Submitted:
27 June 2025
Posted:
01 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extracts
2.2. Metabolomic Profile
2.2.1. Fourier Transform Infrared Spectroscopy (FT-IR)
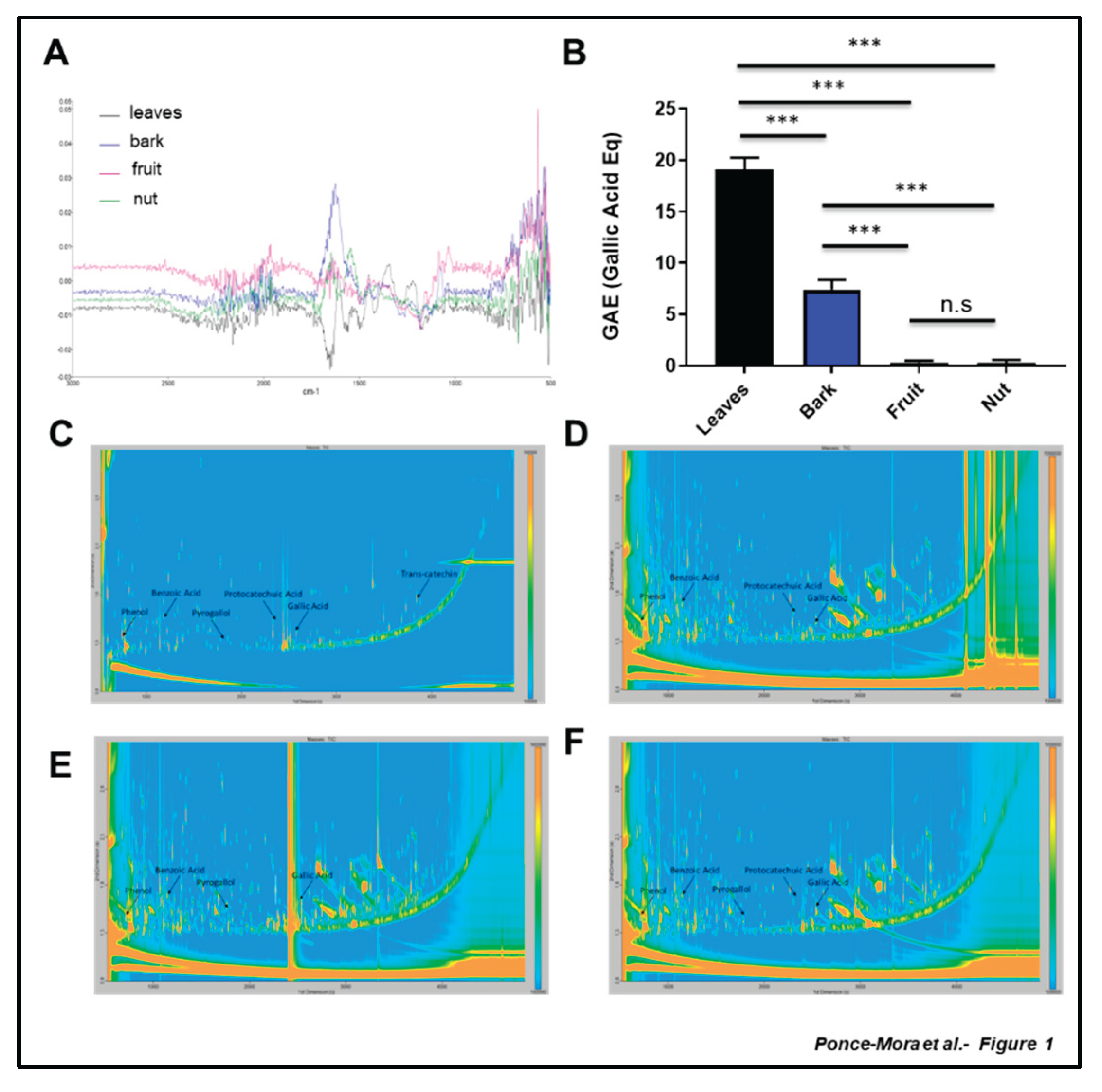
2.2.2. Determination of the Total Polyphenol Content (TPC)
2.2.3. GC×GC-TOFMS Analysis
2.2.3.1. Derivatization Analysis
2.2.3.2. Untargeted GC×GC-TOFMS
2.2.3.3. Data Processing
2.3. Electrochemical Analysis
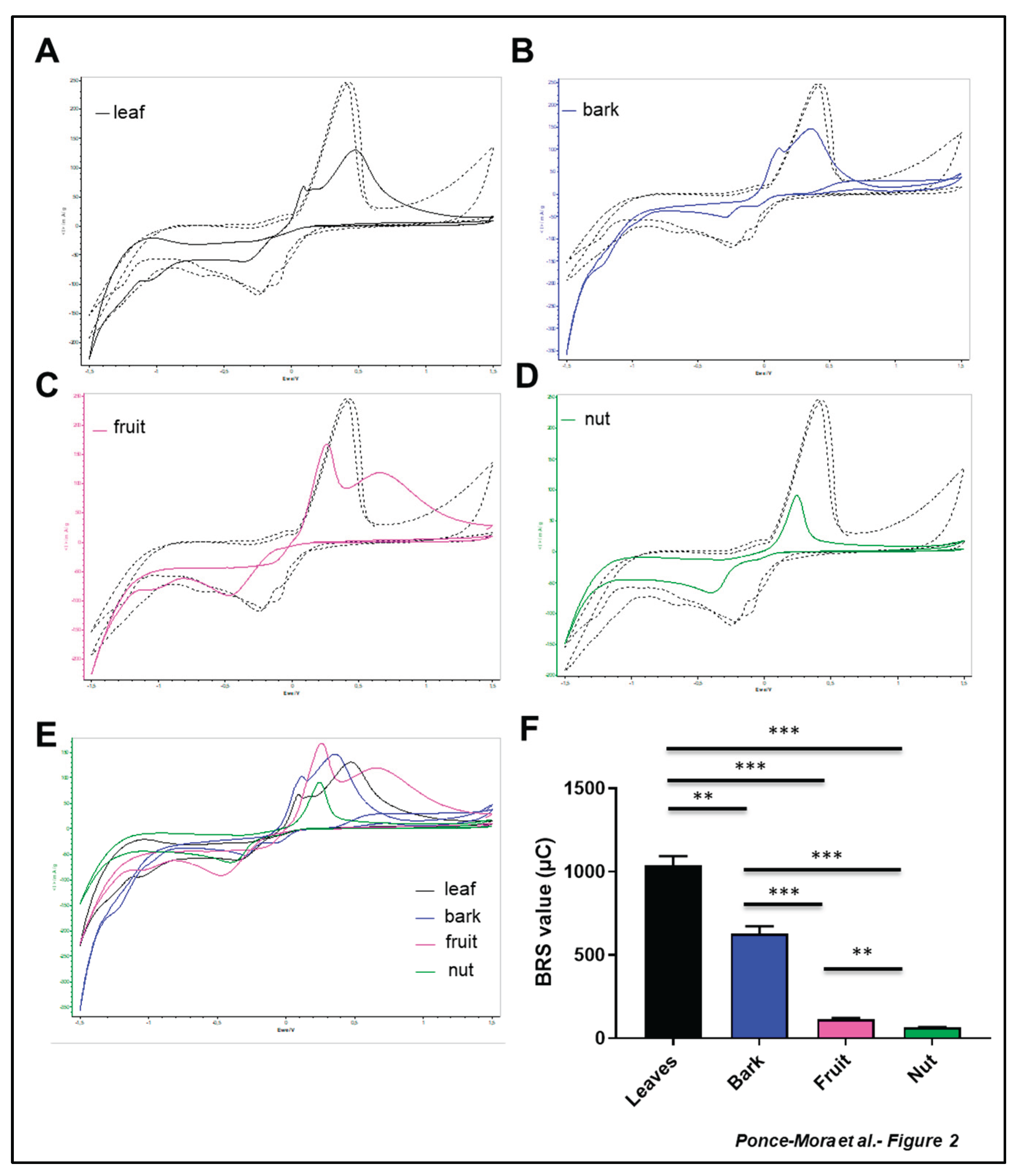
2.3.1. Cyclic Voltammogram
2.3.2. Electrochemical Antioxidant Activity Determination
2.4. Antioxidant Analysis
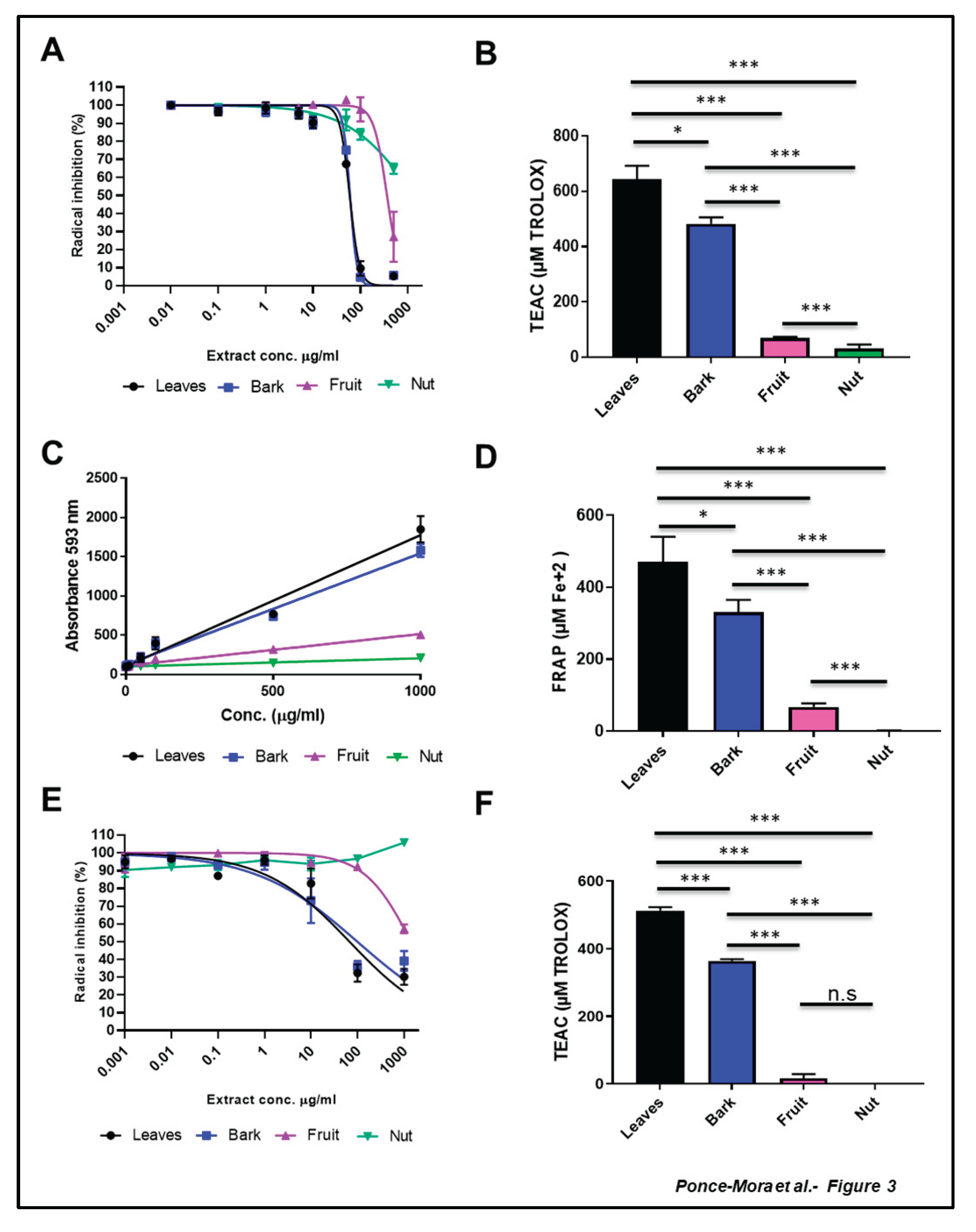
2.4.1. ABTS Assay
2.4.2. FRAP Assay
2.4.3. DPPH Assay
2.5. Cell Culture
2.6. Analysis of Cytotoxicity and Cell Viability Against Oxidative Stress
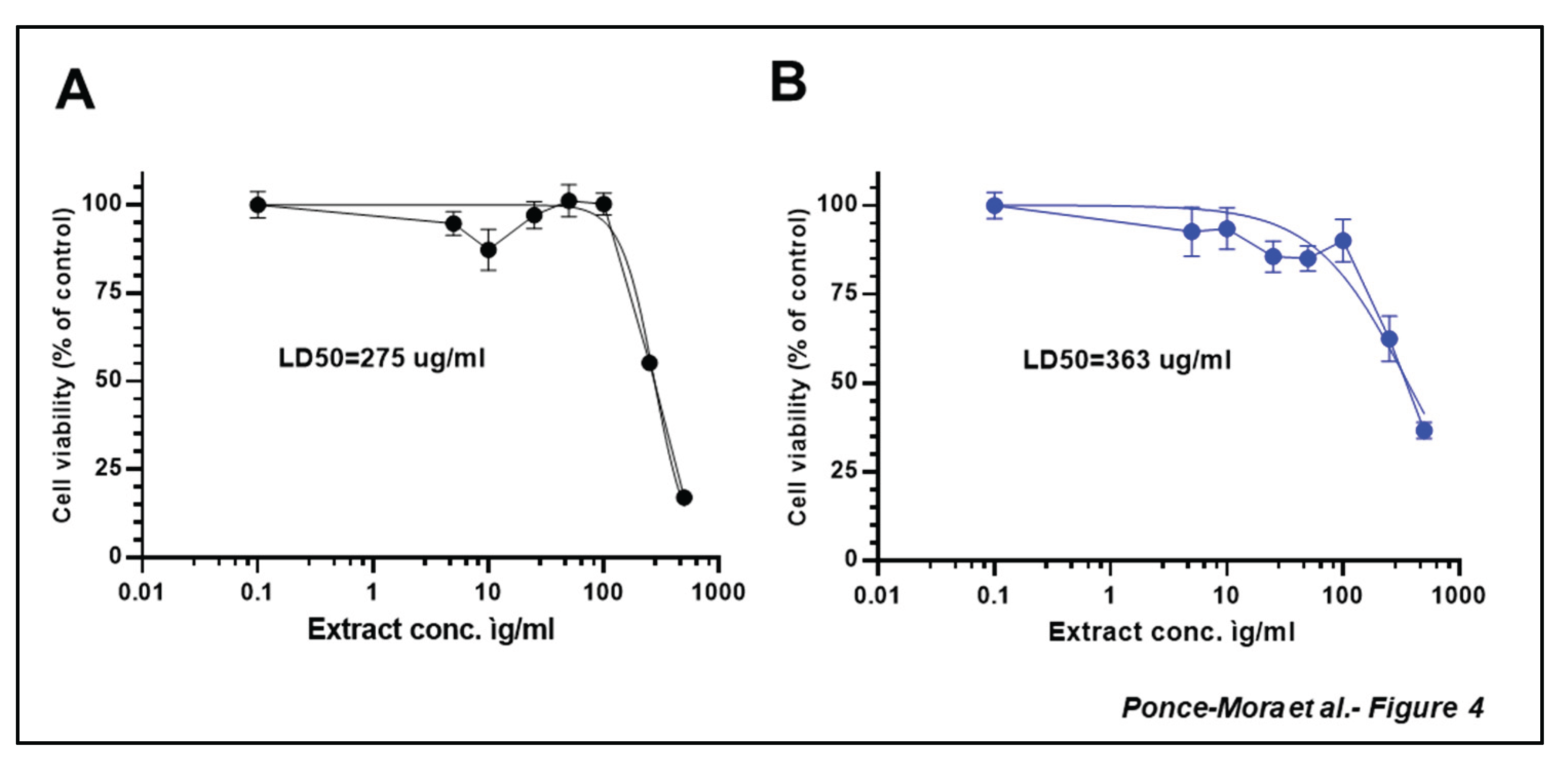
2.6.1. Cytotoxicity Activity
2.6.2. Neuroprotection Assay Against H2O2-Induced Cytotoxicity
2.6.3. Determination of the Levels of Intracellular ROS
2.6.4. Determination of the Mitochondrial Membrane Potential
2.7. Quantitative Real-Time PCR Assays
2.8. Statistical Analysis
3. Results
3.1. Metabolomics Revealed Differential Polyphenolic Content in the Extracts of the Aerial Parts of A. occidentale
3.2. An Array of Antioxidant Capacity Assays Revealed Differential Antioxidant Potential of the Aerial Parts of A. occidentale
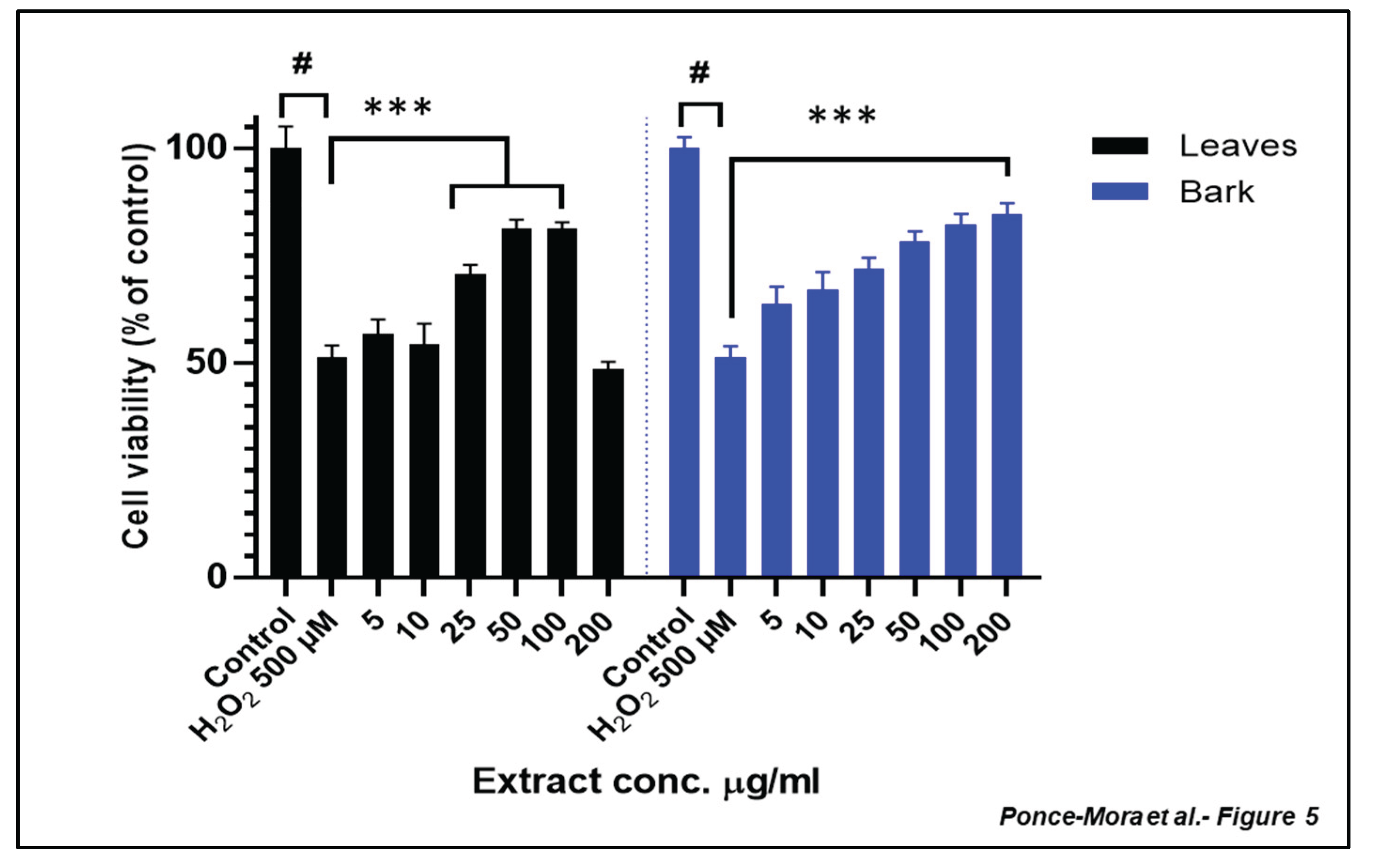
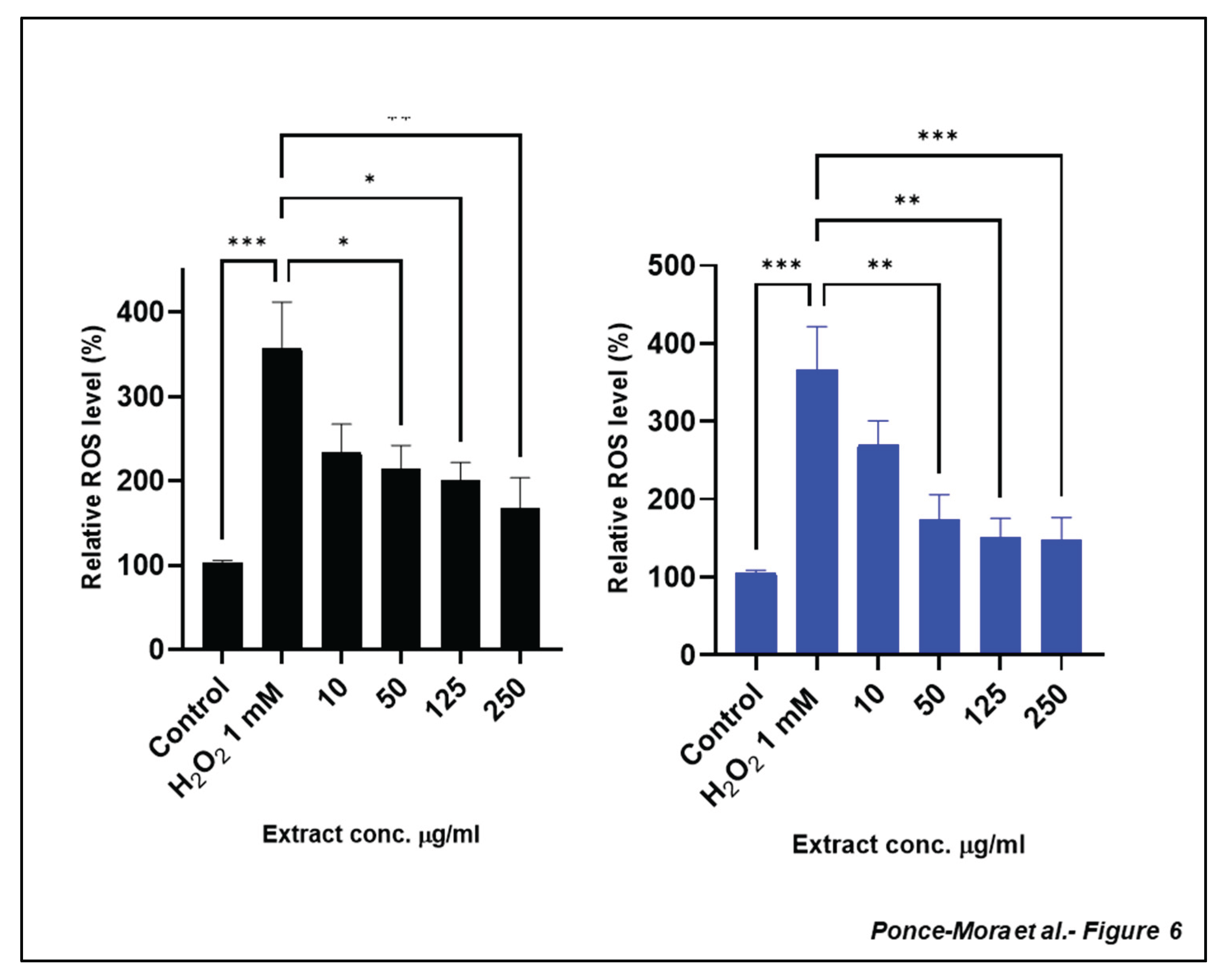
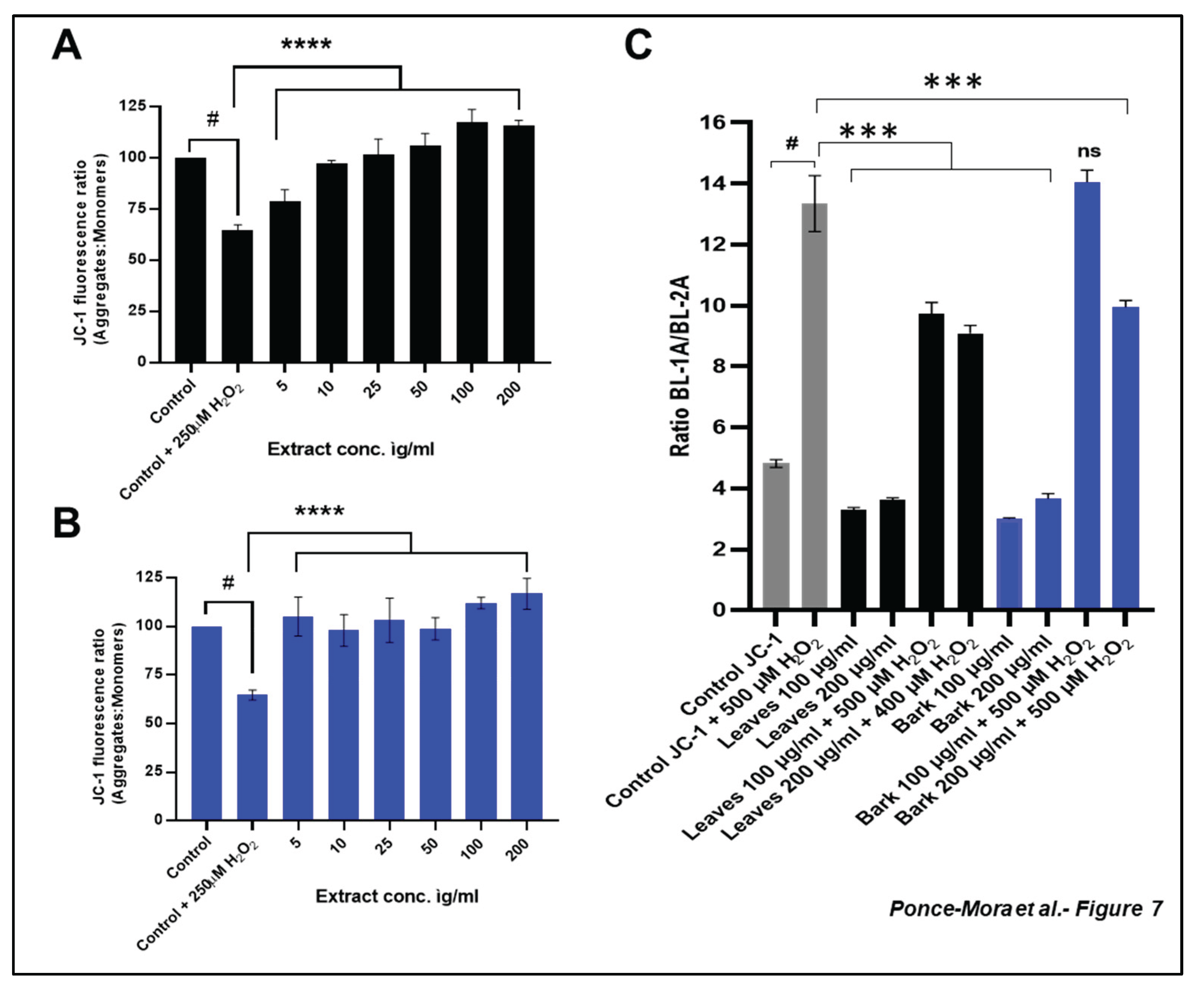
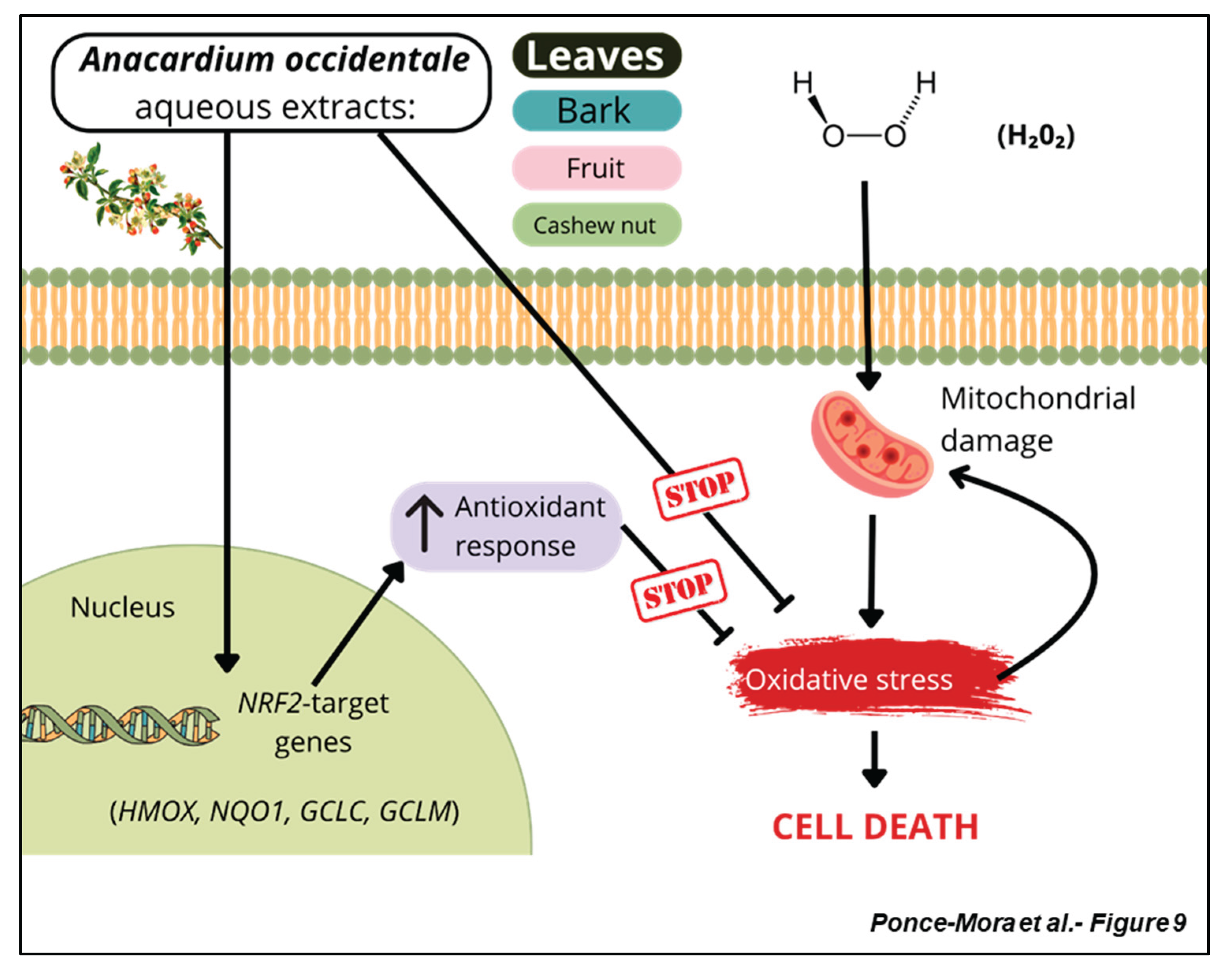
3.3. Leaf and Bark Extracts of A. occidentale Are Protective Against Oxidative Stress in SH-SY5Y Cells
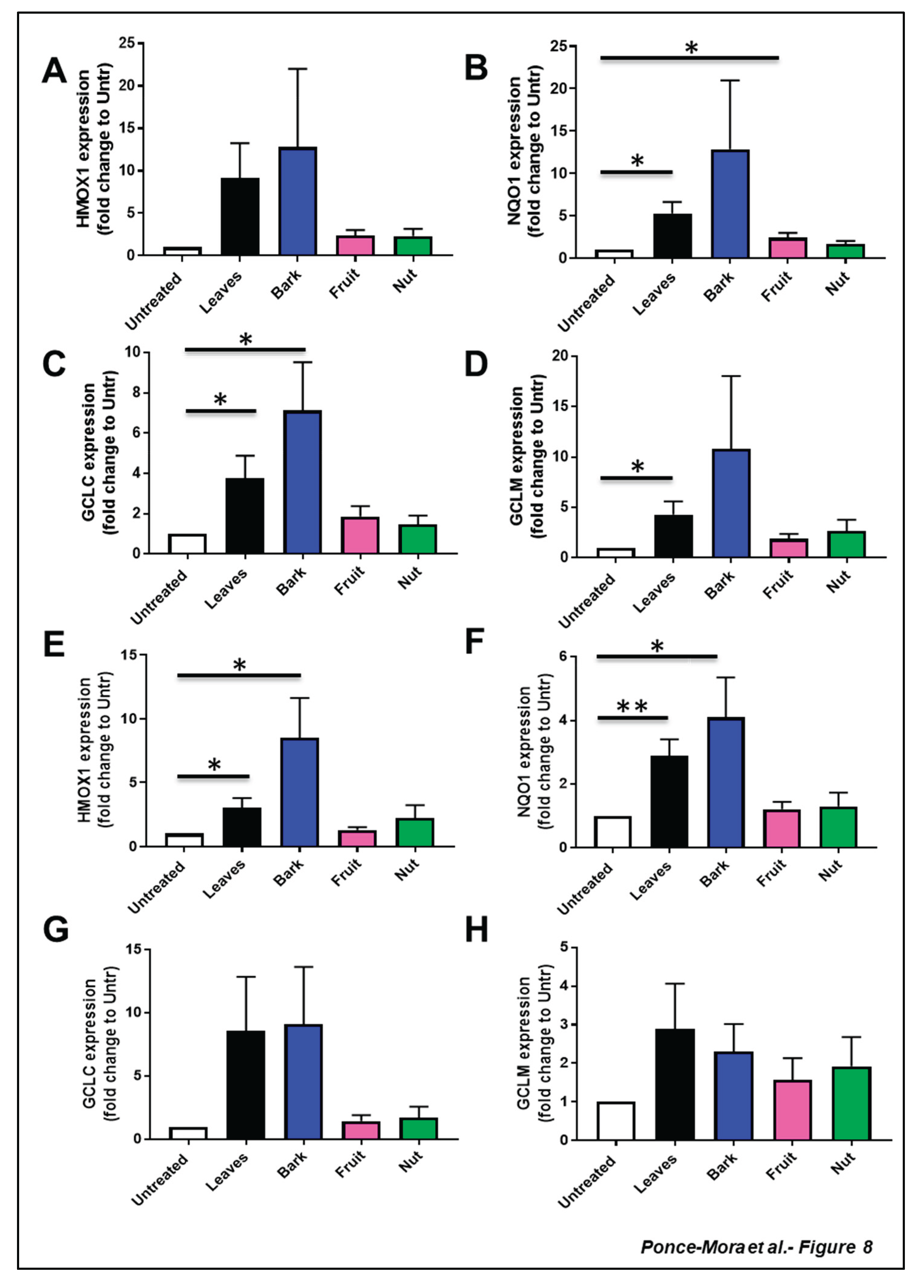
3.4. Leaf and Bark Extracts (But Not Fruit and Nut Extracts) Trigger the Expression of NRF2-Target Cytoprotective Genes Against Oxidative Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free radical biology & medicine 2013, 62, 90–101. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clinical interventions in aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jóźwik, A.; Kühl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive Oxygen Species and Their Impact in Neurodegenerative Diseases: Literature Landscape Analysis. Antioxidants & redox signaling 2021, 34, 402–420. [Google Scholar] [CrossRef]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biology 2023, 68, 102967. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. International journal of molecular sciences 2017, 18. