1. Introduction
Infectious bovine rhinotracheitis is a serious disease of the respiratory and reproductive tract of animals that occurs all over the world and with varying prevalence across wide geographic regions[
1]. Infectious Bovine Rhinotracheitis is caused by bovine herpes virus 1 (BoHV-1), which belongs to the genus Varicellovirus of family herpes viridae [
2,
3]. There are three subtypes of BoHV-1: respiratory (BoHV-1.1), genital (BoHV-1.2a) and encephalic (BoHV-1.2b) [
3,
4]. All three subtypes have major economic consequences for the cattle industry (loss of body weight, reduced milk production, fertility disorders, embryonic mortality, abortion, stillbirth and treatment costs due to secondary bacterial infections) [
5,
6].
The disease is a highly contagious viral disease that affects cattle of all ages. Infection occurs by inhalation and contact between animals, and once it is established within disease-free populations it spreads rapidly from infected cattle to immunocompromised susceptible cattle [
2,
7]. During pick period of virus perpetuation in body of infected animal it excreted through vaginal secretions, nasal discharges or droplets, semen, mucosal discharges, fetal fluid and tissues [
8,
9]. The direct contact of susceptible animal with these contaminated body excretion will lead to transmission of the disease. The virus also transmitted through aerosol infection in the respiratory form [
10,
11]. Latently infected animals are lifelong carriers of BoHV-1 and potential transmitters of the virus between herds [
12].
Natural BoHV-1 infection occurs via contact with contaminated mucous membranes of the respiratory or genital tracts [
13]. Transmission occurs through aerosols, nasal secretions, mating, or infected semen. Virus entry into the respiratory tract can occur via aerosol or by direct contact with virus present in nasal secretions [
14]. It causes two main syndromes: infectious bovine rhinotracheitis (IBR) in the respiratory tract and infectious pustular vulvovaginitis/balanoposthitis (IPV/IPB) in the genital tract. IBR symptoms include fever, lethargy, rapid breathing with harsh cough, anorexia, and reduced milk yield in dairy cows [
13]. Depending on the stage of gestation, the infection can result in early embryonic death and infertility, or late-term abortion. Fetal mummification and stillbirth are also part of the infection sequel[
15,
16].
The occurrence and spread of the disease is influenced by many risk factors. Stress factors such as temperature, nutritional deficiencies, overcrowding, and improper transportation weaken the animal’s immune system, triggering the activation of the latent virus to develop clinical symptoms and transmit the virus to susceptible animals [
17,
18]. Purchasing infected cattle, taking part in agricultural shows, increased herd size, and faulty production system are among the management and environmental risk factors that have contributed to the spread of BoHV-1. The disease also spreads because of uncontrolled movement of animals and guests inside the farm [
19,
20]. Unvaccinated breeding or feedlot cattle are susceptible to epidemics of abortion and respiratory disease. When colostrum antibodies are insufficient or passive immunity is compromised in newborn calves, the disease frequently manifests as the systemic form [
21].
Ethiopia, one of the world’s most populous countries, has the largest livestock population in Africa. It homes nearly about 70.3 million cattle, which supports approximately about 80% of task force and contributes about 45% to the of the agricultural GDP [
22]. BoHV-1 is an economically significant viral disease in the dairy industry worldwide, including sub-Saharan African countries [
15]. Several studies report seroprevalence levels ranging from 35.9–77.5% in Europe and 37–60.8% in Latin America [
23]. Only few studies have been reported from Sub-Sharan Africa with varying level of seroprevalence, the variation is caused by differences in climates, stocking densities and management, among other factors [
24,
25]. The finding in the region includes 48.3% in Southern Zambia [
26], 69% in Ghana [
27], 31.17% in Algeria [
28], and 74.5% in Gauteng province of South Africa [
29].
The disease was first reported in Ethiopia 50 years ago in 1975 in Harrar and Sidamo [
30]. Afterward, it has become one of the major causes of reproductive disorders in dairy cattle, significantly hindering livestock development in recent decades [
31]. Some studies have documented the spread of IBR across different parts of the country. For example, Tesfaye
et al. [
32] reported a 34.7% seroprevalence in central Ethiopia. Asmare
et al. (2018) and Sibhat et al. (2018) confirmed the presence of IBR in central, southern, and southwestern regions. In addition, Wedajo et al. [
33] reported cases in Kombolcha district. More recently, Kolech
et al. [
34] a 72% seroprevalence from Debark and Lay-Armachiho districts of Gonder Zone. In spite of its well-defined economic impacts on dairy industry, yet there is insufficient information available regarding the frequency and spread of IBR in the southern western Oromia region of Ethiopia. Therefore, this cross-sectional study was carried out to estimate seroprevalence of BoHV-1 and identify associated risk factors in small-scale dairy farm in and around Jimma town.
2. Materials and Methods
2.1. Study Design, Study Population and Target Population
A cross-sectional study was conducted from December 2023 to April 2024, targeting dairy cattle in and around Jimma town, southwestern part of Oromia, Ethiopia. The study population consists of dairy herds in kept under both intensive and sem-intensive management in the study area (Figure 1). Since Vaccination against BoHV-1/IBR is not practiced in Ethiopia, the sampled individuals were assumed Unvaccinated. Blood samples were collected from dairy cows within the selected herds, and all sampling procedures were carried out by well-trained veterinary professionals including laboratory experts of serological lab.
2.2. Sample Size Determination and Sampling Methods
The sample size for this study was determined by using the formula given in the Equation (1) [
35].
In this equation, Z is the Z-score for 95% confidence level, which is 1.96. P
exp represents the expected prevalence, set at 34.4% or 0.344 [
32], and d is the precison, determined to be 5% or 0.05. Based on these assumptions, the calculated sample size was 346. However, a total of 439 samples were finally collected to increase the statistical power of the study and enhance the precision of estimates. Out of 65 dairy farms registered at the study area, 39 farms/dairy herds were selected randomly and 30% of individual cattle were recruited randomly from each involved farms to proportionate.
