Submitted:
02 February 2025
Posted:
03 February 2025
You are already at the latest version
Abstract
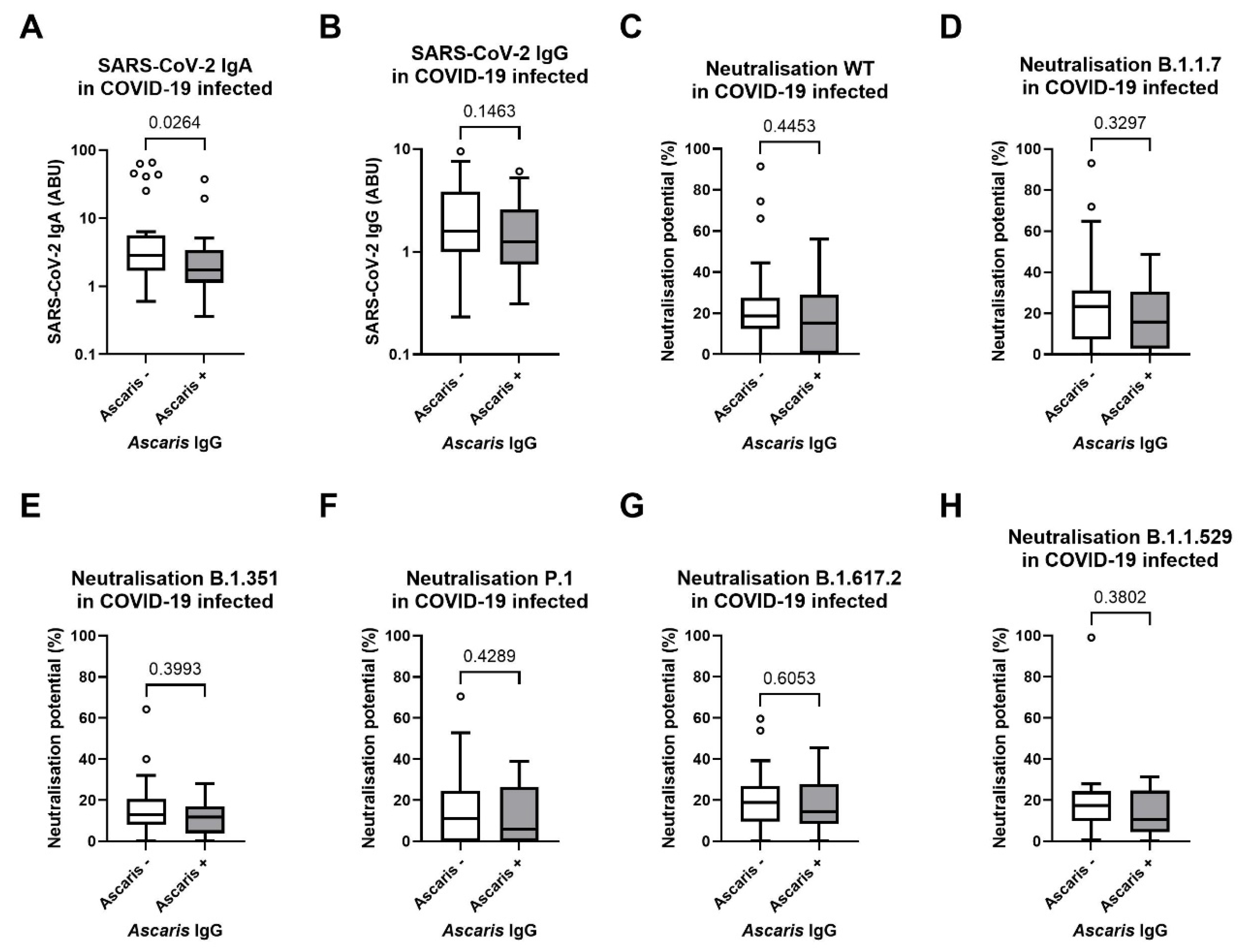
Background/Objectives: Although the COVID-19 pandemic has largely concluded, the varied trajectories it has followed in different regions of the world remain incompletely understood. Intensive research is needed to fully grasp its course and the implications for future global health challenges. Notably, the milder trajectory of the COVID-19 pandemic in Sub-Saharan Africa has defied initial predictions. An emerging body of evidence suggests that, in addition to the continent's younger average age and the lower prevalence of relevant comorbidities, co-infections with helminths may have also impressively shaped the pandemic's milder trajectory in the region. Indeed, helminths are renowned for their ability to modulate human immune responses, which, while potentially beneficial in limiting excessive inflammation, could also diminish vaccine efficacy and impede viral clearance. This study investigated different aspects of the intricate interactions