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. The World Allergy Organization journal 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants (Basel, Switzerland) 2022, 11. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radical Biology and Medicine 2019, 134, 702–707. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food chemistry: X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. Journal of food biochemistry 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Garnatje, T.; Peñuelas, J.; Vallès, J. Ethnobotany, Phylogeny, and ‘Omics’ for Human Health and Food Security. Trends in Plant Science 2017, 22, 187–191. [Google Scholar] [CrossRef]
- Guerra, R.N.M.; Oliveira, A.S.; Farias, J.R.; Franco, D.C.G.; Santos, P.G.; Barbosa, N.T.; Muniz, S.B.; Abreu, A.G.; Nascimento, F.R.F. Anacardiaceae Family: Effect of Isolated Compounds and Other Identified Phytochemicals Against Clinically Relevant Candida Species—A Short Review. Antibiotics 2025, 14, 308. [Google Scholar] [CrossRef]
- Méril-Mamert, V.; Ponce-Mora, A.; Sylvestre, M.; Lawrence, G.; Bejarano, E.; Cebrián-Torrejón, G. Antidiabetic Potential of Plants from the Caribbean Basin. Plants 2022, 11, 1360. [Google Scholar] [CrossRef]
- Ukwenya, V.O.; Alese, M.O.; Ogunlade, B.; Folorunso, I.M.; Omotuyi, O.I. Anacardium occidentale leaves extract and riboceine mitigate hyperglycemia through anti-oxidative effects and modulation of some selected genes associated with diabetes. Journal of diabetes and metabolic disorders 2023, 22, 455–468. [Google Scholar] [CrossRef]
- Thesnor, V.; Molinié, R.; Giebelhaus, R.T.; de la Mata Espinosa, A.P.; Harynuk, J.J.; Bénimélis, D.; Vanhoye, B.; Dunyach-Rémy, C.; Sylvestre, M.; Cheremond, Y.; et al. Antibacterial Activity and Untargeted Metabolomics Profiling of Acalypha arvensis Poepp. Molecules 2023, 28, 7882. [Google Scholar] [CrossRef]
- Giebelhaus, R.T.; Nguyen, G.; Schmidt, S.A.; Wang, S.; Mesfin, E.Y.; Nam, S.L.; de la Mata, A.P.; Harynuk, J.J. GC×GC-TOFMS Analysis of Fecal Metabolome Stabilized Using an At-Home Stool Collection Device. Applied Biosciences 2024, 3, 348–359. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics : Official journal of the Metabolomic Society 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Nam, S.L.; Giebelhaus, R.T.; Tarazona Carrillo, K.S.; de la Mata, A.P.; Harynuk, J.J. Evaluation of normalization strategies for GC-based metabolomics. Metabolomics : Official journal of the Metabolomic Society 