Submitted:
14 August 2025
Posted:
18 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Oxidative Stress and Its Pathological Impact
1.2. Natural Antioxidants: A Safer Alternative
1.3. Phenolic Compounds: Diversity and Function
1.4. Flavonoids: Multifunctional Antioxidants
1.5. Artemisinin: Beyond Antimalarial Properties
1.6. Progress in Antioxidant Research (2015–2025)
1.7. Purpose and Scope of the Review
- To provide an overview of the recent scientific progress in the antioxidant activity and therapeutics of these molecules through in vitro, in vivo, and clinical trial-based studies.
- To investigate underlying mechanisms such as redox modulation, anti-inflammatory effects, and molecular signaling pathways.
- In assessing clinical utility and potential future application, identifying challenges, areas of knowledge gaps, and scope for further research.
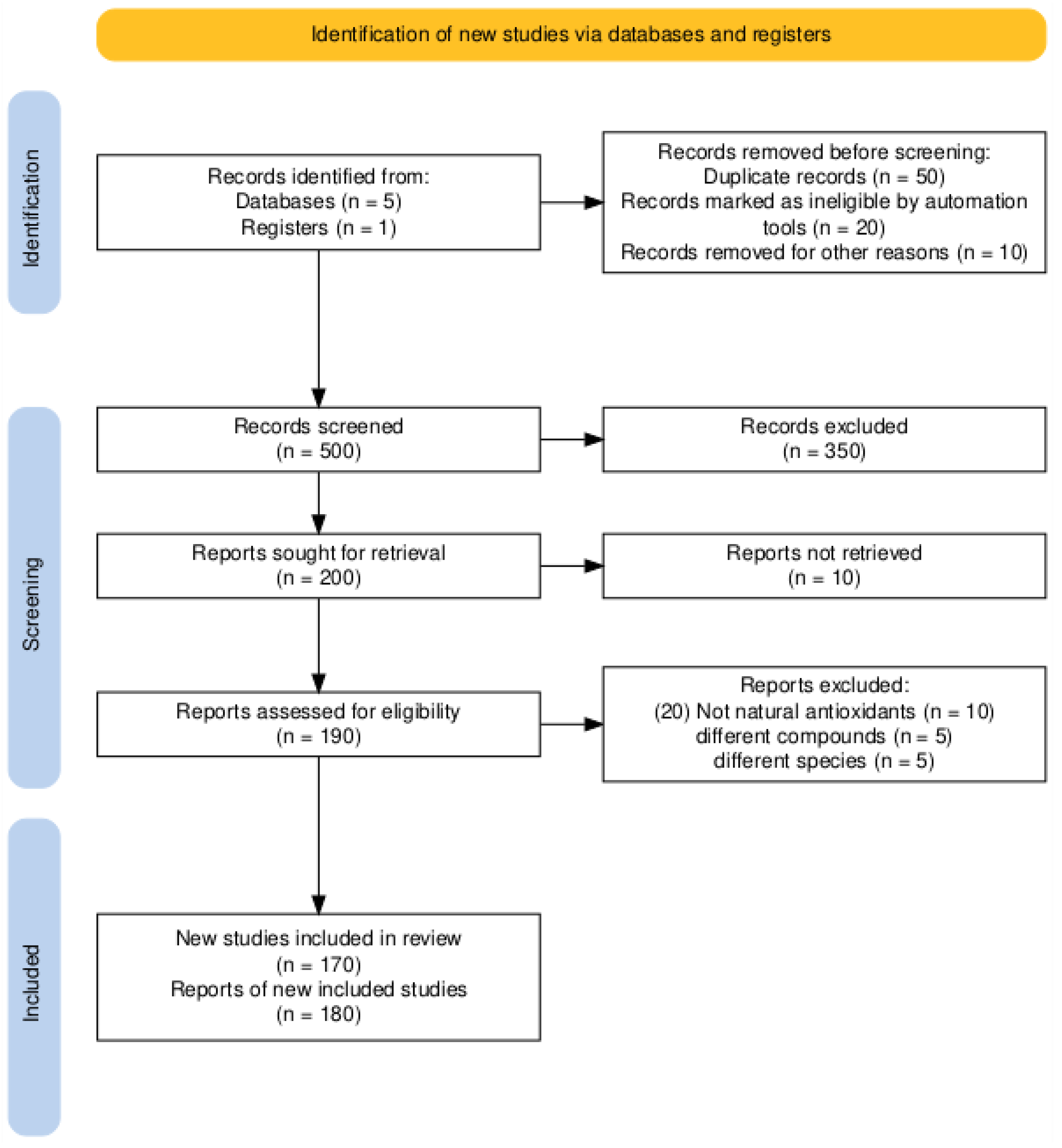
2. Methodology
2.1. Literature Search Strategy
- PubMed / MEDLINE
- Scopus
- Google Scholar
- ScienceDirect
- “Artemisinin antioxidant”
- “Phenolic compounds oxidative stress”
- “Flavonoids free radical scavenging”
- “Natural antioxidants clinical trials”
- “2015–2025 trends in antioxidant research”
2.2. Inclusion and Exclusion Criteria
- Published from 2015 to 2025 in peer reviewed publications.
- Aligned with antioxidant activity of artemisinin, phenolic acids, or flavonoids.
- Combined in vitro, in vivo, clinical, or biotechnological research.
- Provide mechanisms, bioactivity information, or pharmacological uses.
- Conference abstracts, non-peer-reviewed preprints, or editorials.
- Studies out of topic scope (synthetic antioxidants only).
- Non-English publications unless full translations were made available.
2.3. Study Selection Process
- Initial Retrieval: All articles were imported screened by hand and duplicates discarded.
- Title & Abstract Screening: Two independent reviewers screened studies for relevance.
- Full Text Evaluation: Included articles were assessed to confirm inclusion criteria.
2.4. Data Extraction and Organization
- Compound investigated (artemisinin, phenolic, flavonoid)
- Source plant / formulation
- Study type: in vitro, in vivo, or clinical
- Assay / model used (DPPH, ABTS, FRAP, animal model, human trial)
- Primary endpoints (IC₅₀, ROS inhibition, enzymatic activity alteration, clinical outcomes)