between COVID-19 and Lymphatic Filariasis (LF), a helminth infection caused by parasitic worms such as Wuchereria bancrofti, Brugia malayi, and Brugia timori and endemic to various regions in Sub-Saharan Africa and the tropics. Methods: For this purpose, samples were collected from 222 individuals from endemic areas of Ghana, along with comprehensive clinical and demographic data. The samples include LF patients (n=222) grouped according to their Lymphoedema (LE) stages, as well as COVID-19 vaccinated (n=81) and non-vaccinated individuals (n=141). The expressions of SARS-CoV-2 and filarial-specific antibodies (IgG, IgA) were accessed using ELISA, while Luminex-based immunoassays were employed to measure the expression of SARS-CoV-2 variant-specific neutralizing antibodies. The interplay between vaccine responses, and demographic factors was analyzed using group comparisons with the Kruskal-Wallis or Mann-Whitney U tests. Results: The results indicate that a remarkable portion of unvaccinated individuals (56% IgA seropositive, 39% IgG seropositive) developed antibodies against SARS-CoV-2 despite no confirmed infection. Notably, the study identified a robust antibody response to COVID-19 vaccination, which was independent of the degree of LF pathology or parasitic status. An important observation was the reduced SARS-CoV-2 antibody response in individuals seropositive for Ascaris lumbricoides (p=0.0264), highlighting an interaction between roundworm infection and COVID-19. Conclusion: The study concludes that while COVID-19 vaccination triggers a strong immune response in LF patients, filarial seropositivity might influence the immunogenicity and clinical outcomes of COVID-19, emphasizing the complexity of infectious disease dynamics in co-infected populations.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Clinical Characteristics of Participants
| Sample Size (n=) | 222 |
| Age (Mean ± SD, Min-Max) | 46.57 ± 9.21 (17 – 64) |
| Sex (M / F) | 34 (15%) / 188 (85%) |
| BMI (Min-Max, Mean ± SD) | 11.49 – 42.44 (23.32 ± 4.05) |
| LE Staging (0 / 1 / 2 / 3 / 4 / 5 / 6) | 2 / 5 / 130 / 55 / 0 / 1 / 29 0.9% / 2.3% / 58.6% / 24.8% / 0% / 0.5% / 13.1% |
