Submitted:
05 July 2025
Posted:
07 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Characterization of AgNPs/MCFA
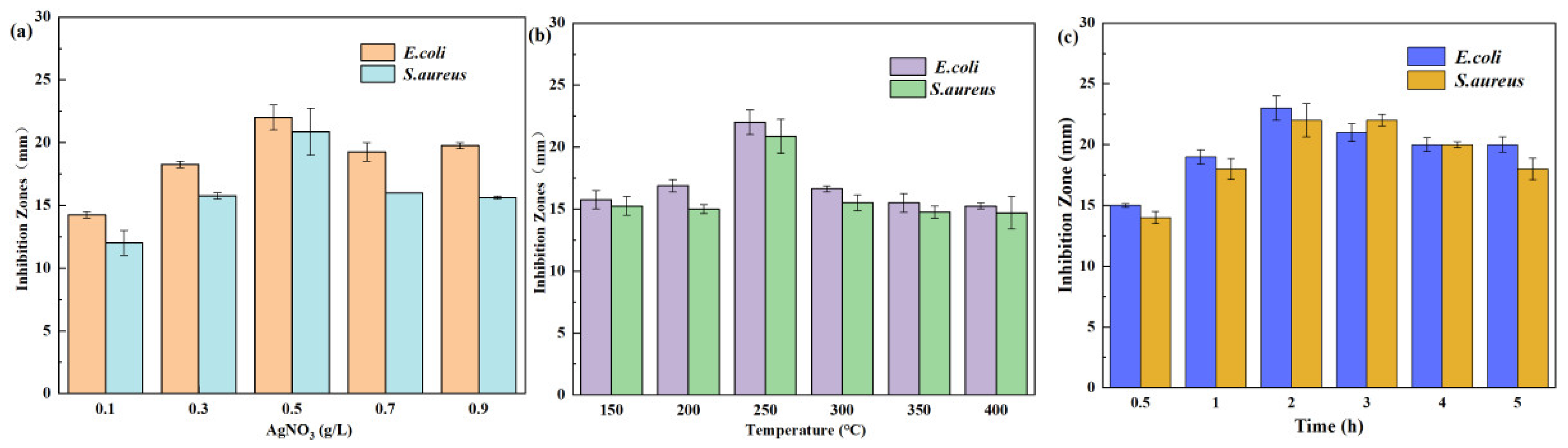
2.2. Effects of AgNO3 Concentration on the Antibacterial Ability