2024, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Brinvillier, D.; Barrast, M.; Couderc-Murillo, P.; Bono-Yagüe, J.; Rousteau, A.; Gómez Escribano, A.P.; Palmeira-Mello, M.V.; Doménech-Carbó, A.; Passe-Coutrin, N.; Sylvestre, M.; et al. Spectroscopic, Electrochemical, and Biological Assays of Copper-Binding Molecules for Screening of Different Drugs and Plant Extracts against Neurodegenerative Disorders. ACS Omega 2022, 7, 16260–16269. [Google Scholar] [CrossRef] [PubMed]
- Matignon, L.; Lo, M.M.; Monpierre, M.; Correia, M.V.; Valencia, D.P.; Palmeira-Mello, M.V.; Sylvestre, M.-N.; Pruneau, L.; Sylvestre, M.; Domenech, A.; et al. Phytochemical and Biological Study of Trophic Interaction between Pseudosphinx Tetrio L. Larvae and Allamanda Cathartica L. Plants 2023, 12, 520. Larvae and Allamanda Cathartica L. Plants 2023, 12, 520. [Google Scholar] [PubMed]
- Corpas, F.J.; Rodríguez-Ruiz, M.; Campos, M.J.; Taboada, J.; Palma, J.M. Electrochemical Detection of Total Antioxidant Capacity (TAC) in Plant Tissues from Different Origins. Methods in molecular biology (Clifton, N.J.) 2024, 2798, 1–9. [Google Scholar] [CrossRef]
- Nieto, C.I.; Cornago, M.P.; Cabildo, M.P.; Sanz, D.; Claramunt, R.M.; Torralba, M.C.; Torres, M.R.; Martínez Casanova, D.; Sánchez-Alegre, Y.R.; Escudero, E.; et al. Evaluation of the Antioxidant and Neuroprotectant Activities of New Asymmetrical 1,3-Diketones. Molecules 2018, 23, 1837. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; T., N.K.V.; S., A.F.; N., A.J.; and Brumaghim, J.L. Reactive oxygen species generation by copper(II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef]
- Pang, Q.Q.; Kim, J.H.; Kim, H.Y.; Kim, J.-H.; Cho, E.J. Protective Effects and Mechanisms of Pectolinarin against H2O2-Induced Oxidative Stress in SH-SY5Y Neuronal Cells. Molecules 2023, 28, 5826. [Google Scholar] [CrossRef]
- Salvioli, S.; Ardizzoni, A.; Franceschi, C.; Cossarizza, A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS letters 1997, 411, 77–82. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Li, X.; Zhang, S.; Gu, H.; Wei, X.; Wang, X.; Xu, Z.; Shen, T. Naturally-derived modulators of the Nrf2 pathway and their roles in the intervention of diseases. Free Radical Biology and Medicine 2024, 225, 560–580. [Google Scholar] [CrossRef]