2.3. Collection of Blood Samples
Approximately about 7-10 ml of blood samples were collected aseptically from the jugular vein using plain vacutainer tubes and an 18 gauge (18G) disposable needle after the animals was well restrained. Each samples were labelled, and kept at inclined position, then transported to Jimma University College of Agriculture and Veterinary Medicine (JUCAVM) and left to stay at room temperature for 2-4 hours to allow clotting of blood and serum separation with centrifugation. Harvested serum samples were stored at -20 °C until further analysis at Animal Health institute, Sebeta.
2.4. Farm- and Cow-Level Data Collection
Farm- and individual-animal level data on potential risk factors were collected using semi-structured questionnaires. Farm/herd level data includes herd size, service type, feeding Place, watering Practice, the number of lactating cows, the annual number of abortions; retention of fetal membrane; daily milk yield, source of semen for breeding and origin of stock replacement. Individual animal level data (animal biodata) includes age, the animal origin, body condition, parity, abortion history, repeat breeder, reproductive disorders, and history of respiratory problem.
2.5. Processing of the Blood Samples
The blood samples were positioned at inclined angle to harvest serum. Then each serum was decanted into 1.5ml labeled Eppendorf tubes and placed in a 96-well plate (no-ELISA plate). Wells A1 and B1 contained positive controls, whereas C1 and D1 contained negative controls. The remaining 92 wells were filled with serum to facilitate the rapid transfer of both controls and samples to the ELISA plate using a 12-channel micropipette.
2.6. Competitive Enzyme Linked Immunosorbent Assay (cELISA)
The collected sera sample were analyzed using Aseptically ID.vet IBR gB antibody competitive ELISA kits ((iD.vet IBR gB Competition ELISA, France), following the manufacturer’s instructions to detect antibodies against BoHV-1. Reagents were brought to room temperature (21°C ± 5°C) before use. The sera were homogenized using Vortex. Then 100 µL of positive control was added to wells Ai and B1, 100 µL of negative control to wells C1 and D1, 100 µL of each serum samples to the remaining wells. The plate were then covered with sterile plastic and incubated for 2hours at 5 °C (±3 °C). Then, the wells emptied and washed three times with washing solution. Ready to use conjugate was added to each wells and incubated at 21°C (±5 °C) for 30 minutes. Unbounded conjugate was washed out by three times by 300 µL of wash solution. To finalize the procedure, a substrate solution was added to each well and incubated at 21°C (±5 °C) for 15 minutes in dark area. Stop solution was added to each well to stop the reaction. The Optical Density (OD) value at 450 nm was read using Spectrophotometer.
2.7. Test Validation and Interpretation
The test was deemed to be valid if the mean value of the negative control OD (ODNC) is greater than 0.7 (ODNC < 0.7) and the mean value of the positive control is less than 30% of the ODNC (ODPC/ODNC < 0.3). The competition percentage (S/N %) for each sample was calculated using the formula S/N%=OD sample/ODNC) x 100. A result was classified as positive if the S/N% was ≤ 45% and negative if it was % ≥ 55%, and the value 45% < S/N % < 55% was regarded as doubtful.
2.8. Data Analysis
2.8.1. Descriptive Statistics
Numerical data such as herd size, and the herd size, service type, the number of lactating cows, the annual number of abortions; retention of fetal membrane; daily milk yield, source of semen for breeding and origin of stock replacement, age, and lactation age were summarized by descriptive statistics function in STATA version 17 ( Statacrop, LLC, Texas 77845, USA). The seroprevalence of IBR antibodies at both the farm and cow levels, along with its 95% confidence interval, was determined using the syntax ‘cii prop’ function in STATA version 17.
2.8.2. Identification of Risk Factors
Herd was classified as IBR seropositive if at least one cattle tested positive for BoHV-1 specific antibodies using the commercial cELISA test. An individual animal was classified as BoHV-1 seropositive if its serum tested positive in the CELISA test. Initially Univariable logistic regression analyses were performed herd level BoHV-1 seropositivity as outcome and each independent variables as a predictor. Variables having a p –value ≤ 0.25 were selected for inclusion in the multivariable logistic regression analysis to assess the strength of associations between two or more risk factors and the outcome variables. Using the variance inflation factor (VIF), multicollinearity was further examined for the explanatory factors (p<0.25). Variance inflation factor >10 or tolerance below 0.1 were regarded as the cut-off points [
36]. The odds ratio (OR) with its 95% confidence interval (CI) was used to see the strength of the association to predict the logistic regression model. The P-value <0.05 was used to rule out the statistical significance value of the associations between the independent and dependent variables.
3. Results
3.1. Summary Statistics
This study involves 39 dairy herds across seven farmer’s associations. Out of the total 439 sera samples tested, highest (28.50%) seroprevalence of BoHV-1 was reported in Ginjo and whereas the least (4.34%) was reported in Bore (Figure 2).
