Submitted:
16 September 2025
Posted:
18 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Composition of I. badionotus
2.2. Preliminary Study
2.3. Collagen Isolation and Characterisation
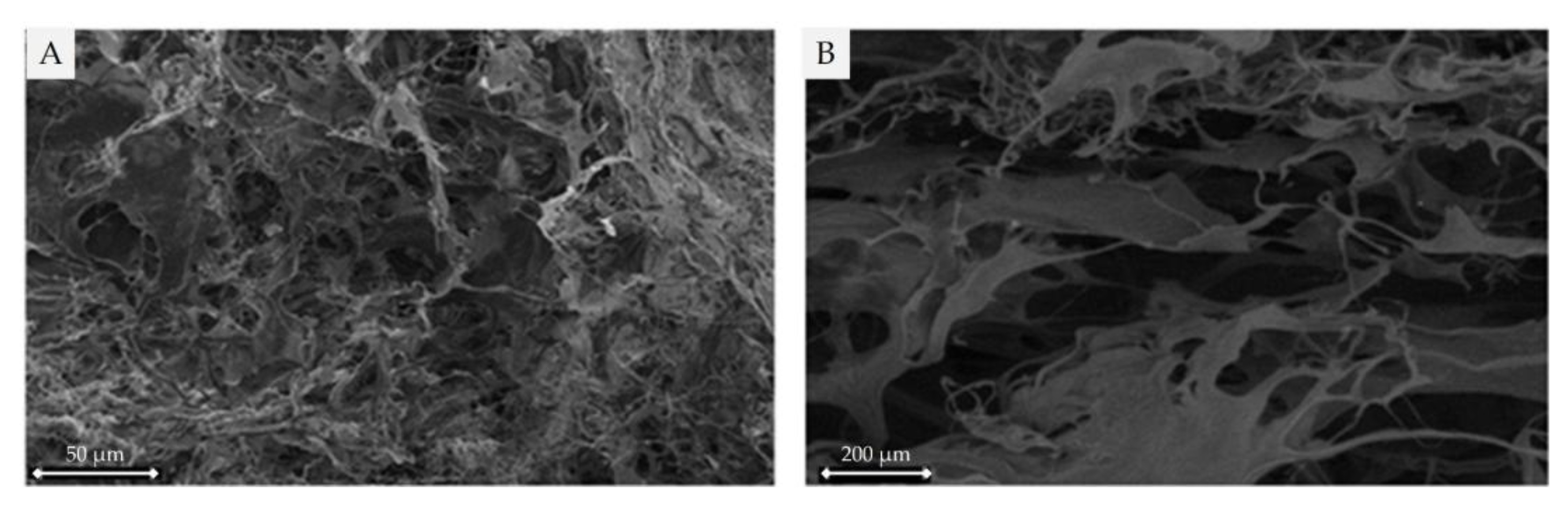
2.3.1. Scanning electron microscope
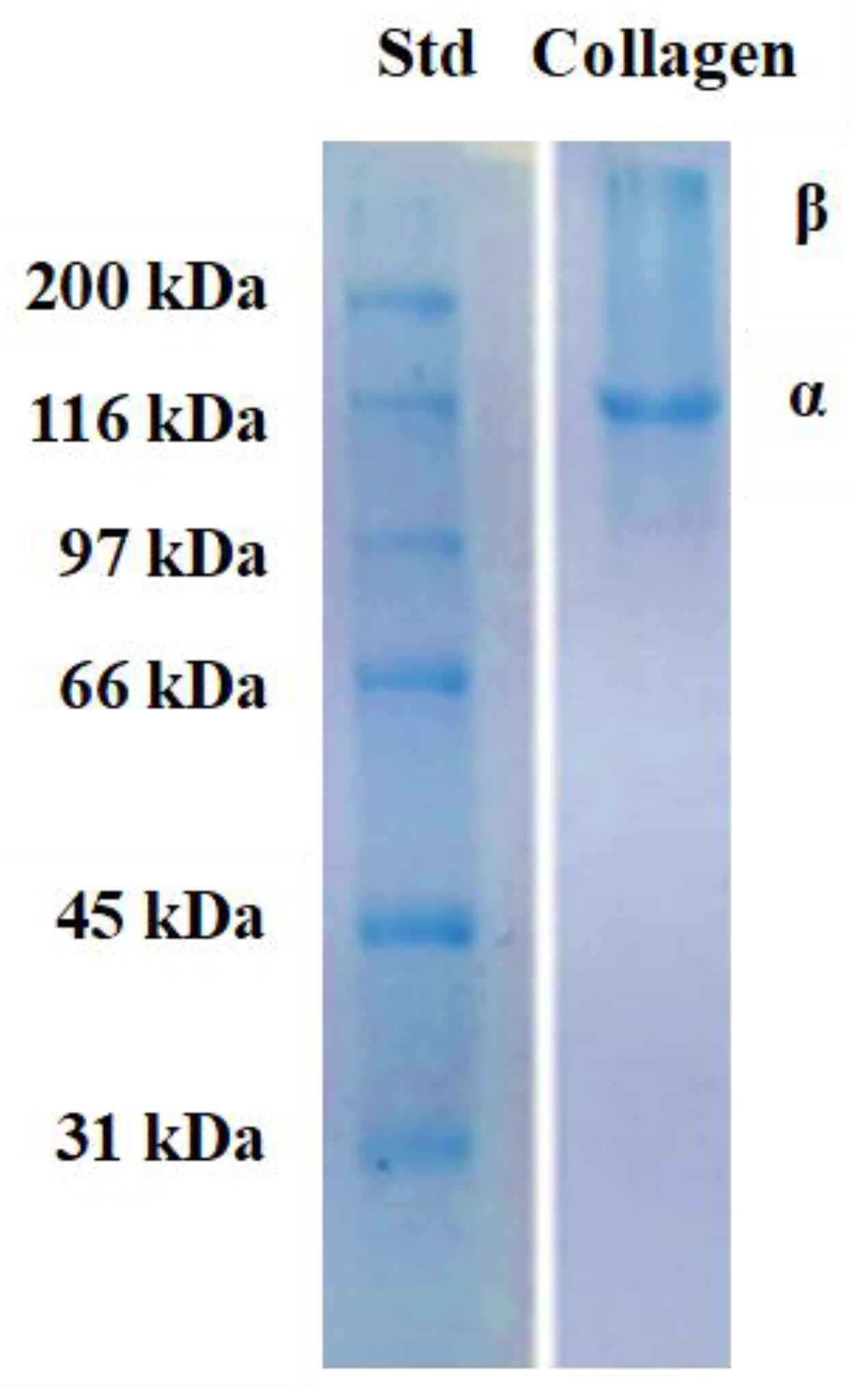
2.3.2. SDS-PAGE
2.3.3. Amino acid composition
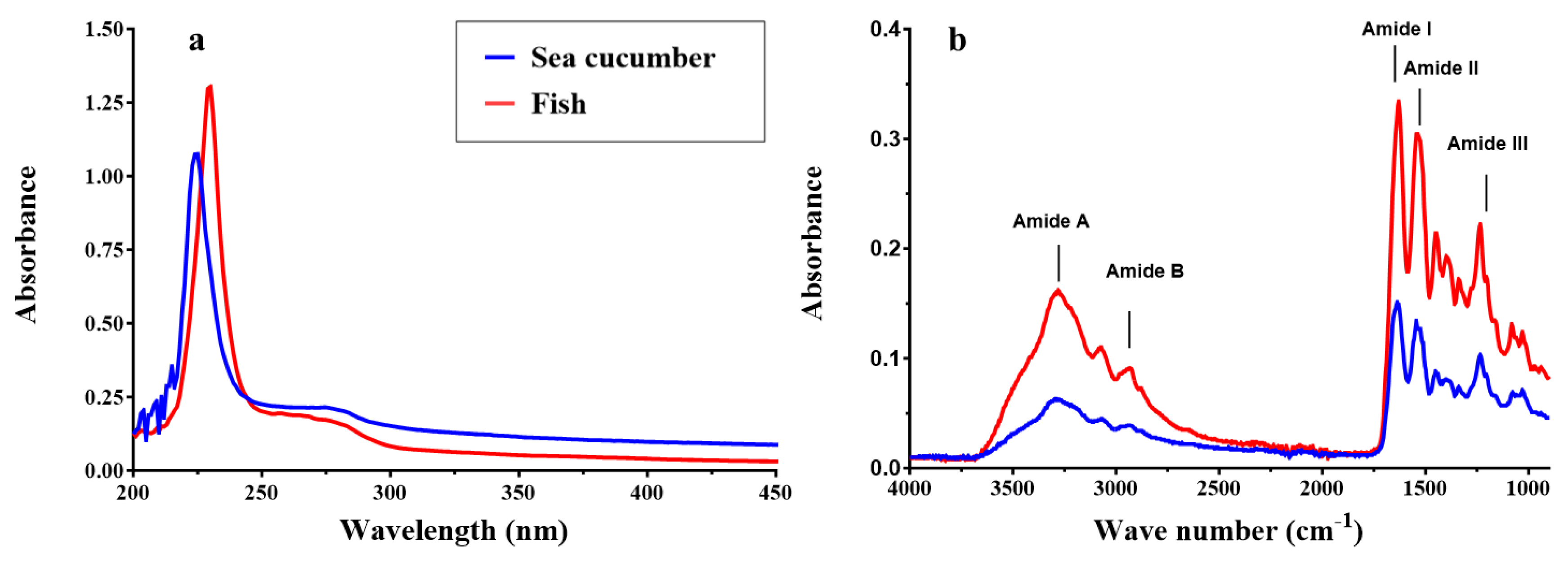
2.3.4. UV-visible spectra and Fourier transform infrared spectroscopy.
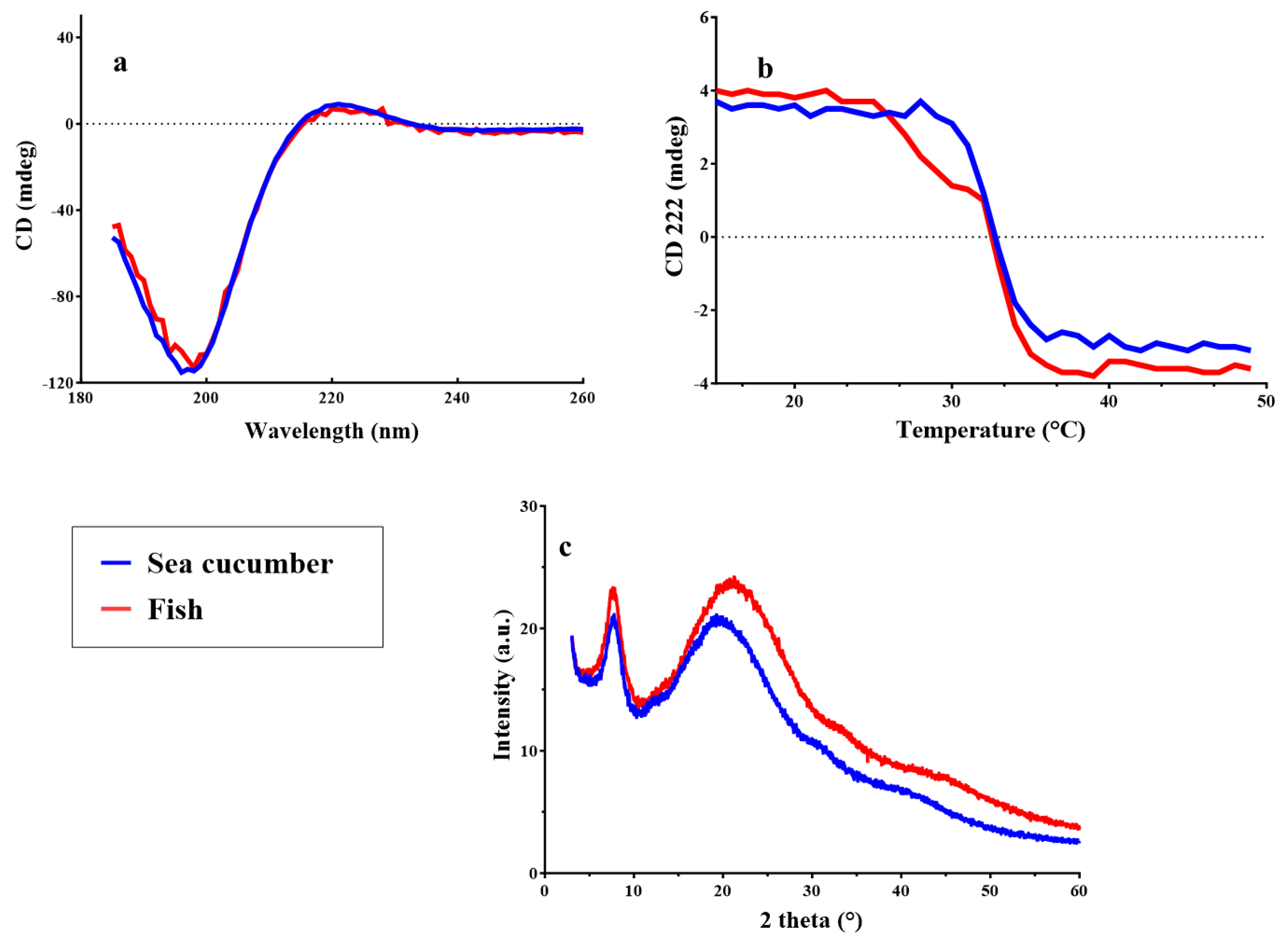
2.3.5. Circular dichroism and x-ray diffraction
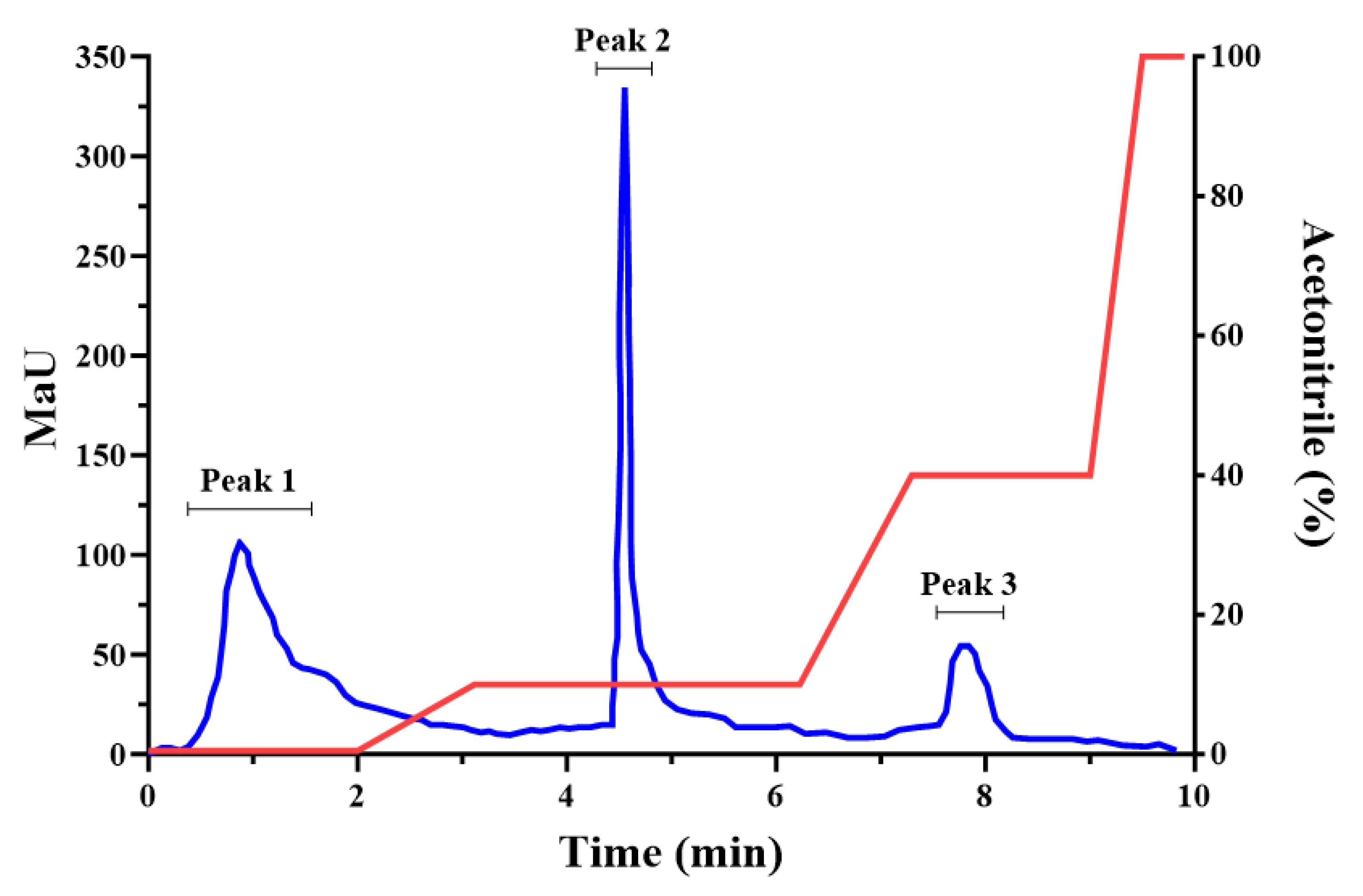
2.3.6. Collagen hydrolysis and ultrafiltration
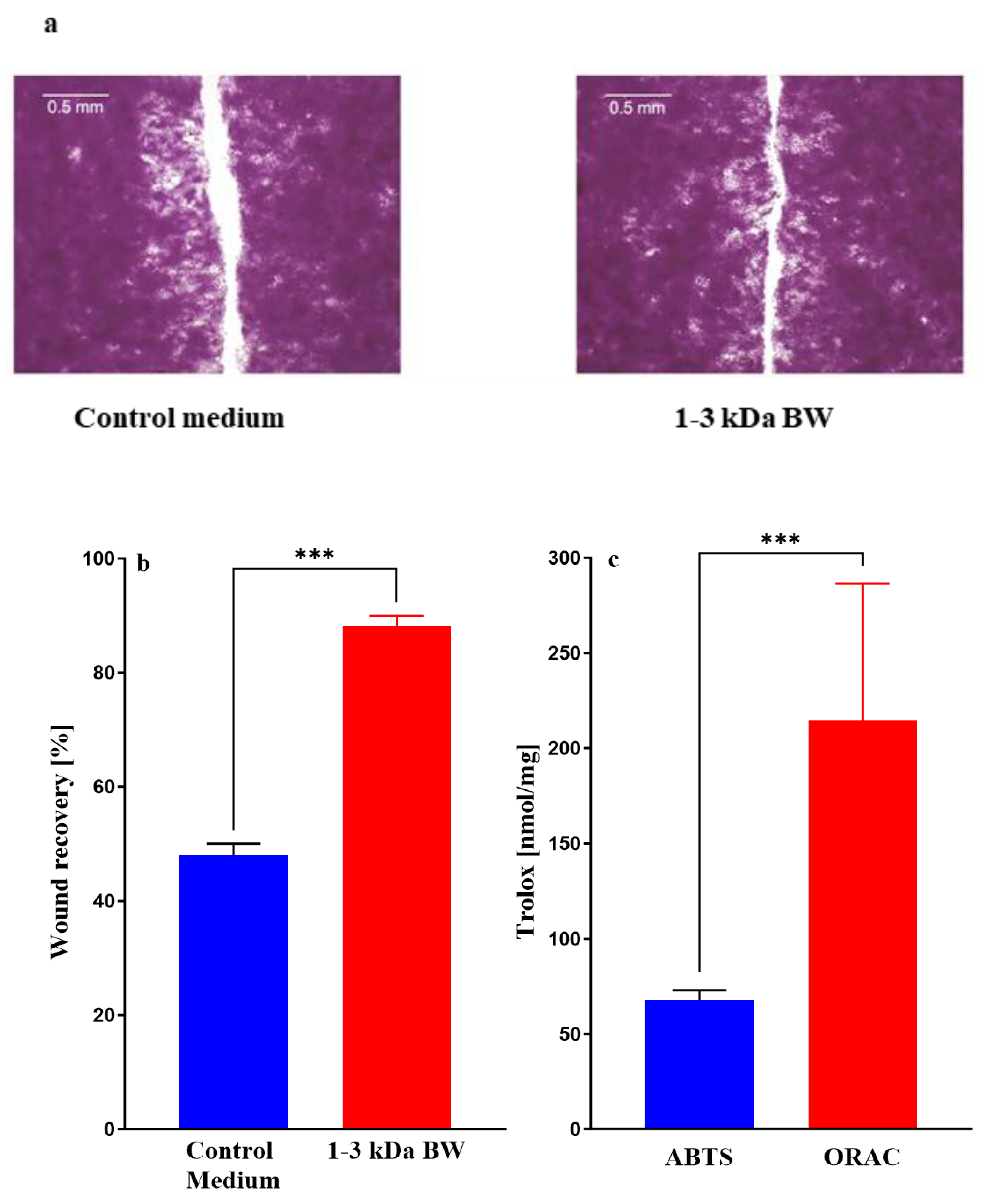