- Menezes, F.; da Cruz Almeida É, T.; da Silva Vieira, A.R.; de Souza Aquino, J.; Dos Santos Lima, M.; Magnani, M.; de Souza, E.L. Impact of Cashew (Anacardium occidentale L.) by-Product on Composition and Metabolic Activity of Human Colonic Microbiota In Vitro Indicates Prebiotic Properties. Current microbiology 2021, 78, 2264–2274. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Peritore, A.F.; Cordaro, M.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. The Antioxidant and Anti-Inflammatory Properties of Anacardium occidentale L. Cashew Nuts in a Mouse Model of Colitis. Nutrients 2020, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.C.Q.; Madruga, M.S.; Pintado, M.M.E.; Almeida, G.H.O.; Alves, A.P.V.; Dantas, F.A.; Bezerra, J.K.G.; de Melo, M.; Viera, V.B.; Soares, J.K.B. Cashew nuts (Anacardium occidentale L.) decrease visceral fat, yet augment glucose in dyslipidemic rats. PloS one 2019, 14, e0225736. [Google Scholar] [CrossRef] [PubMed]
- da Silveira Vasconcelos, M.; Gomes-Rochette, N.F.; de Oliveira, M.L.; Nunes-Pinheiro, D.C.; Tomé, A.R.; Maia de Sousa, F.Y.; Pinheiro, F.G.; Moura, C.F.; Miranda, M.R.; Mota, E.F.; et al. Anti-inflammatory and wound healing potential of cashew apple juice (Anacardium occidentale L.) in mice. Experimental biology and medicine (Maywood, N.J.) 2015, 240, 1648–1655. [Google Scholar] [CrossRef]
- Borges, J. Cashew tree (Anacardium occidentale): Possible applications in dermatology. Clinics in dermatology 2021, 39, 493–495. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Tatke, P.A.; Gabhe, S.Y.; Vaidya, A.B. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. Journal of traditional and complementary medicine 2017, 7, 421–427. [Google Scholar] [CrossRef]
- Kaushik, M.; Hoti, S.L.; Saxena, J.K.; Hingamire, T.; Shanmugam, D.; Joshi, R.K.; Metgud, S.C.; Ungar, B.; Singh, I.; Hegde, H.V. Antimalarial Activity of Anacardium occidentale Leaf Extracts Against Plasmodium falciparum Transketolase (PfTK). Acta parasitologica 2023, 68, 832–841. [Google Scholar] [CrossRef]
- Sunderam, V.; Thiyagarajan, D.; Lawrence, A.V.; Mohammed, S.S.S.; Selvaraj, A. In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi journal of biological sciences 2019, 26, 455–459. [Google Scholar] [CrossRef]
- Sruthi, P.; Roopavathi, C.; Madhava Naidu, M. Profiling of phenolics in cashew nut (Anacardium occidentale L.) testa and evaluation of their antioxidant and antimicrobial properties. Food Bioscience 2023, 51, 102246. [Google Scholar] [CrossRef]
- Désiré, G.N.S.; Simplice, F.H.; Guillaume, C.W.; Kamal, F.Z.; Parfait, B.; Hermann, T.D.S.; Hervé, N.A.H.; Eglantine, K.W.; Linda, D.K.J.; Roland, R.N.; et al. Cashew (Anacardium occidentale) Extract: Possible Effects on Hypothalamic–Pituitary–Adrenal (HPA) Axis in Modulating Chronic Stress. Brain sciences 2023, 13, 1561. [Google Scholar] [CrossRef]
