Submitted:
05 September 2025
Posted:
09 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
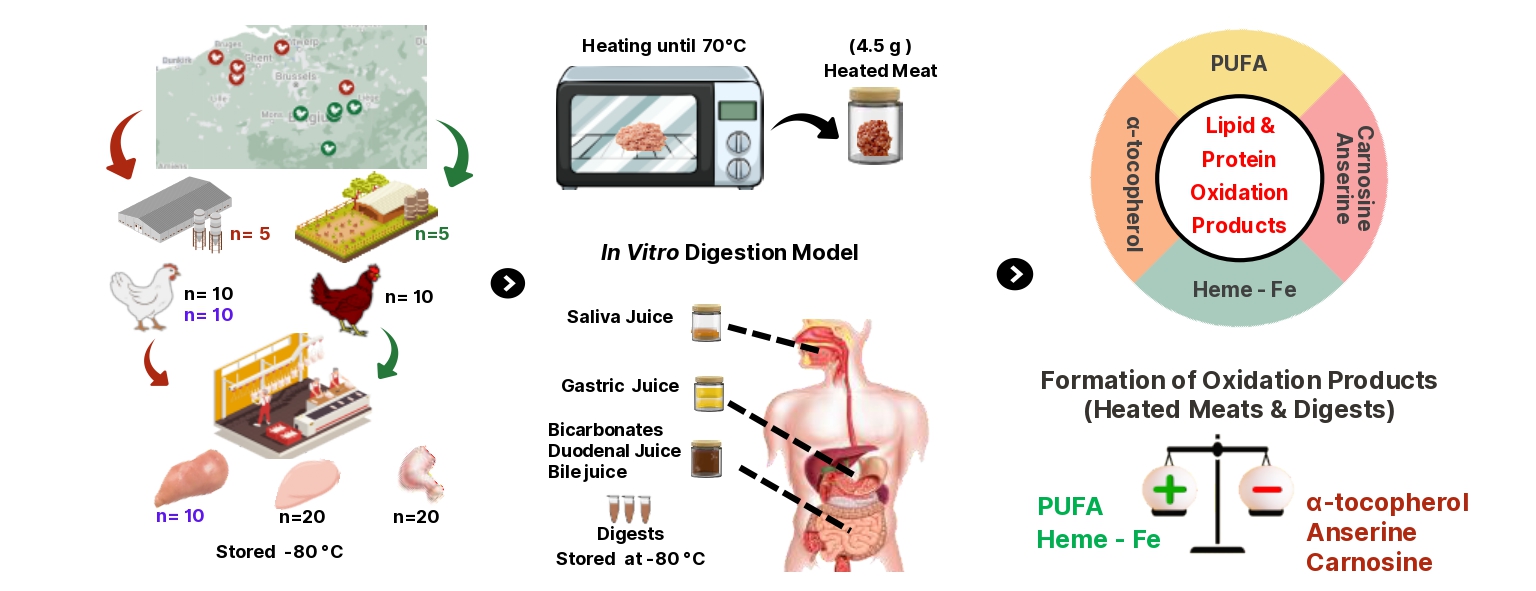
2.2. Meat Sampling and Experimental Design
2.3. In Vitro Digestion
2.4. Meat Composition Analyses
2.4.1. Fatty Acids
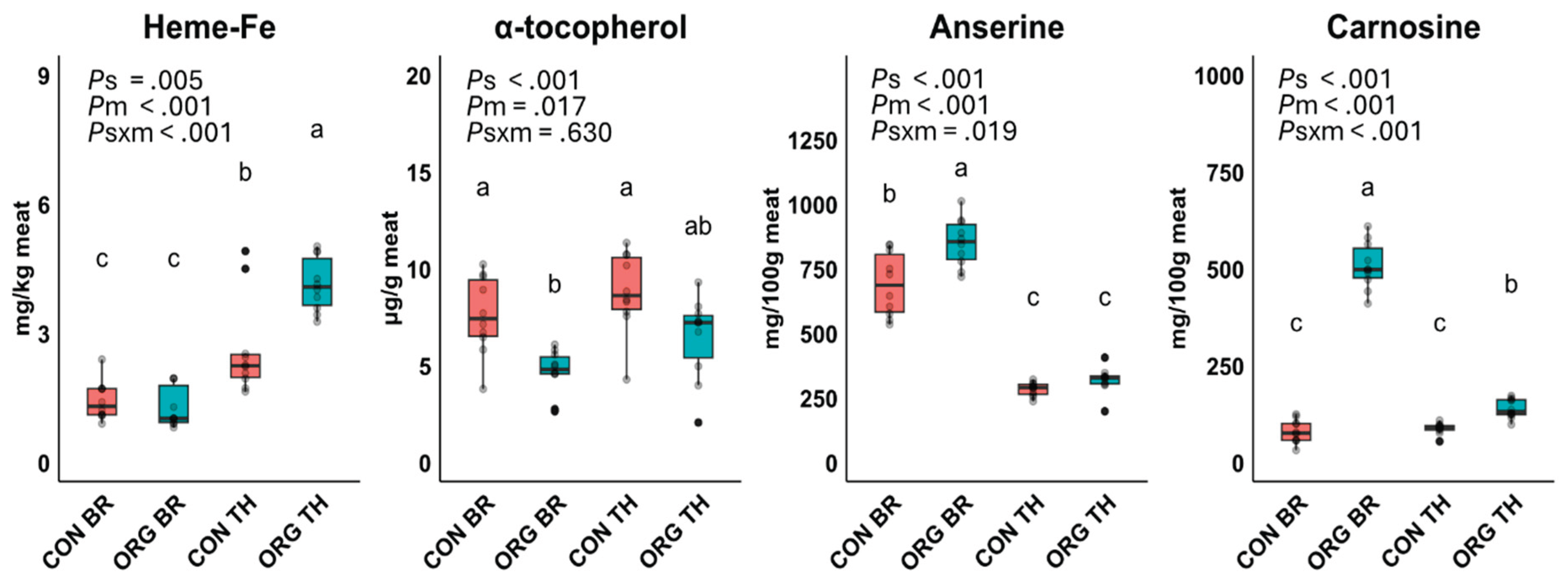
2.4.2. Heme-Fe
2.4.3. α-Tocopherol
2.4.4. Carnosine and Anserine
2.5. Oxidation Products in Meats and Digests
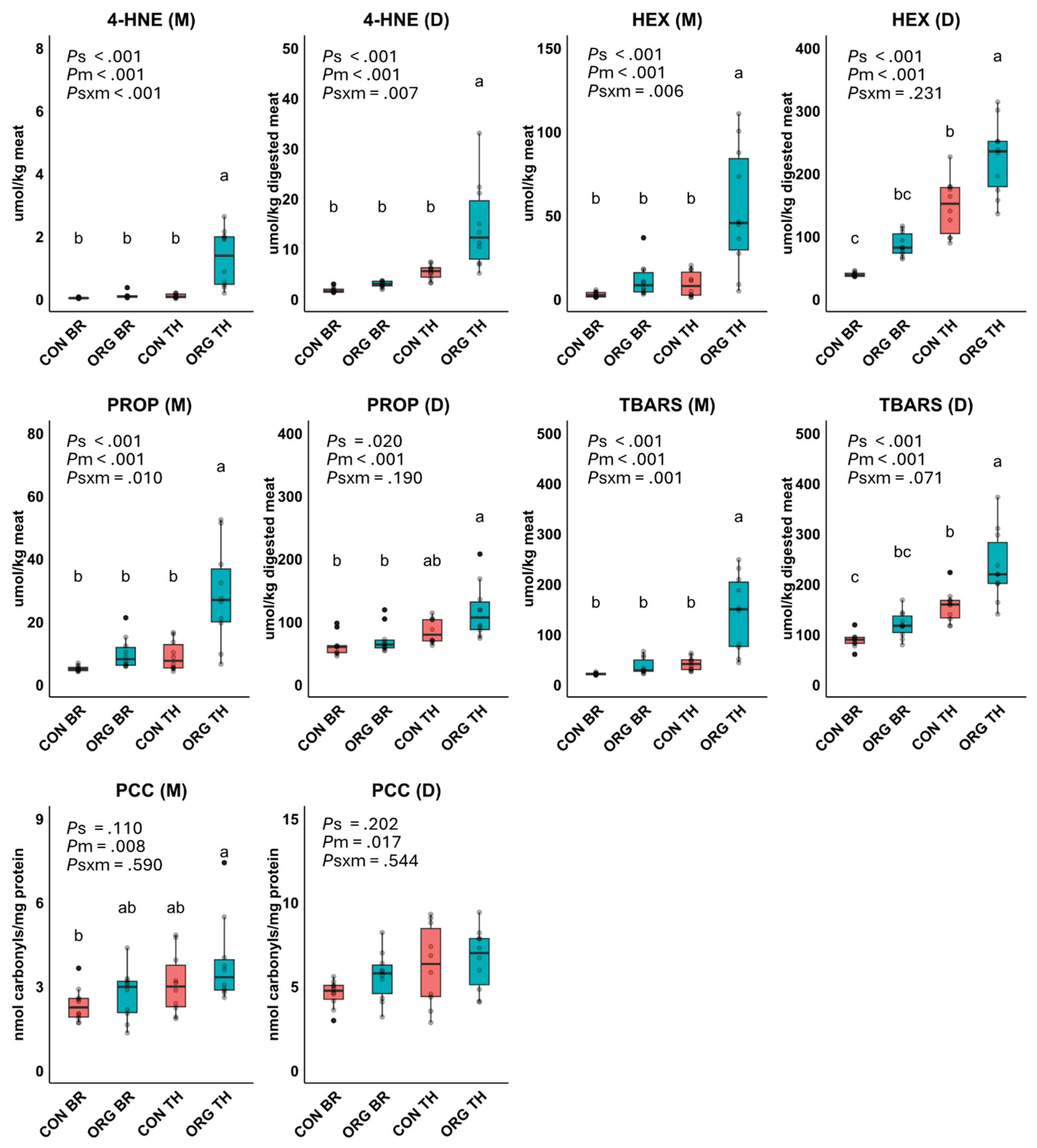
2.5.1. Lipid Oxidation Products
2.5.2. Protein Oxidation Products