- Year and reference
- Comparative properties of selected compound classes
- Properties of Selected Phenolic Compounds
- Properties of Selected Flavonoids
- Selected Properties of Artemisinin and Its Derivatives
- Biotechnological and Clinical Applications
2.5. Synthesis of Data
- Narrative data were summarized in order to highlight trends, gaps, and mechanistic insights.
- Tables and figures were built to display key findings, pathways, and potential future.
- Descriptive analysis rather than meta-analysis was employed because study designs, endpoints, and reporting varied.
- New or emerging themes, e.g., applications of nanotechnology, bioengineering to enhance metabolites, and clinical translation, were emphasized in an attempt to meet review objectives.
2.6. Limitations of Methodology
- Potential underreporting bias from negative results.
- Exclusion of Local studies may miss out some regional data.
- Studies selected were heterogeneous and precluded statistical pooling.
3. Results
3.1. Comparative properties of selected compound classes
3.2. Properties of Selected Phenolic Compounds
3.3. Properties of Selected Flavonoids
3.4. Selected Properties of Artemisinin and Its Derivatives
3.5. Biotechnological and Clinical Applications
4. Discussion
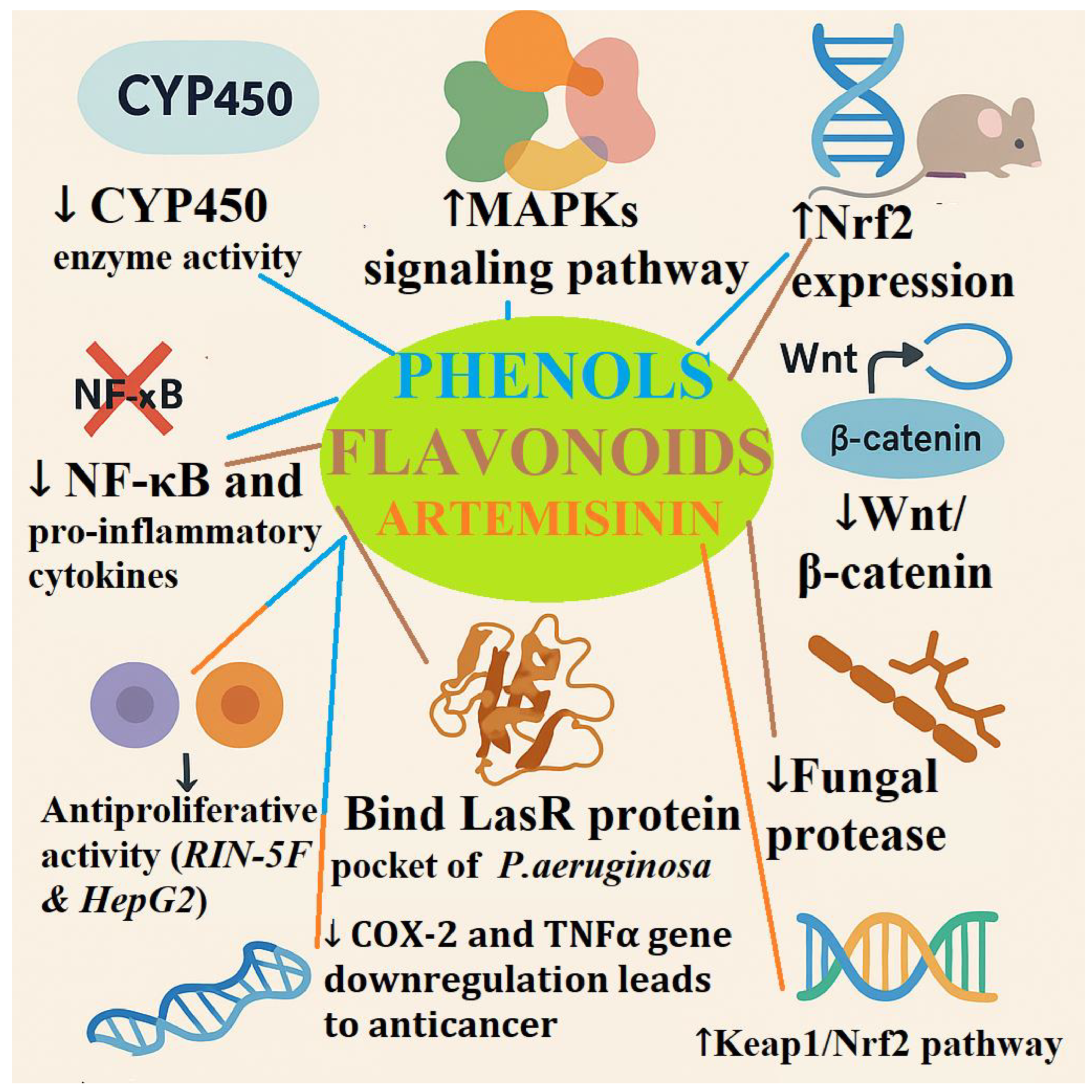
4.1. Comparative Properties of Selected Compound Classes
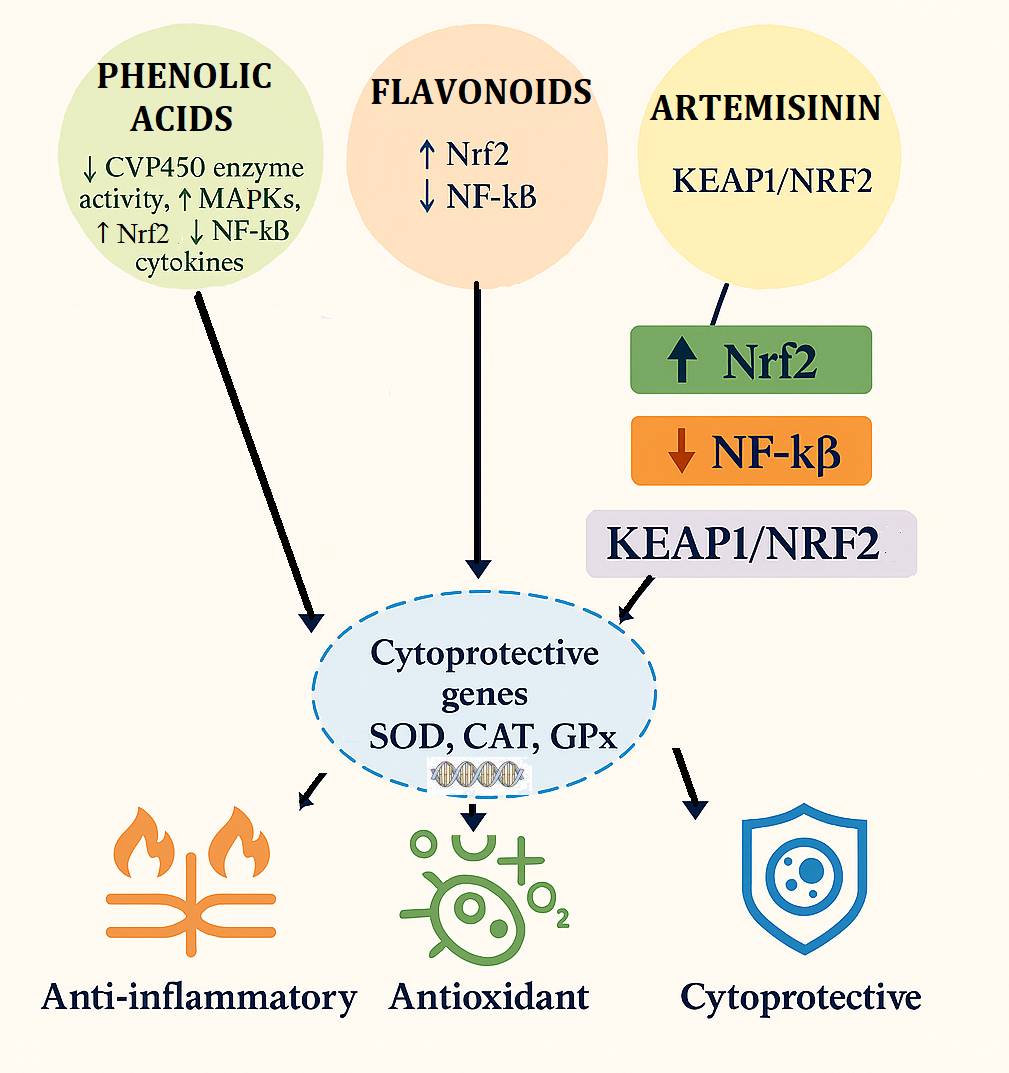
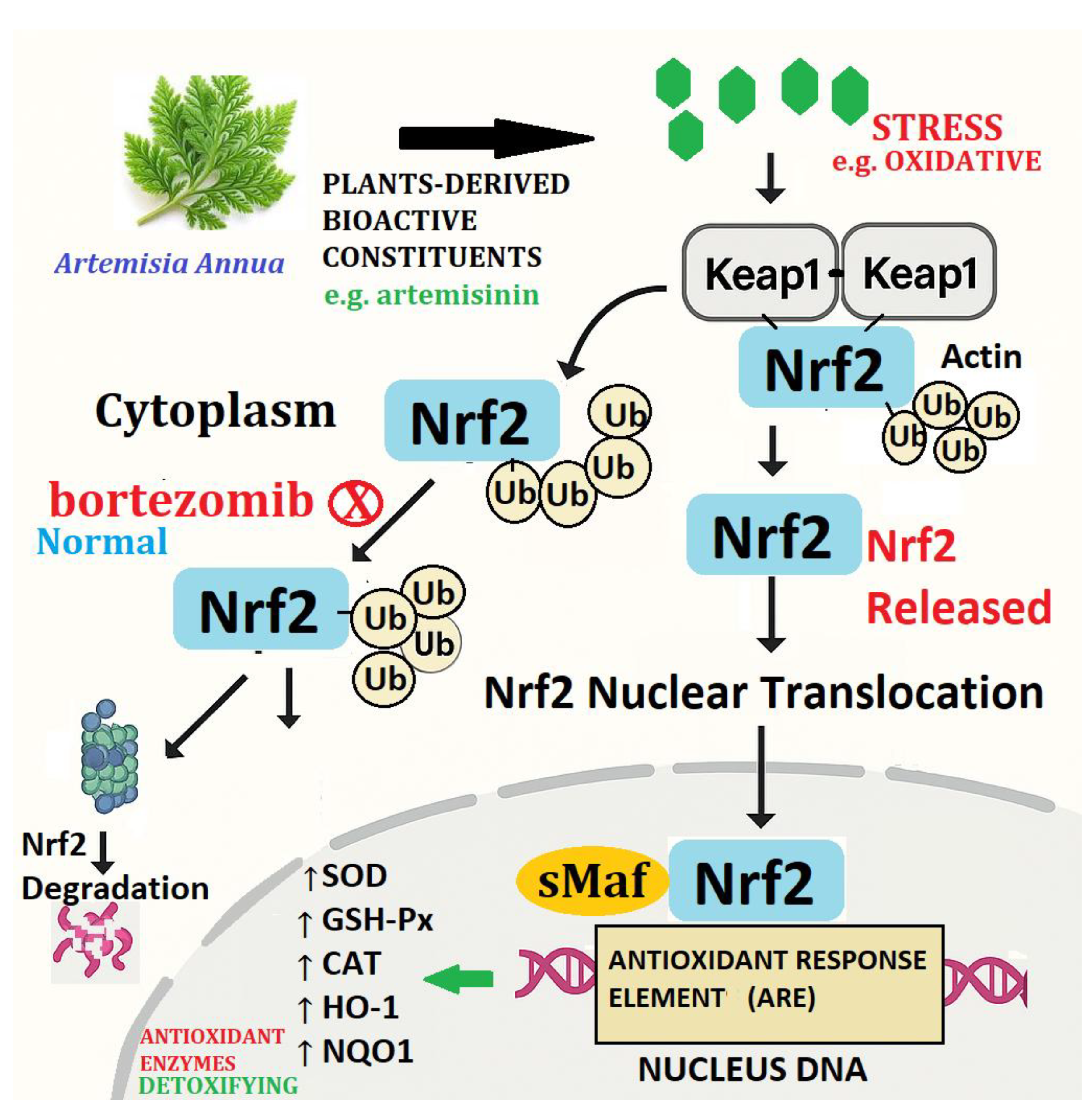
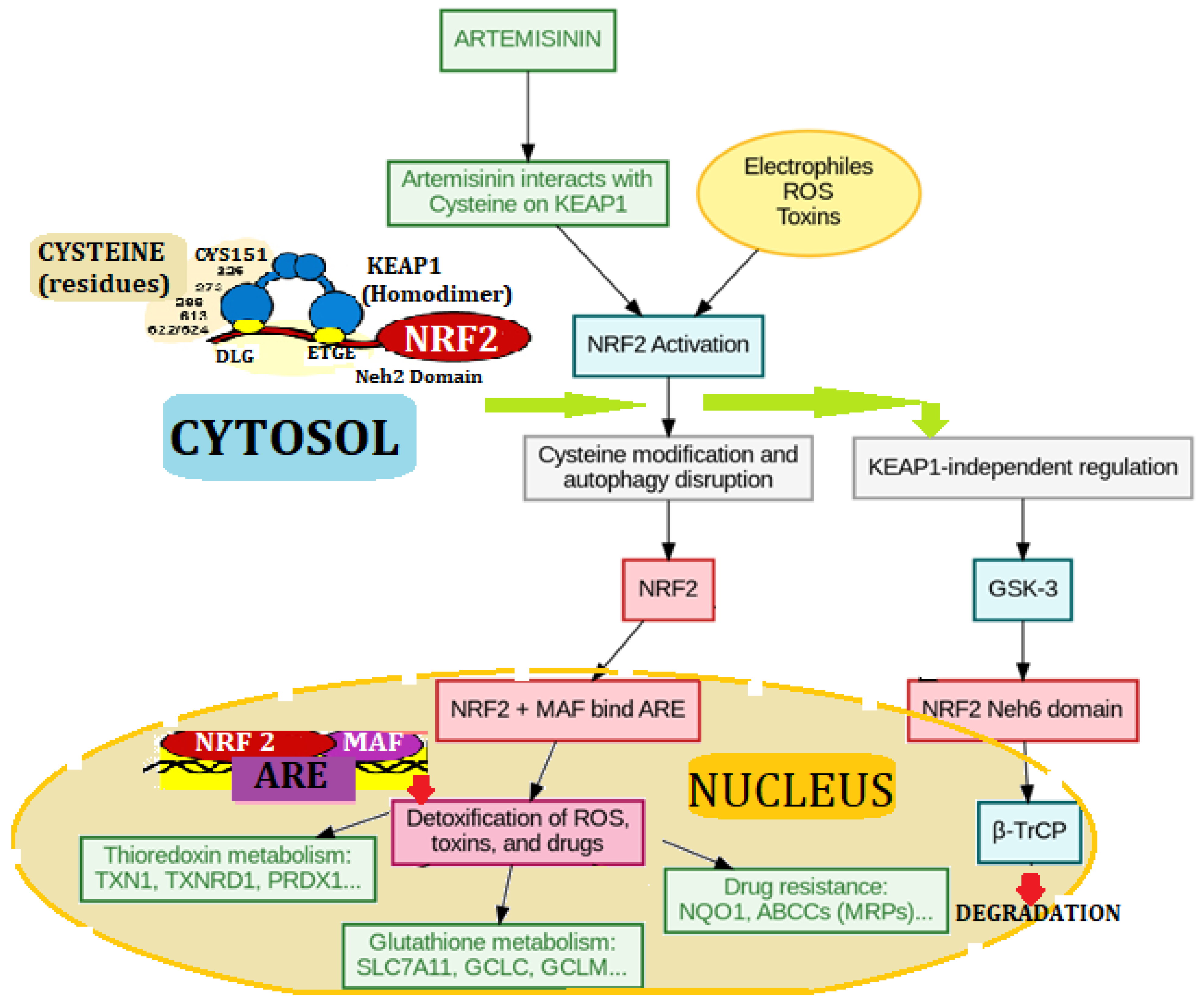
4.2. Artemisinin-Induced Nrf2/Keap1 Pathway Activation Enhances Antioxidant Gene Expression in Cells
- SOD (Superoxide dismutase)
- CAT (Catalase)
- GSH-Px (Glutathione peroxidase)
- HO-1 (Heme oxygenase-1)
- NQO1 (NAD(P)H Quinone Dehydrogenase 1)
4.3. Biotechnology and Gene Studies: Insights into Antioxidant Mechanisms and Therapeutic Targets
4.4. Multitargeted Cellular Effects of Phenols, Flavonoids, and Artemisinin in Oxidative Stress and Disease Modulation
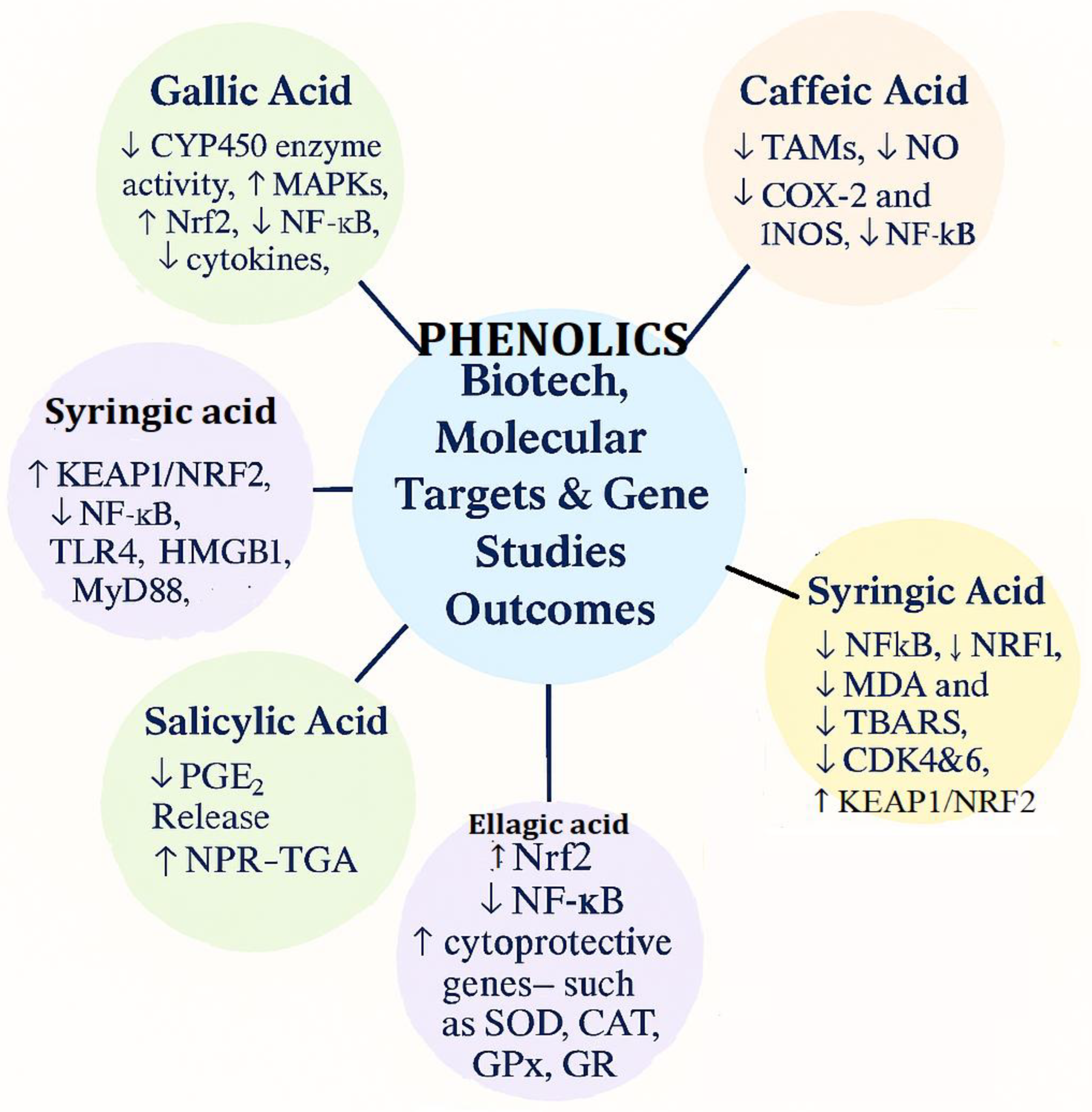
4.5. Bioetch and Gene Target Studies Outcomes of Selected Phenolic acids
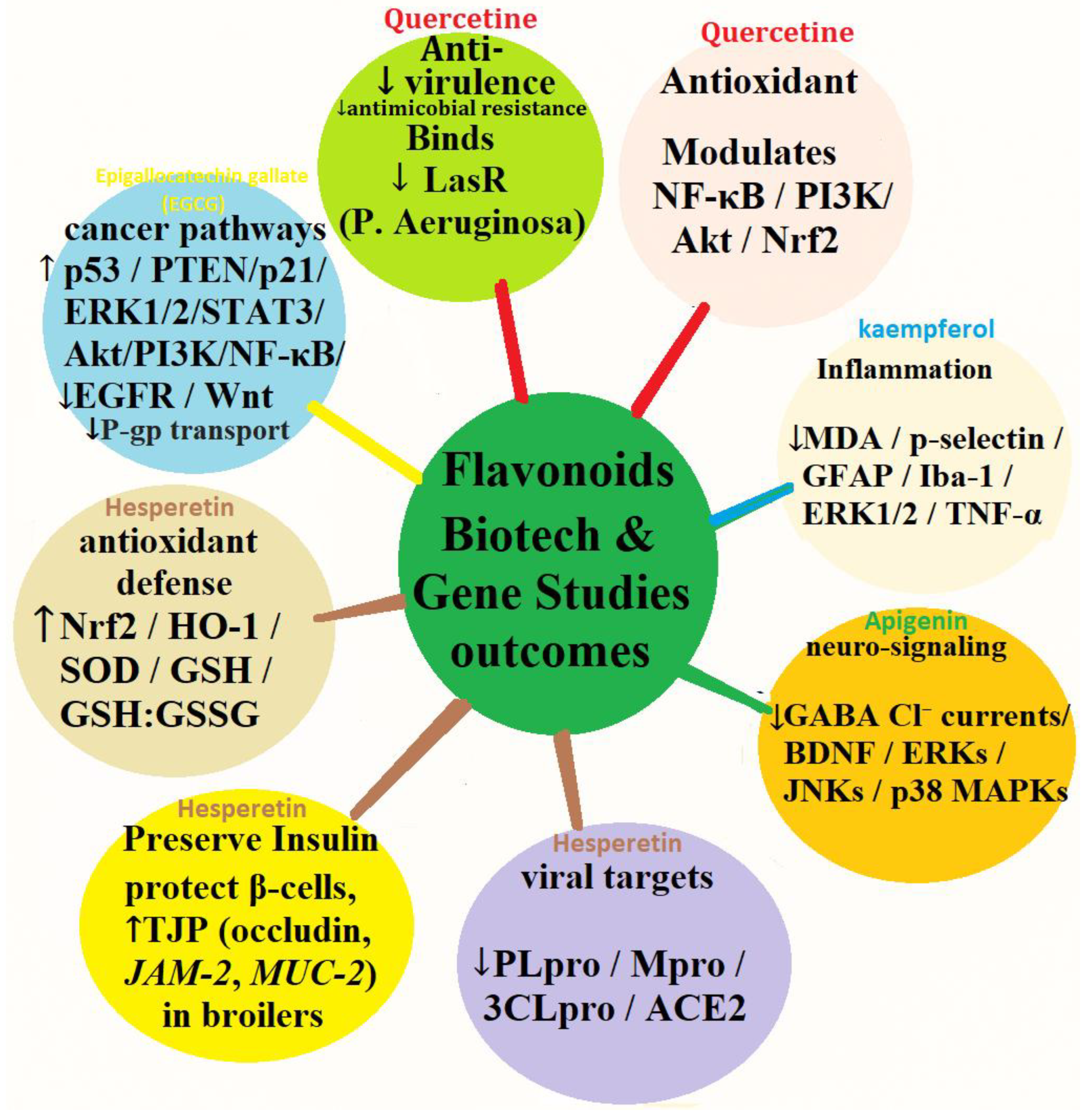
4.6. Bioetch and Gene Target Studies Outcomes of Selected Flavonoids
4.7. Chemistry: From Natural Compound to Enhanced Derivatives