- Lima Júnior, J.P.d.; Franco, R.R.; Saraiva, A.L.; Moraes, I.B.; Espindola, F.S. Anacardium humile St. Hil as a novel source of antioxidant, antiglycation and α-amylase inhibitors molecules with potential for management of oxidative stress and diabetes. Journal of Ethnopharmacology 2021, 268, 113667. [Google Scholar] [CrossRef]
- Amat-ur-Rasool, H.; Symes, F.; Tooth, D.; Schaffert, L.-N.; Elmorsy, E.; Ahmed, M.; Hasnain, S.; Carter, W.G. Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants. Biomolecules 2020, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Masiala, A.; Vingadassalon, A.; Aurore, G. Polyphenols in edible plant leaves: an overview of their occurrence and health properties. Food & function 2024, 15, 6847–6882. [Google Scholar] [CrossRef]
- Yazaki, Y. Utilization of flavonoid compounds from bark and wood: a review. Natural product communications 2015, 10, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Faggian, M.; Bernabè, G.; Ferrari, S.; Francescato, S.; Baratto, G.; Castagliuolo, I.; Dall’Acqua, S.; Peron, G. Polyphenol-Rich Larix decidua Bark Extract with Antimicrobial Activity against Respiratory-Tract Pathogens: A Novel Bioactive Ingredient with Potential Pharmaceutical and Nutraceutical Applications. Antibiotics 2021, 10, 789. [Google Scholar] [CrossRef]
- Wafa, D.; Nicolas, B. Pine Bark Phenolic Extracts, Current Uses, and Potential Food Applications: A Review. Current Pharmaceutical Design 2020, 26, 1866–1879. [Google Scholar] [CrossRef]
- Her, Y.; Lee, T.K.; Sim, H.; Lee, J.C.; Kim, D.W.; Choi, S.Y.; Hong, J.K.; Lee, J.W.; Kim, J.D.; Won, M.H.; et al. Pinus thunbergii bark extract rich in flavonoids promotes hair growth in dorsal skin by regulating inflammatory cytokines and increasing growth factors in mice. Molecular medicine reports 2022, 25. [Google Scholar] [CrossRef]
- Shih, M.-C.; Chang, C.-M.; Kang, S.-M.; Tsai, M.-L. Effect of Different Parts (Leaf, Stem and Stalk) and Seasons (Summer and Winter) on the Chemical Compositions and Antioxidant Activity of Moringa oleifera. International journal of molecular sciences 2011, 12, 6077–6088. [Google Scholar] [CrossRef]
- Sunil, K.J.; Mukesh, K.D.; Sanjeeb, D.; Arti Raj, V.; Ch, V.R. A comparative study on total phenolic content, reducing power and free radical scavenging activity of aerial parts of Barleria prionitis. International Journal of Phytomedicine 2010, 2, 155–159. [Google Scholar]
