett, W. and M.M. Harnett, Helminth-derived immunomodulators: can understanding the worm produce the pill? Nature Reviews Immunology, 2010. 10(4): p. 278-284.
- Eason, R.J., et al., The helminth product, ES-62 modulates dendritic cell responses by inducing the selective autophagolysosomal degradation of TLR-transducers, as exemplified by PKCδ. Scientific Reports, 2016. 6(1): p. 37276.
- Semnani, R.T., et al., Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood, The Journal of the American Society of Hematology, 2008. 112(4): p. 1290-1298.
- Herbert, D.B.R., B. Douglas, and K. Zullo, Group 2 innate lymphoid cells (ILC2): type 2 immunity and helminth immunity. International journal of molecular sciences, 2019. 20(9): p. 2276.
- Cotton, R., et al., Brugia malayi infective larvae fail to activate Langerhans cells and dermal dendritic cells in human skin. Parasite immunology, 2015. 37(2): p. 79-91.
- Li, D. and M. Wu, Pattern recognition receptors in health and diseases. Signal transduction and targeted therapy, 2021. 6(1): p. 291.
- Tawill, S., et al., Both free-living and parasitic nematodes induce a characteristic Th2 response that is dependent on the presence of intact glycans. Infection and immunity, 2004. 72(1): p. 398-407.
- Van Die, I. and R.D. Cummings, Glycan gimmickry by parasitic helminths: a strategy for modulating the host immune response? Glycobiology, 2010. 20(1): p. 2-12.
- Ludin, P., D. Nilsson, and P. Mäser, Genome-wide identification of molecular mimicry candidates in parasites. PLoS One, 2011. 6(3): p. e17546.
- Gomez-Escobar, N., W.F. Gregory, and R.M. Maizels, Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor β, expressed in microfilarial and adult stages of Brugia malayi. Infection and immunity, 2000. 68(11): p. 6402-6410.
- Maizels, R.M., H.H. Smits, and H.J. McSorley, Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity, 2018. 49(5): p. 801-818.
- Shiny, C., et al., Recombinant Wolbachia heat shock protein 60 (HSP60) mediated immune responses in patients with lymphatic filariasis. Microbes and infection, 2011. 13(14-15): p. 1221-1231.
- Dakshinamoorthy, G., et al., Biochemical characterization and evaluation of a Brugia malayi small heat shock protein as a vaccine against lymphatic filariasis. PloS one, 2012. 7(4): p. e34077.
- Liu, L.X. and P.F. Weller, Intravascular filarial parasites inhibit platelet aggregation. Role of parasite-derived prostanoids. The Journal of clinical investigation, 1992. 89(4): p. 1113-1120.
- Kalinski, P., Regulation of immune responses by prostaglandin E2. The Journal of Immunology, 2012. 188(1): p. 21-28.
- Oyesola, O.O. and E.D. Tait Wojno, Prostaglandin regulation of type 2 inflammation: From basic biology to therapeutic interventions. European journal of immunology, 2021. 51(10): p. 2399-2416.
- Medzhitov, R. and C. Janeway Jr, The Toll receptor family and microbial recognition. Trends in microbiology, 2000. 8(10): p. 452-456.
- Medzhitov, R., Toll-like receptors and innate immunity. Nature Reviews Immunology, 2001. 1(2): p. 135-145.
- Lien, E. and R.R. Ingalls, Toll-like receptors. Critical care medicine, 2002. 30(1): p. S1-S11.
- Medzhitov, R., P. Preston-Hurlburt, and C.A. Janeway Jr, A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature, 1997. 388(6640): p. 394-397.
- Takeda, K. and S. Akira, Toll-like receptors. Current protocols in immunology, 2015. 109(1): p. 14.12. 1-14.12. 10.
- Duan, T., et al., Toll-like receptor signaling and its role in cell-mediated immunity. Frontiers in immunology, 2022. 13: p. 812774.
- Akira, S., Mammalian Toll-like receptors. Current opinion in immunology, 2003. 15(1): p. 5-11.
- Kawai, T. and S. Akira, Pathogen recognition with Toll-like receptors. Current opinion in immunology, 2005. 17(4): p. 338-344.
- Akira, S. and K. Takeda, Toll-like receptor signalling. Nature reviews immunology, 2004. 4(7): p. 499-511.