3.2. Animal- and farm- level Seroprevalence
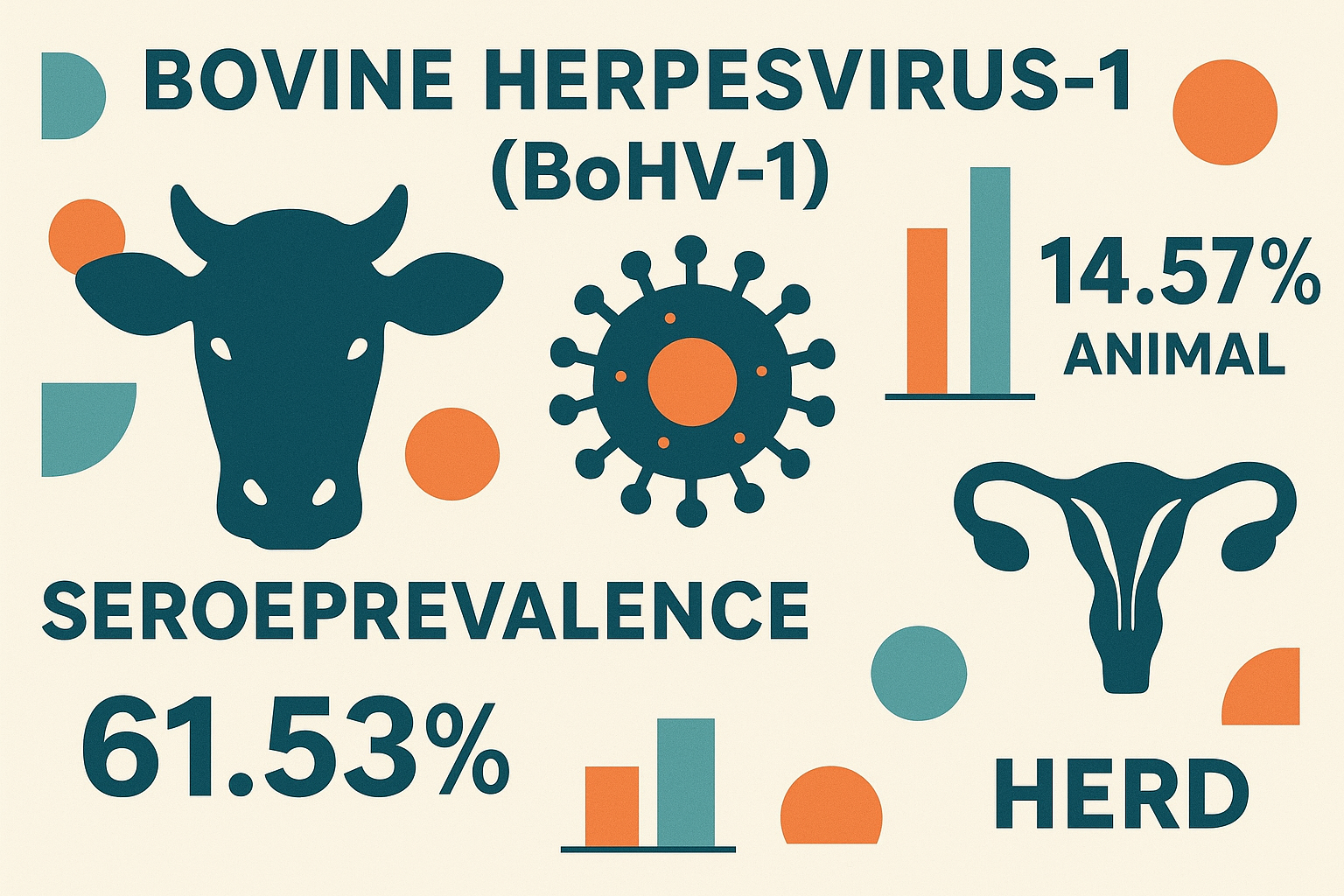
The finding shown individual-level seroprevalence of 14.57% (95% CI: 11.41-18.23), in which 64 animals were found seropositive. The herd-level prevalence of BoHV-1 was 61.53% (95% CI: 44.62-76.64), where the 24 farms that had at least one seropositive BoHV-1 antibody out of 39 sampled dairy farms. There was a difference in seroprevalence of BoHV-1 among herd size, age, animal origin, body condition, parity, repeated breeding, and history of abortion, infertility, and animals with respiratory problem categories (
Table 1).
3.3. Animal Level-Risk Factors Analysis
Logistic regression analysis among the risk factors against BoHV-1 seropositivity was done on age, animal origin, body condition, parity, abortion, repeated breeding, infertility, and respiratory problems. The model shows that the risk/odds of being positive for BoHV-1 were significantly associated with animal origin, where purchased dairy animals had a higher odds ratio (AOR = 94; p-value: 0.0001) than homebred animals. The analysis revealed that dairy animals having respiratory problem (AOR = 20.06; p-value: 0.001) were more likelihood of developing the disease compared to those with no respiratory problem. The present study revealed a strong association between abortion history and BoHV-1 seropositivity, where cows with a history of abortion were 20.06 times more likely to be infected compared to those without abortion. The study also demonstrated a highly significant association between infertility and BoHV-1 seropositivity. Cows with a history of infertility were 37.38 times more likely to be infected with BoHV-1 compared to their fertile counterparts. The rest of the explanatory variables (age, repeated breeding) were not statistically associated with BoHV-1 serostatus (
Table 2).
3.4. Herd Level Risk Factor Analysis for BoHV-1
The seroprevalence occurrence of BoHV-1 with the size of the herd, service type, stock source, feeding, and the watering place was assessed through a semi-structured questionnaire. The higher prevalence of the disease was seen in herds with <10 animals (69.2% (9/13), 100% (2/2) in dairy animals using both AI and bull service, 70% (7/10) in those feeding their dairy animals on the floor, 75% (3/4) in group watering, and 69.2% (9/13) in farms using both home and purchase stock replacement dairy herds as compared to their counterparts. However, the random effect logistic regression analysis of the different management problems and the seroprevalence of BoHV-1 did not show a significant association (
Table 4).
The semi-structured questionnaire was presented to farm owners/animal attendants to assess the previous history of reproductive problem challenges like retained fetal membrane (RFM), abortion history together with experienced abortion stage, and the history of respiratory problems. The highest seroprevalence of BoHV-1 was seen in dairy herds with previous RFM challenges at 64.3% (9/14), dairy herds with the challenges of repeated breeders at 64.7% (23/34), and dairy herds with abortion cases in 5-7 months pregnant animals at 86.6% (13/15), followed by 80% (4/5) in 8-9 months pregnant periods and in herds with respiratory problems at 76% (19/25) as compared to their counterparts. The exact logistic regression analysis shows a significant association for abortion history with OR = 2.2; P < 0.05, while the other categories were not significant (
Table 5).