4.8. The NRF2 Defense Network: From Artemisinin Signals to Detox Genes
5. Future Prospects
6. Conclusion
Acknowledgment
Conflict of Interest
References
- El-Hamid, M.I.A.; El-Malt, R.M.S.; Khater, S.I.; Abdelwarith, A.A.; Khamis, T.; El-Wahab, R.A.A.; Younis, E.M.; Davies, S.J.; Mohamed, D.I.; Mohamed, R.I.; et al. Impact of liposomal hesperetin in broilers: prospects for improving performance, antioxidant potential, immunity, and resistance against Listeria monocytogenes. Avian Pathol. 2024, 54, 120–148. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E. D. N. S., Nam, K., Huang, X., & Ahn, D. U. Plant-and animal-based antioxidants’ structure, efficacy, mechanisms, and applications: A review. Antioxidants 2022, 11, 1025.
- Acquaviva, A.; Nilofar, N.; Bouyahya, A.; Zengin, G.; Di Simone, S.C.; Recinella, L.; Leone, S.; Brunetti, L.; Uba, A.I.; Cakilcioğlu, U.; et al. Chemical Characterization of Different Extracts from Artemisia annua and Their Antioxidant, Enzyme Inhibitory and Anti-Inflammatory Properties. Chem. Biodivers. 2023, 20, e202300547. [Google Scholar] [CrossRef] [PubMed]
- Addissouky, T.A. Artemisinin and its derivatives throughout the therapeutic mechanisms and clinical potential. Discov. Chem. 2025, 2, 1–16. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Nascimento, L.B.d.S.; Gori, A.; Piccolo, E.L.; Tattini, M. Antioxidants by nature: an ancient feature at the heart of flavonoids' multifunctionality. New Phytol. 2024, 245, 11–26. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Pharmacological Significance of Hesperidin and Hesperetin, Two Citrus Flavonoids, as Promising Antiviral Compounds for Prophylaxis Against and Combating COVID-19. Nat. Prod. Commun. 2021, 16, 1934578X211042540. [Google Scholar] [CrossRef]
- Aguilar, K.; Jakubek, P.; Zorzano, A.; Wieckowski, M.R. Primary mitochondrial diseases: The intertwined pathophysiology of bioenergetic dysregulation, oxidative stress and neuroinflammation. Eur. J. Clin. Investig. 2024, 54, e14217. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Abhari, F.M. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Akbarirad, H., Ardabili, A. G., Kazemeini, S. M., & Khaneghah, A. M. An overview on some of important sources of natural antioxidants. International food research journal 2016, 23. http://ifrj.upm.edu.my/23%20%202016/(3).pdf.