2.6. Statistical Analysis
3. Results
3.1. Experiment 1: Meat Composition
3.2. Experiment 1: Lipid and Protein Oxidation Products
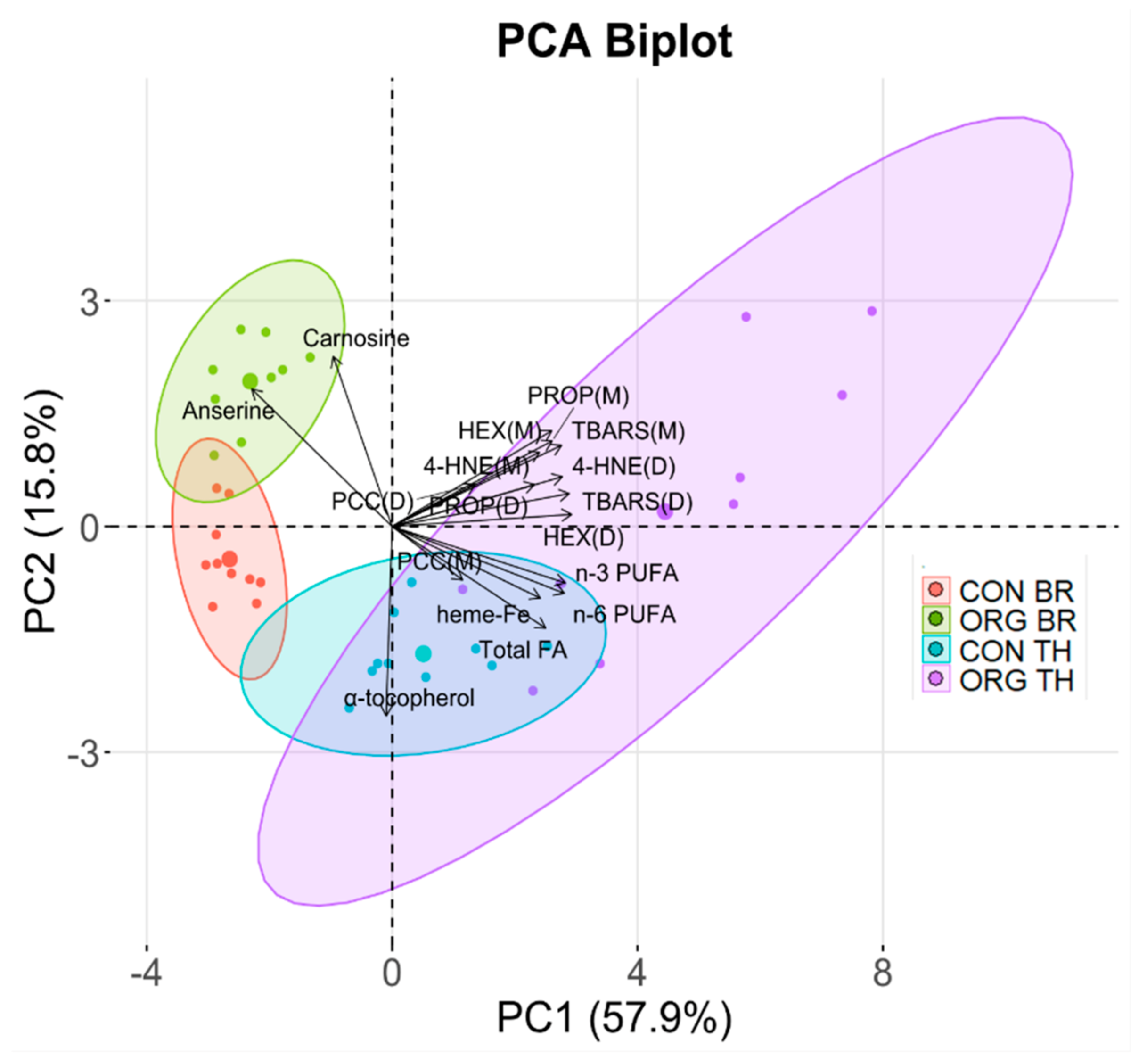
3.3. Experiment 1: Relationships Among Compositional Variables and Oxidation Products
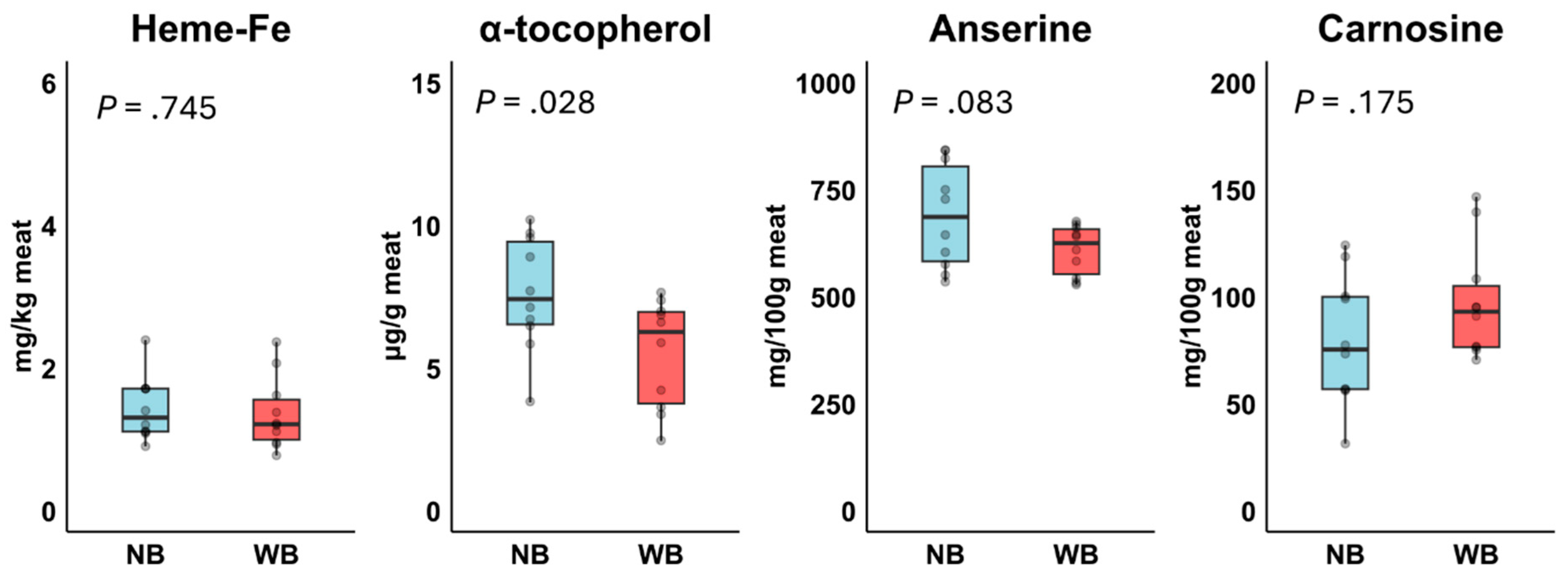
3.4. Experiment 2: Meat Composition
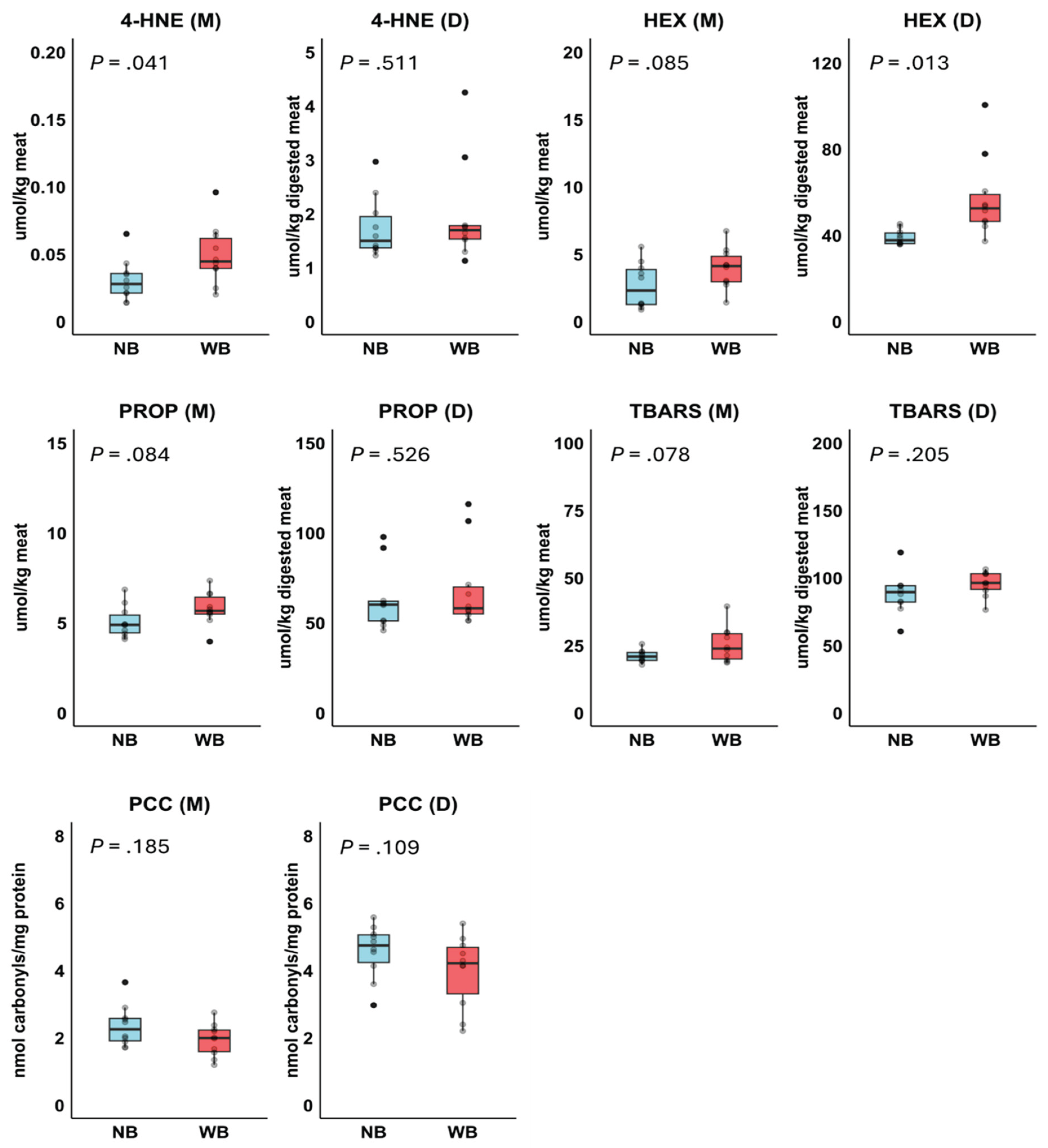
3.5. Experiment 2: Lipid and Protein Oxidation Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| CON | conventional production system |
| DNPH | 2,4-dinitrophenylhydrazine |
| FA | fatty acid |
| HCD | histidine-containing dipeptides |
| HEX | hexanal |
| LDL | low-density lipoproteins |
| MDA | malondialdehyde |
| MUFA | monounsaturated fatty acids |
| ORG | organic production system |
| PCC | protein carbonyl compounds |
| PROP | propanal |
| PUFA | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| SFA | saturated fatty acids |
| TBA | thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
References
- Albrecht, A.; Hebel, M.; Mittler, M.; Hurck, C.; Kustwan, K.; Heitkönig, B.; Bitschinski, D.; Kreyenschmidt, J. Influence of Different Production Systems on the Quality and Shelf Life of Poultry Meat: A Case Study in the German Sector. Journal of Food Quality 2019, 1–11. [CrossRef]
- European Parliament. Directorate General for Parliamentary Research Services. The EU Poultry Meat and Egg Sector: Main Features, Challenges and Prospects: In Depth Analysis.; Publications Office: LU, 2019.