4. Discussion
The current study investigates the seroprevalence of BoHV-1 in and around Jimma town from J The study of BoHV-1 antibody at the individual level seroprevalence was 14.57%. The seroprevalence observed reflect exposure to natural infection, as cattle are not vaccinated against BHV-1 in Ethiopia. The overall seroprevalence of BoHV-1 reported in present study was lower compared to previous studies findings in Ethiopia like the pooled seroprevalence reported Tesfaye et al. [
32] in Bishoftu (23.1%), Bahirdar (28.5%), Nekemte (34.4%) and in Holeta (41.5%). Also, 25.6% [
37] from Desse and Kombolcha towns dairy cattle of the Amhara region; 41% [
31] in the central, southern, and southwestern parts of the Ethiopian country; and 77.6% reported by Zewde et al. [
38]in northwestern and central Ethiopia. On contrary, the current finding was higher relative to findings (8.56%) reported by Jain et al. [
38] from Tamil Nadu and Andhra Pradesh and 10.39% in Garwal district of Uttaranchal State of India [
38]. These discrepancies in seroprevalence could be attributed to factors like management system differences in study animals, study period, breeds of animal, or ecological variation of the study area from other places since Jimma town and zone receive year-round rain.
The present finding almost similar with seroprevalence reported from other countries by Yousef et al. [
39] and Kaddour et al. [
40] with result of 17.4% and 14.16% in Saud Arabian and northeastern part of Algerian dairy cattle respectively. Likewise, different authors obtained relatively lower anti-IBR/BoHV-1 antibodies in dairy cattle: 10.39% and 22.3% in Uttarakhand by Lata et al. [
41], 11.4% in Iran [
42] and 17.37% in Kenya [
43]. The anti-IBR/BoHV-1 antibodies have been identified in other parts of the world ranged from 17.1% to 60.84% [
40,
44,
45].
The herd level seroprevalence of BoHV-1 was notably high, indicating significant presence of the disease with the studied population. It implies the existence of a large pool of latently infected cattle that could potentially serve as a source of infection for other animals, as infected cattle are considered to be infected for life (Muylkens et al., 2007). However, it was relatively low compared with the previous report by Asmare et al.[
15] in dairy herd seroprevalence (81.8%) within the country in three different milk sheds (central, southern and western areas) of Ethiopia. It is comparable with result reported from Southern Mexico by Segura-Correa et al.[
46] with findings of 85.4%; 84.0% IBR/BoHV-1 virus from dairy herds of Belgium by Boelaert et al. [
47], and 90% in Irish cattle population [
48].
On contrary, the present-herd-level serostatus of IBR/BoHV-1 was in agreement with report of 69.8% in central part of Ethiopia [
49] and 68.3% [
50] in Gondar city dairy farms, 54.4% to 60.3% in northeastern and northwestern mesoregions of the State of Rio Grande do Sul, Brazil [
51]. Previous studies conclude that non-vaccination, introducing or mixing unscreened animals, insemination from unscreened bulls, and intensive rearing are key drivers of IBR/BoHV-1 spread and high herd prevalence [
52].
In this study, purchased animals were 94 (AOR=94) times more likely to be infected than homebred animals. This result agrees with the previous findings that reported stock source as the risk factor for BoHV-1 infection [
32,
38,
53]. This reflects a higher possibility of getting the virus due to animal movement from the infected herd to herds free from the disease. This is a reason for the herd-level likely high seroprevalence with few users of purchased stock replacement.
The study revealed a significant association between a history of abortion in cows and seropositivity for BoHV-1 infection (p = 0.005). Cows with a history of abortion were 20.06 times more likely to be seropositive than cows without such a history (OR = 20.06), indicating a strong link between BoHV-1 infection and reproductive losses. This result is consistent with previous studies reporting BoHV-1 as a major cause of abortion in cattle [
53,
54]. BoHV-1 infection can lead to embryonic death or abortion, particularly in the second trimester, due to viral replication in the reproductive tract [
55]. The high odds ratio observed underscores the importance of BoHV-1 in herd reproductive health and highlights the need for control measures such as vaccination, strict biosecurity, and screening of newly introduced animals [
18].
In the present study, reproductive disorders showed a strong association with BoHV-1 infection. Cows with a history of abortion were 20.06 times more likely to be seropositive for BoHV-1, while those with infertility problems were 37.38 times more likely to be infected compared to their counterparts without such histories. These findings highlight the significant reproductive consequences of BoHV-1 infection in dairy herds. Similar associations have been reported elsewhere, where BoHV-1 was linked to abortion, reduced conception rates, and repeat breeding [
53,
56,
57]. The high odds ratios observed in this study suggest that BoHV-1 may play an important role in reproductive inefficiency, leading to considerable economic losses. Therefore, the integration of preventive strategies such as vaccination, herd-level biosecurity, and pre-breeding screening is essential to minimize BoHV-1–related reproductive failures and improve herd productivity.
The study also assessed herd management practices in relation to BoHV-1 seroprevalence. Higher prevalence was observed in small herds (69.2%) compared to large herds (57.6%). This agrees with a previous report [
58] where small herds showed higher prevalence than large ones, but contrasts with another study that found higher seroprevalence in large herds (82.8%) compared to medium (76.2%) and small (70.4%) herds.
Regarding the usage of services for cows during the heat or estrus period about 94.8% of dairy farms breed with AI service, but the seroprevalence of BoHV-1 was higher in farms using both AI and bull (100%) than AI service (59.5%). This study is in line with the finding of
4 that 95.7% in bulls, 76.9% in both AI and Bull, and 70.1% in AI service breeding farms [
58] report 56.25% in bull service than 43.75% in AI service practicing farm. However, the current study differed from the report of
23, which is 25.8% higher in AI service than 25% in bull service. Herd-level seroprevalence of BoHV-1 was markedly higher in herds with respiratory problems (76%) compared to those without respiratory problems (36%). This finding indicates that BoHV-1 infection is strongly associated with both reproductive and respiratory disorders, consistent with previous reports [
58].
5. Conclusions and Recommendation
Among all suspected risk factors, animal origin and animals with respiratory problems were identified as potential risk factors. The dairy farms challenged with retained fetal membrane, repeated breeders, and respiratory problems also had a high prevalence of the disease. The result of this study showed the presence of BoHV-1 antibody among dairy cattle of Jimma town, which helps to prove the circulation of the disease in dairy herds in the country, as many researches have been carried out in different areas with various seroprevalence. Based on the above conclusion, the following recommendations are forwarded.
