Submitted:
15 July 2025
Posted:
16 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
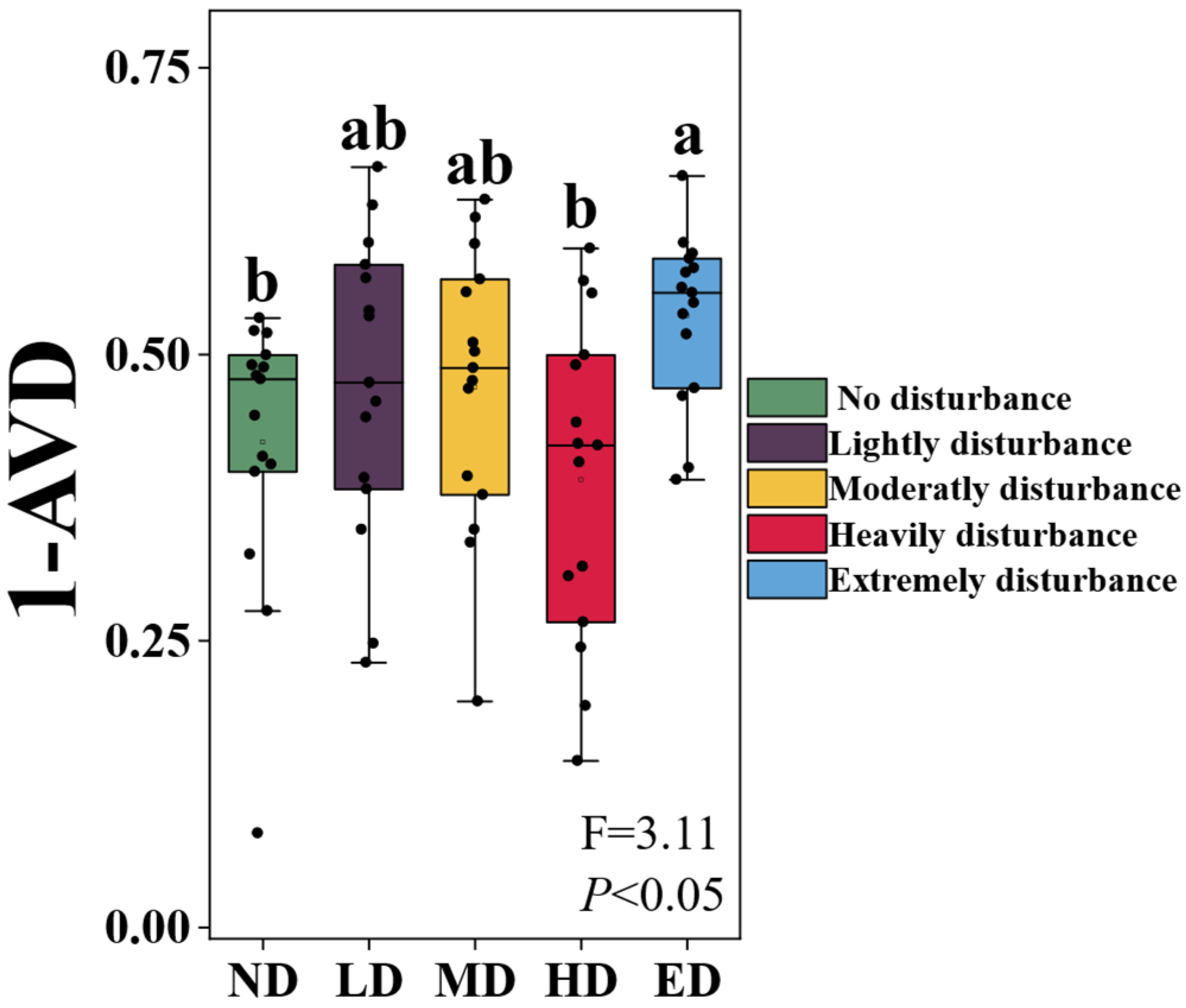
2.1. Variation in Plant Community Stability Across Disturbance Intensities
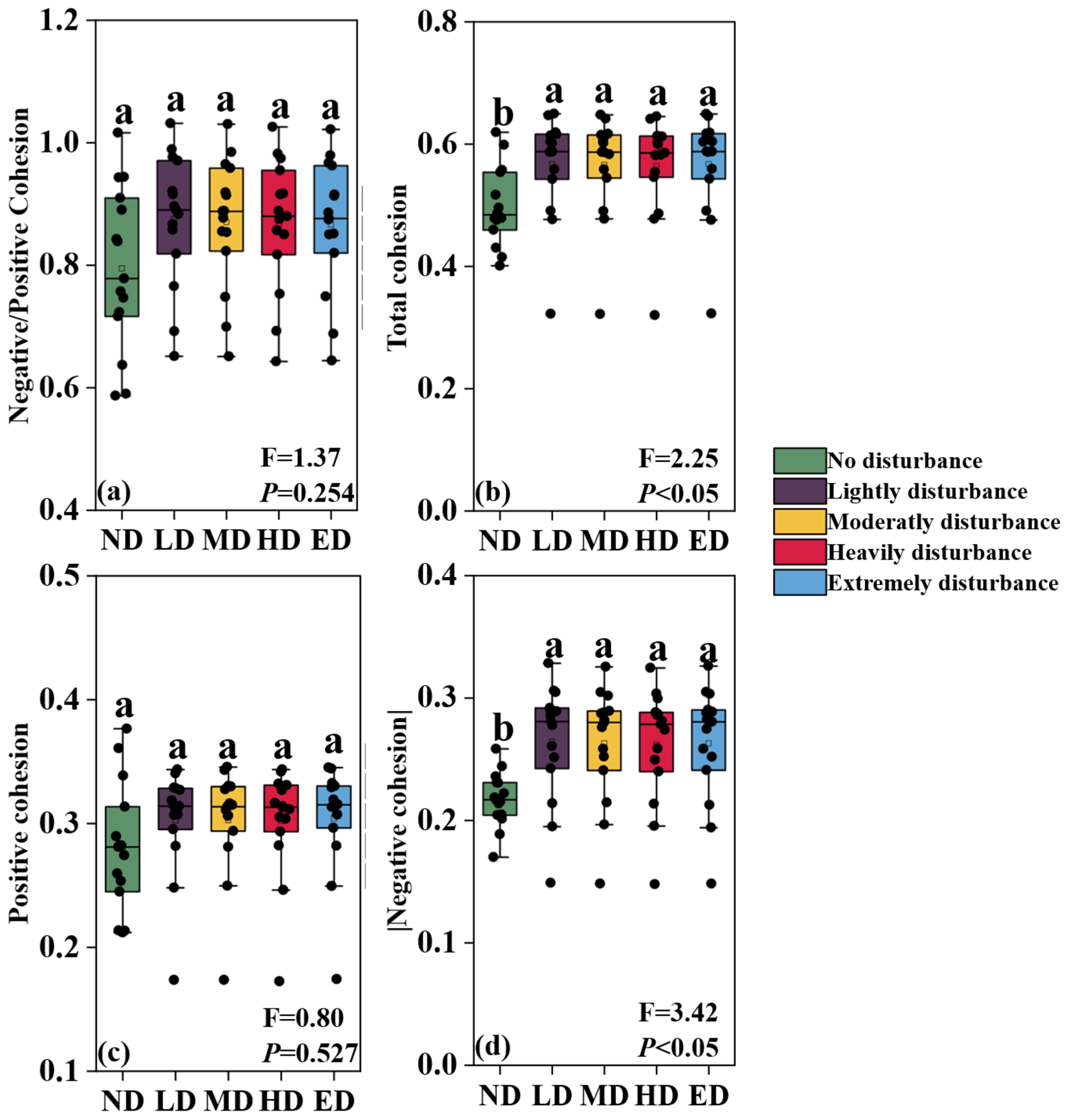
2.2. Cohesion Indices of Plant Communities
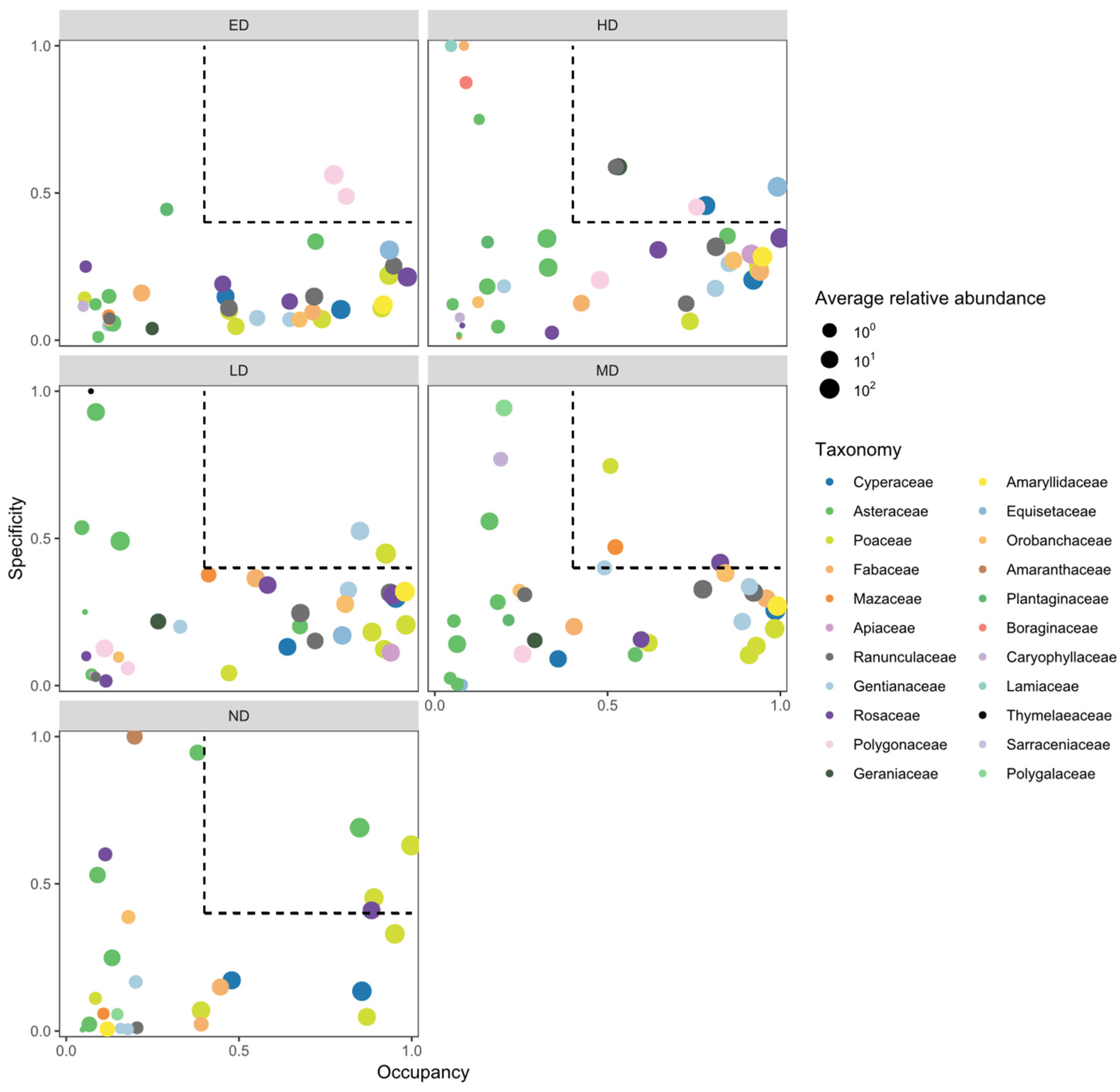
2.3. Indicator Species at Different Disturbance Intensities
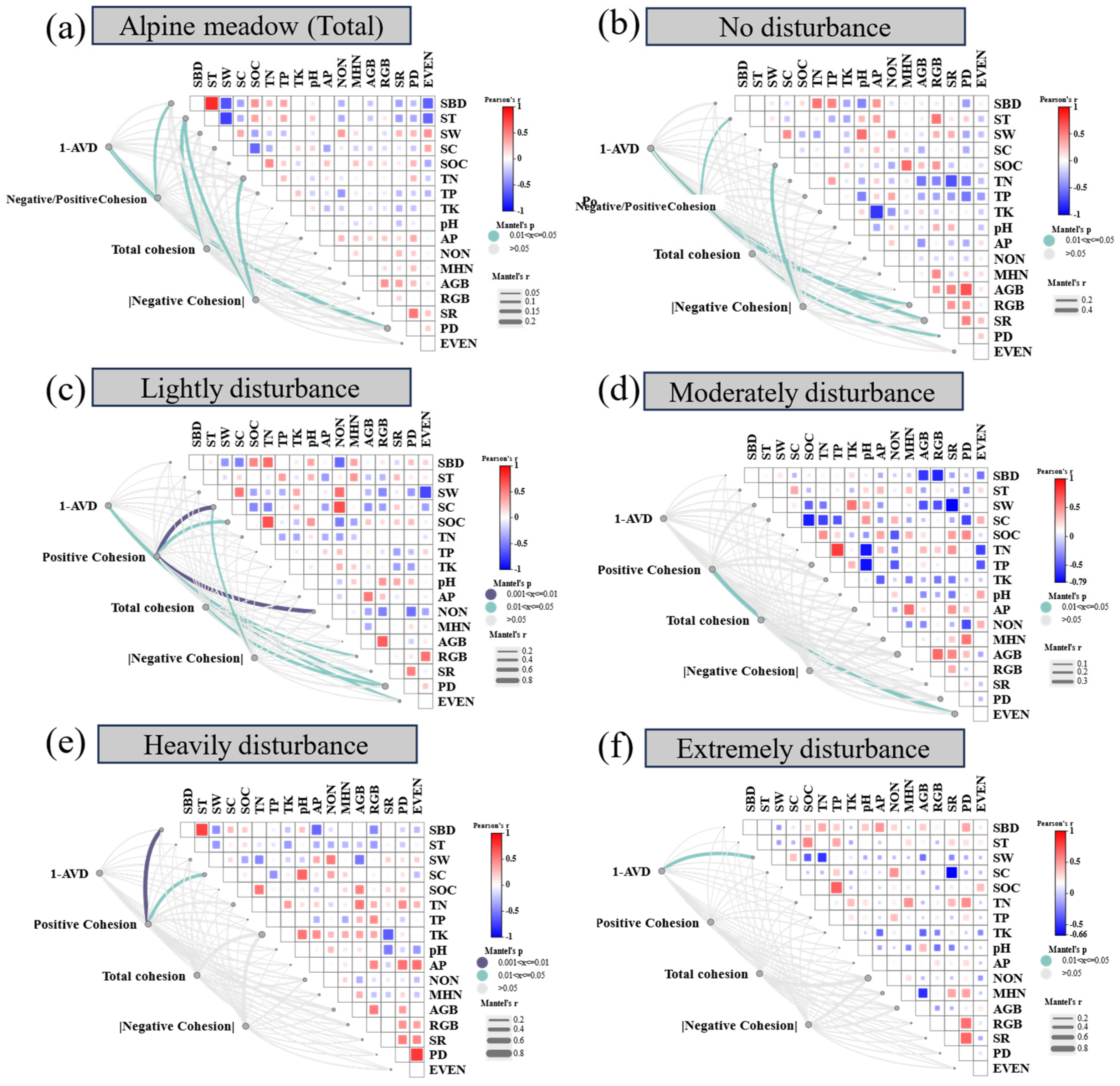
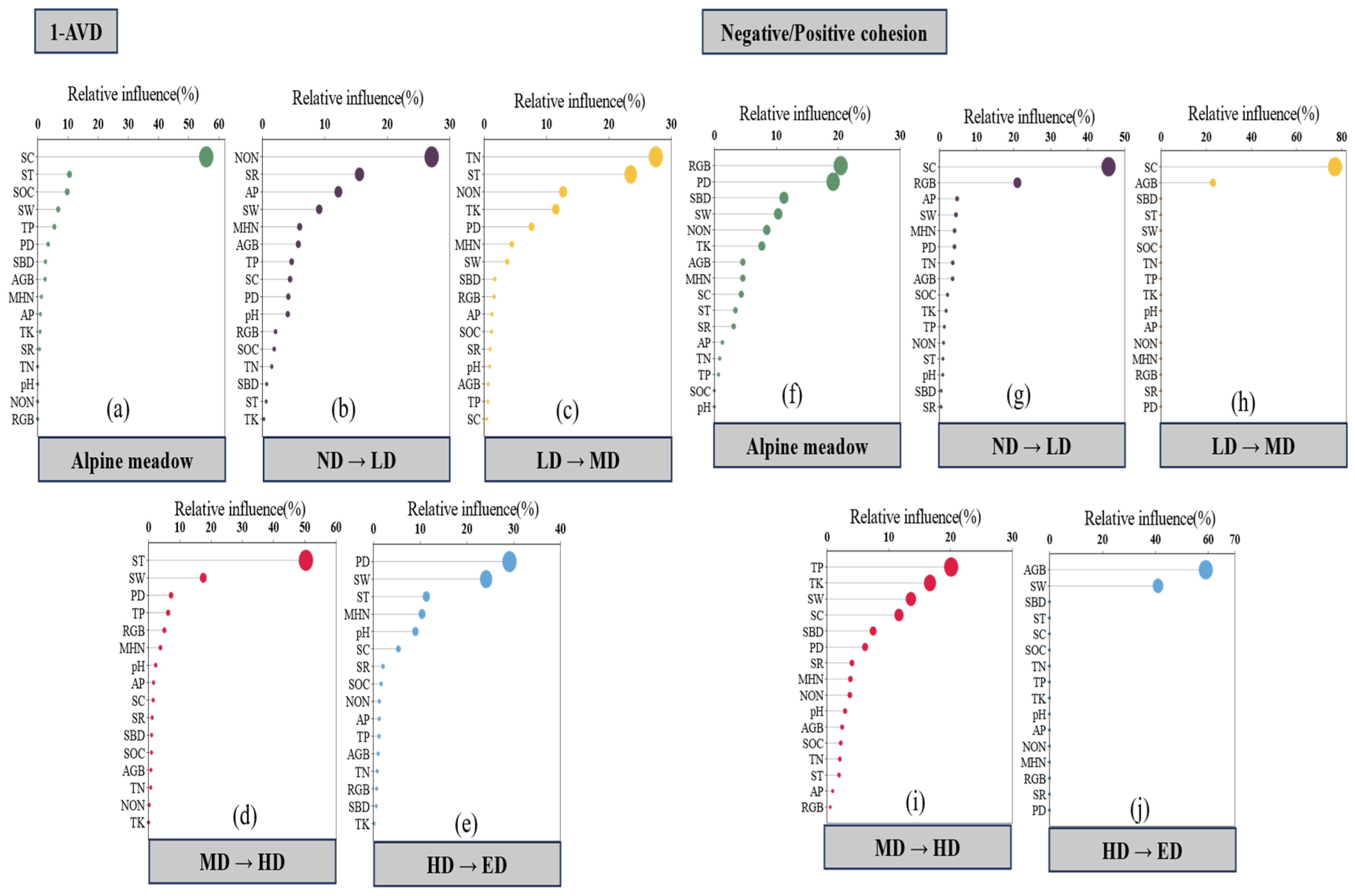
2.4. Environmental Drivers of Plant Community Stability
3. Discussion
3.1. Extreme Zokor Disturbance Enhances Plant Community Stability via Compositional Convergence
3.2. Indicator Species Reflect Disturbance-Induced Regime Shifts in Community Identity
3.3. Disturbance Intensity Mediates a Shift in the Drivers of Community Stability
3.4. Practical Implications for Alpine Grassland Management Under Zokor Disturbance