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res. Notes 2019, 12, 3. [Google Scholar] [CrossRef]
- Ali, W.; Khan, A.; Yousafzai, A.; Rabi, F.; Idrees, M. Nanoparticles Enhancing the Production of Key Phytochemicals: A Comprehensive Review. J. Heal. Wellness Community Res. 2025, e59. [Google Scholar] [CrossRef]
- Amarowicz, R., & Pegg, R. B. (2019). Natural antioxidants of plant origin. In Advances in food and nutrition research (Vol. 90, pp. 1-81). Academic Press. https://www.sciencedirect.com/science/article/abs/pii/S1043452619300269.
- Amin, J., Djajadisastra, J., Syafhan, N. F., Simamora, E. L. P., & Wulandari, K. Green tea [Camellia sinensis (L.) Kuntze] leaves extract and hibiscus (Hibiscus tilliaceus L.) leaves extract as topical hair growth promoter in microemulsion. Agriculture And Natural Resources 2019, 53, 139–147.
- Arif, T. Salicylic acid as a peeling agent: a comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Atta, E. M., Mohamed, N. H., & Abdelgawad, A. A. Antioxidants: An overview on the natural and synthetic types. Eur. Chem. Bull 2017, 6, 365-375. https://pdfs.semanticscholar.org/9583/f82c3cdb5aaea234939b1dbd5806e48be1c5.pdf.
- Babanyaya, A., Mustapha, A. B., Dwanga, D. M., Abubakar, D., Bioltif, Y. E., Abubakar, M. Y., & Adam, A. B. Industrial Scale Production of Phytochemicals for Cancer Prevention: Challenges and Opportunities. Journal Pembelajaran Kimia 2024, 9, 19-39. https://www.researchgate.net/profile/Ansar-Adam/publication/387441413_Industrial_Scale_Production_of_Phytochemicals_for_Cancer_Prevention_Challenges_and_Opportunities/links/676e32c800aa3770e0bd746b/Industrial-Scale-Production-of-Phytochemicals-for-Cancer-Prevention-Challenges-and-Opportunities.pdf.
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Bilia, A. R., Bergonzi, M. C., Boulos, J. C., & Efferth, T. Nanocarriers to enhance solubility, bioavailability, and efficacy of artemisinins. World Journal of Traditional Chinese Medicine 2020, 6, 26-36. https://journals.lww.com/wtcm/fulltext/2020/06010/Nanocarriers_to_Enhance_Solubility,.3.aspx.
- Cao, J.; Han, J.; Xiao, H.; Qiao, J.; Han, M. Effect of Tea Polyphenol Compounds on Anticancer Drugs in Terms of Anti-Tumor Activity, Toxicology, and Pharmacokinetics. Nutrients 2016, 8, 762. [Google Scholar] [CrossRef]
- Cervantes-Anaya, N.; Azpilcueta-Morales, G.; Estrada-Camarena, E.; Ortega, D.R.; de la Cruz, V.P.; González-Trujano, M.E.; López-Rubalcava, C. Pomegranate and Its Components, Punicalagin and Ellagic Acid, Promote Antidepressant, Antioxidant, and Free Radical-Scavenging Activity in Ovariectomized Rats. Front. Behav. Neurosci. 2022, 16, 836681. [Google Scholar] [CrossRef]
- Chandekar, L.; Katgeri, R.; Takke, A. The Potential Clinical Uses and Nanoformulation Strategies of Kaempferol, a Dietary Flavonoid. Rev. Bras. de Farm. 2022, 32, 693–707. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Razis, A.F.A.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Chen, F.; Xiao, M.; Hu, S.; Wang, M. Keap1-Nrf2 pathway: a key mechanism in the occurrence and development of cancer. Front. Oncol. 2024, 14, 1381467. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Guo, Z.; Lei, J.; Zhou, B. Recent advancement in bioeffect, metabolism, stability, and delivery systems of apigenin, a natural flavonoid compound: challenges and perspectives. Front. Nutr. 2023, 10, 1221227. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zou, F.; Liu, W. Recent advancement in prevention against hepatotoxicity, molecular mechanisms, and bioavailability of gallic acid, a natural phenolic compound: challenges and perspectives. Front. Pharmacol. 2025, 16, 1549526. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Choudhary, H.R.; B., D.K.; Kadiri, S.K.; Khobragade, D.S.; Tiwari, P. Integrating Biosensors in Phytochemical Research: Challenges and Breakthroughs. Recent Patents Biotechnol. 2025, 20, 1–16. [CrossRef]
- Ciriminna, R.; Petri, G.L.; Angellotti, G.; Luque, R.; Tixier, A.F.; Meneguzzo, F.; Pagliaro, M. Citrus Flavonoids as Antimicrobials. Chem. Biodivers. 2025, 22, e202403210. [Google Scholar] [CrossRef]
- Črnivec, I.G.O.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Možina, S.S.; et al. Waste streams in onion production: Bioactive compounds, quercetin and use of antimicrobial and antioxidative properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, X.; Chen, W.; Chen, Y.; Zhang, Q.; Mo, S.; Lu, J. Dihydroartemisinin: A Potential Natural Anticancer Drug. Int. J. Biol. Sci. 2021, 17, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kwon, M.; Kim, J.-Y. Enhancement of specialized metabolites using CRISPR/Cas gene editing technology in medicinal plants. Front. Plant Sci. 2024, 15, 1279738. [Google Scholar] [CrossRef] [PubMed]
- Davidova, S.; Galabov, A.S.; Satchanska, G. Antibacterial, Antifungal, Antiviral Activity, and Mechanisms of Action of Plant Polyphenols. Microorganisms 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Cherubim, D.J.d.L.; Martins, C.V.B.; Fariña, L.O.; Lucca, R.A.d.S.d. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- De, R.; Sarkar, A.; Ghosh, P.; Ganguly, M.; Karmakar, B.C.; Saha, D.R.; Halder, A.; Chowdhury, A.; Mukhopadhyay, A.K. Antimicrobial activity of ellagic acid against Helicobacter pylori isolates from India and during infections in mice. J. Antimicrob. Chemother. 2018, 73, 1595–1603. [Google Scholar] [CrossRef]
- Diab, M.K.; Abu-Elsaoud, A.M.; Salama, M.G.; Ghareeb, E.M. Phytochemical treasure troves—insights into bioactivities, phytochemistry, and uses of Artemisia species. Phytochem. Rev. 2025, 1–38. [Google Scholar] [CrossRef]
- Dibal, N.I.; Garba, S.H.; Jacks, T.W. Acute Toxicity of Quercetin From Onion Skin in Mice. Pharm. Biomed. Res. 2020. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, Y. Therapeutic potential of flavonoids in gastrointestinal cancer: Focus on signaling pathways and improvement strategies (Review). Mol. Med. Rep. 2025, 31, 1–34. [Google Scholar] [CrossRef]
- Du, W.; Zhou, M.; Liu, Z.; Chen, Y.; Li, R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control. 2018, 85, 119–126. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [CrossRef]
- El-Rayes, T. K., El Basuini, M. F., Elghioushy, A. B., El-Damrawy, S. Z., Mamdouh, M., Taha, A. E., & Abd El-Hack, M. E. Dietary inclusion of Artemisia annua improves antioxidant performance, immunoglobulin protein levels, lipid profile, carcass characteristics, meat quality, and histomorphometric features of broiler chickens. Annals of Animal Science 2025, 25, 225-238. https://www.proquest.com/openview/c14a38224c48e74ccf76f6c2c1b8cb13/1?pq-origsite=gscholar&cbl=1976406.
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Faria, W.C.S.; da Silva, A.A.; Veggi, N.; Kawashita, N.H.; Lemes, S.A.d.F.; de Barros, W.M.; Cardoso, E.d.C.; Converti, A.; Moura, W.d.M.; Bragagnolo, N. Acute and subacute oral toxicity assessment of dry encapsulated and non-encapsulated green coffee fruit extracts. J. Food Drug Anal. 2020, 28, 14–161. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.-X.; Hong, J.-X.; Wang, Q.; Fan, Y.-Y.; Yuan, C.-T.; Lei, X.-H.; Zhu, M.; Qin, A.; Chen, H.-X.; Hong, D. Dihydroartemisinin prevents breast cancer-induced osteolysis via inhibiting both breast caner cells and osteoclasts. Sci. Rep. 2016, 6, srep19074. [Google Scholar] [CrossRef]
- de Franco, E.P.D.; Contesini, F.J.; da Silva, B.L.; Fernandes, A.M.A.d.P.; Leme, C.W.; Cirino, J.P.G.; Campos, P.R.B.; Carvalho, P.d.O. Enzyme-assisted modification of flavonoids from Matricaria chamomilla: antioxidant activity and inhibitory effect on digestive enzymes. J. Enzym. Inhib. Med. Chem. 2020, 35, 42–49. [Google Scholar] [CrossRef]
- Fuentes, J.; Arias-Santé, M.F.; Atala, E.; Pastene, E.; Kogan, M.J.; Speisky, H. Low nanomolar concentrations of a quercetin oxidation product, which naturally occurs in onion peel, protect cells against oxidative damage. Food Chem. 2020, 314, 126166. [Google Scholar] [CrossRef]
- Furniturewalla, A.; Barve, K. Approaches to overcome bioavailability inconsistencies of epigallocatechin gallate, a powerful anti-oxidant in green tea. Food Chem. Adv. 2022, 1, 100037. [Google Scholar] [CrossRef]
- Gan, L.; Wang, W.; Jiang, J.; Tian, K.; Liu, W.; Cao, Z. Dual role of Nrf2 signaling in hepatocellular carcinoma: promoting development, immune evasion, and therapeutic challenges. Front. Immunol. 2024, 15, 1429836. [Google Scholar] [CrossRef]
- Gang, G.; Gao, R.; Li, R.; Jin, X.; Xing, Y.; Yan, S.; Xu, Y.; Shi, B. Study on the Regulatory Effect of Water Extract of Artemisia annua L. on Antioxidant Function of Mutton Sheep via the Keap1/Nrf2 Signaling Pathway. Antioxidants 2025, 14, 885. [Google Scholar] [CrossRef]
- Gang, G.; Gao, R.; Zhao, H.; Xu, Y.; Xing, Y.; Jin, X.; Hong, L.; Yan, S.; Shi, B. Effects of water extracts of Artemisia annua L. on rumen immune and antioxidative indexes, fermentation parameters and microbials diversity in lambs. Front. Microbiol. 2024, 15, 1485882. [Google Scholar] [CrossRef]
- Gao, H.; Pei, X.; Song, X.; Wang, S.; Yang, Z.; Zhu, J.; Lin, Q.; Zhu, Q.; Yang, X. Application and development of CRISPR technology in the secondary metabolic pathway of the active ingredients of phytopharmaceuticals. Front. Plant Sci. 2025, 15, 1477894. [Google Scholar] [CrossRef] [PubMed]
- Gavarić, N., Aćimović, M., Kladar, N., Hitl, M., Drljača Lero, J., Milić, N., & Radovanović, K. Unlocking the Bioactivity of Sweet Wormwood (Artemisia annua L., Asteraceae) Ethanolic Extract: Phenolics, Antioxidants, and Cytotoxic Effects. Pharmaceutics 2025, 17, 890. https://www.mdpi.com/1999-4923/17/7/890.