Dairy animals with a history of abortion and respiratory problems were better if tested for BoHV-1 and properly managed, as they act as potential sources of infection.
It is better to quarantine the newly purchased animals since they are the potential risk factor for the spread of the disease from one herd to the other.
Farm owner training on good farm management, especially on feeding and watering practices; separation of dairy animals with reproduction and respiratory problems.
Testing and slaughtering of positive animals and the well implementation of biosecurity were the best control strategies for the disease.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no budgetary funding body for reporting and publication of the research work.
Institutional Review Board Statement
This research involved the use of ethical statements of the corresponding university. Ethics approval was obtained from Jimma University in accordance with the principles established by the institution. This study was granted with ethical approval set by Wollega University’s Institutional Ethics Research Review Committee, School of Veterinary Medicine. The approval letter allows me to carrying research work on the currently bears the title “The guide is entitled” Guidelines for Ethics in animal-related research and Teaching involving animals. Animals were approached with great care according to the guidelines for the ethics of animal research.
Data Availability Statement
Acknowledgments
The expression of our gratitude is extended to the Jimma University college of Agriculture and Veterinary Medicine for granting the authorization letter essential for the collection of data. Appreciation was also conveyed to the individuals who possess the dairy animal farm and the Jimma town Agricultural office staff for their support during research work.
Conflicts of Interest
There was no conflict of interest between the authors of this work.
References
- R. Gangil, G. Kaur, and P. N. Dwivedi, “Detection of bovine herpes virus (BOHV-1) infection in respiratory tract of bovines,” Indian J. Anim. Sci., vol. 89, no. 12, pp. 1349–1351, 2019. [CrossRef]
- T. F. Tshinavhe, “Identification and characterisation of the common aetiologies of cattle respiratory diseases in Mahikeng local municipality, South Africa,” 2019, North-West University (South Africa).
- O. I. E. T. Manual, “Infectious Bovine rhinotracheitis/infectious pustular vulvovaginitis,” Man. diagnostic tests vaccines Terr. Anim. World Organ. Anim. Heal., 2010.
- B. Muylkens, J. Thiry, P. Kirten, F. Schynts, and E. Thiry, “Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis,” Vet. Res., vol. 38, no. 2, pp. 181–209, 2007. [CrossRef]
- C. Dima and K. Abdisa, “Diagnostic techniques for infectious Bovine Rhinotracheitis: a review,” J. Appl. Adv. Res., vol. 7, pp. 42–48, 2022. [CrossRef]
- N. Djellata, “Seroprevalence of infectious bovine rhinotracheitis in aborted cows in Algeria.,” Vet. Stanica, vol. 55, no. 3, 2024. [CrossRef]
- S. Biswas, S. Bandyopadhyay, U. Dimri, and P. H. Patra, “Bovine herpesvirus-1 (BHV-1)–a re-emerging concern in livestock: a revisit to its biology, epidemiology, diagnosis, and prophylaxis,” Vet. Q., vol. 33, no. 2, pp. 68–81, 2013. [CrossRef]
- E. Takiuchi, K. C. Médici, A. F. Alfieri, and A. A. Alfieri, “Bovine herpesvirus type 1 abortions detected by a semi-nested PCR in Brazilian cattle herds,” Res. Vet. Sci., vol. 79, no. 1, pp. 85–88, 2005. [CrossRef]
- O. M. Radostits, C. C. Gay, K. W. Hinchcliff, and P. D. Constable, “A textbook of the diseases of cattle, horses, sheep, pigs and goats,” Vet. Med, vol. 10, pp. 2045–2050, 2007.
- M. D. Givens, “Risks of disease transmission through semen in cattle,” Animal, vol. 12, no. s1, pp. s165–s171, 2018. [CrossRef]
- A. Engdawork and H. Aklilu, “Infectious bovine rhinotracheitis: Epidemiology, control, and impacts on livestock production and genetic resources,” Vet. Res. Notes, vol. 4, no. 1, pp. 1–9, 2024. [CrossRef]
- J. B. Ostler and C. Jones, “The bovine herpesvirus 1 latency-reactivation cycle, a chronic problem in the cattle industry,” Viruses, vol. 15, no. 2, p. 552, 2023. [CrossRef]
- R. E. L. Taylor, B. S. Seal, and S. St Jeor, “Isolation of infectious bovine rhinotracheitis virus from the soft-shelled tick, Ornithodoros coriaceus,” Science (80-. )., vol. 216, no. 4543, pp. 300–301, 1982. [CrossRef]
- M. H. Mars, M. C. M. De Jong, C. Van Maanen, J. J. Hage, and J. T. Van Oirschot, “Airborne transmission of bovine herpesvirus 1 infections in calves under field conditions,” Vet. Microbiol., vol. 76, no. 1, pp. 1–13, 2000. [CrossRef]
- K. Asmare, B. Sibhat, G. Ayelet, E. Z. Gebremedhin, K. A. Lidete, and E. Skjerve, “Serological evidence of Bovine herpesvirus-1, Bovine Viral Diarrhea virus and Schmallenberg virus infections in relation to reproductive disorders in dairy cattle in Ethiopia,” Acta Trop., vol. 178, pp. 236–241, 2018. [CrossRef]
- S. M. Bello, A. I. Daneji, U. M. Chafe, M. B. Abubakar, A. H. Jibril, and A. Festus, “Detection of antibodies to bovine viral diarrhea virus in cattle presented for slaughter at Sokoto metropolitan abattoir, Nigeria,” J. Vet. Med. Anim. Heal., vol. 8, no. 2, pp. 11–14, 2016. [CrossRef]
- B. Padalino, “Transportation of horses and the implications for health and welfare,” 2017.
- R. R. G. Sayers, “Biosecurity, bovine viral diarrhoea virus (BVDv), and bovine herpesvirus-1 (BoHV-1): epidemiological investigations in Irish dairy herds,” 2014, University of Limerick.