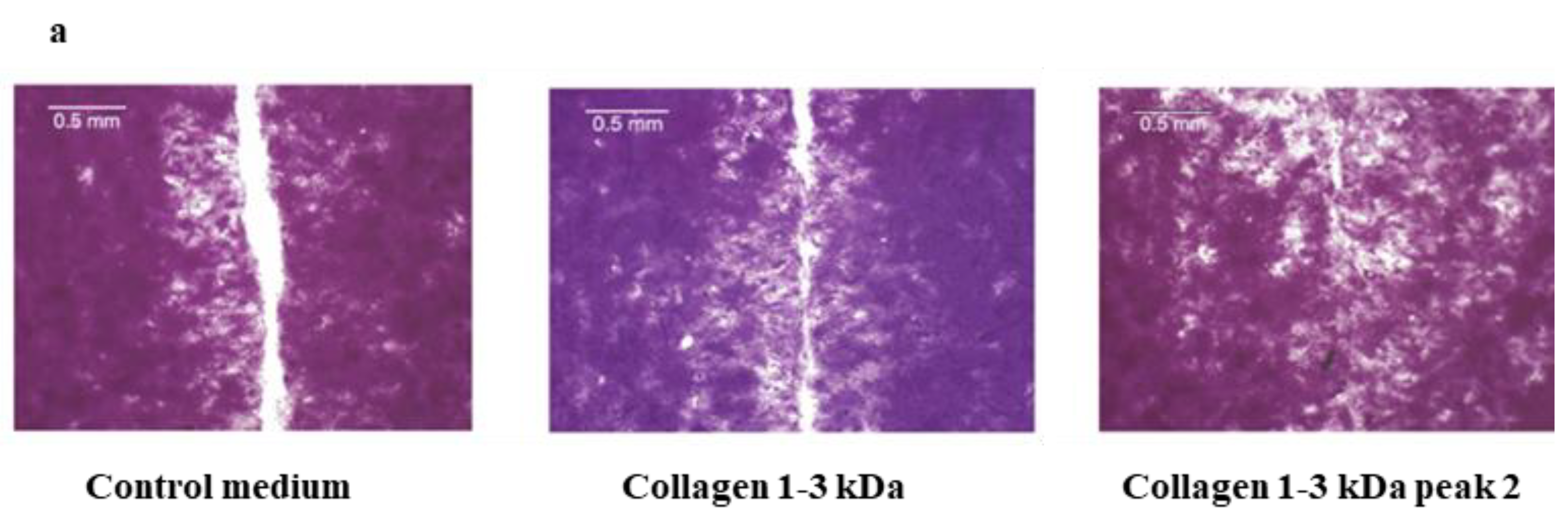
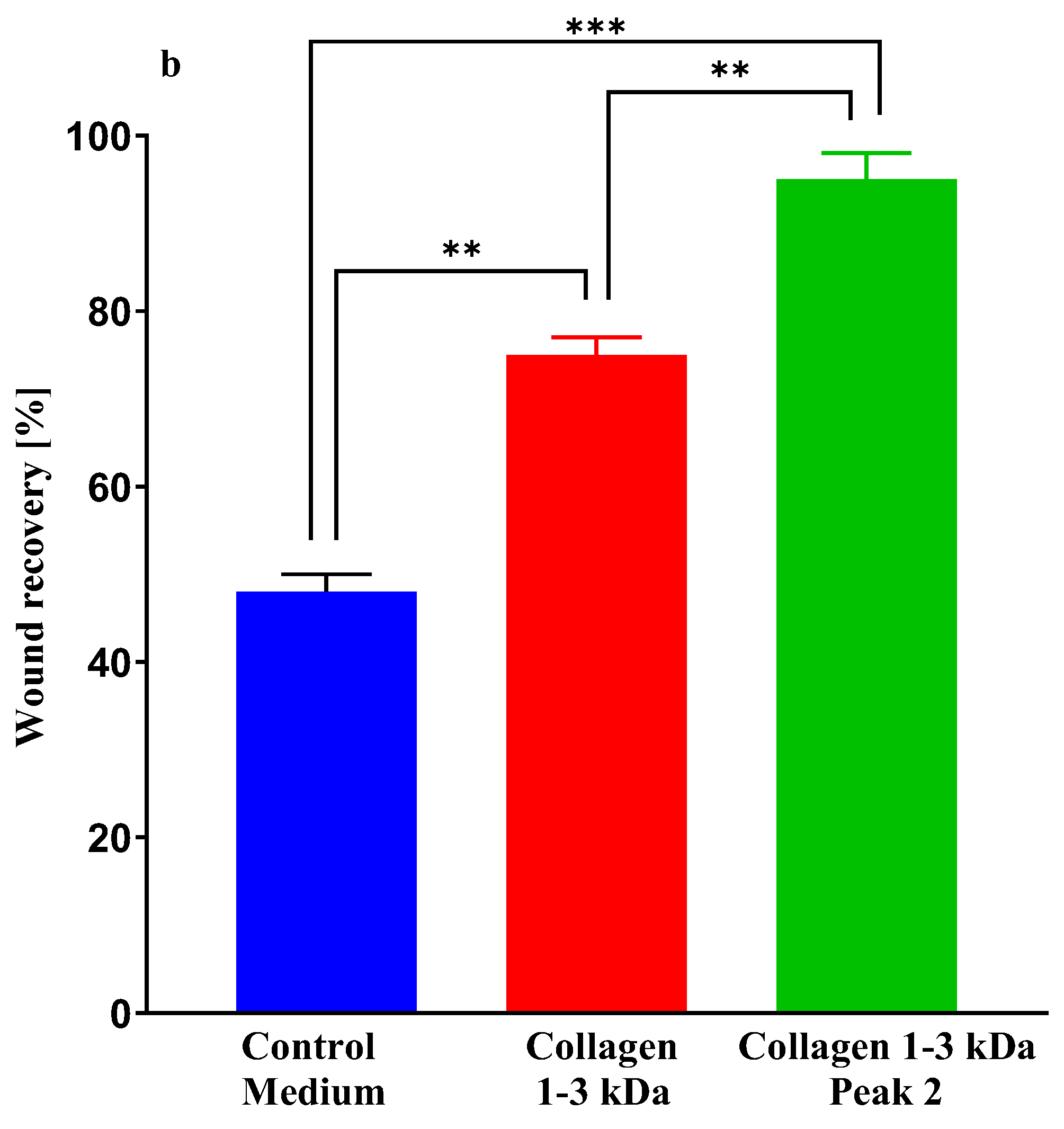
2.3.7. Wound-healing assay
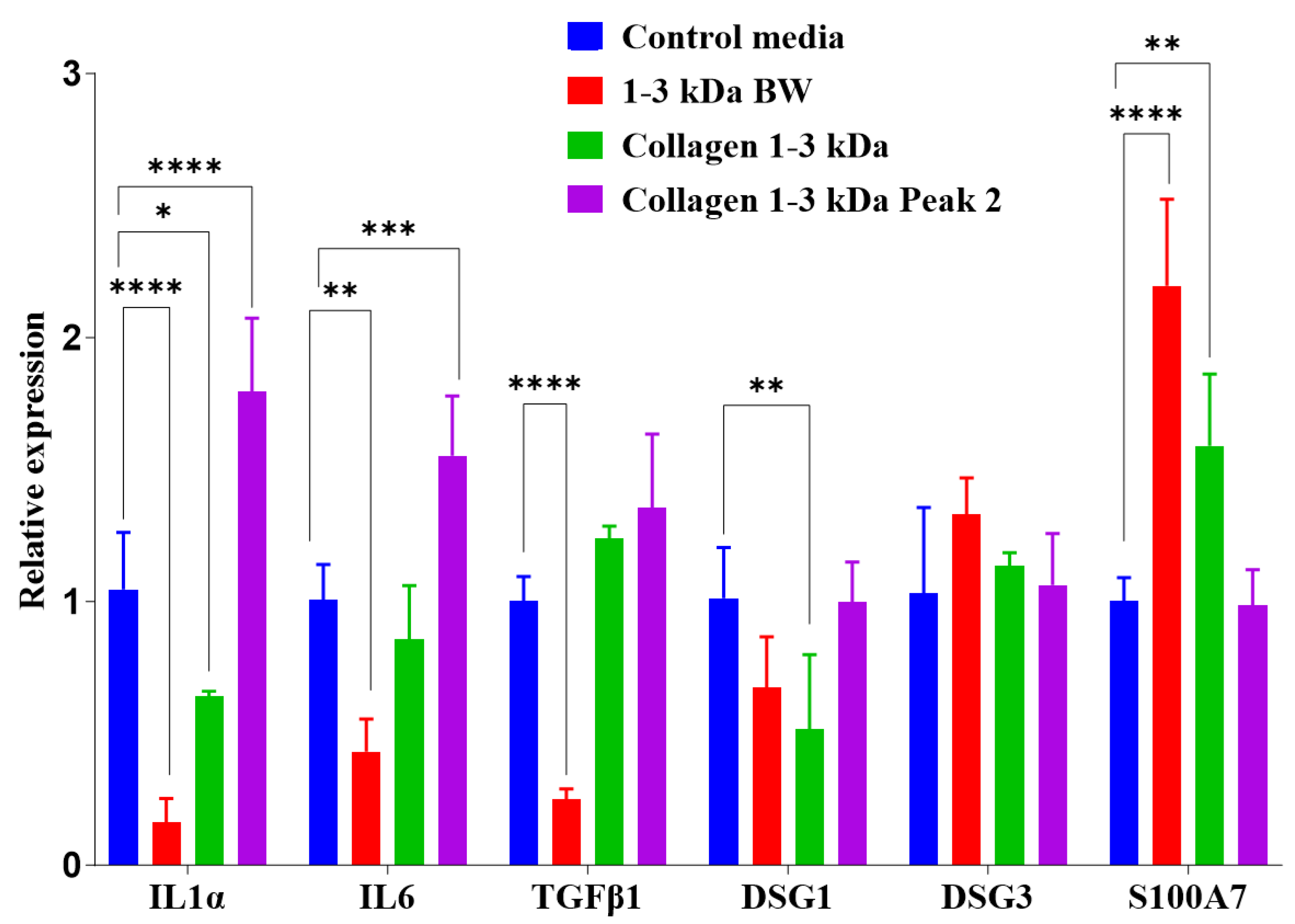
2.3.8. Gene expression analysis
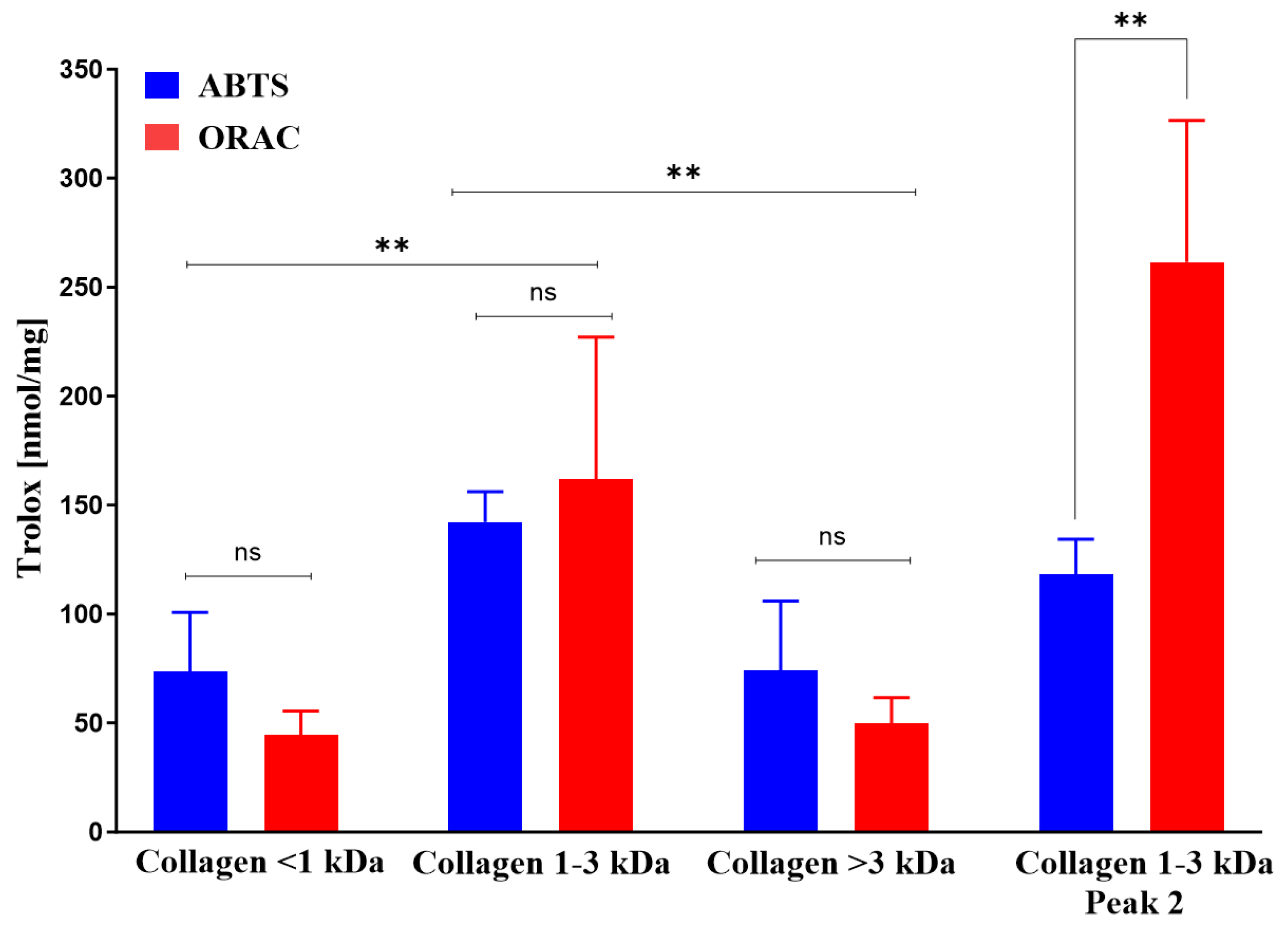
2.3.9. Antioxidant activities of collagen fractions
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Collection and Processing of Sea Cucumber
4.3. Proximate Composition
4.4. Skin-Free Body Wall Digest
4.5. Pepsin-Soluble Collagen Extraction
4.6. Collagen characterization
4.6.1. Electrophoresis
4.6.2. Amino acid composition
4.6.3. X-ray Diffraction (XRD) and Circular Dichroism (CD)
4.6.4. UV-VIS and Fourier Transform Infrared (FTIR) Spectra
4.7. Collagen Hydrolysate Preparation