- Grzelak-Błaszczyk, K.; Milala, J.; Kosmala, M.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Klewicki, R.; Juśkiewicz, J.; Fotschki, B.; Jurgoński, A. Onion quercetin monoglycosides alter microbial activity and increase antioxidant capacity. J. Nutr. Biochem. 2018, 56, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant by-product antioxidants: Control of protein-lipid oxidation in meat and meat products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2023, 25, 13–33. [Google Scholar] [CrossRef]
- Hasan, Z.; Islam, A.; Khan, L.A. Spectroscopic investigations on fungal aspartic protease as target of gallic acid. Int. J. Biol. Macromol. 2023, 228, 333–345. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Huang, Z.; Gan, S.; Zhuang, X.; Chen, Y.; Lu, L.; Wang, Y.; Qi, X.; Feng, Q.; Huang, Q.; Du, B.; et al. Artesunate Inhibits the Cell Growth in Colorectal Cancer by Promoting ROS-Dependent Cell Senescence and Autophagy. Cells 2022, 11, 2472. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H.; Alsharif, K.F.; Khan, A.H.; Aschner, M.; Saso, L. The Therapeutic Potential of Kaemferol and Other Naturally Occurring Polyphenols Might Be Modulated by Nrf2-ARE Signaling Pathway: Current Status and Future Direction. Molecules 2022, 27, 4145. [Google Scholar] [CrossRef]
- Ikawa, M.; Okazawa, H.; Yoneda, M. Molecular imaging for mitochondrial metabolism and oxidative stress in mitochondrial diseases and neurodegenerative disorders. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2021, 1865, 129832. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [CrossRef]
- Jia, X.; Wang, L.; Zhao, H.; Zhang, Y.; Chen, Z.; Xu, L.; Yi, K. The origin and evolution of salicylic acid signaling and biosynthesis in plants. Mol. Plant 2023, 16, 245–259. [Google Scholar] [CrossRef]
- Jomova, K., Alomar, S. Y., Valko, R., Liska, J., Nepovimova, E., Kuca, K., & Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chemico-Biological Interactions 2025, 111489. https://www.sciencedirect.com/science/article/pii/S000927972500119X.
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar] [CrossRef]
- Karalija, E.; Macanović, A.; Ibragić, S. Revisiting Traditional Medicinal Plants: Integrating Multiomics, In Vitro Culture, and Elicitation to Unlock Bioactive Potential. Plants 2025, 14, 2029. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.T.B.; Fatima, H.; Naz, I.; Kanwal, N.; Haq, I.-U. Pre-clinical studies comparing the anti-inflammatory potential of artemisinic compounds by targeting NFκB/TNF-α/NLRP3 and Nrf2/TRX pathways in Balb/C mice. Front. Pharmacol. 2024, 15, 1352827. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Kim, W. S., Choi, W. J., Lee, S., Kim, W. J., Lee, D. C., Sohn, U. D., Shin, H.S., & Kim, W. Anti-inflammatory, antioxidant and antimicrobial effects of artemisinin extracts from Artemisia annua L. The Korean Journal of Physiology and Pharmacology 2015, 19, 21–27.
- Kishi, S.; Nagasu, H.; Kidokoro, K.; Kashihara, N. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat. Rev. Nephrol. 2024, 20, 101–119. [Google Scholar] [CrossRef]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Lipert, A.; Szymańska, G. Yeast Extract Stimulates Ginsenoside Production in Hairy Root Cultures of American Ginseng Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactors. Molecules 2017, 22, 880. [Google Scholar] [CrossRef]
- Korobkova, E.A. Effect of Natural Polyphenols on CYP Metabolism: Implications for Diseases. Chem. Res. Toxicol. 2015, 28, 1359–1390. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Cen, Y.; Qin, R.; Shang, S.; Zhai, Z.; Liu, C.; Pan, X.; Zhou, H. Artesunate Attenuates Pro-Inflammatory Cytokine Release from Macrophages by Inhibiting TLR4-Mediated Autophagic Activation via the TRAF6-Beclin1-PI3KC3 Pathway. Cell. Physiol. Biochem. 2018, 47, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Lala, S. Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: A review. IET Nanobiotechnology 2021, 15, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; de Maio, M.C.; Minniti, G.; de Góes Corrêa, N.; Barbalho, S.M.; Quesada, K.; Guiguer, E.L.; Sloan, K.P.; Detregiachi, C.R.P.; Araújo, A.C.; et al. Effects of Medicinal Plants and Phytochemicals in Nrf2 Pathways during Inflammatory Bowel Diseases and Related Colorectal Cancer: A Comprehensive Review. Metabolites 2023, 13, 243. [Google Scholar] [CrossRef]
- Le, B.; Anh, P.T.N.; Kim, J.-E.; Cheng, J.; Yang, S.H. Rice bran fermentation by lactic acid bacteria to enhance antioxidant activities and increase the ferulic acid, ρ-coumaric acid, and γ-oryzanol content. J. Appl. Biol. Chem. 2019, 62, 257–264. [Google Scholar] [CrossRef]
- Lee, R.; Lee, W.-Y.; Kim, D.-W.; Park, H.-J. Artemisinin alleviates cisplatin-induced damage in GC-1 spermatogonia through ER stress mechanisms. Heliyon 2025, 11, e42579. [Google Scholar] [CrossRef]
- Le, N.P.K.; Herz, C.; Gomes, J.V.D.; Förster, N.; Antoniadou, K.; Mittermeier-Kleßinger, V.K.; Mewis, I.; Dawid, C.; Ulrichs, C.; Lamy, E. Comparative Anti-Inflammatory Effects of Salix Cortex Extracts and Acetylsalicylic Acid in SARS-CoV-2 Peptide and LPS-Activated Human In Vitro Systems. Int. J. Mol. Sci. 2021, 22, 6766. [Google Scholar] [CrossRef]
- Lee, E.J.; Patil, B.S.; Yoo, K.S. Antioxidants of 15 onions with white, yellow, and red colors and their relationship with pungency, anthocyanin, and quercetin. LWT-Food Science and Technology 2015, 63, 108–114. [Google Scholar] [CrossRef]
- Les, F.; Prieto, J.M.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 6, 2049–2057. [Google Scholar] [CrossRef]
- https://pubs.rsc.org/en/content/articlelanding/2015/fo/c5fo00426h/unauth.
- Li, J.; Yu, Q.; Liu, C.; Zhang, N.; Xu, W. Flavonoids as key players in cold tolerance: molecular insights and applications in horticultural crops. Hortic. Res. 2025, 12, uhae366. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, S.; Wei, R.; Cui, Z.; Wu, X.; Wei, R.; Xie, L.; Zhou, Y.; Li, W.; Chen, W. Keap1 Cystenine 151 as a Potential Target for Artemisitene-Induced Nrf2 Activation. BioMed Res. Int. 2019, 2019, 5198138. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Lv, Y.; Li, W.; Liao, W.; Jiang, H.; Liu, Y.; Cao, J.; Lu, W.; Feng, Y. Nano-Drug Delivery Systems Based on Natural Products. Int. J. Nanomed. 2024, ume 19, 541–569. [Google Scholar] [CrossRef]
- Maistro, E.L.; Terrazzas, P.M.; Sawaya, A.C.H.F.; Rosa, P.C.P.; Perazzo, F.F.; Gaivão, I.O.d.M. In vivo toxicogenic potential of Salix alba (Salicaceae) bark extract. J. Toxicol. Environ. Heal. Part A 2021, 85, 121–130. [Google Scholar] [CrossRef]
- Mishra, S.; Khushtar, M.; Ahmad, U. Recent Advances in the Pharmacological Potential of Quercetin and Kaempferol: Mechanisms, Therapeutic Applications, and Future Perspectives- A Comprehensive Review. Cuest. de Fisioter. 2025, 54, 5497–5505. [Google Scholar] [CrossRef]
- Mittal, G.; A, P.; Dhali, A.; Prasad, R.; S, Y.; Nurani, K.M.; Găman, M.-A. Plant extracts with antioxidant and hepatoprotective benefits for liver health: A bibliometric analysis of drug delivery systems. World J. Gastroenterol. 2025, 31, 105836. [Google Scholar] [CrossRef]
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Emwas, A.-H.; Khan, R.A. Salt-Tolerant Plants, Halophytes, as Renewable Natural Resources for Cancer Prevention and Treatment: Roles of Phenolics and Flavonoids in Immunomodulation and Suppression of Oxidative Stress towards Cancer Management. Int. J. Mol. Sci. 2023, 24, 5171. [Google Scholar] [CrossRef]
- Mohammed, M. J., Anand, U., Altemimi, A. B., Tripathi, V., Guo, Y., & Pratap-Singh, A. Phenolic composition, antioxidant capacity and antibacterial activity of white wormwood (Artemisia herba-alba). Plants 2021, 10, 164.
- Molaei, E.; Molaei, A.; Abedi, F.; Hayes, A.W.; Karimi, G. Nephroprotective activity of natural products against chemical toxicants: The role of Nrf2/ARE signaling pathway. Food Sci. Nutr. 2021, 9, 3362–3384. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Wang, J.; Lin, Q.; Ferreira, P.; Avery, M.A.; Elokely, K.; Staines, H.M.; Krishna, S. Selective Inhibition of Plasmodium falciparum ATPase 6 by Artemisinins and Identification of New Classes of Inhibitors after Expression in Yeast. Antimicrob. Agents Chemother. 2022, 66, e0207921. [Google Scholar] [CrossRef] [PubMed]
- Morua, E. , Cuyas, L., & Matías-Hernández, L. The Beneficial Use of Artemisia annua, Artemisinin, and Other Compounds in Animal Health. Animals 2025, 15, 1359. [Google Scholar]
- Mude, H.; Maroju, P.A.; Balapure, A.; Ganesan, R.; Dutta, J.R. Water-soluble caffeic acid-dopamine acid-base complex exhibits enhanced bactericidal, antioxidant, and anticancer properties. Food Chem. 2022, 374, 131830. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Nair, B.; Menon, A.; Kalidas, M.R.; Nath, L.R.; Calina, D.; Sharifi-Rad, J. Modulating the JAK/STAT pathway with natural products: potential and challenges in cancer therapy. Discov. Oncol. 2025, 16, 1–23. [Google Scholar] [CrossRef]
- Nam, D.-G.; Kim, M.; Choi, A.-J.; Choe, J.-S. Health Benefits of Antioxidant Bioactive Compounds in Ginger (Zingiber officinale) Leaves by Network Pharmacology Analysis Combined with Experimental Validation. Antioxidants 2024, 13, 652. [Google Scholar] [CrossRef]
- Naraki, K.; Rameshrad, M.; Hosseinzadeh, H. Protective effects and therapeutic applications of ellagic acid against natural and synthetic toxicants: A review article. Iranian journal of basic medical sciences 2022, 25, 1402. [Google Scholar] [CrossRef]
- Nuryana, I., Ratnakomala, S., Fahrurrozi, A. B. J., Andriani, A., Putra, F. J. N., Rezamela, E., Wulansari, R., Prawira-Atmaja, M.I., & Lisdiyanti, P. Catechin contents, antioxidant and antibacterial activities of different types of Indonesian tea (Camellia sinensis). Ann. Bogor 2020, 24, 107. https://www.researchgate.net/profile/Isa-Nuryana/publication/348264057_Catechin_Contents_Antioxidant_and_Antibacterial_Activities_of_Different_Types_of_Indonesian_Tea_Camellia_sinensis/links/5ff55a90299bf1408874f73c/Catechin-Contents-Antioxidant-and-Antibacterial-Activities-of-Different-Types-of-Indonesian-Tea-Camellia-sinensis.pdf.