4. Materials and Methods
4.1. Sites Description
4.2. Experimental Design
4.3. Field Survey and Sampling
4.4. Soil Physicochemical Analysis
4.5. Community Indicators Calculations
4.5.1. Average Variation Degree (AVD)
4.5.2. Community Cohesion
4.5.3. Specificity and Occupancy
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Supplementary Materials
| Disturbance intensity | Indicator species | Abundance_mean |
| No disturbance | Leymus secalinus | 116.87 |
| Poa araratica | 55.20 | |
| Sibbaldianthe bifurca | 13.93 | |
| Artemisia frigida | 77.20 | |
| Lightly disturbance | Stipa capillata | 148.53 |
| Swertia tetraptera | 23.67 | |
| Moderately disturbance | Cleistogenes squarrosa | 3.13 |
| Lancea tibetica | 2.67 | |
| Ranunculus tanguticus | 16.80 | |
| Sibbaldianthe bifurca | 14.20 | |
| Heavily disturbance | Carex atrata | 48.00 |
| Knorringia sibirica | 6.60 | |
| Geranium pylzowianum | 7.93 | |
| Gymnaconitum gymnandrum | 2.67 | |
| Extremely disturbance | Bistorta vivipara | 68.33 |
| Knorringia sibirica | 7.13 |
References
- Zhao, L.; Li, Y.; Xu, S.; Zhou, H.; Gu, S.; Yu, G.; Zhao, X. Diurnal, Seasonal and Annual Variation in Net Ecosystem CO2 Exchange of an Alpine Shrubland on Qinghai-Tibetan Plateau. Global Change Biol. 2006, 12, 1940–1953. [Google Scholar] [CrossRef]
- Qiu, J. China: The Third Pole. Nature 2008, 454, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, S.; Liu, S.; Su, X.; Wang, X.; Zhang, Y.; Zhao, Z.; Gao, X.; Li, S.; Tang, L. Relationships between Plant Diversity and Biomass Production of Alpine Grasslands Are Dependent on the Spatial Scale and the Dimension of Biodiversity. Ecol. Eng. 2019, 127, 375–382. [Google Scholar] [CrossRef]
