Submitted:
01 August 2025
Posted:
05 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
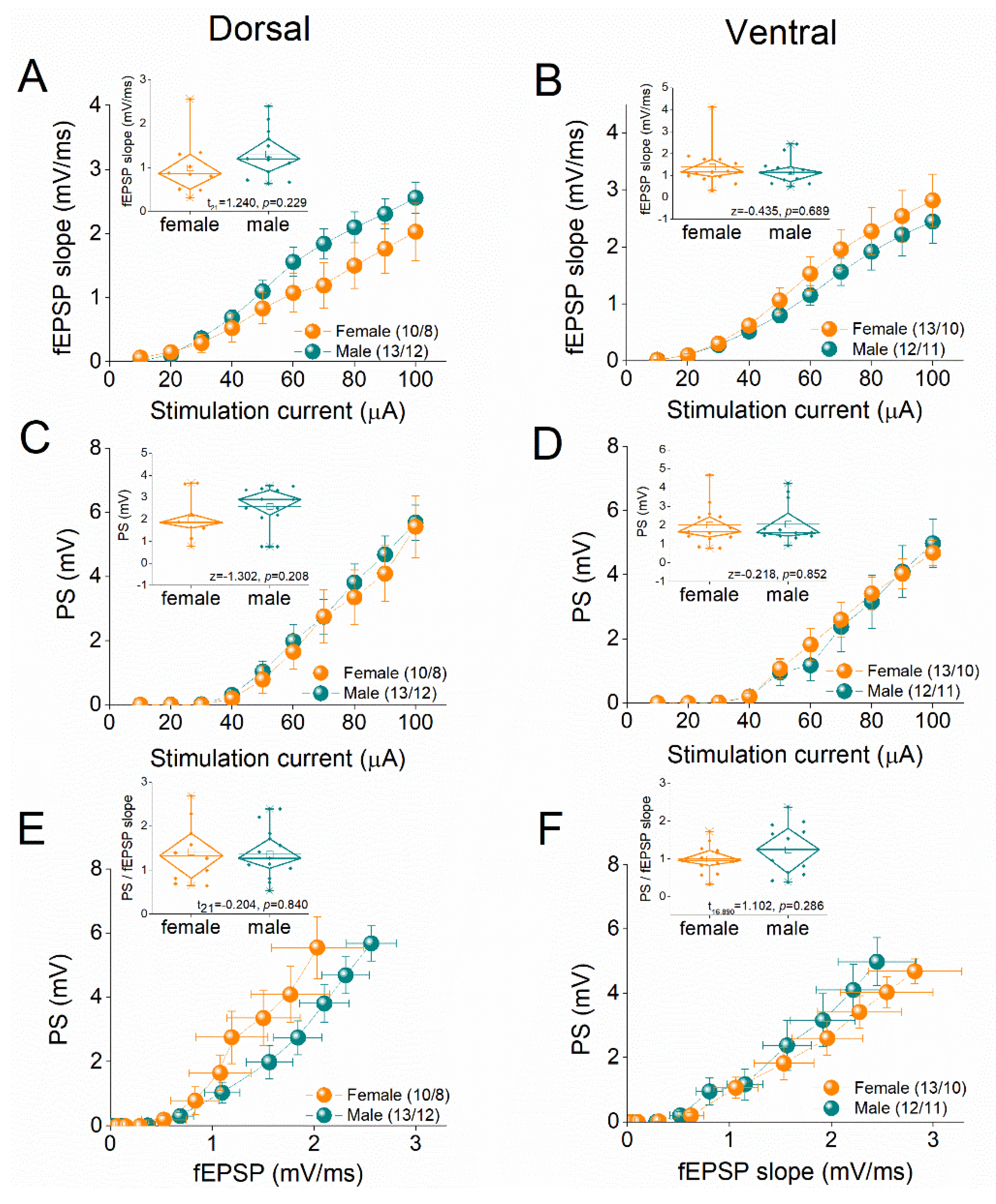
2.1. Similar Synaptic Transmission and Neuronal Excitability in Female amd Male Rats
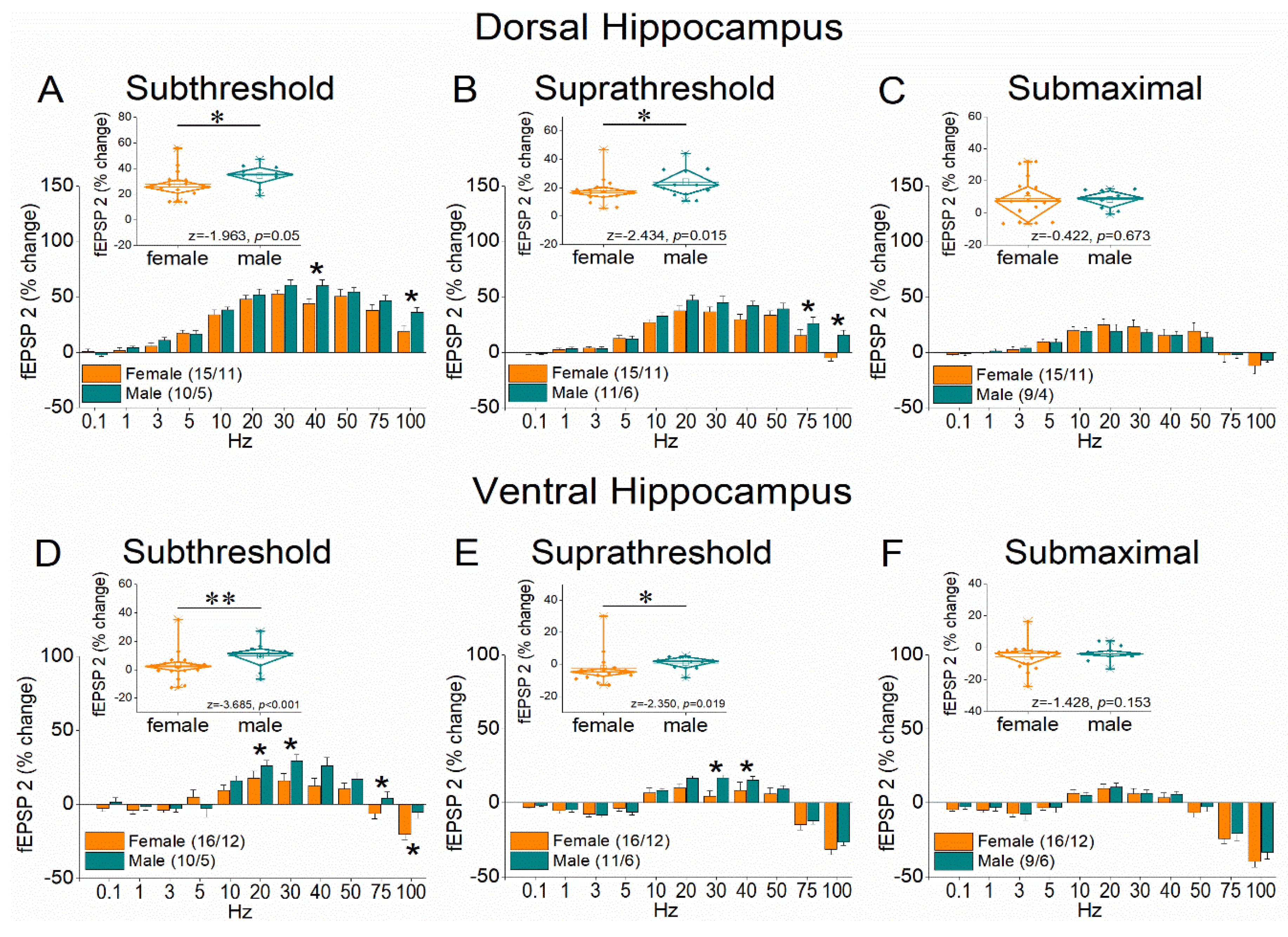
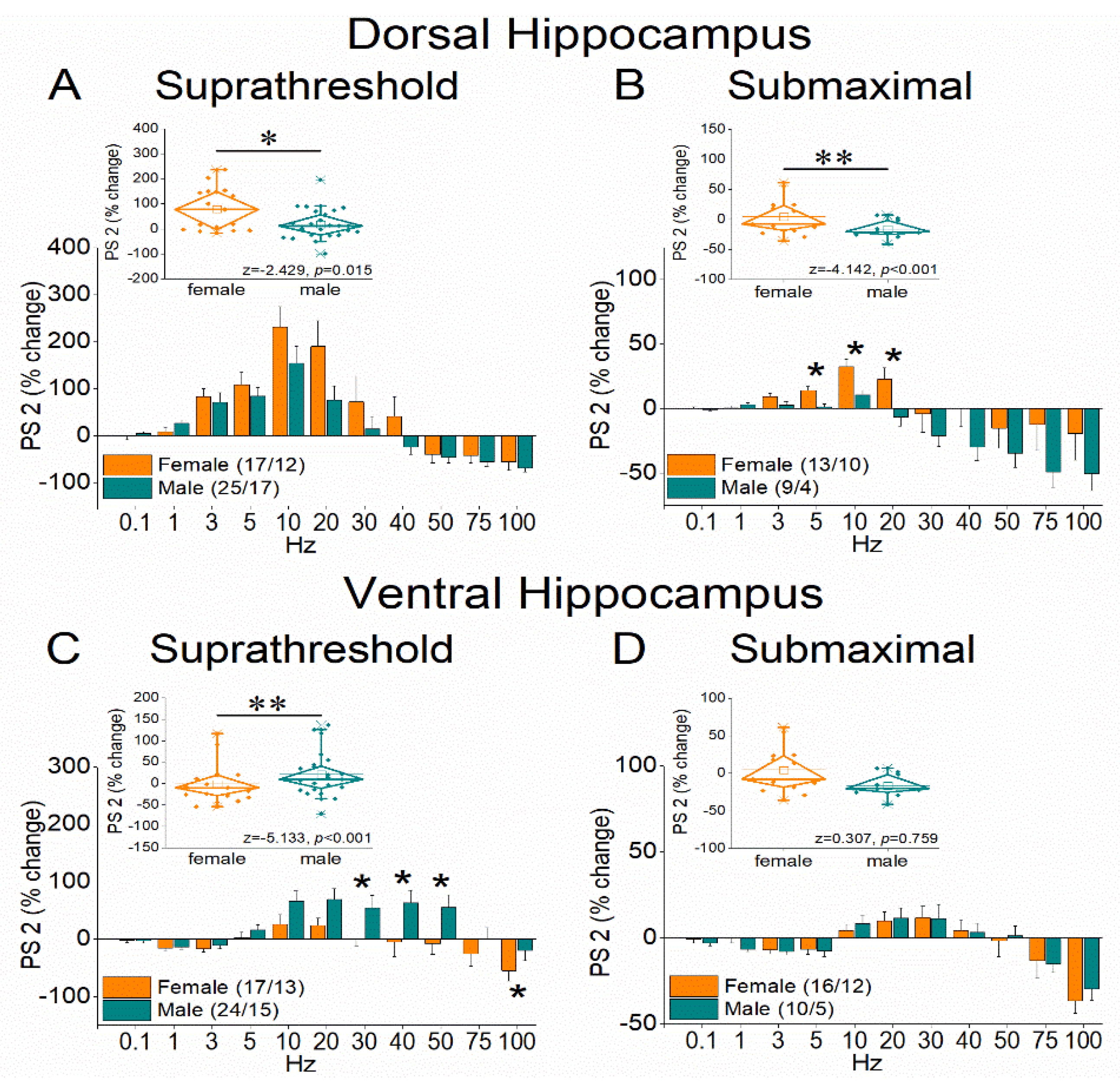
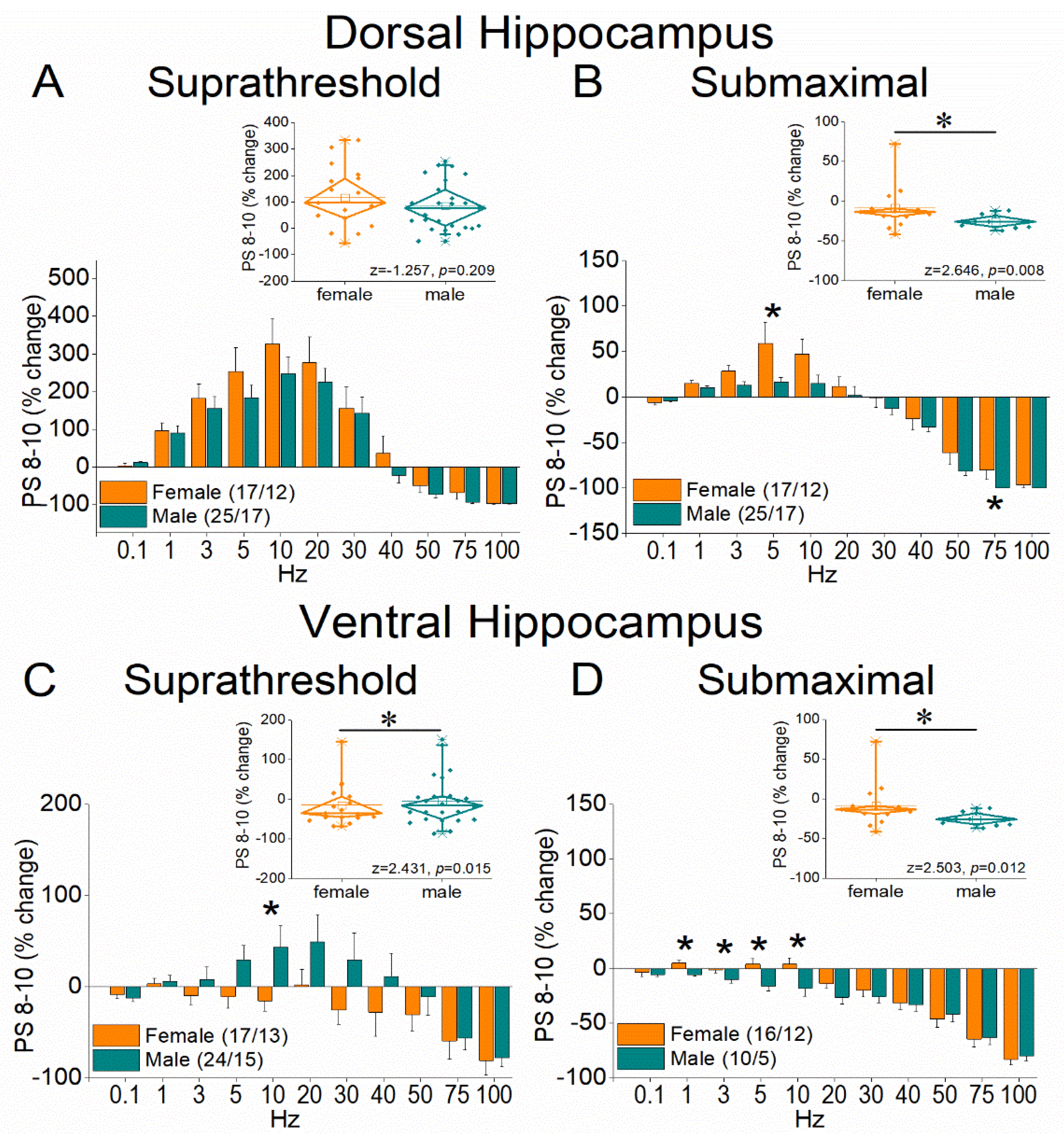
2.2. Short-Term Synaptic Plasticity (STSP) Differs Between Female and Male Hippocampus
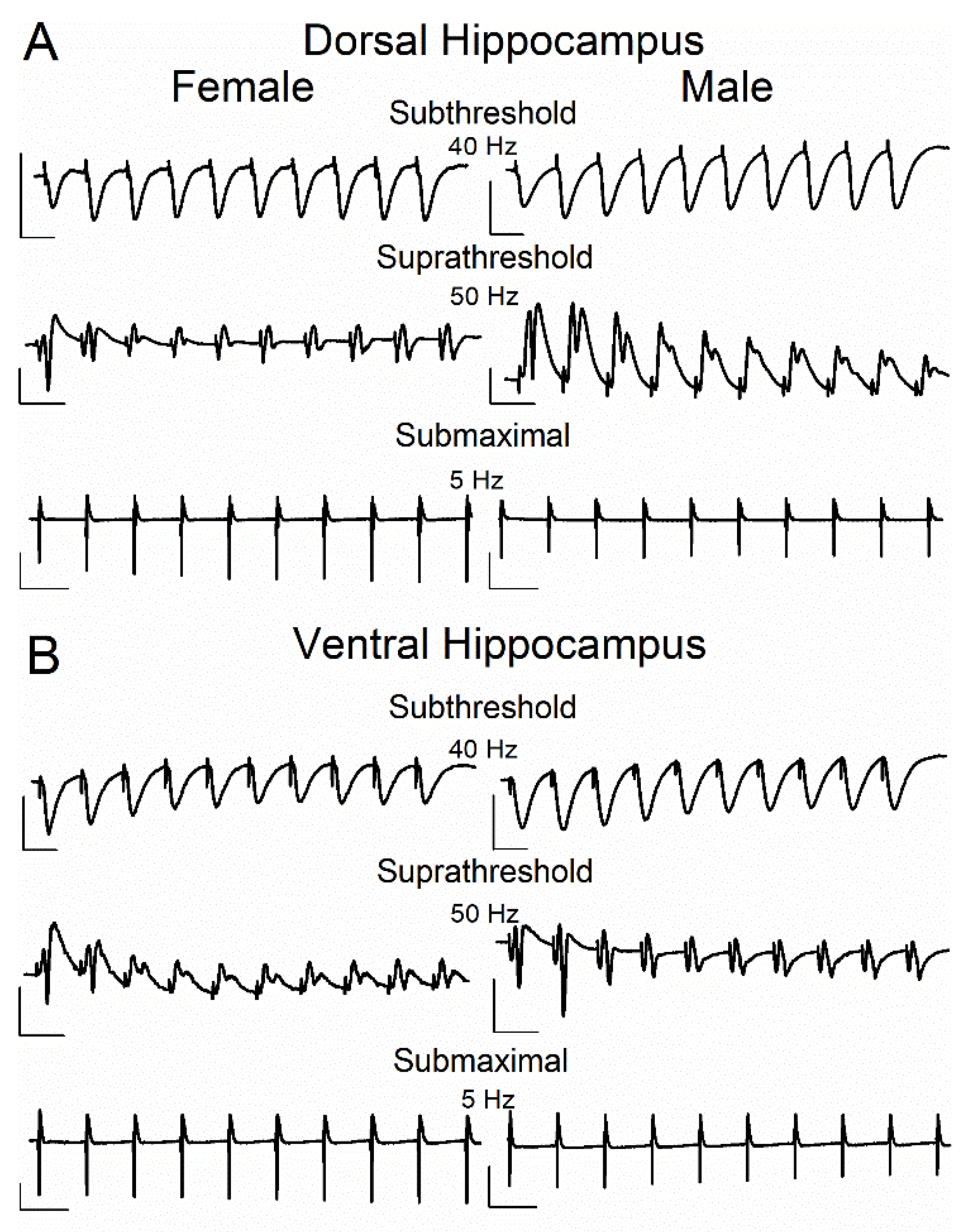
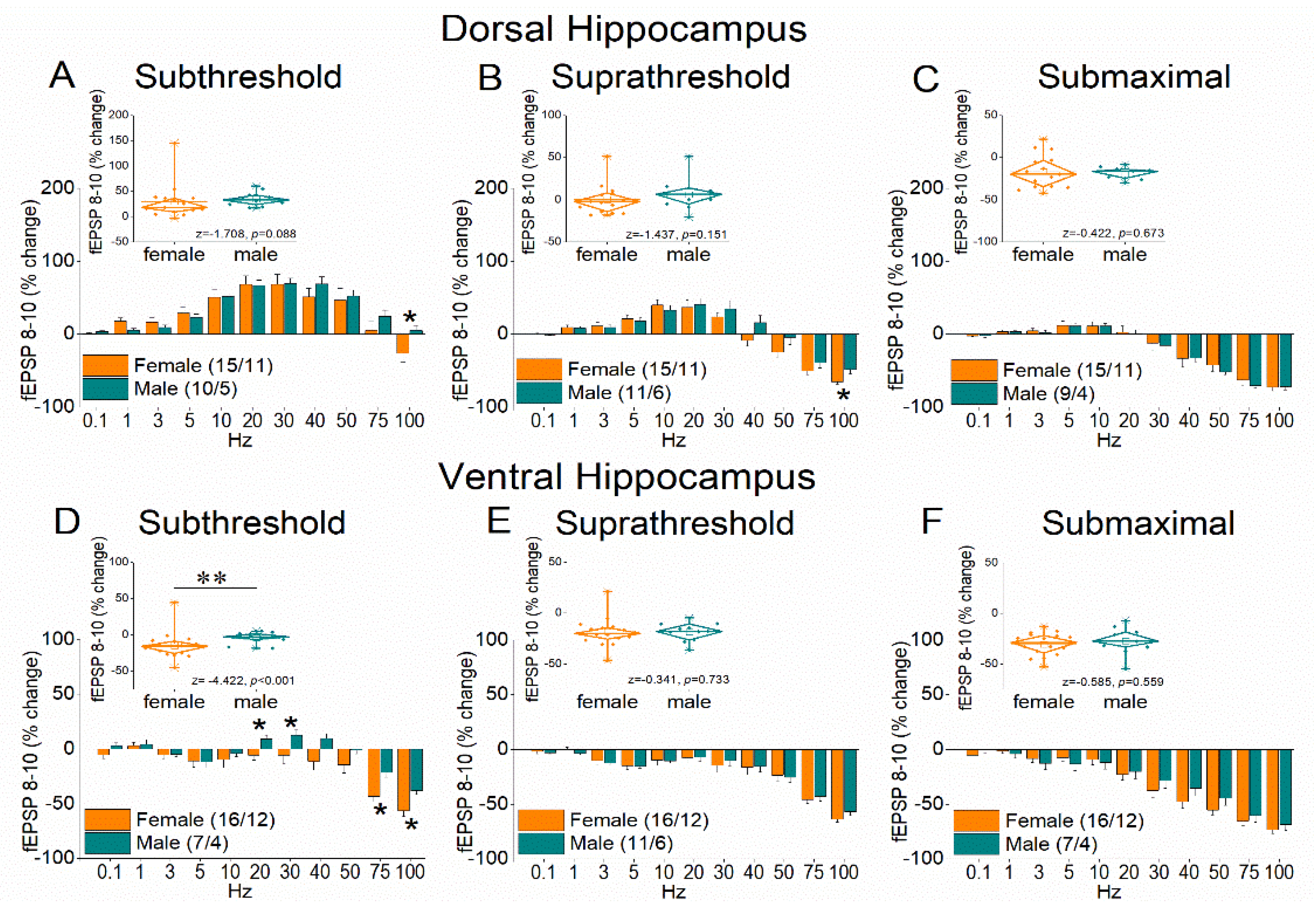
2.3. Short-Term Neuronal Dynamics (STND) Differ Between Female and Male Hippocampus
3. Discussion
3.1. Possible Interpretations
3.2. Implications
5. Materials and Methods
5.1. Animals and Hippocampal Slice Preparation
5.2. Electrophysiology and Data Acquisition
5.3. Stimulation Protocols and Quantification
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zucker, R.S.; Regehr, W.G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef]
- Jackman, S.L.; Regehr, W.G. The Mechanisms and Functions of Synaptic Facilitation. Neuron 2017, 94, 447–464. [Google Scholar] [CrossRef]
- Anwar, H.; Li, X.; Bucher, D.; Nadim, F. Functional roles of short-term synaptic plasticity with an emphasis on inhibition. Curr. Opin. Neurobiol. 2017, 43, 71–78. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Hennig, M.H. Theoretical models of synaptic short term plasticity. Front. Comput. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Devaraju, P.; Yu, J.; Eddins, D.; Mellado-Lagarde, M.M.; Earls, L.R.; Westmoreland, J.J.; Quarato, G.; Green, D.R.; Zakharenko, S.S. Haploinsufficiency of the 22q11.2 microdeletion gene Mrpl40 disrupts short-term synaptic plasticity and working memory