- Barbut, S.; Mitchell, R.; Hall, P.; Bacon, C.; Bailey, R.; Owens, C.M.; Petracci, M. Review: Myopathies in Broilers: Supply Chain Approach to Provide Solutions to Challenges Related to Raising Fast Growing Birds. Poultry Science 2024, 103, 103801. [Google Scholar] [CrossRef]
- Sihvo, H.-K.; Immonen, K.; Puolanne, E. Myodegeneration With Fibrosis and Regeneration in the Pectoralis Major Muscle of Broilers. Vet Pathol 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Gálvez, F.; Domínguez, R.; Maggiolino, A.; Pateiro, M.; Carballo, J.; De Palo, P.; Barba, F.J.; Lorenzo, J.M. Meat Quality of Commercial Chickens Reared in Different Production Systems: Industrial, Range and Organic. Annals of Animal Science 2020, 20, 263–285. [Google Scholar] [CrossRef]
- Brown, S.N.; Nute, G.R.; Baker, A.; Hughes, S.I.; Warriss, P.D. Aspects of Meat and Eating Quality of Broiler Chickens Reared under Standard, Maize-Fed, Free-Range or Organic Systems. British Poultry Science 2008, 49, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Baéza, E.; Guillier, L.; Petracci, M. Review: Production Factors Affecting Poultry Carcass and Meat Quality Attributes. Animal 2022, 16, 100331. [Google Scholar] [CrossRef]
- Husak, R.L.; Sebranek, J.G.; Bregendahl, K. A Survey of Commercially Available Broilers Marketed as Organic, Free-Range, and Conventional Broilers for Cooked Meat Yields, Meat Composition, and Relative Value. Poultry Science 2008, 87, 2367–2376. [Google Scholar] [CrossRef]
- Michalczuk, M.; Zdanowska-Sąsiadek, Ż.; Damaziak, K.; Niemiec, J. Influence of Indoor and Outdoor Systems on Meat Quality of Slow-Growing Chickens. CyTA—Journal of Food 2016, 1–6. [CrossRef]
- Barbaresi, S.; Maertens, L.; Claeys, E.; Derave, W.; De Smet, S. Differences in Muscle Histidine-containing Dipeptides in Broilers. J Sci Food Agric 2019, 99, 5680–5686. [Google Scholar] [CrossRef]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estévez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, Consequences and Consumer Perception of Emerging Broiler Meat Abnormalities. Comp Rev Food Sci Food Safe 2019, 18, 565–583. [Google Scholar] [CrossRef]
- Min, B.; Nam, K.C.; Cordray, J.; Ahn, D.U. Endogenous Factors Affecting Oxidative Stability of Beef Loin, Pork Loin, and Chicken Breast and Thigh Meats. Journal of Food Science 2008, 73, C439–C446. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Goethals, S.; Vossen, E.; De Smet, S. Long-Chain n-3 PUFA Content and n-6/n-3 PUFA Ratio in Mammal, Poultry, and Fish Muscles Largely Explain Differential Protein and Lipid Oxidation Profiles Following In Vitro Gastrointestinal Digestion. Molecular Nutrition Food Res 2019, 63, 1900404. [Google Scholar] [CrossRef]
- Van Hecke, T.; Vanden Bussche, J.; Vanhaecke, L.; Vossen, E.; Van Camp, J.; De Smet, S. Nitrite Curing of Chicken, Pork, and Beef Inhibits Oxidation but Does Not Affect N -Nitroso Compound (NOC)-Specific DNA Adduct Formation during in Vitro Digestion. J. Agric. Food Chem. 2014, 62, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Steppeler, C.; Haugen, J.-E.; Rødbotten, R.; Kirkhus, B. Formation of Malondialdehyde, 4-Hydroxynonenal, and 4-Hydroxyhexenal during in Vitro Digestion of Cooked Beef, Pork, Chicken, and Salmon. J. Agric. Food Chem. 2016, 64, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein Carbonyls in Meat Systems: A Review. Meat Science 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Nègre-Salvayre, A.; Garoby-Salom, S.; Swiader, A.; Rouahi, M.; Pucelle, M.; Salvayre, R. Proatherogenic Effects of 4-Hydroxynonenal. Free Radical Biology and Medicine 2017, 111, 127–139. [Google Scholar] [CrossRef]
- Estévez, M.; Luna, C. Dietary Protein Oxidation: A Silent Threat to Human Health? Critical Reviews in Food Science and Nutrition 2017, 57, 3781–3793. [Google Scholar] [CrossRef]
- European Union. Council Directive 2007/43/EC of 28 June 2007 Laying down Minimum Rules for the Protection of Chickens Kept for Meat Production. Off. J. Eur. Union 2007, L 182, 19–28. Available online: http://data.europa.eu/eli/dir/2007/43/oj (accessed on 28 August 2025).
- European Commission. Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control. Off. J. Eur. Union 2008, L 250, 1–84. Available online: https://eur-lex.europa.eu/eli/reg/2008/889/oj (accessed on 28 August 2025).
- Tijare, V.V.; Yang, F.L.; Kuttappan, V.A.; Alvarado, C.Z.; Coon, C.N.; Owens, C.M. Meat Quality of Broiler Breast Fillets with White Striping and Woody Breast Muscle Myopathies. Poultry Science 2016, 95, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Versantvoort, C.H.M.; Oomen, A.G.; Van De Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in Vitro Digestion Model in Assessing the Bioaccessibility of Mycotoxins from Food. Food and Chemical Toxicology 2005, 43, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Basso, V.; De Smet, S. Lipid and Protein Oxidation during in Vitro Gastrointestinal Digestion of Pork under Helicobacter Pylori Gastritis Conditions. J. Agric. Food Chem. 2018, 66, 13000–13010. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A SIMPLE METHOD FOR THE ISOLATION AND PURIFICATION OF TOTAL LIPIDES FROM ANIMAL TISSUES. Journal of Biological Chemistry 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Raes, K.; Smet, S.D.; Demeyer, D. Effect of Double-Muscling in Belgian Blue Young Bulls on the Intramuscular Fatty Acid Composition with Emphasis on Conjugated Linoleic Acid and Polyunsaturated Fatty Acids. Anim. Sci. 2001, 73, 253–260. [Google Scholar] [CrossRef]