- Nwozo, O.S.; Effiong, E.M.; Aja, P.M.; Awuchi, C.G. Antioxidant, phytochemical, and therapeutic properties of medicinal plants: a review. Int. J. Food Prop. 2023, 26, 359–388. [Google Scholar] [CrossRef]
- Osonwa, U.E.; Hu, M. Bioavailability and Pharmacokinetics of Dihydroartemisinin (DHA) and its Analogs—Mechanistic Studies on its ADME. Curr. Pharmacol. Rep. 2018, 4, 33–44. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Q.; Li, W.; Dong, X.; Xie, S.; Tan, B.; Liu, H.; Wang, Y. Artemisinin supplementation in concentrated cottonseed protein basal diets enhances growth, antioxidant capacity, intestinal immunity and microbiota in hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Aquac. Rep. 2025, 41, 102659. [Google Scholar] [CrossRef]
- Pavlova, E.L.; Zografov, N.N.; Simeonova, L.S. Comparative study on the antioxidant capacities of synthetic influenza inhibitors and ellagic acid in model systems. Biomed. Pharmacother. 2016, 83, 755–762. [Google Scholar] [CrossRef]
- https://www.sciencedirect.com/science/article/abs/pii/S0753332216306886.
- Pawłowska, M.; Nuszkiewicz, J.; Jarek, D.J.; Woźniak, A. Ferroptosis and Metabolic Dysregulation: Emerging Chemical Targets in Cancer and Infection. Molecules 2025, 30, 3020. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Werner, C.M.; Nickel, A.G.; Herrera, M.D.; Motilva, M.-J.; Böhm, M.; de Sotomayor, M.A.; Laufs, U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017, 48, 51–61. [Google Scholar] [CrossRef]
- Parveen, B.; Rajinikanth, V.; Narayanan, M. Natural plant antioxidants for food preservation and emerging trends in nutraceutical applications. Discov. Appl. Sci. 2025, 7, 845. [Google Scholar] [CrossRef]
- Patel, J.; Roy, H.; Chintamaneni, P.K.; Patel, R.; Bohara, R. Advanced Strategies in Enhancing the Hepatoprotective Efficacy of Natural Products: Integrating Nanotechnology, Genomics, and Mechanistic Insights. ACS Biomater. Sci. Eng. 2025, 11, 2528–2549. [Google Scholar] [CrossRef]
- Pham, D.-C.; Shibu, M.A.; Mahalakshmi, B.; Velmurugan, B.K. Effects of phytochemicals on cellular signaling: reviewing their recent usage approaches. Crit. Rev. Food Sci. Nutr. 2020, 60, 3522–3546. [Google Scholar] [CrossRef]
- Piątczak, E., Dybowska, M., Płuciennik, E., Kośla, K., Kolniak-Ostek, J., & Kalinowska-Lis, U. Identification and accumulation of phenolic compounds in the leaves and bark of Salix alba (L.) and their biological potential. Biomolecules 2020, 10, 1391.
- Pillai, R.; Hayashi, M.; Zavitsanou, A.-M.; Papagiannakopoulos, T. NRF2: KEAPing Tumors Protected. Cancer Discov. 2022, 12, 625–643. [Google Scholar] [CrossRef]
- Pooja, G.; Shweta, S.; Patel, P. Oxidative stress and free radicals in disease pathogenesis: a review. Discov. Med. 2025, 2, 104. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Randjelović, P.; Veljković, S.; Stojiljković, N.; Sokolović, D.; Ilić, I.; Laketić, D.; Randjelović, D.; Randjelović, N. The Beneficial Biological Properties of Salicylic Acid. Acta Fac. Medicae Naissensis 2015, 32, 259–265. [Google Scholar] [CrossRef]
- Rasheed, F.; Naeem, M.; Uddin, M.; Khan, M.M.A. Synergistic Effect of Irradiated Sodium Alginate and Methyl Jasmonate on Anticancer Alkaloids Production in Periwinkle (Catharanthus roseus L.). J. Funct. Environ. Bot. 2017, 7, 77–91. [Google Scholar] [CrossRef]
- Rasheed, A.; Kamran, S.H.; Hameed, M.; Siddique, F.; Latif, S.; Bibi, M.; Rizwan, R. Syringic acid loaded chitosan nanoparticles mitigate glycation associated oxidative stress and inflammation in hyperglycaemic rat model. Sci. Rep. 2025, 15, 22778. [Google Scholar] [CrossRef]
- Rithichai, P.; Jirakiattikul, Y.; Nambuddee, R.; Itharat, A.; Addi, M. Effect of Salicylic Acid Foliar Application on Bioactive Compounds and Antioxidant Activity in Holy Basil (Ocimum sanctum L.). Int. J. Agron. 2024, 2024, 8159886. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef]
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef]
- Saikia, L.; Talukdar, N.C.; Dutta, P.P. Exploring the Therapeutic Role of Flavonoids Through AMPK Activation in Metabolic Syndrome: A Narrative Review. Phytotherapy Res. 2025, 39, 1403–1421. [Google Scholar] [CrossRef]
- Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., Lucarini, M., Santini, A., Souto, E.B., Novellino, E. and Antolak, H. & Martins, N. The therapeutic potential of apigenin. International journal of molecular sciences 2019, 20, 1305.
- Salehi, H.; Rad, A.C.; Lucini, L. Different Forms of Manganese Provide Distinctive Metabolomics Signatures and Bioactive Profiles in Artemisia annua. Phytochem. Anal. 2025. [CrossRef]
- Seema, B.R.; Jyothi, Y.; Bhovi, C.N.; Aradhya, M.V.; Lekhak, M.; Mane, S.R. Advanced up and down methodology for acute toxicity assessment with reliable LD50 verified by aqueous extract of curly kale using wistar rats. Res. J. Pharm. Technol. 2023, 16, 4519–4524. [Google Scholar] [CrossRef]
- A Shalaby, E.; Aboul-Enein, A.; El-Shemy, H.; Abou-Elella, F.; Nassrallah, A. Active Ingredients of Some Egyptian Plants Species with Anticancer Activity and Its Mode of Action. Arab. J. Biotechnol. 2025, 24, 18–40. [Google Scholar] [CrossRef]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, S.; Zheng, Z.; Maoz, I.; Zhang, L.; Kai, G. Molecular regulation of the key specialized metabolism pathways in medicinal plants. J. Integr. Plant Biol. 2024, 66, 510–531. [Google Scholar] [CrossRef]
- Shin, J.M.; Son, Y.-J.; Ha, I.J.; Erdenebileg, S.; Jung, D.S.; Song, D.-G.; Kim, Y.S.; Kim, S.M.; Nho, C.W. Artemisia argyi extract alleviates inflammation in a DSS-induced colitis mouse model and enhances immunomodulatory effects in lymphoid tissues. BMC Complement. Med. Ther. 2022, 22, 64. [Google Scholar] [CrossRef]
- Shoaib, M.; Shah, I.; Ali, N.; Adhikari, A.; Tahir, M.N.; Shah, S.W.A.; Ishtiaq, S.; Khan, J.; Khan, S.; Umer, M.N. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2017, 17, 27. [Google Scholar] [CrossRef]
- Shrivastava, S., Uthra, C., Reshi, M., Singh, A., Yadav, D., & Shukla, S. Protective effect of hesperetin against acrylamide induced acute toxicity in rats. Indian J Exp Biol 2018, 56, 164-170. https://d1wqtxts1xzle7.cloudfront.net/77012929/IJEB_20563_20164-170-libre.pdf?1640147462=&response-content-disposition=inline%3B+filename%3DProtective_effect_of_hesperetin_against.pdf&Expires=1754574984&Signature=APPax-hgWB6r1AiJzTJ5bccvN08HKwRkIbD3j50LouNyo6YtD50syth70PPCJvzuqjeVOtbXuUrXWL~HnJhHnhp5xJZCE8Le3NRZdyi7i~guoCIR~611SltGk3yRXmZkL3AE-hutMM6yC9AxehtF64mh37WXn4PWKsgVpVgHJzdxdJfqqwyvsOuY~f5GbUh6a0pz3i6yPRaA8BWmBrOfHwAOtgEZU8mpuKh3Dilf2YgkZao5nWZzLHUD1D2r95ima1ghZKXqK3JgaVtThqgPMgpk6p3aGOaUGIBhtI0Xia3by~fKsypTHrrn1QckoB1oYTIImais82eH1pwFfbHCHg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA.
- Siddiqui, M. F. M. F., Waghmare, S. P., Hajare, S. W., Deshmukh, R. I. S., & Ali, S. C. S. A. Phytochemical analysis and acute toxicity studies of Artemisia annua in Swiss albino mice. Journal of Pharmacognosy and Phytochemistry 2018, 7, 1893-1895. https://www.researchgate.net/profile/Sharad-Chepte/publication/327882389_Phytochemical_analysis_and_acute_toxicity_studies_of_Artemisia_annua_in_Swiss_albino_mice/links/5bab119092851ca9ed25ec4c/Phytochemical-analysis-and-acute-toxicity-studies-of-Artemisia-annua-in-Swiss-albino-mice.pdf.
- Siddiquee, R.; Mahmood, T.; Ansari, V.A.; Ahsan, F.; Bano, S.; Ahmad, S. Apigenin unveiled: an encyclopedic review of its preclinical and clinical insights. Discov. Plants 2025, 2, 11. [Google Scholar] [CrossRef]
- Singh, J., & Kaur, S. Phyllanthus embilica leaves extract: a potential amylase enzyme inhibitor with antioxidant and antimicrobial activity. Int J Pharma Res 2015, 9, 200–206.