- Riva, F. and M. Muzio, Updates on Toll-Like Receptor 10 Research. European Journal of Immunology, 2025. 55(5): p. e202551840.
- Barrat, F.J. and R.L. Coffman, Development of TLR inhibitors for the treatment of autoimmune diseases. Immunological reviews, 2008. 223(1): p. 271-283.
- Franco, L.H., et al., Autophagy downstream of endosomal Toll-like receptor signaling in macrophages is a key mechanism for resistance to Leishmania major infection. Journal of Biological Chemistry, 2017. 292(32): p. 13087-13096.
- Zhou, J., et al., C7ORF41 regulates inflammation by inhibiting NF-κB signaling pathway. BioMed Research International, 2021. 2021(1): p. 7413605.
- Su, C.-M., L. Wang, and D. Yoo, Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Scientific reports, 2021. 11(1): p. 13464.
- Barnabei, L., et al., NF-κB: at the borders of autoimmunity and inflammation. Frontiers in immunology, 2021. 12: p. 716469.
- Inoue, J.-i., et al., IκBγ, a 70 kd protein identical to the C-terminal half of p110 NF-κB: a new member of the IκB family. Cell, 1992. 68(6): p. 1109-1120.
- Solan, N.J., et al., RelB cellular regulation and transcriptional activity are regulated by p100. Journal of Biological Chemistry, 2002. 277(2): p. 1405-1418.
- Oeckinghaus, A. and S. Ghosh, The NF-κB family of transcription factors and its regulation. Cold Spring Harbor perspectives in biology, 2009. 1(4): p. a000034.
- Hotterbeekx, A., et al., The secretome of filarial nematodes and its role in host-parasite interactions and pathogenicity in onchocerciasis-associated epilepsy. Frontiers in Cellular and Infection Microbiology, 2021. 11: p. 662766.
- Venugopal, P.G., T.B. Nutman, and R.T. Semnani, Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunologic research, 2009. 43(1): p. 252-263.
- Bhoj, P., et al., Harnessing immune evasion strategy of lymphatic filariae: A therapeutic approach against inflammatory and infective pathology. Vaccines, 2022. 10(8): p. 1235.
- Rajasekaran, S., R. Anuradha, and R. Bethunaickan, TLR specific immune responses against helminth infections. Journal of Parasitology Research, 2017. 2017(1): p. 6865789.
- Bąska, P. and L.J. Norbury, The role of nuclear factor kappa B (NF-κB) in the immune response against parasites. Pathogens, 2022. 11(3): p. 310.
- McManus, C.M. and R.M. Maizels, Regulatory T cells in parasite infections: susceptibility, specificity and specialisation. Trends in Parasitology, 2023. 39(7): p. 547-562.
- Taylor, M.D., N. van der Werf, and R.M. Maizels, T cells in helminth infection: the regulators and the regulated. Trends in immunology, 2012. 33(4): p. 181-189.
- Steel, C. and T.B. Nutman, CTLA-4 in filarial infections: implications for a role in diminished T cell reactivity. The Journal of Immunology, 2003. 170(4): p. 1930-1938.
- Babu, S., et al., Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. The journal of immunology, 2006. 176(5): p. 3248-3256.
- Semnani, R.T., et al., Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. The Journal of Immunology, 2003. 171(4): p. 1950-1960.
- Semnani, R.T., et al., Filaria-induced immune evasion: suppression by the infective stage of Brugia malayi at the earliest host-parasite interface. The Journal of Immunology, 2004. 172(10): p. 6229-6238.
- Gallagher, I., et al., Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. The Journal of Immunology, 2007. 179(6): p. 3926-3936.
- Georgiev, P., L.-M. Charbonnier, and T.A. Chatila, Regulatory T cells: the many faces of Foxp3. Journal of clinical immunology, 2019. 39(7): p. 623-640.
- Grover, P., P.N. Goel, and M.I. Greene, Regulatory T cells: regulation of identity and function. Frontiers in immunology, 2021. 12: p. 750542.
- Collison, L.W., et al., The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature, 2007. 450(7169): p. 566-569.
- Abbas, A.K., et al., Regulatory T cells: recommendations to simplify the nomenclature. Nature immunology, 2013. 14(4): p. 307-308.