through dysregulation of mitochondrial calcium. Mol. Psychiatry 2016, 22, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Pals, M.; Stewart, T.C.; Akyürek, E.G.; Borst, J.P.; Morrison, A. A functional spiking-neuron model of activity-silent working memory in humans based on calcium-mediated short-term synaptic plasticity. PLOS Comput. Biol. 2020, 16, e1007936. [Google Scholar] [CrossRef]
- Mongillo, G.; Barak, O.; Tsodyks, M. Synaptic Theory of Working Memory. Science 2008, 319, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Rolls, E.T.; Romo, R. Synaptic dynamics and decision making. Proc. Natl. Acad. Sci. 2010, 107, 7545–7549. [Google Scholar] [CrossRef]
- Woolley, C.S. Acute Effects of Estrogen on Neuronal Physiology. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 657–680. [Google Scholar] [CrossRef]
- Gall, C.M.; Le, A.A.; Lynch, G. Sex differences in synaptic plasticity underlying learning. J. Neurosci. Res. 2021, 101, 764–782. [Google Scholar] [CrossRef]
- Kniffin, A.R.; Briand, L.A. Sex differences in glutamate transmission and plasticity in reward related regions. Front. Behav. Neurosci. 2024, 18, 1455478. [Google Scholar] [CrossRef]
- Fester, L.; Rune, G.M. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2015, 1621, 162–169. [Google Scholar] [CrossRef]
- Foy, M.R. Ovarian hormones, aging and stress on hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 2011, 95, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Bi, X.; Aguirre, C. Progesterone–estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience 2013, 239, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Hyer, M.M.; Phillips, L.L.; Neigh, G.N. Sex Differences in Synaptic Plasticity: Hormones and Beyond. Front. Mol. Neurosci. 