- Hornsey, H.C. The Colour of Cooked Cured Pork. I.—Estimation of the Nitric oxide-Haem Pigments. J Sci Food Agric 1956, 7, 534–540. [Google Scholar] [CrossRef]
- Claeys, E.; Vossen, E.; De Smet, S. Determination of A-tocopherol by Reversed-phase HPLC in Feed and Animal-derived Foods without Saponification. J Sci Food Agric 2016, 96, 522–529. [Google Scholar] [CrossRef]
- Kobe, R.; Ishihara, Y.; Takano, J.; Kitami, H. Simultaneous Determination of Anserine and Carnosine in Chicken Meat by Hydrophilic Interaction Chromatography on an Aminopropyl Bonded Silica Gel Column. BUNSEKI KAGAKU 2011, 60, 859–863. [Google Scholar] [CrossRef]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The Potential of Herbs and Spices to Reduce Lipid Oxidation during Heating and Gastrointestinal Digestion of a Beef Product. Food Research International 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Grotto, D.; Santa Maria, L.D.; Boeira, S.; Valentini, J.; Charão, M.F.; Moro, A.M.; Nascimento, P.C.; Pomblum, V.J.; Garcia, S.C. Rapid Quantification of Malondialdehyde in Plasma by High Performance Liquid Chromatography–Visible Detection. Journal of Pharmaceutical and Biomedical Analysis 2007, 43, 619–624. [Google Scholar] [CrossRef]
- Ganhão, R.; Morcuende, D.; Estévez, M. Protein Oxidation in Emulsified Cooked Burger Patties with Added Fruit Extracts: Influence on Colour and Texture Deterioration during Chill Storage. Meat Science 2010, 85, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of Freezing Temperature and Duration of Frozen Storage on Lipid and Protein Oxidation in Chicken Meat. Food Chemistry 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Pellattiero, E.; Tasoniero, G.; Cullere, M.; Gleeson, E.; Baldan, G.; Contiero, B.; Dalle Zotte, A. Are Meat Quality Traits and Sensory Attributes in Favor of Slow-Growing Chickens? Animals 2020, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Berri, C.; Wacrenier, N.; Millet, N.; Le Bihan-Duval, E. Effect of Selection for Improved Body Composition on Muscle and Meat Characteristics of Broilers from Experimental and Commercial Lines. Poultry Science 2001, 80, 833–838. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Mattioli, S.; Rosati, A.; Ruggeri, S.; Ranucci, D.; Castellini, C. Transfer of Bioactive Compounds from Pasture to Meat in Organic Free-Range Chickens. Poultry Science 2016, 95, 2464–2471. [Google Scholar] [CrossRef]
- Michiels, J.; Tagliabue, M.M.; Akbarian, A.; Ovyn, A.; De Smet, S. Oxidative Status, Meat Quality and Fatty Acid Profile of Broiler Chickens Reared under Free-Range and Severely Feed-Restricted Conditions Compared with Conventional Indoor Rearing. Avian Biology Research 2014, 7, 74–82. [Google Scholar] [CrossRef]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of Organic Production System on Broiler Carcass and Meat Quality. Meat Science 2002, 60, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Mancinelli, A.C.; Dal Bosco, A.; Ciarelli, C.; Amato, M.G.; Angelucci, E.; Chiattelli, D.; Castellini, C. Intake of Nutrients (Polyunsaturated Fatty Acids, Tocols, and Carotenes) and Storage Efficiency in Different Slow-Growing Chickens Genotypes Reared in Extensive Systems. PLoS ONE 2022, 17, e0275527. [Google Scholar] [CrossRef] [PubMed]
- Bonnefous, C.; Collin, A.; Guilloteau, L.A.; Germain, K.; Ravon, L.; Bordeau, T.; Chartrin, P.; Godet, E.; Cailleau-Audouin, E.; Couroussé, N.; et al. Performance, Meat Quality and Blood Parameters in Four Strains of Organic Broilers Differ According to Range Use. Sci Rep 2024, 14, 30854. [Google Scholar] [CrossRef]
- Mattioli, S.; Angelucci, E.; Castellini, C.; Cartoni Mancinelli, A.; Chenggang, W.; Di Federico, F.; Chiattelli, D.; Dal Bosco, A. Effect of Genotype and Outdoor Enrichment on Productive Performance and Meat Quality of Slow Growing Chickens. Poultry Science 2024, 103, 104131. [Google Scholar] [CrossRef]
- Perini, F.; Wu, Z.; Cartoni Mancinelli, A.; Soglia, D.; Schiavone, A.; Mattioli, S.; Mugnai, C.; Castellini, C.; Smith, J.; Lasagna, E. RNAseq Reveals Modulation of Genes Involved in Fatty Acid Biosynthesis in Chicken Liver According to Genetic Background, Sex, and Diet. Animal Genetics 2023, 54, 338–354. [Google Scholar] [CrossRef]
- Coudert, E.; Baéza, E.; Chartrin, P.; Jimenez, J.; Cailleau-Audouin, E.; Bordeau, T.; Berri, C. Slow and Fast-Growing Chickens Use Different Antioxidant Pathways to Maintain Their Redox Balance during Postnatal Growth. Animals 2023, 13, 1160. [Google Scholar] [CrossRef]