- Grazia Roncarolo, M., et al., Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunological reviews, 2006. 212(1): p. 28-50.
- Mak, T.W., M.E. Saunders, and B.D. Jett, Primer to the immune response. 2013: Newnes.
- Xing, Y. and K.A. Hogquist, T-cell tolerance: central and peripheral. Cold Spring Harbor perspectives in biology, 2012. 4(6): p. a006957.
- Singh, B., et al., Modulation of autoimmune diseases by interleukin (IL)-17 producing regulatory T helper (Th17) cells. Indian Journal of Medical Research, 2013. 138(5): p. 591-594.
- Pellerin, L., et al., Regulatory T cells and their roles in immune dysregulation and allergy. Immunologic research, 2014. 58(2): p. 358-368.
- Bellemore, S., et al., Preventative role of interleukin-17 producing regulatory T helper type 17 (Treg17) cells in type 1 diabetes in non-obese diabetic mice. Clinical & Experimental Immunology, 2015. 182(3): p. 261-269.
- Sharma, R. and G.R. Kinsey, Regulatory T cells in acute and chronic kidney diseases. American Journal of Physiology-Renal Physiology, 2018. 314(5): p. F679-F698.
- McSorley, H.J., et al., Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. The Journal of Immunology, 2008. 181(9): p. 6456-6466.
- Yurchenko, E., et al., CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. The Journal of experimental medicine, 2006. 203(11): p. 2451-2460.
- Belkaid, Y., Regulatory T cells and infection: a dangerous necessity. Nature Reviews Immunology, 2007. 7(11): p. 875-888.
- Belkaid, Y., R.B. Blank, and I. Suffia, Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunological reviews, 2006. 212(1): p. 287-300.
- Maizels, R. and M. Yazdanbakhsh, Regulation of the immune response by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol, 2006. 3: p. 733.
- Ouaissi, A., Regulatory cells and immunosuppressive cytokines: parasite-derived factors induce immune polarization. BioMed Research International, 2007. 2007(1): p. 094971.
- Metenou, S. and T.B. Nutman, Regulatory T cell subsets in filarial infection and their function. Frontiers in immunology, 2013. 4: p. 305.
- Song, Y., et al., Tr1 cells as a key regulator for maintaining immune homeostasis in transplantation. Frontiers in immunology, 2021. 12: p. 671579.
- Satoguina, J.S., et al., T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. The Journal of Immunology, 2005. 174(8): p. 4718-4726.
- King, C.L., et al., Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. The Journal of clinical investigation, 1993. 92(4): p. 1667-1673.
- Ritter, M., et al., Wuchereria bancrofti-infected individuals harbor distinct IL-10-producing regulatory B and T cell subsets which are affected by anti-filarial treatment. PLoS neglected tropical diseases, 2019. 13(5): p. e0007436.
- Mukherjee, S., et al., Wuchereria bancrofti filaria activates human dendritic cells and polarizes T helper 1 and regulatory T cells via toll-like receptor 4. Communications Biology, 2019. 2(1): p. 169.
- Babu, S., V. Kumaraswami, and T.B. Nutman, Transcriptional control of impaired Th1 responses in patent lymphatic filariasis by T-box expressed in T cells and suppressor of cytokine signaling genes. Infection and immunity, 2005. 73(6): p. 3394-3401.
- Taylor, M.D., et al., Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. The Journal of Immunology, 2005. 174(8): p. 4924-4933.
- Taylor, M.D., et al., CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. The journal of immunology, 2007. 179(7): p. 4626-4634.
- Mariano, F.S., et al., The involvement of CD4+ CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes and Infection, 2008. 10(7): p. 825-833.
- Satoguina, J., et al., Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes and Infection, 2002. 4(13): p. 1291-1300.
- White, M.P., C.M. McManus, and R.M. Maizels, Regulatory T-cells in helminth infection: induction, function and therapeutic potential. Immunology, 2020. 160(3): p. 248-260.
- Babu, S., C.P. Blauvelt, and T.B. Nutman, Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. The Journal of Immunology, 2007. 179(4): p. 2445-2456.
- Narasimhan, P.B., et al., Microfilariae of Brugia malayi inhibit the mTOR pathway and induce autophagy in human dendritic cells. Infection and immunity, 2016. 84(9): p. 2463-2472.