| Treatment Group (A / B) | 198 (89%) / 24 (11%) |
| COVID-19 Positive test (No / Yes) | 222 (100%) / 0 |
| COVID-19 Vaccination (No / 1. Dose / 2. Dose) | 141 (63%) / 63 (28%) / 18 (8%) |
| BCG vaccination (No / Yes / Unknown) | 65 (29%) / 139 (63%) / 18 (8%) |
| Influenza vaccination (No / Yes / Unknown) | 65 (29%) / 139 (63%) / 18 (8%) |
| COVID-19 related symptoms (No / Yes) | 186 (84%) / 36 (16%) |
| SD: Standard Deviation, BMI: Body Mass Index, LE: Lymphoedema, BCG: Bacillus Calmette-Guérin | |
2.2. Quantification of SARS-CoV-2-Specific Antibodies
2.3. Detection of Ascaris Lumbricoides Specific IgG
2.4. Detection of Human Filarial-Specific Antibodies
2.5. Quantification of SARS-CoV-2 Neutralizing Antibody Levels
2.6. Quantification of Systemic Cytokine and Chemokine Levels
2.7. Statistics
3. Results
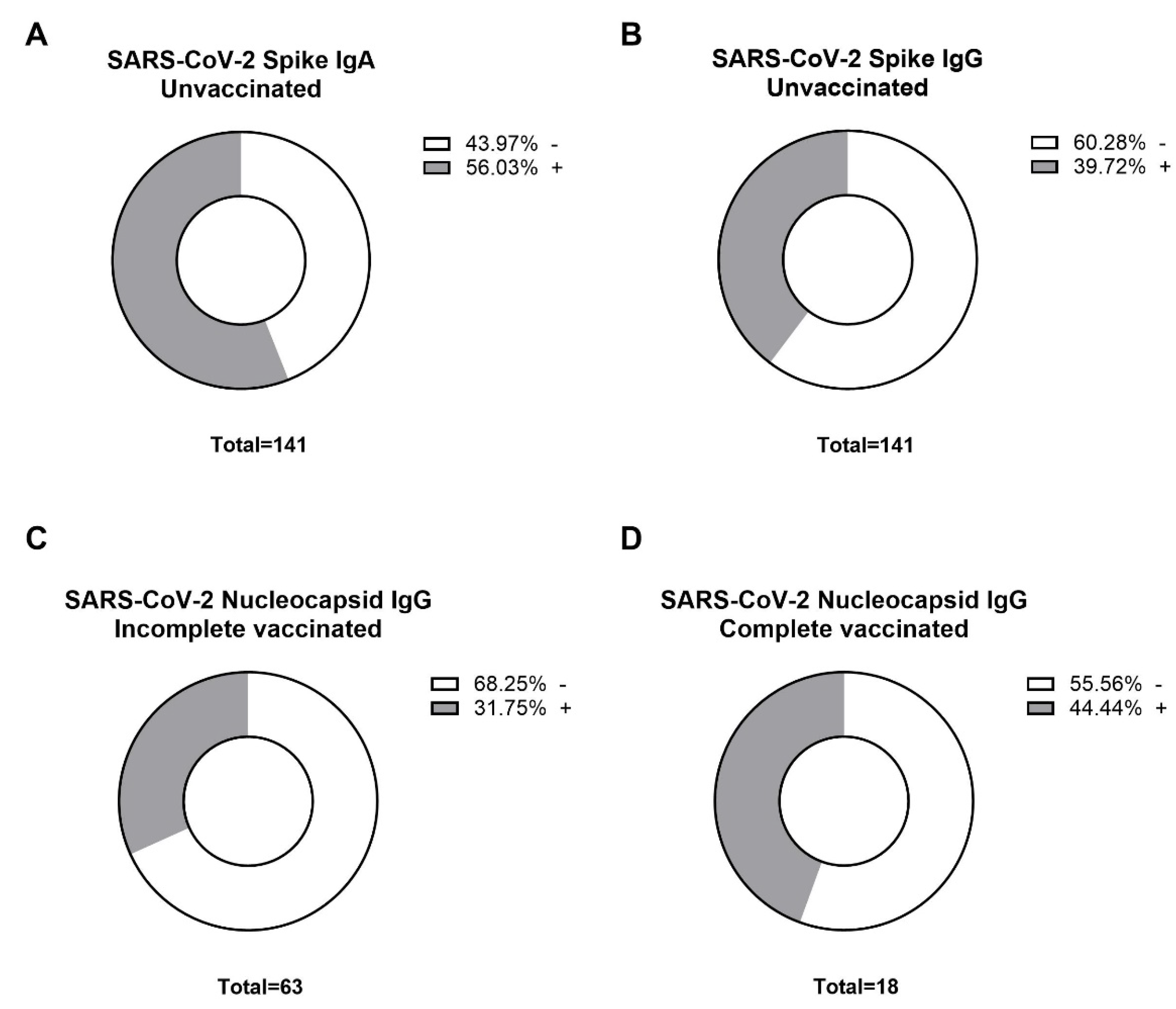
3.1. Elevated SARS-CoV-2 Seroprevalence Among Unvaccinated Individuals in Ghana
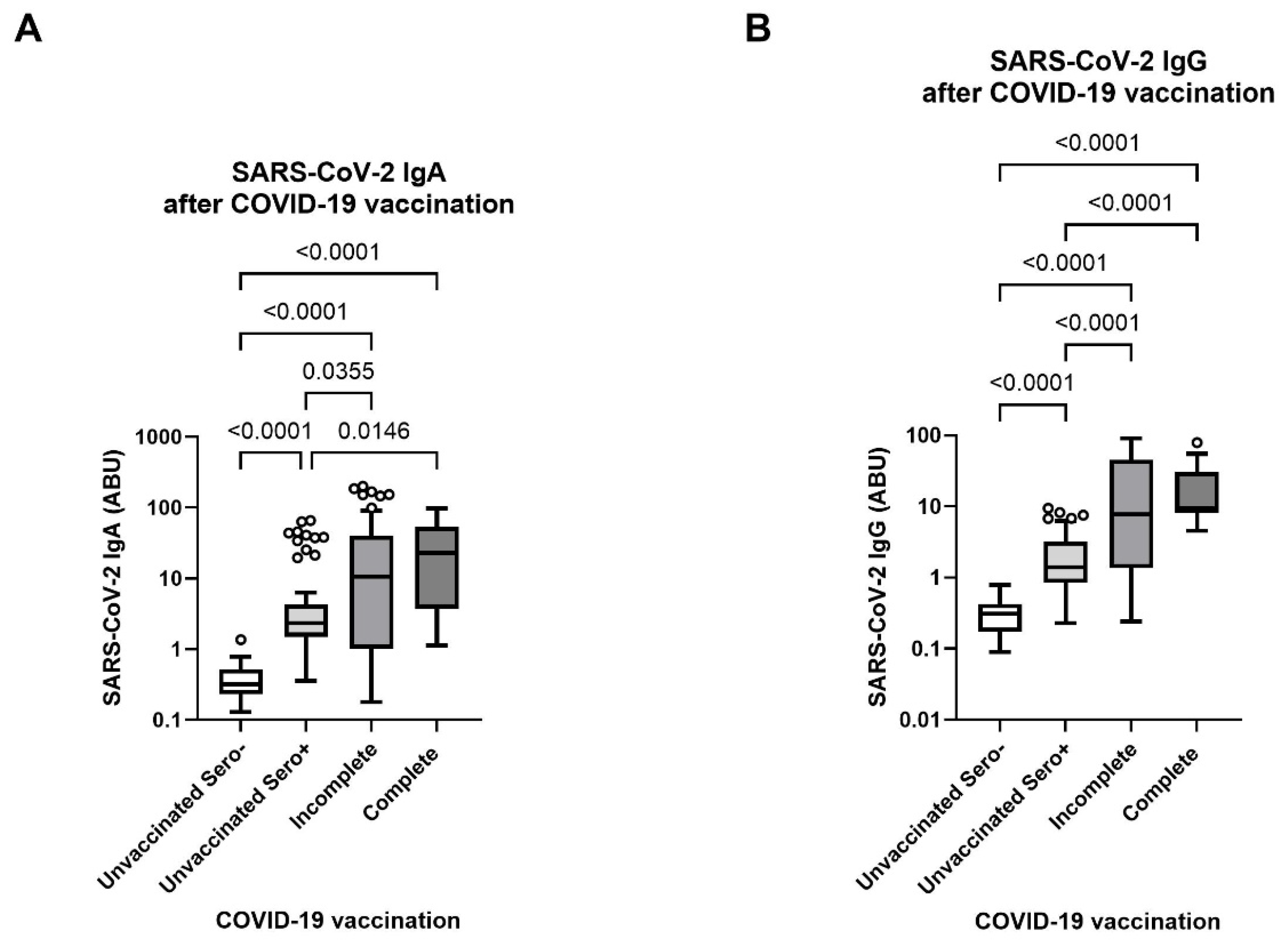
3.2. Robust SARS-CoV-2-Specific Antibody Response in Lymphatic Filariasis Patients after Incomplete and Complete COVID-19 Vaccination