4.7.1. Ultrafiltration
4.7.2. Flash Chromatography of Collagen 1-3 kDa
4.7.3. Antioxidant activity
4.7.3.1. ABTS+ Scavenging Activity
4.7.3.2. Oxygen Radical Absorbance Capacity (ORAC)
4.7.3.3. Further Analyses
4.8. Wound healing In Vitro
4.8.1. Scratch Wound Healing Assay
4.8.2. Gene expression
4.9. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPH | 2,2-Azobis (2-amidinopropane) dihydrochloride |
| ABTS | 2,2-Azinobis[3-ethylbenzothiazoline-6-sulfonic acid] |
| ACE | Angiotensin I-converting enzyme |
| Β-actin | Beta actin |
| BW | Body wall |
| CD | Circular dichroism |
| CFU | Colony-forming units |
| CINVESTAV | Centro de Investigación y de Estudios Avanzados |
| CONAPESCA | Comisión Nacional de Pesca y Acuacultura |
| DGS1 | Desmoglein-1 |
| DGS3 | Desmoglein-3 |
| DH | Degree of hydrolysis |
| DPP4 | Dipeptidyl Peptidase-4 |
| EDTA | Ethylenediamine-tetra-acetic |
| EGF | epidermal growth factor |
| FBS | Foetal bovine serum |
| FL | fluorescein (3,6-dihydroxyspiro [isobenzofuran-1[3H],9[9H]-xanthen]-3-one) |
| FTIR | Fourier transform infrared |
| HaCat | keratinocyte |
| IC50 | Half-maximal inhibitory concentration |
| IL1α | Interleukin 1α |
| IL6 | Interleukin 6 |
| KGF | keratinocyte growth factor |
| MTT | thiazolyl blue tetrazolium bromide |
| NAC | N-Acetyl-L-cysteine |
| ORAC | Oxygen radical absorbance capacity |
| PBS | Phosphate-buffered saline |
| PITC | Phenyl isothiocyanate |
| qPCR | Quantitative polychrome chain reaction |
| RP-UHPLC | -ultra high pressure liquid chromatography |
| S100A7 | S100 calcium-binding protein A7 or psoriasin |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SEM | Scanning electron microscope |
| TE | Trolox equivalents |
| TGFα | Transforming growth factor alpha |
| TGFβ1 | Transforming growth factor beta one |
| XRD | X-ray diffraction |
References
- Mercier, A.; Gebruk, A.; Kremenetskaia, A.; Hamel, J.-F. An Overview of Taxonomic and Morphological Diversity in Sea Cucumbers (Holothuroidea: Echinodermata). In The World of Sea Cucumbers; Mercier, A., Hamel, J.-F., Suhrbier, A., Pearce, C., Eds.; Elsevier, 2024; pp. 3–15.