- Tamiello-Rosa, C.S.; Cantu-Jungles, T.M.; Iacomini, M.; Cordeiro, L.M.C. Pectins from cashew apple fruit (Anacardium occidentale): Extraction and chemical characterization. Carbohydrate research 2019, 483, 107752. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Guo, J.; Ma, Y.; Li, J.; Ji, H.; Chen, Z.; Zheng, J. Anacardic acid improves neurological deficits in traumatic brain injury by anti-ferroptosis and anti-inflammation. Experimental neurology 2023, 370, 114568. [Google Scholar] [CrossRef]
- da Silva, W.M.B.; Pinheiro, S.O.; Alves, D.R.; de Menezes, J.; Magalhães, F.E.A.; Silva, F.C.O.; Marinho, M.M.; Marinho, E.S.; de Morais, S.M. Anacardic Acid Complexes as Possible Agents Against Alzheimer's Disease Through Their Antioxidant, In vitro, and In silico Anticholinesterase and Ansiolic Actions. Neurotoxicity research 2021, 39, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.A.; Shin, P.G.; Yoon, J.S.; Yadunandam, A.K.; Kim, G.D. Induction of the endoplasmic reticulum stress and autophagy in human lung carcinoma A549 cells by anacardic acid. Cell biochemistry and biophysics 2014, 68, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Hollands, A.; Corriden, R.; Gysler, G.; Dahesh, S.; Olson, J.; Raza Ali, S.; Kunkel, M.T.; Lin, A.E.; Forli, S.; Newton, A.C.; et al. Natural Product Anacardic Acid from Cashew Nut Shells Stimulates Neutrophil Extracellular Trap Production and Bactericidal Activity. The Journal of biological chemistry 2016, 291, 13964–13973. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutrition Bulletin 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Martins, I.K.; de Carvalho, N.R.; Macedo, G.E.; Rodrigues, N.R.; Ziech, C.C.; Vinadé, L.; Filho, V.M.B.; Menezes, I.A.; Franco, J.; Posser, T. Anacardium microcarpum Promotes Neuroprotection Dependently of AKT and ERK Phosphorylation but Does Not Prevent Mitochondrial Damage by 6-OHDA. Oxidative Medicine and Cellular Longevity 2018, 2018, 2131895. [Google Scholar] [CrossRef]
- Müller, K.R.; Martins, I.K.; Rodrigues, N.R.; da Cruz, L.C.; Barbosa Filho, V.M.; Macedo, G.E.; da Silva, G.F.; Kamdem, J.P.; de Menezes, I.R.A.; Franco, J.L.; et al. Anacardium microcarpum extract and fractions protect against paraquat-induced toxicity in Drosophila melanogaster. EXCLI journal 2017, 16, 302–312. [Google Scholar] [CrossRef]