- M. A. S. Moreira, A. S. Júnior, M. C. Lima, and S. L. da Costa, “Infectious diseases in dairy cattle,” in Raw Milk, Elsevier, 2019, pp. 235–258.
- P. Hostnik, D. Černe, J. Mrkun, J. Starič, and I. Toplak, “Review of infections with bovine herpesvirus 1 in Slovenia,” Front. Vet. Sci., vol. 8, p. 676549, 2021. [CrossRef]
- A. H. Jaramillo, “Immune Response and Protection Against BVDV-2 and BHV-1 Infection Elicited by Modified-Live Virus Vaccination in Dairy Calves. Effects of Vaccination Route and Trace Mineral Injection,” 2021, University of Georgia.
- G. Leggesse et al., “Ethiopia national dairy development strategy 2022–2031,” 2023.
- K. Raaperi, T. Orro, and A. Viltrop, “Epidemiology and control of bovine herpesvirus 1 infection in Europe,” Vet. J., vol. 201, no. 3, pp. 249–256, 2014. [CrossRef]
- M. Ackermann and M. Engels, “Pro and contra IBR-eradication,” Vet. Microbiol., vol. 113, no. 3–4, pp. 293–302, 2006. [CrossRef]
- Í. C. de Almeida et al., “Seroprevalence and associated factors of infectious bovine rhinotracheitis and bovine viral diarrhea in dairy cows in the Caparaó region, Espírito Santo, Brazil,” Ciência Rural, vol. 51, no. 12, p. e20200220, 2021. [CrossRef]
- A. S. Mweene et al., “The prevalence of bovine herpesvirus-1 in traditional cattle in Southern Province, Zambia.,” Rev. Sci. Tech., vol. 22, no. 3, pp. 873–877, 2003. [CrossRef]
- B. Adu-Addai, E. B. Koney, P. Addo, J. Kaneene, C. Mackenzie, and D. W. Agnew, “Importance of infectious bovine reproductive diseases: an example from Ghana,” Vet. Rec., vol. 171, no. 2, p. 47, 2012. [CrossRef]
- S. Derrar et al., “Seroprevalence and risk factors associated with bovine herpesvirus-1 infection in the region of Tiaret, Algeria.,” Vet., vol. 68, no. 3, pp. 127–132, 2019.
- A. S. Hamdy, A. Selim, S. A. Shoulah, and A. M. M. Ibrahim, “Sero-surveillance infectious bovine rhinotracheitis in ruminants and assessment the associated risk factors,” Benha Vet. Med. J., vol. 42, no. 2, pp. 160–163, 2022. [CrossRef]
- P.-C. Lefèvre, F. Roger, and J.-J. Tulasne, “Mission d’appui technique à l’OUA/IBAR-PARC. Rapport de synthèse,” 1998.
- B. S. Berhanu Sibhat, G. A. Gelagay Ayelet, E. S. Eystein Skjerve, E. Z. Gebremedhin, and K. A. Kassahun Asmare, “Bovine herpesvirus-1 in three major milk sheds of Ethiopia: serostatus and association with reproductive disorders in dairy cattle.,” 2018. [CrossRef]
- A. Tesfaye, S. Guta, B. Urge, and F. Gutema, “Epidemiology and Associated Risk Factors of Bovine Viral Disease (Bvd) and Infectious Bovine Rhinotracheitis Virus (Ibr) in Dairy Farms,” Livest. Res. Results, p. 62, 2022.
- G. K. Wedajo, M. K. Muleta, and B. G. Awoke, “Performance evaluation of multiple satellite rainfall products for Dhidhessa River Basin (DRB), Ethiopia,” Atmos. Meas. Tech. Discuss., vol. 2020, pp. 1–31, 2020. [CrossRef]
- T. A. Kolech, Y. K. Kebede, and S. A. Mekonnen, “Seroprevalence and associated risk factors of bovine herpesvirus 1 in smallholder dairy farms in two districts of Gondar zones, North-West Ethiopia,” Prev. Vet. Med., vol. 234, p. 106367, 2025. [CrossRef]
- M. Thrusfield, Veterinary epidemiology. John Wiley & Sons, 2018.
- L. Simon, D. Young, and I. Pardoe, “10.7—Detecting Multicollinearity Using Variance Inflation Factors,” Stat, vol. 462, 2018.
- W. M. Tadeg, A. Lemma, T. Yilma, H. Asgedom, and A. A. Reda, “Seroprevalence of infectious bovine rhinotracheitis and brucellosis and their effect on reproductive performance of dairy cattle,” J. Vet. Med. Anim. Heal., vol. 13, no. 2, pp. 106–113, 2021. [CrossRef]
- D. Zewde, T. Tadesse, and S. Alemu, “Sero Status and Presumed Risk Factors Assessment for Bovine Herpesvirus-1 in North Western, Ethiopia,” Austin J. Vet. Sci. Anim. Husb., vol. 8, no. 2, p. 1080, 2021. [CrossRef]
- M. R. Yousef, M. A. E. F. Mahmoud, S. M. Ali, and M. H. Al-Blowi, “Seroprevalence of some bovine viral respiratory diseases among non vaccinated cattle in saudi arabia,” Vet. World, vol. 6, no. 1, pp. 1–4, 2013. [CrossRef]
- A. Kaddour, A. Bouyoucef, G. Fernandez, A. Prieto, F. Geda, and N. Moula, “Bovine herpesvirus 1 in the northeast of Algiers, Algeria: Seroprevalence and associated risk factors in dairy herd,” J. Adv. Vet. Anim. Res., vol. 6, no. 1, p. 60, 2019. [CrossRef]
- L. J. Lata Jain, A. N. Kanani, V. K. Vinay Kumar, C. G. Joshi, and J. H. Purohit, “Detection of bovine herpesvirus 1 infection in breeding bulls by ELISA and PCR assay.,” 2009.