- Mercier, A.; Purcell, S.W.; Montgomery, E.M.; Kinch, J.; Byrne, M.; Hamel, J.-F. Revered and Reviled: The Plight of the Vanishing Sea Cucumbers. Ann Rev Mar Sci 2025, 17, 115–142. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and Health Benefit Effects of Functional Sea Cucumbers. J Tradit Complement Med 2018, 8, 341–351. [Google Scholar] [CrossRef]
- Okada, A.; Udagawa, S.; Kohtsuka, H.; Hayashi, Y.; Miura, T. Gene-Expression Patterns during Regeneration of the Multi-Organ Complex after Evisceration in the Sea Cucumber Eupentacta Quinquesemita. Front Mar Sci 2024, 11. [Google Scholar] [CrossRef]
- Liu, R.; Ren, X.; Wang, J.; Chen, T.; Sun, X.; Lin, T.; Huang, J.; Guo, Z.; Luo, L.; Ren, C.; et al. Transcriptomic Analysis Reveals the Early Body Wall Regeneration Mechanism of the Sea Cucumber Holothuria Leucospilota after Artificially Induced Transverse Fission. BMC Genomics 2023, 24, 766. [Google Scholar] [CrossRef] [PubMed]
- Maskur, M.; Sayuti, M.; Widyasari, F.; Haryo Bimo Setiarto, R. Bioactive Compound and Functional Properties of Sea Cucumbers as Nutraceutical Products. Reviews in Agricultural Science 2024, 12, 45–64. [Google Scholar] [CrossRef]
- Shou, Y.; Feng, C.; Lu, Q.; Mao, X.; Huang, H.; Su, Z.; Guo, H.; Huang, Z. Research Progress on the Chemical Components and Biological Activities of Sea Cucumber Polypeptides. Front Pharmacol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ahmed, F.; Zhang, M.; Sperou, N.; Franco, C.M.M.; Feng, Q.; Zhang, W. In Vivo and Clinical Studies of Sea Cucumber-Derived Bioactives for Human Health and Nutrition From 2012-2021. Front Mar Sci 2022, 9. [Google Scholar] [CrossRef]
- Das, A.; Hossain, A.; Dave, D. The Effect of Pre-Treatment and the Drying Method on the Nutritional and Bioactive Composition of Sea Cucumbers—A Review. Applied Sciences 2024, 14, 6475. [Google Scholar] [CrossRef]
- Sales, S.; Lourenço, H.M.; Bandarra, N.M.; Afonso, C.; Matos, J.; Botelho, M.J.; Pessoa, M.F.; Félix, P.M.; Veronez, A.; Cardoso, C. How Biological Activity in Sea Cucumbers Changes as a Function of Species and Tissue. Foods 2023, 13, 35. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, L.; Xia, X.; Hu, W.; Zhou, P. Effect of Geographic Variation on the Proteome of Sea Cucumber (Stichopus Japonicus). Food Research International 2020, 136, 109498. [Google Scholar] [CrossRef]
- Nuringtyas, T.R.; Hidayati, L.; Rohmah, Z.; Paramita, D.K.; Suparmin, A.; Prinanda, H.H.; Febryzalita, Q.N.; Utami, S.L.; Zulfa, L.F.; Ardiansyah, B.K.; et al. Bioactive Peptides from Sea Cucumbers and Sea Urchins: Therapeutic Roles and Mechanistic Insights. Trends in Sciences 2025, 22, 9513. [Google Scholar] [CrossRef]
- Popov, A.; Kozlovskaya, E.; Rutckova, T.; Styshova, O.; Makhankov, V.; Vakhrushev, A.; Hushpulian, D.; Gazaryan, I.; Son, O.; Tekutyeva, L. Matrikines of Sea Cucumbers: Structure, Biological Activity and Mechanisms of Action. Int J Mol Sci 2024, 25, 12068. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Castillo, L.; Grant, G.; Kantún-Moreno, N.; Barrera-Pérez, H.A.; Montero, J.; Olvera-Novoa, M.A.; Carrillo-Cocom, L.M.; Acevedo, J.J.; Puerto-Castillo, C.; May Solís, V.; et al. A Glycosaminoglycan-Rich Fraction from Sea Cucumber Isostichopus Badionotus Has Potent Anti-Inflammatory Properties In Vitro and In Vivo. Nutrients 2020, 12, 1698. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Castillo, L.; Grant, G.; Kantún-Moreno, N.; Acevedo-Fernández, J.J.; Puc-Sosa, M.; Montero, J.; Olvera-Novoa, M.A.; Negrete-León, E.; Santa-Olalla, J.; Ceballos-Zapata, J.; et al. Sea Cucumber (Isostichopus Badionotus ) Body-Wall Preparations Exert Anti-Inflammatory Activity in Vivo. PharmaNutrition 2018, 6, 74–80. [Google Scholar] [CrossRef]
- Wiegand, C.; Dirksen, A.; Tittelbach, J. Treatment with a Red-laser-based Wound Therapy Device Exerts Positive Effects in Models of Delayed Keratinocyte and Fibroblast Wound Healing. Photodermatol Photoimmunol Photomed 2024, 40. [Google Scholar] [CrossRef]
- Kotian, S.R.; Bhat, K.M.R.; Padma, D.; Pai, K.S.R. Influence of Traditional Medicines on the Activity of Keratinocytes in Wound Healing: An in-Vitro Study. Anat Cell Biol 2019, 52, 324. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Solís, M.J.; Gullian-Klanian, M.; Toledo-López, V.; Lora-Vilchis, M.C. Proximate composition and fatty acid profile of the sea cucumber isostichopus badionotus and holothuria floridana Food Sci Technol Res 2021, 27, 319–327. [CrossRef]
- Acosta, E.J.; Rodríguez-Forero, A.; Werding, B.; Kunzmann, A. Ecological and Reproductive Characteristics of Holothuroids Isostichopus Badionotus and Isostichopus Sp. in Colombia. PLoS One 2021, 16, e0247158. [Google Scholar] [CrossRef]
- Farooq, S.; Ahmad, M.I.; Zheng, S.; Ali, U.; Li, Y.; Shixiu, C.; Zhang, H. A Review on Marine Collagen: Sources, Extraction Methods, Colloids Properties, and Food Applications. Collagen and Leather 2024, 6, 11. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Gkalogianni, E.Z.; Apostologamvrou, C.; Voulgaris, K.; Varkoulis, A.; Vafidis, D. Proximate Compositions and Fatty Acid Profiles of Raw and Processed Holothuria Polii and Holothuria Tubulosa from the Aegean Sea. Sustainability 2024, 16, 6048. [Google Scholar] [CrossRef]
- Talab et al., A.S. Proximate Composition and Quality Properties of Some Egyptian Sea Cucumber Species. Egypt J Aquat Biol Fish 2024, 28, 379–396. [CrossRef]
- Wen, Y.; Dong, X.; Zamora, L.N.; Jeffs, A.G.; Quek, S.Y. Physicochemical Properties, Functionalities, and Antioxidant Activity of Protein Extracts from New Zealand Wild Sea Cucumbers (Australostichopus Mollis). Foods 2024, 13, 2735. [Google Scholar] [CrossRef] [PubMed]
- Muhsin, M.F.; Fujaya, Y.; Hidayani, A.A.; Fazhan, H.; Wan Mahari, W.A.; Lam, S.S.; Shu-Chien, A.C.; Wang, Y.; Afiqah-Aleng, N.; Rukminasari, N.; et al. Bridging the Gap between Sustainability and Profitability: Unveiling the Untapped Potential of Sea Cucumber Viscera. PeerJ 2023, 11, e16252. [Google Scholar] [CrossRef] [PubMed]
- Sugesti Yogi Pamungkas; Florensius Eko Dwi Haryono Bioprospecting of Sea Cucumber (Holothuria Sp. ) as Industries and Functional Foods for Human Health. International Journal of Science and Research Archive 2023, 10, 669–690. [Google Scholar] [CrossRef]
- Purcell, S.W.; Lovatelli, A.; González-Wangüemert, M.; Solís-Marín, F.A.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World; No. 6, Rev. 1.; FAO: Rome, 2023; ISBN 978-92-5-137793-2. [Google Scholar]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-Collagen-Hydroxyapatite Membranes for Tissue Engineering. J Mater Sci Mater Med 2022, 33, 18. [Google Scholar] [CrossRef]
- Teng, S.; Lee, E.; Wang, P.; Shin, D.; Kim, H. Three-layered Membranes of Collagen/Hydroxyapatite and Chitosan for Guided Bone Regeneration. J Biomed Mater Res B Appl Biomater 2008, 87B, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sun, N.; Lu, Z.; Zheng, J.; Zhang, S.; Lin, S. The Potential Mechanisms of Skin Wound Healing Mediated by Tetrapeptides from Sea Cucumber. Food Biosci 2023, 53, 102742. [Google Scholar] [CrossRef]
- Ibrahim, N. ‘Izzah; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int J Environ Res Public Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Pérez-Vega, J.A.; Olivera-Castillo, L.; Gómez-Ruiz, J.Á.; Hernández-Ledesma, B. Release of Multifunctional Peptides by Gastrointestinal Digestion of Sea Cucumber (Isostichopus Badionotus). J Funct Foods 2013, 5, 869–877. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lim, H.K.; Lee, S.; Hwang, H.C.; Cho, S.K.; Cho, M. Pepsin-Solubilised Collagen (PSC) from Red Sea Cucumber (Stichopus Japonicus) Regulates Cell Cycle and the Fibronectin Synthesis in HaCaT Cell Migration. Food Chem 2012, 132, 487–492. [Google Scholar] [CrossRef]