- Santo, G.D.; de Veras, B.O.; Rico, E.; Magro, J.D.; Agostini, J.F.; Vieira, L.D.; Calisto, J.F.F.; Mocelin, R.; de Sá Fonseca, V.; Wanderley, A.G. Hexane extract from SpoSndias mombin L. (Anacardiaceae) prevents behavioral and oxidative status changes on model of Parkinson's disease in zebrafish. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP 2021, 241, 108953. [Google Scholar] [CrossRef]
- Zhong, T.; Li, M.; Wu, H.; Wang, D.; Liu, J.; Xu, Y.; Fan, Y. Novel Flavan-3,4-diol vernicidin B from Toxicodendron Vernicifluum (Anacardiaceae) as potent antioxidant via IL-6/Nrf2 cross-talks pathways. Phytomedicine : international journal of phytotherapy and phytopharmacology 2022, 100, 154041. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Kolawole, A.A.; Elizabeth, A.O.; Oluwaseun, A.K.; Racheal, F.O.; Olanrewaju, E.O.; Olabisi, A.T.; Seun, O.B.; Olubunmi, F.O.; Omolola, O.I.; et al. Leaf extract of Anacardium occidentale ameliorates biomarkers of neuroinflammation, memory loss, and neurobehavioral deficit in N(ω)-nitro-L-arginine methyl ester (L-NAME) treated rats. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals 2023, 28, 263–272. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Wink, M.; Tencomnao, T. Anacardium Occidentale L. Leaf Extracts Protect Against Glutamate/H(2)O(2)-Induced Oxidative Toxicity and Induce Neurite Outgrowth: The Involvement of SIRT1/Nrf2 Signaling Pathway and Teneurin 4 Transmembrane Protein. Frontiers in pharmacology 2021, 12, 627738. [Google Scholar] [CrossRef]
- Ponce-Mora, A.; Salazar, N.A.; Domenech-Bendaña, A.; Locascio, A.; Bejarano, E.; Gimeno-Mallench, L. Interplay Between Polyphenols and Autophagy: Insights From an Aging Perspective. Frontiers in bioscience (Landmark edition) 2025, 30, 25728. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Riera-Ponsati, L.; Kauppinen, S.; Klitgaard, H.; Erler, J.T.; Hansen, S.N. Targeting the NRF2 pathway for disease modification in neurodegenerative diseases: mechanisms and therapeutic implications. Frontiers in pharmacology 2024, 15, 1437939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, G.; Yang, S.; Liu, S.; Wu, Y.; Miao, Y.; Liang, Z.; Hua, Y.; Zhang, J.; Shi, J.; et al. The plant extract PNS mitigates atherosclerosis via promoting Nrf2-mediated inhibition of ferroptosis through reducing USP2-mediated Keap1 deubiquitination. British journal of pharmacology 2024, 181, 4822–4844. [Google Scholar] [CrossRef] [PubMed]