- Ricciardi, A., et al., Extracellular vesicles released from the filarial parasite Brugia malayi downregulate the host mTOR pathway. PLoS neglected tropical diseases, 2021. 15(1): p. e0008884.
- Mishra, R., et al., Increased Fas ligand expression of peripheral B-1 cells correlated with CD 4+ T-cell apoptosis in filarial-infected patients. Parasite Immunology, 2017. 39(4): p. e12421.
- Kalyanasundaram, R., V. Khatri, and N. Chauhan, Advances in vaccine development for human lymphatic filariasis. Trends in parasitology, 2020. 36(2): p. 195-205.
- Won, K.Y., et al., Diagnostics to support elimination of lymphatic filariasis—Development of two target product profiles. PLoS neglected tropical diseases, 2021. 15(11): p. e0009968.
- Kumar, V., A. Mishra, and A. Singh, Identification of promising nutraceuticals against filarial immune-modulatory proteins: insights from in silico and ex vivo studies. RSC advances, 2022. 12(35): p. 22542-22554.
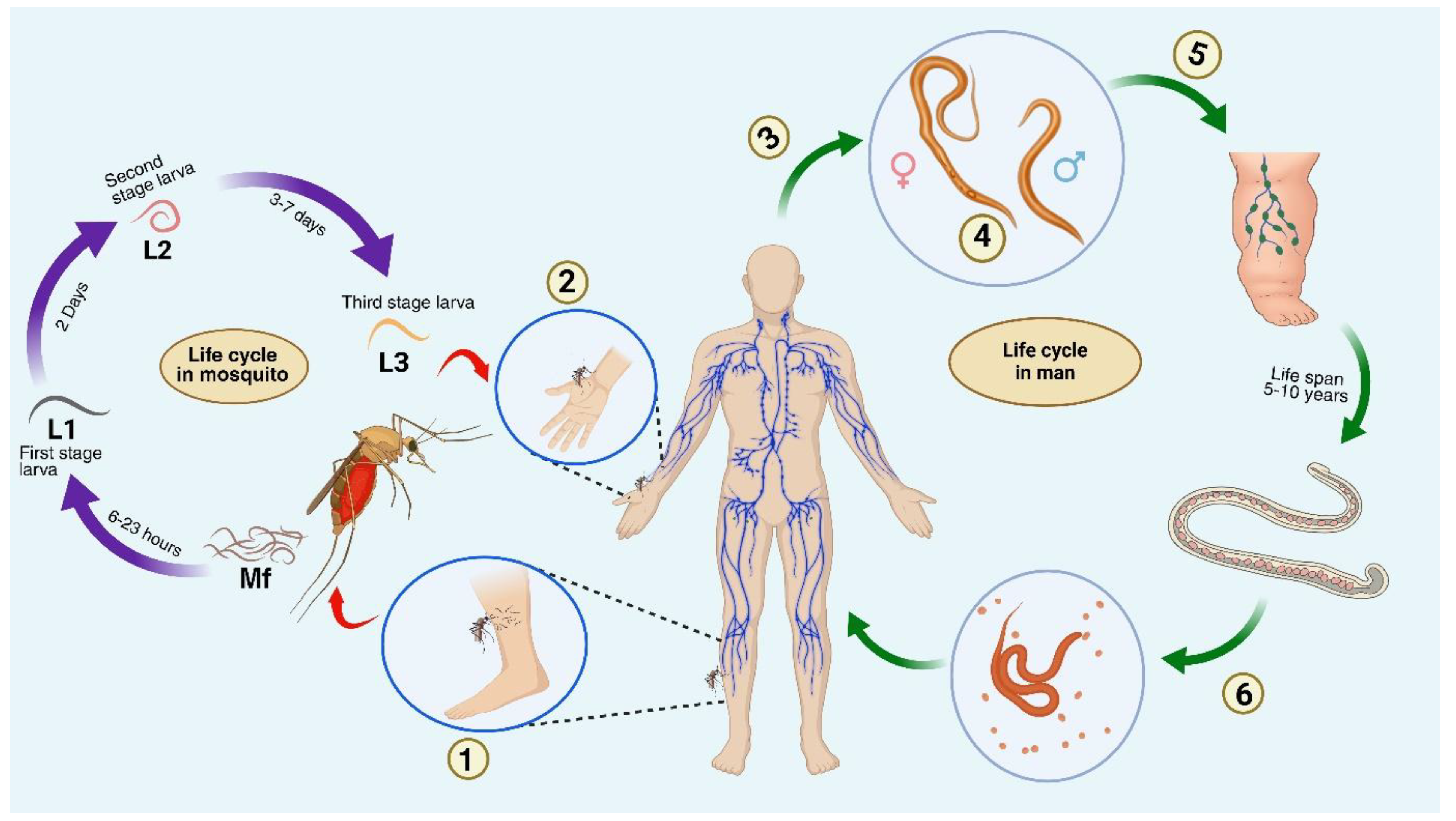
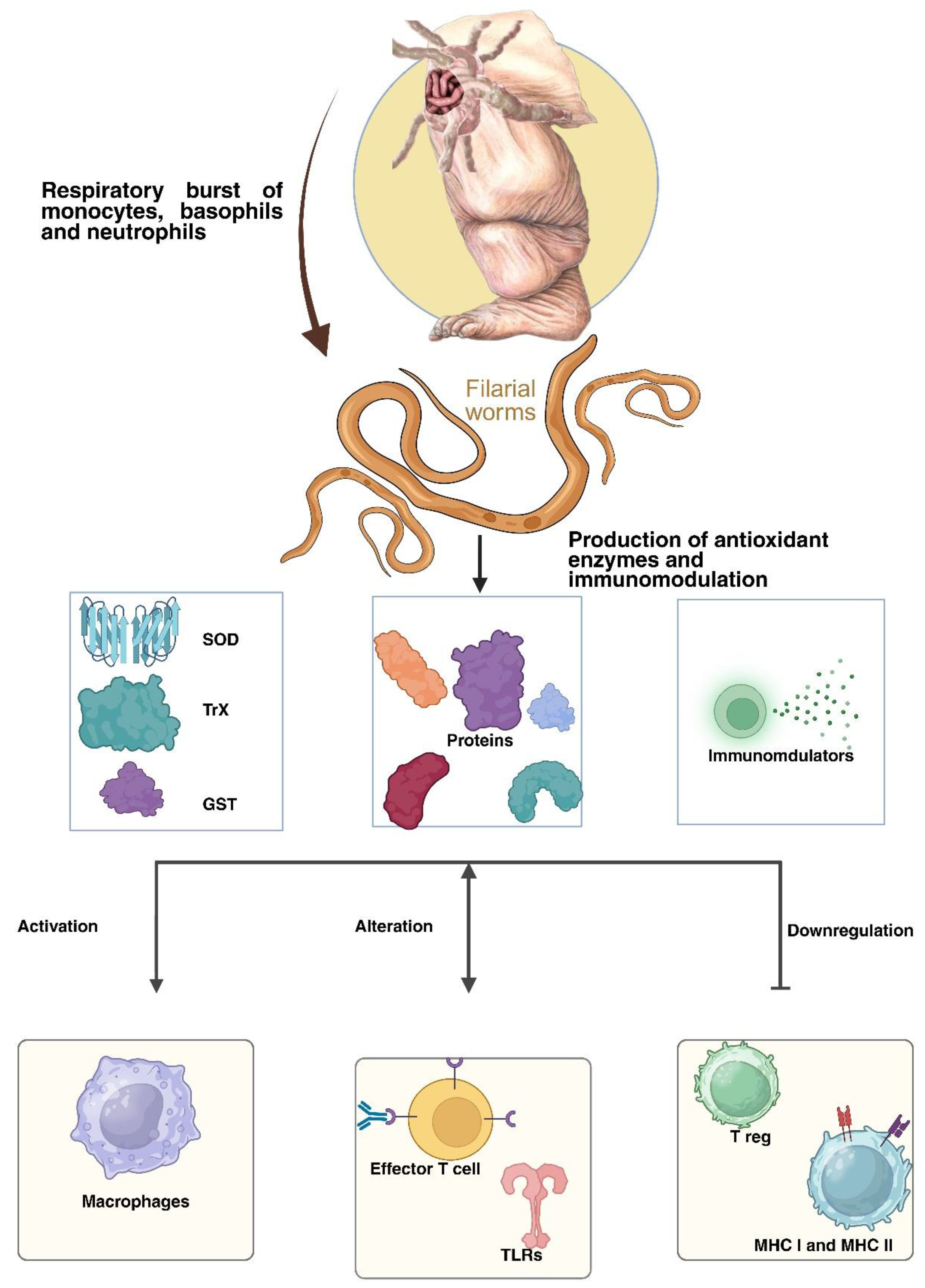
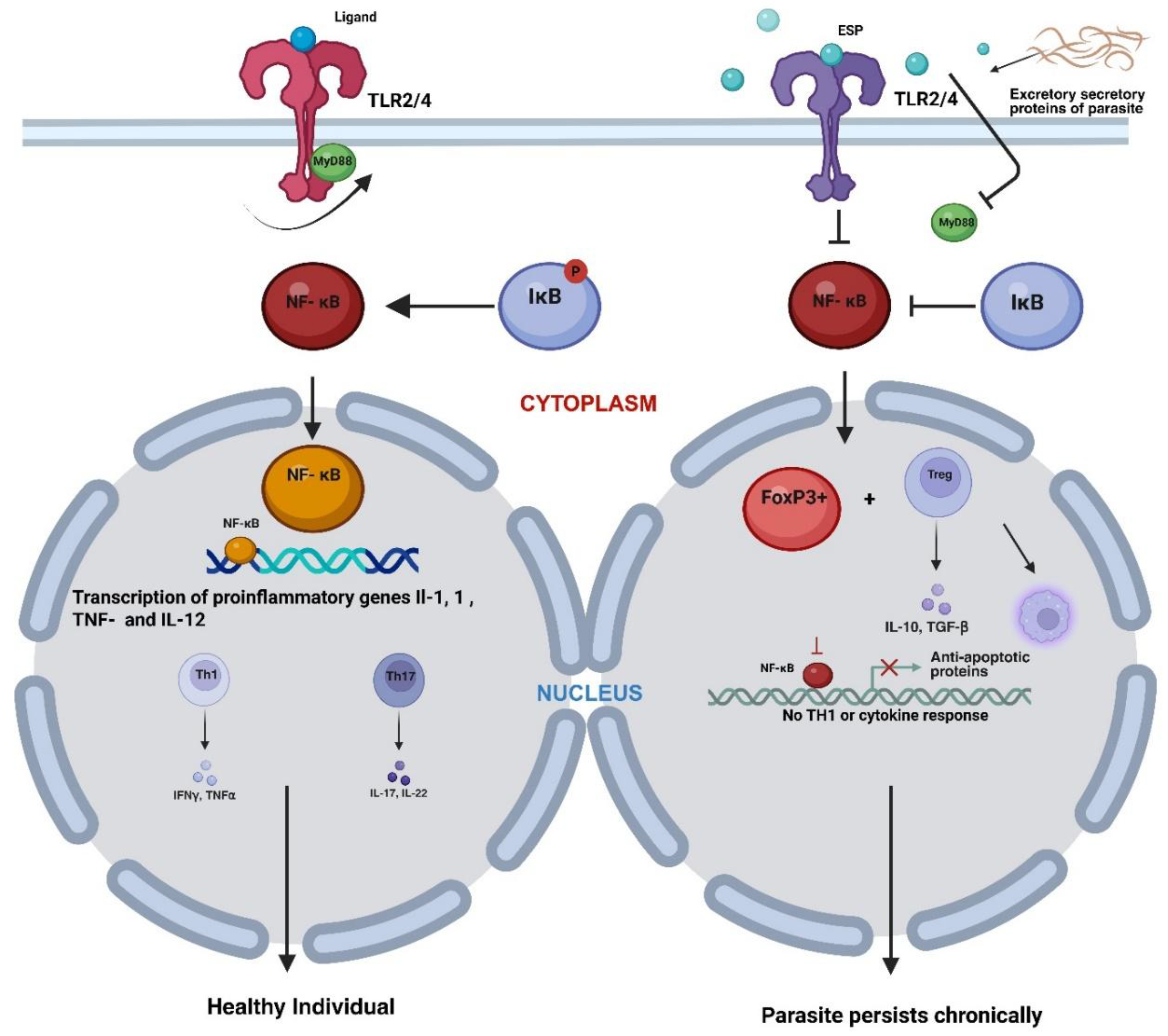
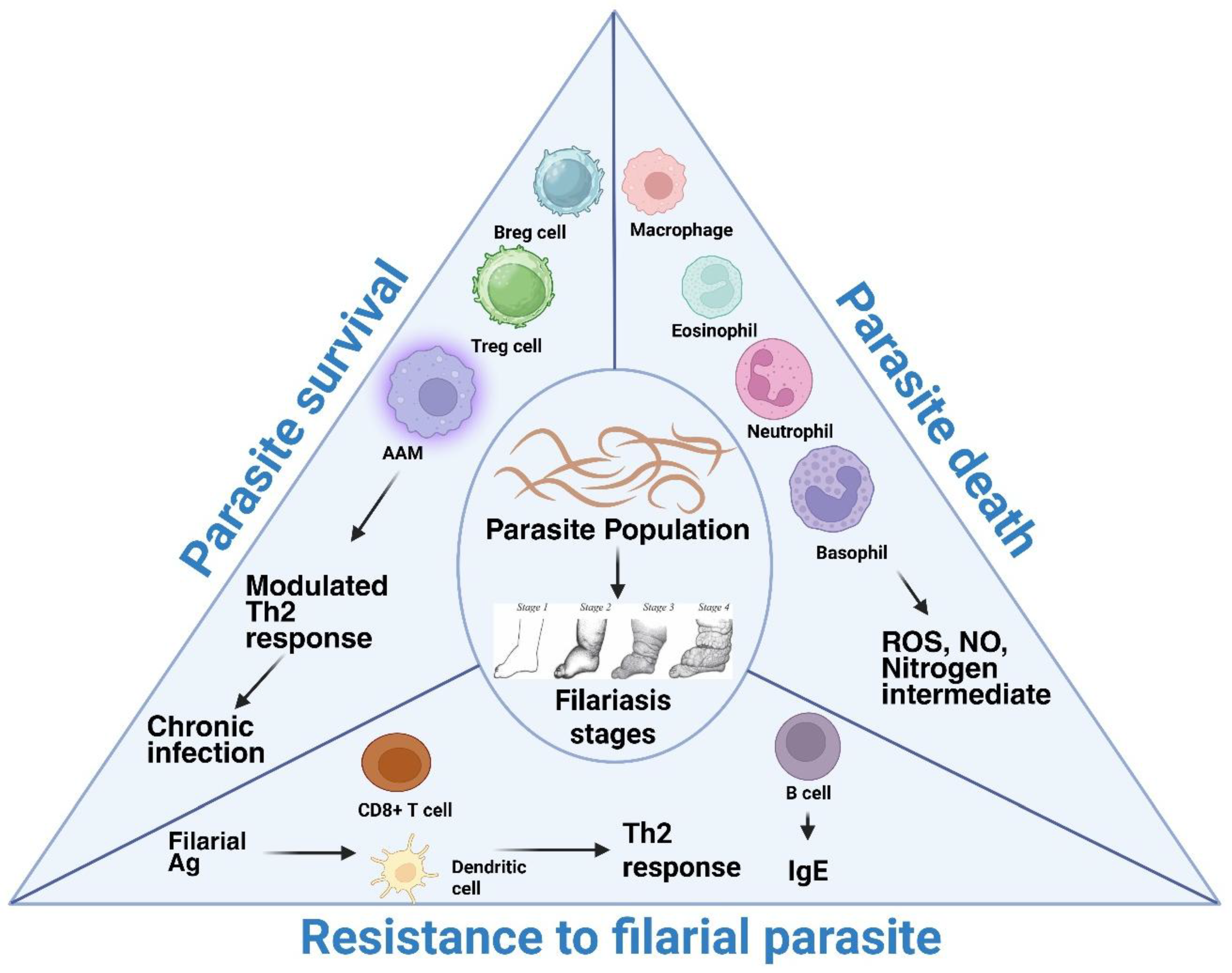