- Atanassova, M.R.; Mildenberger, J.; Hansen, M.D.; Tamm, T. Microstructure of Sea Cucumber Parastichopus Tremulus Peptide Hydrogels and Bioactivity in Caco-2 Cell Culture Model. Gels 2025, 11, 280. [Google Scholar] [CrossRef]
- Man, J.; Abd El-Aty, A.M.; Wang, Z.; Tan, M. Recent Advances in Sea Cucumber Peptide: Production, Bioactive Properties, and Prospects. Food Front 2023, 4, 131–163. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Antioxidant Potential and Physicochemical Properties of Protein Hydrolysates from Body Parts of North Atlantic Sea Cucumber (Cucumaria Frondosa). Food Production, Processing and Nutrition 2021, 3, 3. [Google Scholar] [CrossRef]
- Barzkar, N.; Attaran-Fariman, G.; Taheri, A.; Venmathi Maran, B.A. Extraction and Characterization of Collagen and Gelatin from Body Wall of Sea Cucumbers Stichopus Horrens and Holothuria Arenicola. PeerJ 2024, 12, e18149. [Google Scholar] [CrossRef] [PubMed]
- Nabilla, N.; Shofiyah, I. ; Sugiharto; Alvitasari, D.; Sumarsih, S.; Khaleyla, F.; Wirawati, I.; Winarni, D. Organization, Density, and Content of Collagen in the Body Wall of Sea Cucumbers Acaudina Rosettis and Phyllophorus Sp. Aquac Fish. [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea Cucumber Derived Type I Collagen: A Comprehensive Review. Mar Drugs 2020, 18, 471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Q.; Tan, M.; Chen, Z.; Zheng, H.; Gao, J.; Lin, H.; Zhu, G.; Cao, W. Characterization and Film-Forming Properties of Collagen from Three Species of Sea Cucumber from the South China Sea: Emphasizing the Effect of Transglutaminase. Int J Biol Macromol 2025, 294, 139321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, W.; Li, H.; Che, H.; Xie, W.; Ju, W.; Qi, H.; Dong, X. Effect of Ca2+ on the Structure of Collagen Fibers in Sea Cucumber (Apostichopus Japonicus) under Low-Temperature Tenderization Condition. Food Chem X 2025, 27, 102450. [Google Scholar] [CrossRef]
- Cruz-López, H.; Rodríguez-Morales, S.; Enríquez-Paredes, L.M.; Villarreal-Gómez, L.J.; True, C.; Olivera-Castillo, L.; Fernández-Velasco, D.A.; López, L.M. Swim Bladder of Farmed Totoaba Macdonaldi: A Source of Value-Added Collagen. Mar Drugs 2023, 21, 173. [Google Scholar] [CrossRef]
- Cruz-López, H.; Rodríguez-Morales, S.; Enríquez-Paredes, L.M.; Villarreal-Gómez, L.J.; Olivera-Castillo, L.; Cortes-Santiago, Y.; López, L.M. Comparison of Collagen Characteristic from the Skin and Swim Bladder of Gulf Corvina (Cynoscion Othonopterus). Tissue Cell 2021, 72, 101593. [Google Scholar] [CrossRef]
- Indriani, S.; Benjakul, S.; Quan, T.H.; Sitanggang, A.B.; Chaijan, M.; Kaewthong, P.; Petcharat, T.; Karnjanapratum, S. Effect of Different Ultrasound-Assisted Process Modes on Extraction Yield and Molecular Characteristics of Pepsin-Soluble Collagen from Asian Bullfrog Skin. Food Bioproc Tech 2023, 16, 3019–3032. [Google Scholar] [CrossRef]
- Li, P.-H.; Lu, W.-C.; Chan, Y.-J.; Ko, W.-C.; Jung, C.-C.; Le Huynh, D.T.; Ji, Y.-X. Extraction and Characterization of Collagen from Sea Cucumber (Holothuria Cinerascens) and Its Potential Application in Moisturizing Cosmetics. Aquaculture 2020, 515, 734590. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, T.; Hu, C.; Ren, C. Isolation and Characterization of Collagen from the Body Wall of Sea Cucumber Stichopus Monotuberculatus. J Food Sci 2015, 80. [Google Scholar] [CrossRef]
- Liu, Z.; Oliveira, A.C.M.; Su, Y.-C. Purification and Characterization of Pepsin-Solubilized Collagen from Skin and Connective Tissue of Giant Red Sea Cucumber (Parastichopus Californicus). J Agric Food Chem 2010, 58, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, W.; Meng, Y.; Tian, Q.; Hao, L.; Hou, H. Thermal Stability of Sea Cucumber Collagen and Effects of Gallic Acid Crosslinking. Int J Food Sci Technol 2023, 58, 2280–2288. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J Agric Food Chem 2022, 70, 7607–7625. [Google Scholar] [CrossRef]
- Yang, F.; Bai, X.; Dai, X.; Li, Y. The Biological Processes During Wound Healing. Regenerative Med 2021, 16, 373–390. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. In Methods Mol Biol; 2019; Vol. 2109, pp. 225–229. [CrossRef]
- Aw, Y.B.; Chen, S.; Yeo, A.; Dangerfield, J.A.; Mok, P. Development and Functional Testing of a Novel in Vitro Delayed Scratch Closure Assay. Histochem Cell Biol 2024, 162, 245–255. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C.; Elsner, P.; Tittelbach, J. Keratinocyte and Fibroblast Wound Healing In Vitro Is Repressed by Non-Optimal Conditions but the Reparative Potential Can Be Improved by Water-Filtered Infrared A. Biomedicines 2021, 9, 1802. [Google Scholar] [CrossRef]
- Farhangniya, M.; Samadikuchaksaraei, A. A Review of Genes Involved in Wound Healing. Med J Islam Repub Iran 2023. [CrossRef]
- Morgner, B.; Husmark, J.; Arvidsson, A.; Wiegand, C. Effect of a DACC-Coated Dressing on Keratinocytes and Fibroblasts in Wound Healing Using an in Vitro Scratch Model. J Mater Sci Mater Med 2022, 33, 22. [Google Scholar] [CrossRef]
- Ågren, M.S.; Litman, T.; Eriksen, J.O.; Schjerling, P.; Bzorek, M.; Gjerdrum, L.M.R. Gene Expression Linked to Reepithelialization of Human Skin Wounds. Int J Mol Sci 2022, 23, 15746. [Google Scholar] [CrossRef]
- Costantini, E.; Aielli, L.; Serra, F.; De Dominicis, L.; Falasca, K.; Di Giovanni, P.; Reale, M. Evaluation of Cell Migration and Cytokines Expression Changes under the Radiofrequency Electromagnetic Field on Wound Healing In Vitro Model. Int J Mol Sci 2022, 23, 2205. [Google Scholar] [CrossRef]
- Marinelli, L.; Cacciatore, I.; Costantini, E.; Dimmito, M.P.; Serra, F.; Di Stefano, A.; Reale, M. Wound-Healing Promotion and Anti-Inflammatory Properties of Carvacrol Prodrugs/Hyaluronic Acid Formulations. Pharmaceutics 2022, 14, 1468. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound Healing Promotion by Hyaluronic Acid: Effect of Molecular Weight on Gene Expression and In Vivo Wound Closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, S.N.; Dikmen, M.; Canturk, Z. Evaluation of Real Time Cell Proliferation, Anti-Inflammatory and Wound Healing Potential of Helenalin on HaCaT Keratinocytes Treated with Lipopolysaccharide Stimulated Monocytes. Indian J Pharm Sci 2021. [CrossRef]
- Shamilov, R.; Ackley, T.W.; Aneskievich, B.J. Enhanced Wound Healing- and Inflammasome-Associated Gene Expression in TNFAIP3-Interacting Protein 1- (TNIP1-) Deficient HaCaT Keratinocytes Parallels Reduced Reepithelialization. Mediators Inflamm 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Ferrone, A.; Costantini, E.; Franceschelli, S.; Pesce, M.; Speranza, L.; Amerio, P.; D’Angelo, C.; Felaco, M.; Grilli, A.; et al. Extremely Low-frequency Electromagnetic Fields Accelerates Wound Healing Modulating <scp>MMP</Scp> -9 and Inflammatory Cytokines. Cell Prolif 2018, 51. [Google Scholar] [CrossRef]
- Peplow, P. V.; Chatterjee, M.P. A Review of the Influence of Growth Factors and Cytokines in In Vitro Human Keratinocyte Migration. Cytokine 2013, 62, 1–21. [Google Scholar] [CrossRef]
- Koivisto, L.; Jiang, G.; Häkkinen, L.; Chan, B.; Larjava, H. HaCaT Keratinocyte Migration Is Dependent on Epidermal Growth Factor Receptor Signaling and Glycogen Synthase Kinase-3α. Exp Cell Res 2006, 312, 2791–2805. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J Clin Med 2021, 10, 3558. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int J Mol Sci 2021, 22, 4945. [Google Scholar] [CrossRef]
- Yang, C.S.; Ho, C.-T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing Meanings in Food Science and Health Science. J Agric Food Chem 2018, 66, 3063–3068. [Google Scholar] [CrossRef] [PubMed]
- Mfotie Njoya, E. Medicinal Plants, Antioxidant Potential, and Cancer. In Cancer; Preedy, V.R., Patel, V.B., Eds.; Elsevier, 2021; pp. 349–357.