- Jung, S.; Bae, Y.S.; Kim, H.J.; Jayasena, D.D.; Lee, J.H.; Park, H.B.; Heo, K.N.; Jo, C. Carnosine, Anserine, Creatine, and Inosine 5′-Monophosphate Contents in Breast and Thigh Meats from 5 Lines of Korean Native Chicken. Poultry Science 2013, 92, 3275–3282. [Google Scholar] [CrossRef]
- Banerjee, R.; Verma, A.K.; Siddiqui, M.W. Applications in Foods of Animal Origin; Apple Academic Press: New York, NY, USA, 2017; ISBN 978-1-315-36591-6. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Charoensin, S.; Laopaiboon, B.; Boonkum, W.; Phetcharaburanin, J.; Villareal, M.O.; Isoda, H.; Duangjinda, M. Thai Native Chicken as a Potential Functional Meat Source Rich in Anserine, Anserine/Carnosine, and Antioxidant Substances. Animals 2021, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhuang, H.; Zhou, G.; Zhang, J. Investigation of Inhibition of Lipid Oxidation by L-Carnosine Using an Oxidized-Myoglobin-Mediated Washed Fish Muscle System. LWT 2018, 97, 703–710. [Google Scholar] [CrossRef]
- Gorelik, S.; Kanner, J.; Schurr, D.; Kohen, R. A Rational Approach to Prevent Postprandial Modification of LDL by Dietary Polyphenols. Journal of Functional Foods 2013, 5, 163–169. [Google Scholar] [CrossRef]
- Van Hecke, T.; Goethals, S.; Vossen, E.; De Smet, S. Long-Chain n-3 PUFA Content and n-6/n-3 PUFA Ratio in Mammal, Poultry, and Fish Muscles Largely Explain Differential Protein and Lipid Oxidation Profiles Following In Vitro Gastrointestinal Digestion. Molecular Nutrition Food Res 2019, 63, 1900404. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by Polyphenols of Postprandial Human Plasma and Low-Density Lipoprotein Modification: The Stomach as a Bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef]
- Kenmogne-Domguia, H.B.; Meynier, A.; Boulanger, C.; Genot, C. Lipid Oxidation in Food Emulsions Under Gastrointestinal-Simulated Conditions: The Key Role of Endogenous Tocopherols and Initiator. Food Dig. 2012, 3, 46–52. [Google Scholar] [CrossRef]
- Larsson, K.; Cavonius, L.; Alminger, M.; Undeland, I. Oxidation of Cod Liver Oil during Gastrointestinal in Vitro Digestion. J. Agric. Food Chem. 2012, 60, 7556–7564. [Google Scholar] [CrossRef]
- Kenmogne-Domguia, H.B.; Moisan, S.; Viau, M.; Genot, C.; Meynier, A. The Initial Characteristics of Marine Oil Emulsions and the Composition of the Media Inflect Lipid Oxidation during in Vitro Gastrointestinal Digestion. Food Chemistry 2014, 152, 146–154. [Google Scholar] [CrossRef]
- Mahecha, L.; Dannenberger, D.; Nuernberg, K.; Nuernberg, G.; Hagemann, E.; Martin, J. Relationship between Lipid Peroxidation and Antioxidant Status in the Muscle of German Holstein Bulls Fed n -3 and n -6 PUFA-Enriched Diets. J. Agric. Food Chem. 2010, 58, 8407–8413. [Google Scholar] [CrossRef]
- Van Hecke, T.; Wouters, A.; Rombouts, C.; Izzati, T.; Berardo, A.; Vossen, E.; Claeys, E.; Van Camp, J.; Raes, K.; Vanhaecke, L.; et al. Reducing Compounds Equivocally Influence Oxidation during Digestion of a High-Fat Beef Product, Which Promotes Cytotoxicity in Colorectal Carcinoma Cell Lines. J. Agric. Food Chem. 2016, 64, 1600–1609. [Google Scholar] [CrossRef]
- Barski, O.A.; Xie, Z.; Baba, S.P.; Sithu, S.D.; Agarwal, A.; Cai, J.; Bhatnagar, A.; Srivastava, S. Dietary Carnosine Prevents Early Atherosclerotic Lesion Formation in Apolipoprotein E–Null Mice. ATVB 2013, 33, 1162–1170. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yaylayan, V.; Palin, M.; Ngapo, T.M.; Cliche, S.; Sabik, H.; Gariépy, C. Dual Effects of Dietary Carnosine during in Vitro Digestion of a Western Meal Model with Added Ascorbic Acid. Journal of Food Science 2024, 89, 710–726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Yaylayan, V.; Palin, M.; Sullivan, B.; Fortin, F.; Cliche, S.; Sabik, H.; Gariépy, C. Protective Effects of Dietary Carnosine during In-vitro Digestion of Pork Differing in Fat Content and Cooking Conditions. J. Food Biochem. 2021, 45, e13624. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Critical Reviews in Food Science and Nutrition 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Santé-Lhoutellier, V.; Engel, E.; Aubry, L.; Gatellier, P. Effect of Animal (Lamb) Diet and Meat Storage on Myofibrillar Protein Oxidation and in Vitro Digestibility. Meat Science 2008, 79, 777–783. [Google Scholar] [CrossRef]
- Soglia, F.; Laghi, L.; Canonico, L.; Cavani, C.; Petracci, M. Functional Property Issues in Broiler Breast Meat Related to Emerging Muscle Abnormalities. Food Research International 2016, 89, 1071–1076. [Google Scholar] [CrossRef]
- Thanatsang, K.V.; Malila, Y.; Arayamethakorn, S.; Srimarut, Y.; Tatiyaborworntham, N.; Uengwetwanit, T.; Panya, A.; Rungrassamee, W.; Visessanguan, W. Nutritional Properties and Oxidative Indices of Broiler Breast Meat Affected by Wooden Breast Abnormality. Animals 2020, 10, 2272. [Google Scholar] [CrossRef] [PubMed]