- Silva, J.D.R.; Arruda, H.S.; Andrade, A.C.; Berilli, P.; Borsoi, F.T.; Monroy, Y.M.; Rodrigues, M.V.N.; Sampaio, K.A.; Pastore, G.M.; Junior, M.R.M. Eugenia calycina and Eugenia stigmatosa as Promising Sources of Antioxidant Phenolic Compounds. Plants 2024, 13, 2039. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.; Parween, G.; Khator, R.; Monga, V. A comprehensive review of apigenin a dietary flavonoid: biological sources, nutraceutical prospects, chemistry and pharmacological insights and health benefits. Crit. Rev. Food Sci. Nutr. 2024, 1–37. [Google Scholar] [CrossRef]
- Song, C.-H.; Kim, N.; Nam, R.H.; Choi, S.I.; Kang, C.; Jang, J.Y.; Nho, H.; Shin, E.; Lee, H.-N. Nuclear Factor Erythroid 2-related Factor 2 Knockout Suppresses the Development of Aggressive Colorectal Cancer Formation Induced by Azoxymethane/Dextran Sulfate Sodium-Treatment in Female Mice. J. Cancer Prev. 2021, 26, 41. [Google Scholar] [CrossRef]
- Jeong, D.E.; Song, H.J.; Lim, S.; Lee, S.J.; Lim, J.E.; Nam, D.-H.; Joo, K.M.; Jeong, B.C.; Jeon, S.S.; Choi, H.Y.; et al. Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget 2015, 6, 33046. [Google Scholar] [CrossRef]
- Sousa, C., Gabriel, C., Cerqueira, F., Manso, M. C., & Vinha, A. F. (2015). Coffee industrial waste as a natural source of bioactive compounds with antibacterial and antifungal activities. https://core.ac.uk/reader/301338150.
- Srinivasulu, C., Ramgopal, M., Ramanjaneyulu, G., Anuradha, C. M., & Kumar, C. S. Syringic acid (SA)‒a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomedicine & Pharmacotherapy 2018, 108, 547–557.
- Sultana, R.; Imam, Z.; Kumar, R.R.; Banu, V.S.; Nahakpam, S.; Bharti, R.; Bharadwaj, C.; Singh, A.K.; Pasala, R.K.; Singh, D.R.; et al. Signaling and Defence Mechanism of Jasmonic and Salicylic Acid Response in Pulse Crops: Role of WRKY Transcription Factors in Stress Response. J. Plant Growth Regul. 2025, 44, 5–21. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Sun, J.; Xufeng, J.; Qiu, Z.; Gao, Q.; Zhu, Z.; He, J.; Gao, S.; Sui, X. Artemisiae Annuae Herba: from anti-malarial legacy to emerging anti-cancer potential. Theranostics 2025, 15, 7346. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Fatimawali; Kepel, B.J.; Idroes, R.; Effendi, Y.; Alam Sakib, S.; Bin Emran, T.; Riganti, C. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica 2020, 2020, 6307457. [CrossRef]
- Thanikachalam, P.V.; Devaraji, M.; Jayaprakash, N.; Sivakumar, H.; Balamurugan, D.; Shanmugan, H.K. Phytochemical innovations in oral cancer therapy: targeting oncogenic pathways with natural compounds. J. Complement. Integr. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Pojskic, L.; Tomic, N.; Kalajdzic, A.; Ramic, J.; Kadric, N.L.; Ikanovic, T.; Maksimovic, M.; Pojskic, N. Screening of preferential binding affinity of selected natural compounds to SARS-CoV-2 proteins using in silico methods. Eurasian J. Med. Oncol. 2020, 4, 319–323. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef]
- Velumani, K.; Rajan, P.S.; Shaik, M.R.; Hussain, S.A.; Shaik, B.; Guru, A.; Issac, P.K. Protective Effect of Artemisinin Against Luperox Induced Oxidative Stress and Insulin Resistance via Pi3k/Akt Pathway in Zebrafish Larvae. Cell Biochem. Biophys. 2025, 1–13. [Google Scholar] [CrossRef]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Li, R.; Zhao, Q. Unraveling the specialized metabolic pathways in medicinal plant genomes: a review. Front. Plant Sci. 2024, 15, 1459533. [Google Scholar] [CrossRef]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef]
- Wei, T.; Liu, J. Anti-angiogenic properties of artemisinin derivatives (Review). Int. J. Mol. Med. 2017, 40, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Li, Z.; Zhang, Y.; Wu, H.; Zhang, T.; Wang, W. Artemisinin and its derivatives as promising therapies for autoimmune diseases. Heliyon 2024, 10, e27972. [Google Scholar] [CrossRef] [PubMed]
- Xin, T. A. O., & Tian, W. A. N. G. In vitro and in vivo antioxidant activities of three major polyphenolic compounds in pomegranate peel: Ellagic acid, punicalin, and punicalagin. Journal of integrative agriculture 2017, 16, 1808–1818.
- Yang, Y.; Liu, M.; Liu, C.; Tang, S.; Gu, D.; Tian, J.; Huang, D.; He, F. Ellagic acid from pomegranate peel: Consecutive countercurrent chromatographic separation and antioxidant effect. Biomed. Chromatogr. 2023, 37, e5662. [Google Scholar] [CrossRef]
- Zduńska, K., Dana, A., Kolodziejczak, A., & Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin pharmacology and physiology 2018, 31, 332–336.
- Zhao, S.; Nan, Y.; Yao, R.; Wang, L.; Zeng, X.; Aadil, R.M.; Shabbir, M.A. Antibacterial Activity and Transcriptomic Analysis of Hesperetin against Alicyclobacillus acidoterrestris Vegetative Cells. Foods 2023, 12, 3276. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Q.; Sun, Y.; Ruan, X. Unveiling the antioxidant and anti-inflammatory potential of syringic acid: mechanistic insights and pathway interactions. Front. Pharmacol. 2025, 16, 1615294. [Google Scholar] [CrossRef]
- Zheng, L.; Jacquier, J.-C.; Harbourne, N. Preparation of Polyphenol-Rich Herbal Beverages from White Willow (Salix alba) Bark with Potential Alzheimer’s Disease Inhibitory Activity In Silico. Beverages 2024, 10, 75. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Wang, S.; Wang, K.; Tao, L.; Zou, M.; Chen, N.; Xu, J.; Liu, S.; Li, X. Artesunate suppresses RANKL-induced osteoclastogenesis through inhibition of PLCγ1-Ca 2+ –NFATc1 signaling pathway and prevents ovariectomy-induced bone loss. Biochem. Pharmacol. 2017, 124, 57–68. [Google Scholar] [CrossRef]
- Zhuang, W.-B.; Li, Y.-H.; Shu, X.-C.; Pu, Y.-T.; Wang, X.-J.; Wang, T.; Wang, Z. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef]
- Zhou, F.; Li, G.; Tan, R.; Wu, G.; Deng, C. Artemisinins in autoimmune diseases: effects and mechanisms in systemic lupus erythematosus and rheumatoid arthritis. Br. J. Pharmacol. 2025, 182, 3411–3427. [Google Scholar] [CrossRef]
- Zufarov, O., & Serkayev, K. Natural antioxidants. Technical science and innovation 2024, 2024, 19–25.

| Property / Compound | Gallic Acid | Quercetin | Not Available | Artemisinin and Solution |
|---|---|---|---|---|
| Chemical Class | Phenolics | Flavanols / Flavonoids (phenols) | Phenolics and unknown | Sesquiterpenes |
| Plant Sources | Phyllanthus emblica i(Amla) | Allium cepa (Onion) | Artemisia spp. roots | Artemisia annua |
| Antioxidant Assays | Free radical scavenging | DPPH, ABTS | DPPH | DPPH |
| DPPH Activity (%) | 68.53 | 79.8 | – | 91.0 ± 3.2 |
| MIC (mg/L) Against M.Os | 125 | 1000 | >256 | 14 |
| Enzyme Inhibition | Modifies hepatic drug metabolizing enzymes | Anti-cholinesterase activity | Inhibits α-amylase, α-glucosidase, tyrosinase, and cholinesterases | Acetylcholine esterase, digestive α-glucosidase |
| Biological Activities | Anti-tumor, antihepatotoxic, anti-inflammatory, antioxidant | Antioxidant, anticancer | Antioxidant, antimicrobial, anti-inflammatory, anti-Mycobacterium activity | Antimalarial, anti-inflammatory, antimicrobial, antibacterial, antioxidant |
| Absorption Route | Oral | Oral | Oral | Oral |
| Bioavailability | Low | Low | Low | Low |
| Toxicity (LD₅₀ mg/kg) | Rare, 5,000 in rabbits | 3807 | N.A. | >5,000 in rats |
| Solubility | Slightly soluble in water | Insoluble in water | Highly water-soluble | Sparingly soluble in water |
| Common Formulations | Nanosuspensions | Extract or waste-skin tablets | – | Artemisinin-containing solution or water extract and tablets |
| Research Status (2025) | Preclinical / clinical trials | Clinical | In vivo studies needed | For dental M.Os disease; clinical trials needed |
| Biotech & Gene Studies Outcomes | ↓ CYP450 enzyme activity, ↑ MAPKs, ↑ Nrf2, ↓ NF-κB, ↓ cytokines, ↓ Wnt/β-catenin, ↓ fungal protease | Binds LasR protein of P. aeruginosa, ↓ Violacein pigment, modulates NF-κB, PI3K/Akt, and Nrf2 | ↑ BZF metabolite, effects on COX-2 and TNF-α expression; antiproliferative (Rin-5F & HepG2 cells) | ↑ Keap1/Nrf2 pathway in sheep and humans |
| References | Chen et al., 2025; Hasan et al., 2023; Singh and Kaur, 2015 | Quecan et al., 2019; Grzelak-Błaszczyk et al., 2018; Lee et al., 2015; Fuentes et al., 2020; Črnivec et al., 2021; Kandemir et al., 2024; Mishra et al., 2025; Dibal et al., 2020 | Trifan et al., 2022; Acquaviva et al., 2023 | Kim et al., 2015; Siddiqui et al., 2018; Morua et al., 2025; Gang et al., 2025; Chen et al., 2024; Gavarić et al., 2025 |
| Property / Compound | Gallic Acid | Caffeic Acid | Ellagic Acid | Syringic Acid | Salicylic Acid |