- Sakurai, S.; Kawakami, Y.; Kuroki, M.; Gotoh, H. Structure–Antioxidant Activity (Oxygen Radical Absorbance Capacity) Relationships of Phenolic Compounds. Struct Chem 2022, 33, 1055–1062. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.T.; Meli, V.S.; Wu-Woods, N.J.; Chen, E.Y.; Liu, W.F.; Wang, S.-W. Effects of Surface-Bound Collagen-Mimetic Peptides on Macrophage Uptake and Immunomodulation. Front Bioeng Biotechnol 2020, 8. [Google Scholar] [CrossRef]
- Szarka, E.; Neer, Z.; Balogh, P.; Ádori, M.; Angyal, A.; Prechl, J.; Kiss, A.; Kövesdi, D.; Sármay, G. Exacerbation of Collagen Induced Arthritis by FcΓ Receptor Targeted Collagen Peptide Due to Enhanced Inflammatory Chemokine and Cytokine Production. Biologics 2012, 6, 101–115. [Google Scholar] [CrossRef]
- Pilus, N.S.M.; Muhamad, A.; Shahidan, M.A.; Yusof, N.Y.M. Potential of Epidermal Growth Factor-like Peptide from the Sea Cucumber Stichopus Horrens to Increase the Growth of Human Cells: In Silico Molecular Docking Approach. Mar Drugs 2022, 20, 596. [Google Scholar] [CrossRef]
- Sun, J.-H.; Song, S.; Yang, J.-F. Oral Administration of Sea Cucumber ( Stichopus Japonicus ) Protein Exerts Wound Healing Effects via the PI3K/AKT/MTOR Signaling Pathway. Food Funct 2022, 13, 9796–9809. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hagiwara, M. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blotting Analyses via Colored Stacking Gels. Anal Biochem 2022, 652, 114751. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of Hydrolyzed Collagen from Defatted Sea Bass Skin on Proliferation and Differentiation of Preosteoblast MC3T3-E1 Cells. Foods 2021, 10, 1476. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mourão, P.A. Occurrence of a Unique Fucose-Branched Chondroitin Sulfate in the Body Wall of a Sea Cucumber. Journal of Biological Chemistry 1988, 263, 18176–18183. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J Food Sci 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and Exopeptidase Hydrolysis of Whey Protein: Comparison of the TNBS, OPA and PH Stat Methods for Quantification of Degree of Hydrolysis. Int Dairy J 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Miralles, B.; Amigo, L.; Ramos, M.; Recio, I. Identification of Antioxidant and ACE-inhibitory Peptides in Fermented Milk. J Sci Food Agric 2005, 85, 1041–1048. [Google Scholar] [CrossRef]
- Garrett, A.R.; Weagel, E.G.; Martinez, A.D.; Heaton, M.; Robison, R.A.; O’Neill, K.L. A Novel Method for Predicting Antioxidant Activity Based on Amino Acid Structure. Food Chem 2014, 158, 490–496. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G. In Vitro Cytotoxicity Analysis: MTT/XTT, Trypan Blue Exclusion. In; 2023; pp. 267–284.
- Nagy, G.; Kiraly, G.; Banfalvi, G. Optimization of Cell Cycle Measurement by Time-Lapse Microscopy. In Methods in Cell Biology; P. Michael Conn, Ed.; Academic Press, 2012; Vol. 112, pp. 143–161.
- Wallace, S.E.; Wilcox, W.R. Camurati-Engelmann Disease; Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, Eds.; GeneReviews® [Internet]: Seattle (WA): , 2023; In Camurati-Engelmann Disease.
- Rebrikov, D. V.; Trofimov, D.Yu. Real-Time PCR: A Review of Approaches to Data Analysis. Appl Biochem Microbiol 2006, 42, 455–463. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res 2001, 29, 45e–445. [Google Scholar] [CrossRef] [PubMed]
- Artika, I.M.; Dewi, Y.P.; Nainggolan, I.M.; Siregar, J.E.; Antonjaya, U. Real-Time Polymerase Chain Reaction: Current Techniques, Applications, and Role in COVID-19 Diagnosis. Genes (Basel) 2022, 13, 2387. [Google Scholar] [CrossRef] [PubMed]
| Amino acid | Residues* | Amino acid | Residues* |
| Aspartic acid | 93 | Tyrosine | 6 |
| Glutamine | 157 | Valine | 20 |
| Hydroxyproline | 97 | Methionine | 5 |
| Serine | 21 | Cysteine | 1 |
| Glycine | 289 | Isoleucine | 10 |
| Histidine | 3 | Leucine | 15 |
| Arginine | 54 | Hydroxylysine | 5 |
| Threonine | 23 | Phenylalanine | 9 |
| Alanine | 110 | Lysine | 5 |
| Proline | 75 | Imino acids** | 172 |
| Target gene | Forward 5’ - 3’ Reverse 3’ - 5’ | Reference |
|---|---|---|
| ILlα | F: CGCCAATGACTCAGAGGAAGA R: AGGGCGTCATTCAGGATGAA |
Wiegand et al, 2021 [52] |
| IL6 | F: AGACAGCCACTCACCTCTTCAG R: TTCTGCCAGTGCCTCTTTGCTG |
NM_000600.5 |
| TGFβ1 | F: GAGCCCTGGATACCAACTATT R: AGGACCTTGCTGTACTGTGTG |
Wallace et al., 2023 [85] |
| DDSG1 | F: TCCCCACATTTCGGCACTAC R: GCCCAGAGGATCGAGAATAGG |
Wiegand et al, 2021 [52] |
| DSG3 | F: GTCAGAACAATCGGTGTGAGATG R: TGCGGCCTGCCATACCT |
Wiegand et al, 2021 [52] |
| SI00A7 | F: GTCCAAACACACACATCTCACT R: TCATCATCGTCAGCAGGCTT |
Wiegand et al, 2021 [52] |
|
β-actin |
F: GATCATTGCTCCTCCTGAGC R: GTCATAGTCCGCCTAGAAGCAT |
NM_001101.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