| S.No | Filarial parasite | Associated disease |
Vector involved | Cause of symptoms | Recommended treatment | Affected regions | Experimental model | References |
|---|---|---|---|---|---|---|---|---|
| 1. |
Oncocerca volvulus, Oncocerca ochengi |
Onchocerciasis | Blackflies (Simulium spp.) | MF-related immune response | Ivermectin ( generally in co-endemic areas), not advised in case of areas co-endemic with loiasis | Sub-Saharan Africa, Yemen, Uganda, Cameroon, small foci in South America | Mice in the case of Oncocerca ochengi | [6] |
| 2. | Wuchereria bancrofti, Brugia malayi, Brugia timori | Lymphatic filariasis (Elephantiasis) |
Mosquito species (Aedes, Anopheles, Culex, Mansonia, etc.) |
Adult worm-specific immune responses TPE: lung mf trapping |
Diethylcarbamazine citrate (DEC), Ivermectin, Albendazole ( Different combinations of these drugs are given in areas with co-endemicity) | Tropical regions of Asia, America, the Pacific, Africa, and countries like Indonesia, Malaysia, Thailand | Ferrets, Mice, and Jerds | [15] |
| 3. | Loa loa | Loiasis | Chrysops flies | Due to the migration of adult worms, Calabar swelling, severe reactions to DEC treatment, and hypereosinophilia | DEC or Albendazole (Due to the possibility of SAEs, treatment is not always advised) | West and Central Africa | Primates (Baboons) and rodents | [16] |
| 4. | Mansonella perstans, Mansonella ozzardi, Mansonella streptocerca | Mansonellosis | Midges of the genus Culicoides | Due to adult worm migration, ocular symptoms brought on by MF migration | Doxycycline | Eastern, Western, and Central Africa, parts of South and Central America, Caribbean islands | NA | [16] |
| S.N. | Clinical Stages in individuals | Healthy/Diseased | Parasitic stages | Circulating filarial antigens | Symptoms | References |
|---|---|---|---|---|---|---|
| 1. | Normal | Healthy | None | Absent | Nil | [25] |
| 2. | Endemic Normals | Healthy | None | Absent | Nil | [3] |
| 3. | Microfilaraemic /Asymptomatic | Diseased | Microfilariae in blood, live adult worms in lymphatics | Present | Clinically asymptomatic | [26] |
| 4. | Acute Clinical Disease | Diseased | Adult worms in lymphatics | Present | Episodes of lymphangitis, filarial fever, lymph nodes, localized inflammation | [27] |