2018, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.G.; Humphreys, A.G.; Juraska, J.M.; Greenough, W.T. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995, 703, 26–30. [Google Scholar] [CrossRef]
- Good, M.; Day, M.; Muir, J.L. Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur. J. Neurosci. 1999, 11, 4476–4480. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Foy, M.R.; Vouimba, R.-M.; Thompson, R.F.; Baudry, M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc. Natl. Acad. Sci. 2001, 98, 13391–13395. [Google Scholar] [CrossRef]
- Kramár, E.; Babayan, A.; Gall, C.; Lynch, G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience 2013, 239, 3–16. [Google Scholar] [CrossRef]
- Kramár, E.A.; Chen, L.Y.; Brandon, N.J.; Rex, C.S.; Liu, F.; Gall, C.M.; Lynch, G. Cytoskeletal Changes Underlie Estrogen's Acute Effects on Synaptic Transmission and Plasticity. J. Neurosci. 2009, 29, 12982–12993. [Google Scholar] [CrossRef]
- Montoya, D.C.; Carrer, H. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res. 1997, 778, 430–438. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Hojo, Y.; Kojima, H.; Ikeda, M.; Hotta, K.; Sato, R.; Ooishi, Y.; Yoshiya, M.; Chung, B.-C.; Yamazaki, T.; et al. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res. 2015, 1621, 147–161. [Google Scholar] [CrossRef]
- Maren, S.; Baudry, M. Properties and Mechanisms of Long-Term Synaptic Plasticity in the Mammalian Brain: Relationships to Learning and Memory. Neurobiol. Learn. Mem. 1995, 63, 1–18. [Google Scholar] [CrossRef]