- Dong, S.; Gao, H.; Xu, G.; Hou, X.; Long, R.; Kang, M.; Lassoie, J. Farmer and Professional Attitudes to the Large-Scale Ban on Livestock Grazing of Grasslands in China. Environ. Conserv. 2007, 34, 246–254. [Google Scholar] [CrossRef]
- Lu, Q.; Fan, H.; Yan, B.; Zhao, D.; Wei, X. Soil C, N, and P and C: N: P Stoichiometry Associated with Environmental Factors in Two Typical Alpine Grasslands in Northern Tibet. J. Soils Sediments 2023, 23, 3735–3747. [Google Scholar] [CrossRef]
- Loreau, M.; De Mazancourt, C. Biodiversity and Ecosystem Stability: A Synthesis of Underlying Mechanisms. Ecol. Lett. 2013, 16, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Ives, A.R.; Carpenter, S.R. Stability and Diversity of Ecosystems. Science 2007, 317, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.; Shen, Z.; Shi, P.; Yu, C.; Chen, B. Effects of Livestock Exclusion and Climate Change on Aboveground Biomass Accumulation in Alpine Pastures across the Northern Tibetan Plateau. Chin. Sci. Bull. 2014, 59, 4332–4340. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J. Effects of Plateau Zokors (Myospalax Fontanierii) on Plant Community and Soil in an Alpine Meadow. J. Mammal. 2003, 84, 644–651. [Google Scholar] [CrossRef]
- Chu, B.; Ye, G.; Yang, S.; Zhou, F.; Zhang, F.; Zhou, J.; Hua, L. Effect of Plateau Zokor (Myospalax Fontanierii) Disturbance on Plant Community Structure and Soil Properties in the Eastern Qinghai-Tibet Plateau, China. Rangeland Ecol. Manage. 2020, 73, 520–530. [Google Scholar] [CrossRef]
- Pickett, S.T.; Pickett, S.T.; White, P. The Ecology of Natural Disturbance and Patch Dynamics; Academic press, 1985; ISBN 978-0-08-050495-7.
- Hutchings, M.; John, E.; Stewart, A. The Ecological Consequences of Environmental Heterogeneity: 40th Symposium of the British Ecological Society; Cambridge University Press, 2000.