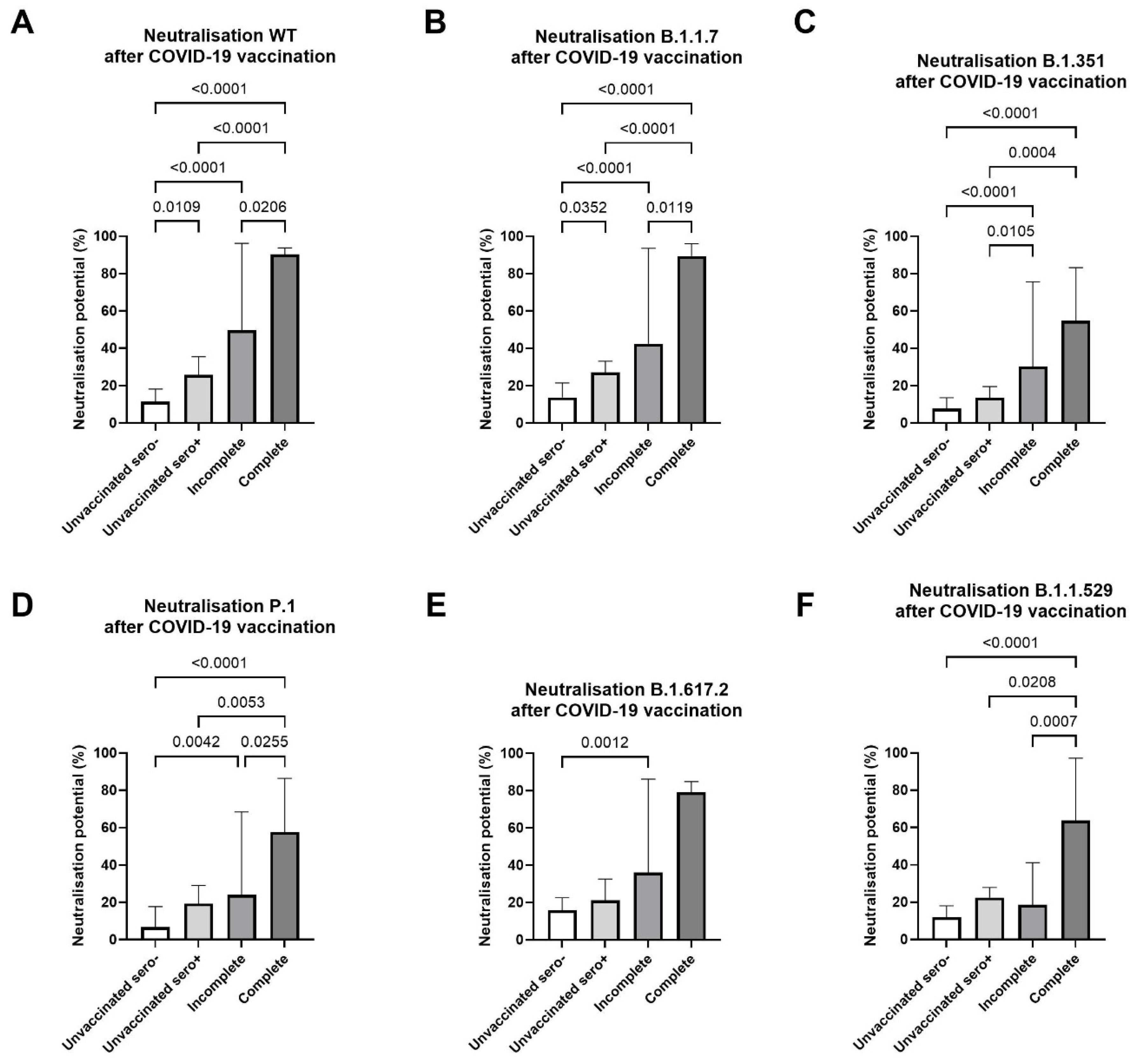
3.3. Elevated Neutralizing Antibody Levels Towards Variants of Concern in COVID-19 Vaccinated Filaria Seropositive Individuals
3.4. Diminished SARS-CoV-2-Specific IgA Response in Ascaris Seropositive Individuals
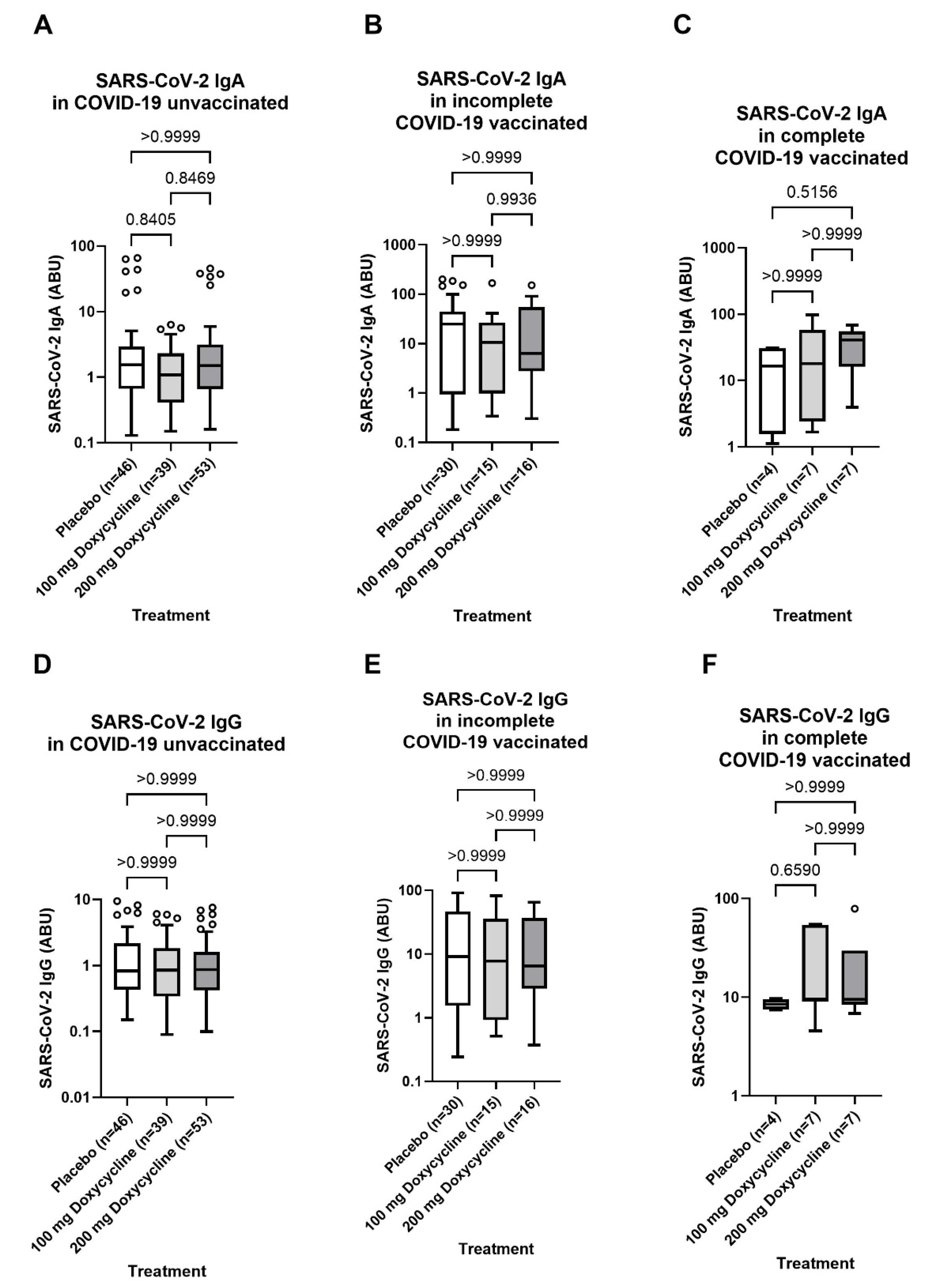
3.5. No Influence of Doxycycline Treatment 36-Months Before Blood Sampling of Lymphatic Filariasis Individuals with Lymphoedema Pathology Compared to the Placebo Control Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFA | Circulating Filarial Antigen |
| COVID-19 | Coronavirus Disease 2019 |
| LE | Lymphoedema |
| LF | Lymphatic Filariasis |
| NCP | Nucleocapsid |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| VoC | Variant of concern |
| WT | Wildtype |
References
- Venkatesh, A.; Edirappuli, S. Social distancing in covid-19: What are the mental health implications? Bmj 2020, 369. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.; et al. Illustrating the impact of commercial determinants of health on the global COVID-19 pandemic: Thematic analysis of 16 country case studies. Health Policy 2023, 134, 104860. [Google Scholar] [CrossRef]
- Hsieh, E.; et al. Global Perspective on the Impact of the COVID-19 Pandemic on Rheumatology and Health Equity. Arthritis Care Res (Hoboken) 2024, 76, 22–31. [Google Scholar] [CrossRef]
- Lau, J.; et al. Understanding the mental health impact of COVID-19 in the elderly general population: A scoping review of global literature from the first year of the pandemic. Psychiatry Res 2023, 329, 115516. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; et al. The WHO has terminated global public health emergency for COVID-19 by the IHR Emergency Committee recommendation: Potential impact analysis. Ann Med Surg (Lond) 2023, 85, 3755–3756. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, B.T.; et al. COVID-19 Pandemic and Its Global Impact on the Accessibility and Provision of Maternal and Child Health Care Services. Asia Pac J Public Health 2024, 10105395241250120. [Google Scholar] [CrossRef]
- (WHO), W.H.O. COVID-19 Deaths Dashbord. 2024. Available online: https://data.who.int/dashboards/covid19/deaths?n=c.