- Monfort, P.; Gomez-Gimenez, B.; Llansola, M.; Felipo, V. Gender Differences in Spatial Learning, Synaptic Activity, and Long-Term Potentiation in the Hippocampus in Rats: Molecular Mechanisms. ACS Chem. Neurosci. 2015, 6, 1420–1427. [Google Scholar] [CrossRef]
- Safari, S.; Ahmadi, N.; Mohammadkhani, R.; Ghahremani, R.; Khajvand-Abedeni, M.; Shahidi, S.; Komaki, A.; Salehi, I.; Karimi, S.A. Sex differences in spatial learning and memory and hippocampal long-term potentiation at perforant pathway-dentate gyrus (PP-DG) synapses in Wistar rats. Behav. Brain Funct. 2021, 17, 1–11. [Google Scholar] [CrossRef]
- Jain, A.; Huang, G.Z.; Woolley, C.S. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci. 2018, 39, 1897–18. [Google Scholar] [CrossRef] [PubMed]
- Shughrue, P.; Merchenthaler, I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience 2000, 99, 605–612. [Google Scholar] [CrossRef]
- Hart, S.A.; Patton, J.D.; Woolley, C.S. Quantitative analysis of ERα and GAD colocalization in the hippocampus of the adult female rat. J. Comp. Neurol. 2001, 440, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, A.U.; Kirkland, J.M.; Briand, L.A. Adolescent social isolation induced alterations in nucleus accumbens glutamate signalling. Addict. Biol. 2021, 27, e13077–e13077. [Google Scholar] [CrossRef] [PubMed]
- Knouse, M.C.; McGrath, A.G.; Deutschmann, A.U.; Rich, M.T.; Zallar, L.J.; Rajadhyaksha, A.M.; Briand, L.A. Sex differences in the medial prefrontal cortical glutamate system. Biol. Sex Differ. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Luine, V.; Frankfurt, M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 2013, 239, 34–45. [Google Scholar] [CrossRef]