- Abasht, B.; Mutryn, M.F.; Michalek, R.D.; Lee, W.R. Oxidative Stress and Metabolic Perturbations in Wooden Breast Disorder in Chickens. PLoS ONE 2016, 11, e0153750. [Google Scholar] [CrossRef]
- Xing, T.; Zhao, X.; Xu, X.; Li, J.; Zhang, L.; Gao, F. Physiochemical Properties, Protein and Metabolite Profiles of Muscle Exudate of Chicken Meat Affected by Wooden Breast Myopathy. Food Chemistry 2020, 316, 126271. [Google Scholar] [CrossRef]
- Sihvo, H.-K.; Airas, N.; Lindén, J.; Puolanne, E. Pectoral Vessel Density and Early Ultrastructural Changes in Broiler Chicken Wooden Breast Myopathy. Journal of Comparative Pathology 2018, 161, 1–10. [Google Scholar] [CrossRef]
- Li, B.; Dong, X.; Puolanne, E.; Ertbjerg, P. Effect of Wooden Breast Degree on Lipid and Protein Oxidation and Citrate Synthase Activity of Chicken Pectoralis Major Muscle. LWT 2022, 154, 112884. [Google Scholar] [CrossRef]
- Estévez, M. Oxidative Damage to Poultry: From Farm to Fork. Poultry Science 2015, 94, 1368–1378. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Rocha, T.C.; Delgado, J.; Díaz-Velasco, S.; Madruga, M.S.; Estévez, M. Deciphering the Underlying Mechanisms of the Oxidative Perturbations and Impaired Meat Quality in Wooden Breast Myopathy by Label-Free Quantitative MS-Based Proteomics. Food Chemistry 2023, 423, 136314. [Google Scholar] [CrossRef]
- Prache, S.; Lebret, B.; Baéza, E.; Martin, B.; Gautron, J.; Feidt, C.; Médale, F.; Corraze, G.; Raulet, M.; Lefèvre, F.; et al. Review: Quality and Authentication of Organic Animal Products in Europe. Animal 2022, 16, 100405. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
| Production system | ||
|---|---|---|
| Characteristics | Conventional | Organic |
| Breed | Ross 308 | Sasso (Ruby XL) |
| Age at slaughter (days) | 39.00–42.00 | 73.00–76.00 |
| Finishing phase (days) | 14.00 | 21.00 |
| Live weight at slaughter (kg) | 2.63–2.85 | 2.30–2.50 |
| Stocking density (kg/m2) | 42.00 | 21.00 |
| Outdoor access | No | Yes |
| Indoor enrichments | No | Yes |
| Diet composition (finisher phase) | ||
| Protein % | 21.33 | 20.56 |
| Fat % | 7.40 | 4.00 |
| Ash % | 5.60 | 5.40 |
| Fiber % | 2.60 | 2.90 |
| Methionine % | 0.75 | 0.30 |
| Lysine % | 1.25 | 0.94 |
| Calcium % | 0.73 | 0.8 |
| Phosphorus % | 0.43 | 0.60 |
| Na % | 0.16 | 0.20 |
| Vitamin A (IU/kg) | 13200.00 | 10000.00 |
| Vitamin D3 (IU/kg) | 3630.00 | 3000.00 |
| Vitamin E (mg/kg) | 50.00 | 55.00 |
| Se (mg/kg) | 0.52 | 0.30 |
| Iron (mg/kg) | 19.80 | 70.00 |
| Zn (mg/kg) | 66.00 | 70.00 |
| Cu (mg/kg) | 19.84 | 15.40 |
| Trait | Unit | Breast | Thigh | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| CON | ORG | CON | ORG | RMSE | S | M | S×M | ||
| Total FA | g/100g | 1.06 ± 0.23b | 0.77 ± 0.07b | 2.79 ± 0.80a | 3.05 ± 0.62a | 0.49 | 0.921 | <0.001 | 0.102 |
| SFA | mg/100g | 343.10 ± 80.17b | 243.55 ± 23.79b | 896.28 ± 261.54a | 875.34 ± 187.78a | 158.54 | 0.263 | <0.001 | 0.458 |
| MUFA | mg/100g | 316.06 ± 91.00b | 183.01± 35.06b | 906.07 ± 286.64a | 878.45 ± 234.79a | 182.34 | 0.191 | <0.001 | 0.398 |
| PUFA | mg/100g | 267.45 ± 51.32c | 234.65 ± 24.43c | 684.95 ± 193.32b | 980.76 ± 164.29a | 123.09 | 0.002 | <0.001 | <0.001 |
| n-3 PUFA | mg/100g | 22.74 ± 5.63c | 21.80 ± 2.55c | 56.94 ± 16.37b | 85.54 ± 17.07a | 11.60 | <0.001 | <0.001 | <0.001 |
| ALA | mg/100g | 10.67 ± 4.02c | 7.68 ± 1.19c | 41.90 ± 13.65b | 63.42 ± 16.74a | 10.44 | 0.011 | <0.001 | 0.001 |
| EPA | mg/100g | 2.21 ± 0.87ab | 1.39 ± 0.45b | 2.68 ± 1.27a | 2.28 ± 0.64ab | 0.81 | 0.031 | 0.017 | 0.447 |
| DHA | mg/100g | 2.84 ± 0.88c | 4.94 ± 1.15b | 3.42 ± 1.18c | 7.52 ± 1.39a | 1.10 | <0.001 | <0.001 | 0.010 |
| n-6 PUFA | mg/100g | 244.68 ± 49.77c | 213.75 ± 22.26c | 627.23 ±184.65b | 894.48 ± 148.52a | 115.65 | 0.004 | <0.001 | <0.001 |
| LA | mg/100g | 197.10 ± 46.70c | 157.42 ± 18.9c | 565.42 ± 176.54b | 803.28 ± 145.32a | 111.42 | 0.011 | <0.001 | <0.001 |
| AA | mg/100g | 33.17 ± 3.70c | 45.30 ± 4.20b | 44.93 ± 9.38b | 71.62 ± 10.13a | 7.08 | <0.001 | <0.001 | 0.004 |
| n-6/n-3 PUFA | ratio | 11.35 ± 3.79 | 9.82 ± 0.69 | 11.53 ± 3.20 | 10.54 ± 0.71 | 2.40 | 0.126 | 0.596 | 0.741 |
| Trait | Unit | Normal Breast | Wooden Breast | RMSE | P-value |
|---|---|---|---|---|---|
| Total FA | g/100g | 1.06 ± 0.23 | 1.42 ± 0.70 | 0.49 | 0.156 |
| SFA | mg/100g | 343.10 ± 80.17 | 476.43 ± 244.78 | 173.34 | 0.131 |
| MUFA | mg/100g | 316.06 ± 91.00 | 430.10 ± 233.32 | 167.43 | 0.173 |
| PUFA | mg/100g | 267.45 ± 51.32 | 353.12 ± 162.57 | 114.78 | 0.140 |
| n-3 PUFA | mg/100g | 22.74 ± 5.63 | 32.91 ± 17.06 | 12.27 | 0.090 |
| ALA | mg/100g | 10.67 ± 4.02 | 21.70 ± 14.83 | 10.23 | 0.043 |
| EPA | mg/100g | 2.21 ± 0.87 | 2.15 ± 0.81 | 0.79 | 0.878 |
| DHA | mg/100g | 2.84 ± 0.88 | 2.63 ± 0.78 | 0.78 | 0.592 |
| n-6 PUFA | mg/100g | 244.68 ± 49.77 | 320.34 ± 146.45 | 103.46 | 0.149 |
| LA | mg/100g | 197.10 ± 46.70 | 277.17 ± 137.60 | 96.94 | 0.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