- Li, Q.; et al. Immune response in COVID-19: What is next? Cell Death Differ 2022, 29, 1107–1122. [Google Scholar] [CrossRef]
- Noor, F.M.; Islam, M.M. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J Community Health 2020, 45, 1270–1282. [Google Scholar] [CrossRef]
- Fang, X.; et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: A systematic review and meta-analysis. Aging (Albany NY) 2020, 12, 12493–12503. [Google Scholar] [CrossRef]
- Peckham, H.; et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020, 11, 6317. [Google Scholar] [CrossRef]
- Herrera-Esposito, D.; de Los Campos, G. Age-specific rate of severe and critical SARS-CoV-2 infections estimated with multi-country seroprevalence studies. BMC Infect Dis 2022, 22, 311. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida. PLoS ONE 2021, 16, e0249038. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; et al. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Vabret, N.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef]
- Coveney, C.; et al. Innate immunology in COVID-19-a living review. Part I: Viral entry, sensing and evasion. Oxf Open Immunol 2020, 1, iqaa004. [Google Scholar] [CrossRef]
- Li, G.; et al. Coronavirus infections and immune responses. J Med Virol 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Vafaeinezhad, A.; Atashzar, M.R.; Baharlou, R. The Immune Responses against Coronavirus Infections: Friend or Foe? Int Arch Allergy Immunol 2021, 182, 863–876. [Google Scholar] [CrossRef]
- Okuyama, R. mRNA and Adenoviral Vector Vaccine Platforms Utilized in COVID-19 Vaccines: Technologies, Ecosystem, and Future Directions. Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef]
- Adjobimey, T.; et al. Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization With Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm's COVID-19 Vaccines. Front Immunol 2022, 13, 917905. [Google Scholar] [CrossRef]
- Edouard Mathieu, H.R.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; Roser, M. Coronavirus Pandemic (COVID-19). 2020. Available online: https://ourworldindata.org/covid-vaccinations.
- Oduro-Mensah, D.; et al. Explaining the unexpected COVID-19 trends and potential impact across Africa. F1000Res 2021, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Bwire, G.; et al. The COVID-19 pandemic in the African continent. BMC Med 2022, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, F. Malaria and the incidence of COVID-19 in Africa: An ecological study. BMC Infect Dis 2023, 23, 66. [Google Scholar] [CrossRef]
- Adjobimey, T.; et al. Negative association between ascaris lumbricoides seropositivity and Covid-19 severity: Insights from a study in Benin. Front Immunol 2023, 14, 1233082. [Google Scholar] [CrossRef]
- Adjobimey, T.; et al. Helminth antigens differentially modulate the activation of CD4(+) and CD8(+) T lymphocytes of convalescent COVID-19 patients in vitro. BMC Med 2022, 20, 241. [Google Scholar] [CrossRef]
- Tan, L.Y.; Komarasamy, T.V.; Balasubramaniam, V.R. Hyperinflammatory Immune Response and COVID-19: A Double Edged Sword. Front Immunol 2021, 12, 742941. [Google Scholar] [CrossRef]
- Natukunda, A.; et al. The effect of helminth infection on vaccine responses in humans and animal models: A systematic review and meta-analysis. Parasite Immunol 2022, 44, e12939. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.F.; et al. Recombinant antigens used as diagnostic tools for lymphatic filariasis. Parasit Vectors 2021, 14, 474. [Google Scholar] [CrossRef]
- (WHO), W.H.O. Lymphatic Filariasis. 2024.