| 5. | Chronic Pathology | Diseased | Usually, dead adult worms are present in the lymphatics | Present | Lymphedema, elephantiasis, and hydrocele | [28] |
| 6. | Occult | Diseased | Adult worms present, but no circulating microfilariae | May or may not be present | Symptoms include Tropical Pulmonary Eosinophilia, restrictive pulmonary changes, filarial arthritis, glomerulonephritis, and breast abscesses, among others. | [29] |
| S.No. | Cell type | Location | Functions and roles | Reference |
|---|---|---|---|---|
| 1. | Regulatory T cells | Thymus and Periphery | Maintains tolerance and prevents pathologies. Present in high levels in the asymptomatic stage and low levels in the chronic stage | [35,36] |
| 2. | Regulatory B cells | Blood circulation and inflammation site | Perform Immune regulatory mechanisms by secreting immunosuppressive cytokines. Induce Treg cells, suppressing CD4+, CD8+ T cells and NK cells | [18] |
| 3. | Eosinophils | Derived from bone marrow, later migrates to tissue | Contributes both to the protection and development of filarial pathology | [6] |
| 4. | Neutrophils | Circulates in blood later migrate to tissue | Release toxins to eliminate parasites. Involved in protective immune responses and pathological aggravation of disease. | [37] |
| 5. | Alternatively Activated Macrophages(AAMs) | Reside in Blood and Tissue | Blood-derived AAMs perform immune regulatory roles whereas tissue-resident AAMs responsible for fibrosis seen during chronic infections | [3] |
| 6. | Dendritic cells | Present in epithelial tissues | Parasite-derived products interact with Dendritic cells to initiate profound changes in immune responses leading to suppressed inflammation suitable for prolonged survival of parasites characteristic of chronic infections. | [38] |
| 7. | CD4+ T cells | Thymus and peripheral blood circulation | Involved in parasite clearance along with type-2 cytokines. Presence of Treg balance Th1/Th2 responses. | [39] |
| 8. | CD8+ T cells | Thymus and peripheral blood circulation | Involved in the cytotoxic killing of filarial parasites with the help of type-2 cytokines, persistence of filaria antigens contributes to chronic pathology | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