- Niu, Y.; Yang, S.; Zhu, H.; Zhou, J.; Chu, B.; Sujie Ma; Rui Hua; Limin Hua Cyclic Formation of Zokor Mounds Promotes Plant Diversity and Renews Plant Communities in Alpine Meadows on the Tibetan Plateau. Plant and Soil 2020, 446, 65–79. [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs: High Diversity of Trees and Corals Is Maintained Only in a Nonequilibrium State. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Bhatt, A.; Tang, Z.; Peng, Y.; Wu, W. ; Jiaxin Zhang; Jingxuan Wang; David Gallacher; Saixia Zhou Disturbance of Plateau Zokor-Made Mound Stimulates Plant Community Regeneration in the Qinghai-Tibetan Plateau, China. J. Arid Land 2021, 13, 1054–1070. [Google Scholar] [CrossRef]
- Mouquet, N.; Loreau, M. Community Patterns in Source-Sink Metacommunities. Am. Nat. 2003, 162, 544–557. [Google Scholar] [CrossRef]
- Thébault, E.; Fontaine, C. Stability of Ecological Communities and the Architecture of Mutualistic and Trophic Networks. Science 2010, 329, 853–856. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.; Villéger, S.; Mason, N.W.; Bellwood, D.R. A Functional Approach Reveals Community Responses to Disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xiong, K.; Chi, Y.; Song, S.; He, C.; He, S. Research Advancement in Grassland Ecosystem Vulnerability and Ecological Resilience and Its Inspiration for Improving Grassland Ecosystem Services in the Karst Desertification Control. Plants 2022, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Miao, H.; Zhang, C.; Zou, H.; Yang, Y.; Zhang, Z.; Liu, J. Appropriate Livestock Grazing Alleviates the Loss of Plant Diversity and Maintains Community Resistance in Alpine Meadows. J. Environ. Manage. 2024, 351, 119850. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, G.; Liang, Y.; Wang, H. Impacts of Locust Feeding on Interspecific Relationships and Niche of the Major Plants in Inner Mongolia Grasslands. Global Ecol. Conserv. 2024, 51, e02913. [Google Scholar] [CrossRef]
- Donohue, I.; Hillebrand, H.; Montoya, J.M.; Petchey, O.L.; Pimm, S.L.; Fowler, M.S.; Healy, K.; Jackson, A.L.; Lurgi, M.; McClean, D.; et al. Navigating the Complexity of Ecological Stability. Ecol. Lett. 2016, 19, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L. The Complexity and Stability of Ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consequences of Dominance: A Review of Evenness Effects on Local and Regional Ecosystem Processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Dı́az, S.; Cabido, M. Vive La Différence: Plant Functional Diversity Matters to Ecosystem Processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting Changes in Community Composition and Ecosystem Functioning from Plant Traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin Iii, F.; Cornelissen, J.H.; DIAz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.-L. Scaling Environmental Change through the Community-Level: A Trait-Based Response-and-Effect Framework for Plants. Global Change Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons, 2006.
- Bardgett, R.D.; Van Der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Valencia, E.; De Bello, F.; Galland, T.; Adler, P.B.; Lepš, J.; E-Vojtkó, A.; van Klink, R.; Carmona, C.P.; Danihelka, J.; Dengler, J.; et al. Synchrony Matters More than Species Richness in Plant Community Stability at a Global Scale. Proc. Natl. Acad. Sci. 2020, 117, 24345–24351. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Langenheder, S.; Lebret, K.; Lindström, E.; Östman, Ö.; Striebel, M. Decomposing Multiple Dimensions of Stability in Global Change Experiments. Ecol. Lett. 2017, 21, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized Metabolic Functions of Keystone Taxa Sustain Soil Microbiome Stability. Microbiome 2021, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Loreau, M. Biodiversity and Ecosystem Stability across Scales in Metacommunities. Ecol. Lett. 2016, 19, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Pu, Z. Different Effects of Species Diversity on Temporal Stability in Single-Trophic and Multitrophic Communities. Am. Nat. 2009, 174, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, J.M.; Laliberté, E.; Nielsen, A.; Bascompte, J. Conservation of Species Interaction Networks. Biol. Conserv. 2010, 143, 2270–2279. [Google Scholar] [CrossRef]
- Delmas, E.; Besson, M.; Brice, M.-H.; Burkle, L.A.; Dalla Riva, G.V.; Fortin, M.-J.; Gravel, D.; Guimarães Jr, P.R.; Hembry, D.H.; Newman, E.A.; et al. Analysing Ecological Networks of Species Interactions. Biol. Rev. 2018, 94, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Bascompte, J. Disentangling the Web of Life. Science 2009, 325, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Chiu, C.-H. ; Others Species Richness: Estimation and Comparison. Wiley Statsref: Stat. Ref. Online 2016, 1, 1–26. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A Working Guide to Boosted Regression Trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Oppel, S.; Meirinho, A.; Ramı́rez, I.; Gardner, B.; O’Connell, A.F.; Miller, P.I.; Louzao, M. Comparison of Five Modelling Techniques to Predict the Spatial Distribution and Abundance of Seabirds. Biol. Conserv. 2012, 156, 94–104. [Google Scholar] [CrossRef]
- Jucker, T.; Bongalov, B.; Burslem, D.F.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Coomes, D.A. Topography Shapes the Structure, Composition and Function of Tropical Forest Landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Edwards Jr, T.C.; Hastie, T. Generalized Linear and Generalized Additive Models in Studies of Species Distributions: Setting the Scene. Ecol. Modell. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Tilman, D. Biodiversity: Population versus Ecosystem Stability. Ecology 1996, 77, 350–363. [Google Scholar] [CrossRef]
- Grime, J.P.; Brown, V.K.; Thompson, K.; Masters, G.J.; Hillier, S.H.; Clarke, I.P.; Askew, A.P.; Corker, D.; Kielty, J.P. The Response of Two Contrasting Limestone Grasslands to Simulated Climate Change. Science 2000, 289, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Evidence for the Existence of Three Primary Strategies in Plants and Its Relevance to Ecological and Evolutionary Theory. The american naturalist 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Keddy, P.A. Assembly and Response Rules: Two Goals for Predictive Community Ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the Stress-Gradient Hypothesis for Competition and Facilitation in Plant Communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- De Bello, F.; Lavorel, S.; Dı́az, S.; Harrington, R.; Cornelissen, J.H.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an Assessment of Multiple Ecosystem Processes and Services via Functional Traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Wilkinson, D.M. The Disturbing History of Intermediate Disturbance. Oikos 1999, 84, 145. [Google Scholar] [CrossRef]
- Roxburgh, S.H.; Shea, K.; Wilson, J.B. The Intermediate Disturbance Hypothesis: Patch Dynamics and Mechanisms of Species Coexistence. Ecology 2004, 85, 359–371. [Google Scholar] [CrossRef]
- Turner, M.G. Disturbance and Landscape Dynamics in a Changing World. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M. Resource Strategies of Wild Plants; Princeton University Press, 2011; Vol. 36.