- A. M. Erfani, M. Bakhshesh, M. H. Fallah, and M. Hashemi, “Seroprevalence and risk factors associated with bovine viral diarrhea virus and bovine herpes virus-1 in Zanjan Province, Iran,” Trop. Anim. Health Prod., vol. 51, no. 2, pp. 313–319, 2019. [CrossRef]
- S. E. Kipyego and E. Serem, “Sero-Prevalence and Risk Factors of Infectious Bovine Rhinotracheitis in the Smallholder Dairy Farms of Naari Sub-Location of Meru County, Kenya,” 2019.
- B. J. Trangadia, S. K. Rana, K. Nagmani, and V. A. Srinivasan, “Serological Investigation of Bovine Brucellosis, Johneâ€TM s Disease and Infectious Bovine Rhinotracheitis in Two States of India,” J. Adv. Vet. Res., vol. 2, no. 1, pp. 38–41, 2012.
- G. Nikbakht, S. Tabatabaei, S. Lotfollahzadeh, B. Nayeri Fasaei, A. Bahonar, and M. Khormali, “Seroprevalence of bovine viral diarrhoea virus, bovine herpesvirus 1 and bovine leukaemia virus in Iranian cattle and associations among studied agents,” J. Appl. Anim. Res., vol. 43, no. 1, pp. 22–25, 2015. [CrossRef]
- J. C. Segura-Correa, D. Domínguez-Díaz, R. Avalos-Ramírez, and J. Argaez-Sosa, “Intraherd correlation coefficients and design effects for bovine viral diarrhoea, infectious bovine rhinotracheitis, leptospirosis and neosporosis in cow–calf system herds in North-eastern Mexico,” Prev. Vet. Med., vol. 96, no. 3–4, pp. 272–275, 2010. [CrossRef]
- F. Boelaert et al., “Prevalence of bovine herpesvirus-1 in the Belgian cattle population,” Prev. Vet. Med., vol. 45, no. 3–4, pp. 285–295, 2000. [CrossRef]
- D. Barrett et al., “Prevalence of bovine viral diarrhoea virus (BVDV), bovine herpes virus 1 (BHV 1), leptospirosis and neosporosis, and associated risk factors in 161 Irish beef herds,” BMC Vet. Res., vol. 14, no. 1, p. 8, 2018. [CrossRef]
- B. Sibhat, G. Ayelet, E. Skjerve, E. Z. Gebremedhin, and K. Asmare, “Bovine herpesvirus-1 in three major milk sheds of Ethiopia: Serostatus and association with reproductive disorders in dairy cattle,” Prev. Vet. Med., vol. 150, no. May 2017, pp. 126–132, 2018. [CrossRef]
- E. Demil et al., “Prevalence of bovine viral diarrhea virus antibodies and risk factors in dairy cattle in Gondar city, Northwest Ethiopia,” Prev. Vet. Med., vol. 191, p. 105363, 2021. [CrossRef]
- G. Van Schaik, Y. H. Schukken, M. Nielen, A. A. Dijkhuizen, H. W. Barkema, and G. Benedictus, “Probability of and risk factors for introduction of infectious diseases into Dutch SPF dairy farms: a cohort study,” Prev. Vet. Med., vol. 54, no. 3, pp. 279–289, 2002. [CrossRef]
- G. Kaur and M. Chandra, “Herpesvirus in Bovines: Importance of Bovine Herpesvirus Type 1,” Herpesviridae, p. 219, 2016.
- D. D. Shewie, C. Dima, A. Garoma, Y. Getachew, and H. Negussie, “Seroepidemiological study of bovine alphaherpesvirus 1 in the dairy cattle herds of Addis Ababa, Ethiopia,” Prev. Vet. Med., vol. 216, p. 105947, 2023. [CrossRef]
- A. Khaneabad, T. Taktaz, S. Goodarzi, and H. Momtaz, “BoHV--1 affects abortion and progesterone in dairy cows Bovine alphaherpesvirus 1 (BoHV--1) seropositivity, progesterone levels and embryo loss of 30--day--old pregnant dairy cows in Zagros Industrial Dairy Farm in Shahrekord: Examination and analysis,” Vet. Med. Sci., vol. 9, no. 4, pp. 1934–1939, 2023. [CrossRef]
- D. A. Graham, “Bovine herpes virus-1 (BoHV-1) in cattle–a review with emphasis on reproductive impacts and the emergence of infection in Ireland and the United Kingdom,” Ir. Vet. J., vol. 66, no. 1, p. 15, 2013. [CrossRef]
- H. Yildiz and A. R. BABAOĞLU, “Molecular Investigation of Bovine Viral Diarrhea Virus, Bovine Herpes Virus-1 and Bovine Herpes Virus-4 Infections in Abortion Cases of Cattle in Van District, Turkey.,” Van Vet. J., vol. 33, no. 3, 2022. [CrossRef]
- H. U. Rehman, M. Rabbani, A. Ghafoor, A. Riaz, F. N. Awan, and S. Raza, “First isolation and genetic characterization of bovine herpesvirus 1 from cattle in Pakistan,” Pak. Vet. J, vol. 41, pp. 163–165, 2020. [CrossRef]
- C. Dima, K. Abdisa, and D. Zewde, “Bovine Herpesvirus-1 Seroprevalence and its Associated Risk Factors in Dairy Farms in Holeta Town , Oromia Region , Ethiopia,” vol. 7, no. 2, pp. 32–41, 2024.
Table 1.
Seroprevalence of BoHV-1 antibodies in Jimma town dairy cattle of different categories.
Table 1.