- Babu, S.; Nutman, T.B. Immunology of lymphatic filariasis. Parasite Immunol 2014, 36, 338–346. [Google Scholar] [CrossRef]
- Debrah, L.B.; et al. Adherence to Hygiene Protocols and Doxycycline Therapy in Ameliorating Lymphatic Filariasis Morbidity in an Endemic Area Post-Interruption of Disease Transmission in Ghana. Am J Trop Med Hyg 2024, 111 4_Suppl, 66–82. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc Natl Acad Sci U S A 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- Mohammed, I.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum Vaccin Immunother 2022, 18, 2027160. [Google Scholar] [CrossRef]
- Watson, O.J.; et al. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect Dis 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 2016, 138, 666–675. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.S.; Nutman, T.B. Perspective: Prospects for development of vaccines against human helminth infections. J Infect Dis 1996, 174, 1384–1390. [Google Scholar] [CrossRef]
- Storey, H.L.; et al. Soil transmitted helminth infections are not associated with compromised antibody responses to previously administered measles and tetanus vaccines among HIV-1 infected, ART naïve Kenyan adults. Parasite Epidemiol Control 2017, 2, 13–20. [Google Scholar] [CrossRef]
- (WHO), W.H.O. Soil-transmitted helminth infections. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections.
- Adetifa, I.M.O.; et al. Temporal trends of SARS-CoV-2 seroprevalence during the first wave of the COVID-19 epidemic in Kenya. Nat Commun 2021, 12, 3966. [Google Scholar] [CrossRef]
- Uyoga, S.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science 2021, 371, 79–82. [Google Scholar] [CrossRef]
- Voysey, M.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Ewer, K.J.; et al. Author Correction: T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med 2021, 27, 1116. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Hvidt, A.K.; et al. Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines. Front Med (Lausanne) 2022, 9, 994160. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Aniagyei, W.; et al. Doxycycline Treatment of Mansonella perstans-Infected Individuals Affects Immune Cell Activation and Causes Long-term T-cell Polarization. Clin Infect Dis 2023, 76, e1399–e1407. [Google Scholar] [CrossRef]
- Debrah, A.Y.; et al. Doxycycline Leads to Sterility and Enhanced Killing of Female Onchocerca volvulus Worms in an Area With Persistent Microfilaridermia After Repeated Ivermectin Treatment: A Randomized, Placebo-Controlled, Double-Blind Trial. Clin Infect Dis 2015, 61, 517–526. [Google Scholar] [CrossRef]
- Dhar, R.; et al. Doxycycline for the prevention of progression of COVID-19 to severe disease requiring intensive care unit (ICU) admission: A randomized, controlled, open-label, parallel group trial (DOXPREVENT.ICU). PLoS ONE 2023, 18, e0280745. [Google Scholar] [CrossRef]
- Johannesen, C.K.; et al. Risk Factors for Being Seronegative following SARS-CoV-2 Infection in a Large Cohort of Health Care Workers in Denmark. Microbiol Spectr 2021, 9, e0090421. [Google Scholar] [CrossRef]
- Ding, J.; et al. Characteristics and Prognosis of Antibody Non-responders With Coronavirus Disease 2019. Front Med (Lausanne) 2022, 9, 813820. [Google Scholar] [CrossRef]
- Hamady, A.; Lee, J.; Loboda, Z.A. Waning antibody responses in COVID-19: What can we learn from the analysis of other coronaviruses? Infection 2022, 50, 11–25. [Google Scholar] [CrossRef]
- Adepoju, P. Africa's struggle with inadequate COVID-19 testing. Lancet Microbe 2020, 1, e12. [Google Scholar] [CrossRef] [PubMed]
- Moyazzem Hossain, M.; Abdulla, F.; Rahman, A. Challenges and difficulties faced in low- and middle-income countries during COVID-19. Health Policy Open 2022, 3, 100082. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