- Dı́az, S.; Noy-Meir, I.; Cabido, M. Can Grazing Response of Herbaceous Plants Be Predicted from Simple Vegetative Traits? J. Appl. Ecol. 2001, 38, 497–508. [Google Scholar] [CrossRef]
- Klimešová, J.; Martı́nková, J.; Ottaviani, G. Belowground Plant Functional Ecology: Towards an Integrated Perspective. Funct. Ecol. 2018, 32, 2115–2126. [Google Scholar] [CrossRef]
- Ottaviani, G.; Keppel, G.; Götzenberger, L.; Harrison, S.; Opedal, Ø.H.; Conti, L.; Liancourt, P.; Klimešová, J.; Silveira, F.A.; Jiménez-Alfaro, B.; et al. Linking Plant Functional Ecology to Island Biogeography. Trends Plant Sci. 2020, 25, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Weiher, E.; Freund, D.; Bunton, T.; Stefanski, A.; Lee, T.; Bentivenga, S. Advances, Challenges and a Developing Synthesis of Ecological Community Assembly Theory. Philos. Trans. R. Soc. B: Biol. Sci. 2011, 366, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.T.; Blois, J.L. Community Ecology in a Changing Environment: Perspectives from the Quaternary. Proc. Natl. Acad. Sci. 2015, 112, 4915–4921. [Google Scholar] [CrossRef] [PubMed]
- Herren, C.M.; McMahon, K.D. Cohesion: A Method for Quantifying the Connectivity of Microbial Communities. ISME J. 2017, 11, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.; Arnoldi, J.-F.; Bunin, G.; Loreau, M. Generic Assembly Patterns in Complex Ecological Communities. Proc. Natl. Acad. Sci. 2018, 115, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.R. Ecosystems of Disturbed Ground; Elsevier, 2000; Vol. 11.
- Cingolani, A.M.; Noy-Meir, I.; Dı́az, S. Grazing Effects on Rangeland Diversity: A Synthesis of Contemporary Models. Ecol. Appl. 2005, 15, 757–773. [Google Scholar] [CrossRef]
- Adler, P.B.; Milchunas, D.G.; Lauenroth, W.K.; Sala, O.E.; Burke, I.C. Functional Traits of Graminoids in Semi-Arid Steppes: A Test of Grazing Histories. J. Appl. Ecol. 2004, 41, 653–663. [Google Scholar] [CrossRef]
- Isbell, F.; Cowles, J.; Dee, L.E.; Loreau, M.; Reich, P.B.; Gonzalez, A.; Hector, A.; Schmid, B. Quantifying Effects of Biodiversity on Ecosystem Functioning across Times and Places. Ecol. Lett. 2018, 21, 763–778. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic Shifts in Ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Suding, K.N.; Hobbs, R.J. Threshold Models in Restoration and Conservation: A Developing Framework. Trends Ecol. Evol. 2009, 24, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Bestelmeyer, B.T.; Ellison, A.M.; Fraser, W.R.; Gorman, K.B.; Holbrook, S.J.; Laney, C.M.; Ohman, M.D.; Peters, D.P.; Pillsbury, F.C.; Rassweiler, A.; et al. Analysis of Abrupt Transitions in Ecological Systems. Ecosphere 2011, 2, art129. [Google Scholar] [CrossRef]
- Fukami, T.; Nakajima, M. Community Assembly: Alternative Stable States or Alternative Transient States? Ecol. Lett. 2011, 14, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response Diversity, Ecosystem Change, and Resilience. Front. Ecol. Environ. 2003, 1, 488. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Chown, S.L. Scaling up the Value of Bioindicators. Trends Ecol. Evol. 1998, 13, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Mouquet, N.; Gonzalez, A. Biodiversity as Spatial Insurance in Heterogeneous Landscapes. Proc. Natl. Acad. Sci. 2003, 100, 12765–12770. [Google Scholar] [CrossRef] [PubMed]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global Ecosystem Thresholds Driven by Aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.P.; Pielke Sr, R.A.; Bestelmeyer, B.T.; Allen, C.D.; Munson-McGee, S.; Havstad, K.M. Cross-Scale Interactions, Nonlinearities, and Forecasting Catastrophic Events. Proc. Natl. Acad. Sci. 2004, 101, 15130–15135. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos (cph. Den.) 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Carpenter, S.; Walker, B.; Anderies, J.M.; Abel, N. From Metaphor to Measurement: Resilience of What to What? Ecosyst. (n. Y. N.Y.) 2001, 4, 765–781. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Holling, C.S.; Carpenter, S.R.; Kinzig, A. Resilience, Adaptability and Transformability in Social–Ecological Systems. Ecol. Soc. 2004, 9. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Norton, D.A. Towards a Conceptual Framework for Restoration Ecology. Restor. Ecol. 1996, 4, 93–110. [Google Scholar] [CrossRef]
- Suding, K.N. Toward an Era of Restoration in Ecology: Successes, Failures, and Opportunities Ahead. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 465–487. [Google Scholar] [CrossRef]
- Harris, J.A.; Hobbs, R.J.; Higgs, E.; Aronson, J. Ecological Restoration and Global Climate Change 2006, 14, 170–176.