Seroprevalence of BoHV-1 antibodies in Jimma town dairy cattle of different categories.
| Risk factors |
Categories |
No of examined Animals |
No of positives |
Prevalence
(95% CI)
|
| Herd size |
≤10 Animals |
363 |
53 |
14.60% (11.13-18.65) |
| >10 Animals |
76 |
11 |
14.66% (7.55 – 24.72) |
| Age |
> 6Year |
137 |
32 |
23.35% (16.55-31.34) |
| 3-6 Year |
174 |
27 |
15.51% (10.48-21.76) |
| < 3 Year |
128 |
5 |
3.90% (1.28 -8.88) |
| Animal Origin |
Homebred |
355 |
41 |
11.50% (8.58 -14.8) |
| Purchased |
84 |
23 |
27.4% (17.9 - 36.9) |
| Body Condition |
Poor |
133 |
31 |
23.30% (16.9-31.4) |
| Medium |
238 |
28 |
11.70% (7.96 -16.5) |
| Good |
68 |
5 |
7.35% (5.46 -23.3) |
| Parity |
Multiparous |
131 |
32 |
24.42% (16.9-31.57) |
| Uniparous |
98 |
19 |
19.38% (11.5-27.16) |
| Pregnant |
13 |
2 |
15.38% (-4.26-34.5) |
| Non pregnant |
197 |
11 |
5.58% (2.53-9.06) |
| Abortion history |
Aborted |
74 |
37 |
50.0% (38.6-6.85) |
| Not Aborted |
365 |
27 |
8.12% (5.37-11.67) |
| Repeated Breeding |
Present |
302 |
55 |
18.21% (14.02-23.05) |
| Absent |
137 |
9 |
6.56% (3.04- 12.10) |
| Infertility |
Yes |
118 |
42 |
35.59% (26.99- 44.93) |
| No |
321 |
22 |
7.63% (4.33-10.92) |
| Respiratory Problem |
Present |
93 |
38 |
40.8% (30.8-50.78) |
| Absent |
346 |
26 |
7.50% (5.17-10.8) |
Table 2.
Association of risk factors with BoHV-1 antibodies seropositivity in the study area.
Table 2.
Association of risk factors with BoHV-1 antibodies seropositivity in the study area.
| Risk factors |
Categories |
Univariable analysis |
Multivariable analysis |
| COR |
P-value |
AOR |
P-value |
| Age |
≥ 6Year |
7.37 (2.77-19.63) |
.0001 |
1.30 (0.30-5.62) |
|
| 3-6 Year |
4.38 (1.63-11.74) |
.003 |
0.542 (0.12-2.39) |
0.420 |
| < 3 Year |
* |
|
* |
|
| Animal Origin |
Purchased |
2.88 (1.61-5.15) |
.0001 |
94 (11.05 -810.25) |
0.0001 |
| Homebred |
* |
|
* |
|
| Body Condition |
Poor |
3.82 (1.41-10.38) |
.008 |
5.79 (.49.48- 67.83 |
0.162 |
| Medium |
1.68 (0.62-4.53) |
.306 |
|
|
| |
Good |
* |
|
* |
|
| Parity |
Multiparous |
5.46 (2.64-11.30) |
.0001 |
2.77 (1.04-7.37) |
|
| Uniparous |
4.06 (1.84-8.94) |
.0001 |
2.30 (1.41-11.83) |
0.071 |
| Pregnant |
3.07(0.60-15) |
.176 |
1.30 (0.20-8.34) |
|
| |
Heifers |
* |
|
* |
|
| Abortion history |
Aborted |
12.90 (7.05-23.61) |
.0001 |
20.06 (2.53-159.00) |
0.005 |
| Not Aborted |
* |
|
* |
|
| Repeat Breeder |
Yes |
3.16 (1.51 -6.61) |
.002 |
0.99 (0.08-11.79) |
0.999 |
| No |
* |
|
* |
|
| Infertility |
Present |
7.51 (4.22-13.34)0 |
.0001 |
37.38 (4.39-317.83) |
0.001 |
| Absent |
* |
|
|
|
| Respiratory problems |
Present |
8.50 (4.78-15.11) |
.000 |
0.38 (0.38-386) |
0.417 |
| Absent |
* |
|
* |
|
Table 4.
Logistic regression analysis of BoHV-1 in relation to herd level farm managements.
Table 4.
Logistic regression analysis of BoHV-1 in relation to herd level farm managements.
| Key questions |
Categories |
No of respondents (%) |
No of positive herd |
Odds Ratio |
P-value |
| Herd size |
< 10 animals |
13 (33.3%) |
69.2% (9/13) |
0.26 (0 -2.30) |
0.24 |
| ≥ 10 animals |
26 (66.6%) |
57.6% (15/26) |
| Service type |
AI |
37 (94.8%) |
59.45 (22/37) |
1 (0.025-+Inf) |
1.00 |
| Both AI and Bull |
2 (5.12%) |
100% (2/2) |
| Feeding Place |
On feeder |
29 (74.3%) |
58.6% (17/29) |
1.95 (0.28-17.1) |
0.70 |
| On floor |
10 (25.6%) |
70% (7/10) |
| Watering Practice |
Individual |
35 (89.7%) |
60% (21/35) |
0.90 (0.009-83) |
1.00 |
| Group watering |
4 (10.25%) |
75% (3/4) |
| Stock Source |
Home breed |
26 (66.6%) |
57.6% (15/26) |
5.35(0.61-+Inf) |
0.13 |
| Home & Purchase |
13 (33.3%) |
69.2% (9/13) |
Table 5.
The exact logistic regression analysis of BoHV-1 in relation to herd level health problems.
Table 5.
The exact logistic regression analysis of BoHV-1 in relation to herd level health problems.
| Key questions/ variables |
Categories |
No of respondents |
NPH (%) |
Odds Ratio |
P-value |
| Problem of RFM |
No |
25 (64.1%) |
15 (60% ) |
0.59 (0.063-4.11) |
0.868 |
| Yes |
14 (35.8%) |
9 (64.3%) |
| Presence of repeated breeder |
Absent |
5 (12.8%) |
2(40%) |
2.62 (0.08-90) |
0.974 |
| Present |
34 (87.1%) |
22 (64.7%) |
| Abortion stage |
No Abortion |
11 (28.2%) |
4 (36.3%) |
.22 (1.12-5.15) |
0.017 |
| 1-4 month |
8 (20.5%) |
3 (37.5%) |
| 8-9 month |
5 (12.8%) |
4 (80%0 |
| 5-7 months |
15 (38.4%) |
13 86.6%) |
| Respiratory problem |
Absent |
14 (35.8%) |
5 (35.7%) |
3.32 (0.53-23.58) |
0.258 |
| Present |
25 (64.1%) |
19 (76%) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).