- Li, C.; Brake, W.G.; Romeo, R.D.; Dunlop, J.C.; Gordon, M.; Buzescu, R.; Magarinos, A.M.; Allen, P.B.; Greengard, P.; Luine, V.; et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. 2004, 101, 2185–2190. [Google Scholar] [CrossRef]
- Tuscher, J.J.; Luine, V.; Frankfurt, M.; Frick, K.M. Estradiol-Mediated Spine Changes in the Dorsal Hippocampus and Medial Prefrontal Cortex of Ovariectomized Female Mice Depend on ERK and mTOR Activation in the Dorsal Hippocampus. J. Neurosci. 2016, 36, 1483–1489. [Google Scholar] [CrossRef]
- Castillo-Fernández, S.; Silva-Gómez, A.B. Changes in dendritic arborization related to the estrous cycle in pyramidal neurons of layer V of the motor cortex. J. Chem. Neuroanat. 2021, 119, 102042. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.A.S.; Choleris, E.; Galea, L.A.M. Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol. Brain 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Branco, T.; Häusser, M. Synaptic Integration Gradients in Single Cortical Pyramidal Cell Dendrites. Neuron 2011, 69, 885–892. [Google Scholar] [CrossRef]
- Poirazi, P.; Brannon, T.; Mel, B.W. Arithmetic of Subthreshold Synaptic Summation in a Model CA1 Pyramidal Cell. Neuron 2003, 37, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.F.; Dobrunz, L.E. Short-term plasticity regulates the excitation/inhibition ratio and the temporal window for spike integration in CA1 pyramidal cells. Eur. J. Neurosci. 2015, 41, 1402–1415. [Google Scholar] [CrossRef]
- Carvalho, T.P.; Buonomano, D.V. Differential Effects of Excitatory and Inhibitory Plasticity on Synaptically Driven Neuronal Input-Output Functions. Neuron 2009, 61, 774–785. [Google Scholar] [CrossRef]
- Galarreta, M.; Hestrin, S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat. Neurosci. 1998, 1, 587–594. [Google Scholar] [CrossRef]