- Likens, G.; Lindenmayer, D. Effective Ecological Monitoring; CSIRO publishing, 2018; ISBN 978-1-4863-0893-4.
- Standish, R.J.; Hobbs, R.J.; Mayfield, M.M.; Bestelmeyer, B.T.; Suding, K.N.; Battaglia, L.L.; Eviner, V.; Hawkes, C.V.; Temperton, V.M.; Cramer, V.A.; et al. Resilience in Ecology: Abstraction, Distraction, or Where the Action Is? Biol. Conserv. 2014, 177, 43–51. [Google Scholar] [CrossRef]
- Miehe, G.; Schleuss, P.-M.; Seeber, E.; Babel, W.; Biermann, T.; Braendle, M.; Chen, F.; Coners, H.; Foken, T.; Gerken, T.; et al. The Kobresia Pygmaea Ecosystem of the Tibetan Highlands–Origin, Functioning and Degradation of the World’s Largest Pastoral Alpine Ecosystem: Kobresia Pastures of Tibet. Sci. Total Environ. 2019, 648, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhou, J.; Yang, S.; Chu, B.; Zhu, H.; Zhang, B.; Fang, Q.; Tang, Z.; Hua, L. Plant Diversity Is Closely Related to the Density of Zokor Mounds in Three Alpine Rangelands on the Tibetan Plateau. PeerJ 2019, 7, e6921. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Gao, J.; Ma, G.; Qi, X. ; Others Micro-Scale Fragmentation of the Alpine Meadow Landscape on the Qinghai-Tibet Plateau under External Disturbances. Catena 2021, 201, 105220. [Google Scholar] [CrossRef]
- Ye, G.; Chu, B.; Tang, Z.; Hu, G.; Bao, D.; Hua, R.; Pfeiffer, M.; Hua, L.; Niu, Y. Soil Microbial and Macroinvertebrate Functional Diversity in Response to Zokor Disturbance in Tibetan Alpine Meadow. Catena 2023, 225, 107014. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. Methods Soil Anal.: 3 Chem. Methods 2018, 5, 961–1010. [Google Scholar]
- Pei, L.; Wu, Z.; Qian, Y.; Li, X.; Zhang, J.; Sun, J.; Wang, Y. Plant Community Stability, Indicator Species and Their Driving Factors at a Gradient of Grazing Intensity in an Alpine Meadow. Ecol. Indic. 2024, 162, 112012. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate Warming Enhances Microbial Network Complexity and Stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Jia, Z.; Zhai, L.; Zhang, B.; Grüters, U.; Ma, S.; Qian, J.; Liu, X.; Zhang, J.; et al. Meta-Analysis Reveals the Effects of Microbial Inoculants on the Biomass and Diversity of Soil Microbial Communities. Nat. Ecol. Evol. 2024, 8, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Gweon, H.S.; Bowes, M.J.; Moorhouse, H.L.; Oliver, A.E.; Bailey, M.J.; Acreman, M.C.; Read, D.S. Contrasting Community Assembly Processes Structure Lotic Bacteria Metacommunities along the River Continuum. Environ. Microbiol. 2020, 23, 484–498. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Mallery, P. IBM SPSS Statistics 26 Step by Step: A Simple Guide and Reference; Routledge, 2019.
- Gardener, M. Beginning R: The Statistical Programming Language; John Wiley & Sons, 2012.
- Cao, T.; Li, Q.; Huang, Y.; Li, A. plotnineSeqSuite: A Python Package for Visualizing Sequence Data Using Ggplot2 Style. BMC Genomics 2023, 24, 585. [Google Scholar] [CrossRef] [PubMed]
| Index | Lightly disturbance | Moderately disturbance | Heavily disturbance | Extremely disturbance |
| Total area of patch / m2 | 64.82 ± 1.894 c | 94.46 ± 4.441 bc | 117.3 ± 3.765 ab | 142.81 ± 2.578 a |
| Total edge of patch / m | 174.37 ± 6.451 c | 248.01 ± 4.443 b | 297.15 ± 7.546 b | 411.38 ± 6.591 a |
| Splitting index | 1.46 ± 0.016 c | 1.66 ± 0.038 bc | 1.94 ± 0.042 ab | 2.18 ± 0.038 a |
| Shape index | 1.13 ± 0.008 a | 1.15 ± 0.002 a | 1.16 ± 0.001 a | 1.17 ± 0.010 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