|---|---|---|---|---|---|
| Chemical Class | Phenolics, hydroxybenzoic | Phenolics/phenolic acidsm Hydroxycinnamic | Polyphenolic | Dimethoxybenzoic | Monohydroxybenzoic |
| Plant Sources | Phyllanthus emblica (Amla) |
Coffea arabica (Coffee) |
Punica granatum (Pomegranate) | Vitis vinifea (Grapes) | Salix alba Willow |
| Antioxidant Assays | DPPH, FRAP | DPPH, ABTS | DPPH, FRAP, LPO | DPPH, ABTS | DPPH, FRAP |
| DPPH Activity (%) | 68.53 | 93.4 | ~88 | 24.5 | 28.23(leaf) 48 (bark) |
| MIC (mg/L) Against M.Os | 125 | 1000 | 5-30 (H. pylori) | 625 | 500 |
| Enzyme Inhibition | Modifies hepatic drug metabolizing enzymes, ↓α-amylase |
inhibitor for low-density lipoprotein oxidative modification | ↓COX-2 & MAO-A enzymes | ↓NADPH oxidase, ↓iNOS, ↓COX-2, ↓Catalase, ↓ SOD, ↓ GPx |
↓AChE & BuChE, |
| Biological Activities | Anti-tumor, antihepatotoxic, anti-inflammatory, antioxidant | Antitumour, antioxidant, anti-inflammatory, anti cancer, antiaging, antiUVB damage, pathogenesis of atherosclerosis | Antioxidant, antidepressant, Antiapoptotic, anti-mutagenic, Antiviral, Hepatoprotective | antioxidant and anti-inflammatory, anticancer, ↓TNF-α, IL-6, IL-1β, IFN-γ | analgesic, antipyretic, antiinflammatory and antirheumatic, antimicrobial |
| Absorption Route | Oral | Oral | Oral | Oral | Absorbs readily in skin |
| Bioavailability | Low | Low | low | poor | poor |
| Toxicity (LD₅₀ mg/kg) | Rare, 5,000 in rabbits | 5000 in mice | >2000 in mice | >1,000 in rats | > 2000 in mice |
| Solubility | Slightly | Low | low | moderate | poor |
| Common Formulations | Nanosuspensions | encapsulation | Complex EA with cyclodextrins | polymer microcapsules | Aspirin, capsules |
| Research Status (2025) | Preclinical / clinical trials | In vivo | Pre-clinical | In vivo, preclinical, clinical trials needed | limited clinical studies |
| Biotech, molecular targets& Gene Studies Outcomes | ↓ CYP450 enzyme activity, ↑ MAPKs, ↑ Nrf2, ↓ NF-κB, ↓ cytokines, ↓ Wnt/β-catenin, ↓ fungal protease | ↓TAMs, ↓ NO ↓COX -2 and iNOS, ↓NF-κB, |
↑ Nrf2, ↓NF-κB, ↑cytoprotective genes—such as SOD, CAT, GPx, GR, GGT, GST, NQO1, and HO-1, ↓PGE₂, ↓TNF-α, ↓ IL-6, ↓IL-1β, ↓Bax/Bcl-2 ratio |
↑ KEAP1/NRF2, ↓ NF-κB, TLR4, HMGB1, MyD88, and TRAF6, ↑Ppara, ↑Cpt1, Cpt2, ↓ NF-κB, ↓ NRF1, ↓ MDA and ↓ TBARS, ↓CDK4&6, ↓Cyclins, ↓Bax, ↓Bak, ↓Bcl-2, ↓cIAP1&2, role in bioremediation | ↓ PGE2 Release, ↑NPR–TGA |
| References | Chen et al., 2025; Hasan et al., 2023; Singh and Kaur, 2015 | Khan et al., 2016; Alam et al., 2022b; Sousa et al., 2015; Faria et al., 2020; Mude et al., 2020 | Yang et al.,2023; Xin et al.,2017; Cervantes-Anaya et al., 2022; Evtyugin et al., 2020; verotta et al.,2018; De et al., 2018; Les et al., 2015; Pavlova et al., 2016; Naraki et al., 2022; Wojtunik-Kulesza et al.,2025 | Zhao et al., 2025; Srinivasulu et al., 2018; Rasheeed et al., 2025 | Le et al., 2021; Shalaby et al., 2025; Sultana et al., 2025;Zheng et al., 2024; Piątczak et al, 2020;Maistro et al.,2022; Randjelović et al., 2015; Jia et al, 2023; Chaudhary et al.,2023; Arif, 2015; Davidova et al ., 2024 |
| Property / Compound | Quercetin | Kaempferol | Apigenin | Hesperetin | EGCG |
|---|---|---|---|---|---|
| Chemical Class | Flavanols / Flavonoids (phenols) | Flavonol | Flavone | Flavanone | Flavonol |
| Plant Sources |
Allium cepa (Onion) |
Brassica oleracea (Kale) | Matricaria chamomilla, Chamomile, parsley | Citrus fruits (Orange) | Camellia sinensis (Green tea) |
| Antioxidant Assays | DPPH, ABTS | DPPH, ABTS |
DPPH | DPPH, ABTS | DPPH |
| DPPH Activity (%) | 79.8 | ~55 | 94.8 | 70 | 67.3 |
| MIC (mg/L) Against M.Os | 1000 | 256 | 2 | 62 & 500 | 200-400 |
|
Enzyme (inhibition) Studies |
Anti-cholinesterase activity | ↓AChE | ↓pancreatic lipase, ↓CD38 | ↓Mpro, PLpro, RdRp, & NSP15 |
↑caspases, ↓matrix metalloproteinases, ↓DHFR, ↓Telomerase, ↓MMPs in cancer cells |
| Biological Activities | Antioxidant, anticancer | antioxidant, antimicrobial, anticancer, neuroprotective, and hepatoprotective, Antigenotoxic, Antitumour | anti-inflammatory, antioxidant, analgesic, antimicrobial, hepatoprotective, anti-allergic, anticancer, and anti-hypertensive agent | Neuroprotective, antioxidative, antiviral, antidiabetic effects, preventing hepatic, renal and cerebral damage | anti-cancer, anti-oxidant, anti-inflammatory, anti-angiogenesis, anti apoptotic effects, anti bacterial |
| Absorption Route | Oral | Oral | Oral, GIT | orally & intraperitoneally | Oral, GIT |
| Bioavailability | Low | low | Low needs improvement | low | Low |
| Toxicity (LD₅₀ mg/kg) | 3807 | >2000 in rats | low | >4000 | > 45 in mice |
| Solubility | Insoluble | Low | diminished solubility | low | slight |
| Common Formulations | Extract or waste-skin tablets | nanoformulations | Nanoformulations, nanoemulsions, energy drinks | nanocomplexes | Extract, tea |
| Research Status (2025) | Clinical | In vivo, invitro | Animal, clinical | Clinical bioavailability improvement needed | Clinical |
| Biotech & Gene Studies Outcomes | Binds LasR protein of P. aeruginosa, ↓ Violacein pigment, modulates NF-κB, PI3K/Akt, and Nrf2 | ↓MDA, ↓p-selectin, GFAP, Iba-1, ERK1/2, & TNF-α, ↑Nrf2 |
↓ GABA (gamma-aminobutyric acid)-activated Cl− currents in rats, ↓BDNF, ↓ERKs, JNKs, and p38 MAPKs, | ↑Nrf2 & HO-1, protected RPE-19 cells from apoptosis, ↑SOD & GSH, ↑GSH/GSSG ratio, ↓PLpro , Mpro inhibition, ↓3CLpro main protease, Bind to ACE2, protect β-cells, ↑TJP (occludin, JAM-2, MUC-2) in broilers | ↑ p53 and PTEN/ p21, ↓ERK1/2, ↓ STAT3, ↓Akt/PI3K, NF-κB, ↓EGFR and ↓Wnt pathways , ↓DNA methylation in cancer, ↓P-gp transport |
| References | Quecan et al., 2019; Grzelak-Błaszczyk et al., 2018; Lee et al., 2015; Fuentes et al., 2020; Črnivec et al., 2021; Kandemir et al., 2024; Mishra et al., 2025; Dibal et al., 2020 | Bangar et al., 2023; Seema et al., 2023; Chandekar et al., 2022; Sharma et al., 2021; Hussain et al., 2022; Molaei et al., 2021; Jan et al., 2022 | Al-Dabbagh et al., 2019; Sah et al., 2022; Singh et al., 2024; Wang et al., 2019; Salehi et al., 2019; Siddiquee et al. 2025; Chen et al.¸2023a; Franco et al., 2020 | Khan et al., 2020; Tallei et al., 2020; Tomic et al., 2020; Agrawal et al., 2021; Wdowiak et al., 2022; Ciriminna et al., 2025; Zhao et al.,2023; Shrivastava et al., 2018; Choi et al., 2022 | Alam et al., 2022a; Cao et al.,2026; Amin et al.,2019; Nuryana et al.,2020; Furniturewall & Barve, 2022; Wang et al., 2015; Du et al., 2018 |
| Property / Compound | Artemisinin | Semi-synthetic Artemisin derivatives (Dihydroartemisinin (DHA), Artesunate, Artemether, Arteether) |
|---|---|---|
| Chemical Class | Sesquiterpenes | Reduced artemisinin derivative |
| Plant/synthetic Sources | Artemisia annua | Metabolite of artemsinin, semi-syntheitic |
| Antioxidant Assays | DPPH, ABTS | N.A |
| DPPH Activity (%) | 91.0 ± 3.2 | N.A |
| IC₅₀ for DPPH (µg/mL) | 5.17 | N.A |
| MIC (mg/L) Against M.Os | 14 | N.A |
|
Enzyme (inhibition) Studies |
Acetylcholine esterase, digestive α-glucosidase, ↓PfATP6 | ↓PfATP6, modulates ↑ antioxidative enzymes |
| Biological Activities | Antimalarial, anti-inflammatory, antimicrobial, antibacterial, antioxidant, anti viral, antineoplastic activity against pancreatic, leukemic, osteosarcoma, and lung cancer cells | Anticancer, antitumor, anti-angiogenenic in cancer cells |
| Absorption Route | Oral | N.A |
| Bioavailability | Low | poor |
| Toxicity (LD₅₀ mg/kg) | >5,000 in rats | N.A |
| Toxicity | Neurotoxicity>28days in animals | N.A |
| Solubility | Sparingly soluble in water | Lipophilic, enhanced water solubility than artemisinin but still low |
| Common Formulations | Artemisinin-containing solution or water extract and tablets | |
| Research Status (2025) | For dental M.Os disease; clinical trials needed | Preclinical/clinical |
| Biotech, molecular targets & Gene Studies Outcomes | ↑ Keap1/Nrf2 pathway in sheep and humans, ↓IRE1α phosphorylation, Direct DNA damage to cancer, ↓ ERK1/2, ↓VEGFR2, ↑ antioxidative enzymes | ↑ ratio Bax/Bcl-2 ↑ caspase 3 & cytochrome c, ↓ AKT/GSK3β/cyclin D1 pathway, ↓TCTP, ↓HSP70, ↓Bcl-xL & Bcl-2, ↓AKT/SRC pathways inn breast cancer, ↓mTORC1, ↑ Nrf-2 and TRX |
| References | Kim et al., 2015; Siddiqui et al., 2018; Morua et al., 2025; Gang et al., 2025; Chen et al., 2024; Gavarić et al., 2025; Kuang et al.,2018; Wei and Liu, 2017; Moore et al., 2022 | Dai et al., 2021; Osonwa & Hu, 2018; Addissouky, 2025; Feng et al., 2016; Wei and Liu, 2017; Moore et al., 2022; Kazmi et al., 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