- Abbott, L.F.; Varela, J.A.; Sen, K.; Nelson, S.B. Synaptic Depression and Cortical Gain Control. Science 1997, 275, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.A. Neuronal arithmetic. Nat. Rev. Neurosci. 2010, 11, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumpa, A.; Papatheodoropoulos, C. Short-term dynamics of input and output of CA1 network greatly differ between the dorsal and ventral rat hippocampus. BMC Neurosci. 2019, 20, 1–30. [Google Scholar] [CrossRef]
- Tsotsokou, G.; Fassea, M.; Papatheodoropoulos, C. Muscarinic Modulation of Network Excitability and Short-Term Dynamics in the Dorsal and Ventral Hippocampus. . 2024, 2024. [Google Scholar] [CrossRef]
- Tsotsokou, G.; Sotiropoulou, I.-M.; Stampolitis, K.; Oikonomou, G.D.; Avdi, A.-P.; Papatheodoropoulos, C. Cannabinoid Modulation of Excitability and Short-Term Neuronal Dynamics in the Dorsal and Ventral Hippocampus. Biology 2025, 14, 642. [Google Scholar] [CrossRef]
- Miliou, A.; Papaleonidopoulos, V.; Trompoukis, G.; Papatheodoropoulos, C. Septotemporal variation in beta-adrenergic modulation of short-term dynamics in the hippocampus. IBRO Neurosci. Rep. 2021, 11, 64–72. [Google Scholar] [CrossRef]
- Trompoukis, G.; Tsotsokou, G.; Koutsoumpa, A.; Tsolaki, M.; Vryoni, G.; Papatheodoropoulos, C. Age-dependent modulation of short-term neuronal dynamics in the dorsal and ventral rat hippocampus. Int. J. Dev. Biol. 2022, 66, 285–296. [Google Scholar] [CrossRef]
- Kandilakis, C.L.; Papatheodoropoulos, C. Serotonin Modulation of Dorsoventral Hippocampus in Physiology and Schizophrenia. Int. J. Mol. Sci. 2025, 26, 7253. [Google Scholar] [CrossRef]