- HAS, A.L.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2019, 111, 676–685. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Yang, S.; Cui, S.W.; Wang, M.; Hao, W. Rosemary extract reverses oxidative stress through activation of Nrf2 signaling pathway in hamsters fed on high fat diet and HepG2 cells. Journal of Functional Foods 2020, 74, 104136. [Google Scholar] [CrossRef]
| Gene | Sequence (5’-3’) | Length (bp) | Tm (oC) | GC content (%) | Species |
|---|---|---|---|---|---|
| Nrf2 FW | CAGAAGGAACAGGAGAAGGC | 20 | 55,3 | 55 | Mus musculus |
| Nrf2 REV | CTTGTTTGGGAATGTGGGC | 19 | 54,6 | 52,6 | Mus musculus |
| Hmox FW | TGCTCGAATGAACACTCTGG | 20 | 54,9 | 50 | Mus musculus |
| Hmox1 REV | TGGTCTTTGTGTTCCTCTGTC | 21 | 54,7 | 47,6 | Mus musculus |
| Gclm FW | ATGACCCGAAAGAACTGCTC | 20 | 55 | 50 | Mus musculus |
| Gclm REV | ATGATTCCCCTGCTCTTCAC | 20 | 54,6 | 50 | Mus musculus |
| Gclc FW | ACCATCACTTCATTCCCCAG | 20 | 54,6 | 50 | Mus musculus |
| Gclc REV | TTCTTGTTAGAGTACCGAAGCG | 22 | 54,5 | 45,5 | Mus musculus |
| Nqo1 FW | GGATTTGCCTACACATATGCTG | 22 | 53,9 | 45,5 | Mus musculus |
| Nqo1 REV | TGAATCGGCCAGAGAATGAC | 20 | 54,8 | 50 | Mus musculus |
| Gapdh FW | CCTGCTTCACCACCTTCTTGA | 21 | 57,2 | 52,4 | Mus musculus |
| Gapdh REV | TGTGTCCGTCGTGGATCTGA | 20 | 58,1 | 55 | Mus musculus |
| NRF2 FW | ATGACAATGAGGTTTCTTCGG | 21 | 52,9 | 42,9 | Homo sapiens |
| NRF2 REV | CAATGAAGACTGGGCTCTC | 19 | 52,9 | 52,6 | Homo sapiens |
| HMOX1 FW | AACTCCCTGGAGATGACTC | 19 | 53,3 | 52,6 | Homo sapiens |
| HMOX1 REV | CTCAAAGAGCTGGATGTTGAG | 21 | 53,4 | 47,6 | Homo sapiens |
| GCLM FW | GTTGACATGGCCTGTTCAG | 19 | 53,9 | 52,6 | Homo sapiens |
| GCLM REV | AACTCCATCTTCAATAGGAGGT | 22 | 53,1 | 40,9 | Homo sapiens |
| GCLC FW | AAGTGGATGTGGACACCAG | 19 | 54,7 | 52,6 | Homo sapiens |
| GCLC REV | CTGTCATTAGTTCTCCAGATGC | 22 | 53,1 | 45,5 | Homo sapiens |
| NQO1 FW | ACATCACAGGTAAACTGAAGG | 21 | 52,3 | 42,9 | Homo sapiens |
| NQO1 REV | TCAGATGGCCTTCTTTATAAGC | 22 | 52,5 | 40,9 | Homo sapiens |
| GAPDH FW | TCCTTCCTGGGCATGGAG | 18 | 56,9 | 61,1 | Homo sapiens |
| GAPDH REV | AGGAGGAGCAATGATCTTGATCTT | 24 | 55,8 | 41,7 | Homo sapiens |
| Sample | IC50 (µg/ml) (ABTS) | TEAC50 (ABTS) | Trolox equivalents (FRAP) | IC50 (DPPH) | |
|---|---|---|---|---|---|
| Trolox | 5.8 ± 1.3 | 1 | 1 | 5.62 | |
| Leaves | 12.3 ± 2.1 | 0.5 | 0.13 | 58.67 | |
| Bark | 13.8 ± 4.1 | 0.4 | 0.12 | 79.52 | |
| Fruit | 447.2 ± 15.0 | 0.01 | 0.03 | > 1000 | |
| Nut | >1000 | n.d | 0.01 | > 1000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