- Deng, P.-Y.; Sojka, D.; Klyachko, V.A. Abnormal Presynaptic Short-Term Plasticity and Information Processing in a Mouse Model of Fragile X Syndrome. J. Neurosci. 2011, 31, 10971–10982. [Google Scholar] [CrossRef] [PubMed]
- Leontiadis, L.J.; Felemegkas, P.; Trompoukis, G.; Tsotsokou, G.; Miliou, A.; Karagianni, E.; Rigas, P.; Papatheodoropoulos, C. Septotemporal Variation of Information Processing in the Hippocampus of Fmr1 KO Rat. Dev. Neurosci. 2024, 46, 353–364. [Google Scholar] [CrossRef]
- Bannerman, D.M.; Sprengel, R.; Sanderson, D.J.; McHugh, S.B.; Rawlins, J.N.; Monyer, H.; Seeburg, P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci 2014, 15, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Strange, B.A.; Witter, M.P.; Lein, E.S.; Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Sertel, S.M.; Blumenstein, W.; Mandad, S.; Shomroni, O.; Salinas, G.; Rizzoli, S.O. Differences in synaptic vesicle pool behavior between male and female hippocampal cultured neurons. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Stevens, C.F.; Wesseling, J.F. Activity-Dependent Modulation of the Rate at which Synaptic Vesicles Become Available to Undergo Exocytosis. Neuron 1998, 21, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Denker, A.; O Rizzoli, S. Synaptic vesicle pools: an update. Front. Synaptic Neurosci. 2010, 2, 135. [Google Scholar] [CrossRef]
- Neher, E.; Brose, N. Dynamically Primed Synaptic Vesicle States: Key to Understand Synaptic Short-Term Plasticity. Neuron 2018, 100, 1283–1291. [Google Scholar] [CrossRef]
- Tsotsokou, G.; Miliou, A.; Trompoukis, G.; Leontiadis, L.J.; Papatheodoropoulos, C. Region-Related Differences in Short-Term Synaptic Plasticity and Synaptotagmin-7 in the Male and Female Hippocampus of a Rat Model of Fragile X Syndrome. Int. J. Mol. Sci. 2024, 25, 6975. [Google Scholar] [CrossRef]
- Day, H.L.L.; Stevenson, C.W. The neurobiological basis of sex differences in learned fear and its inhibition. Eur. J. Neurosci. 2019, 52, 2466–2486. [Google Scholar] [CrossRef]
- Abbott, L.F.; Regehr, W.G. Synaptic computation. Nature 2004, 431, 796–803. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.-W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Mottron, L.; Duret, P.; Mueller, S.; Moore, R.D.; D’aRc, B.F.; Jacquemont, S.; Xiong, L. Sex differences in brain plasticity: a new hypothesis for sex ratio bias in autism. Mol. Autism 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Bagot, R.C.; Parise, E.M.; Peña, C.J.; Zhang, H.-X.; Maze, I.; Chaudhury, D.; Persaud, B.; Cachope, R.; Bolaños-Guzmán, C.A.; Cheer, J.F.; et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 2015, 6, 7062–7062. [Google Scholar] [CrossRef]
- Papp, M.; Gruca, P.; Lason, M.; Litwa, E.; Solecki, W.; Willner, P. Insufficiency of ventral hippocampus to medial prefrontal cortex transmission explains antidepressant non-response. J. Psychopharmacol. 2021, 35, 1253–1264. [Google Scholar] [CrossRef]
- Fenton, A.A.; Muller, R.U. Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proc. Natl. Acad. Sci. 1998, 95, 3182–3187. [Google Scholar] [CrossRef]
- Koutsoumpa, A.; Papatheodoropoulos, C. Frequency-dependent layer-specific differences in short-term synaptic plasticity in the dorsal and ventral CA1 hippocampal field. Synapse 2021, 75, e22199. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