2.3. Sintering Temperature and Sintering Time on the Antibacterial Ability
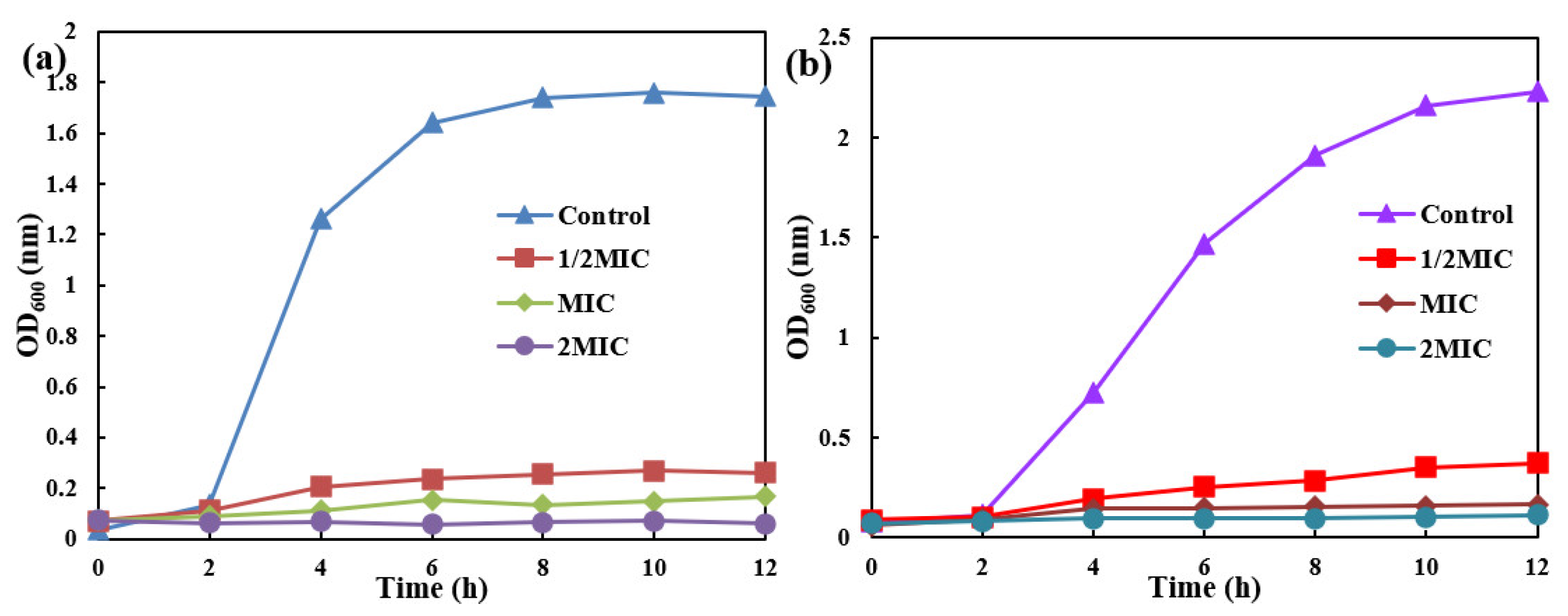
2.4. The MIC of AgNPs/MCFA
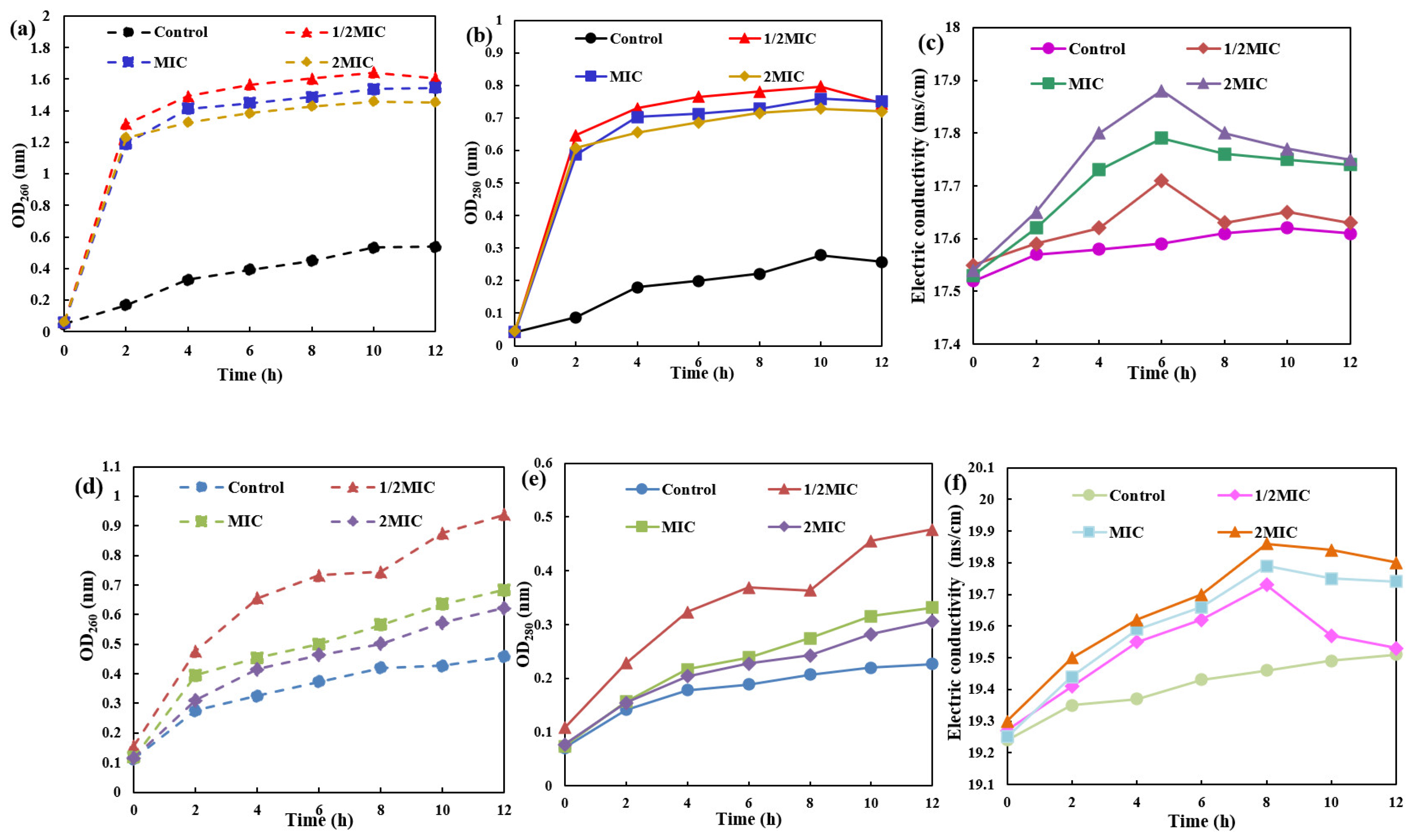
2.5. Impact of AgNPs/MCFA on Bacterial Nucleic Acid Leakage, Protein Leakage and Electrical Con Ductivity
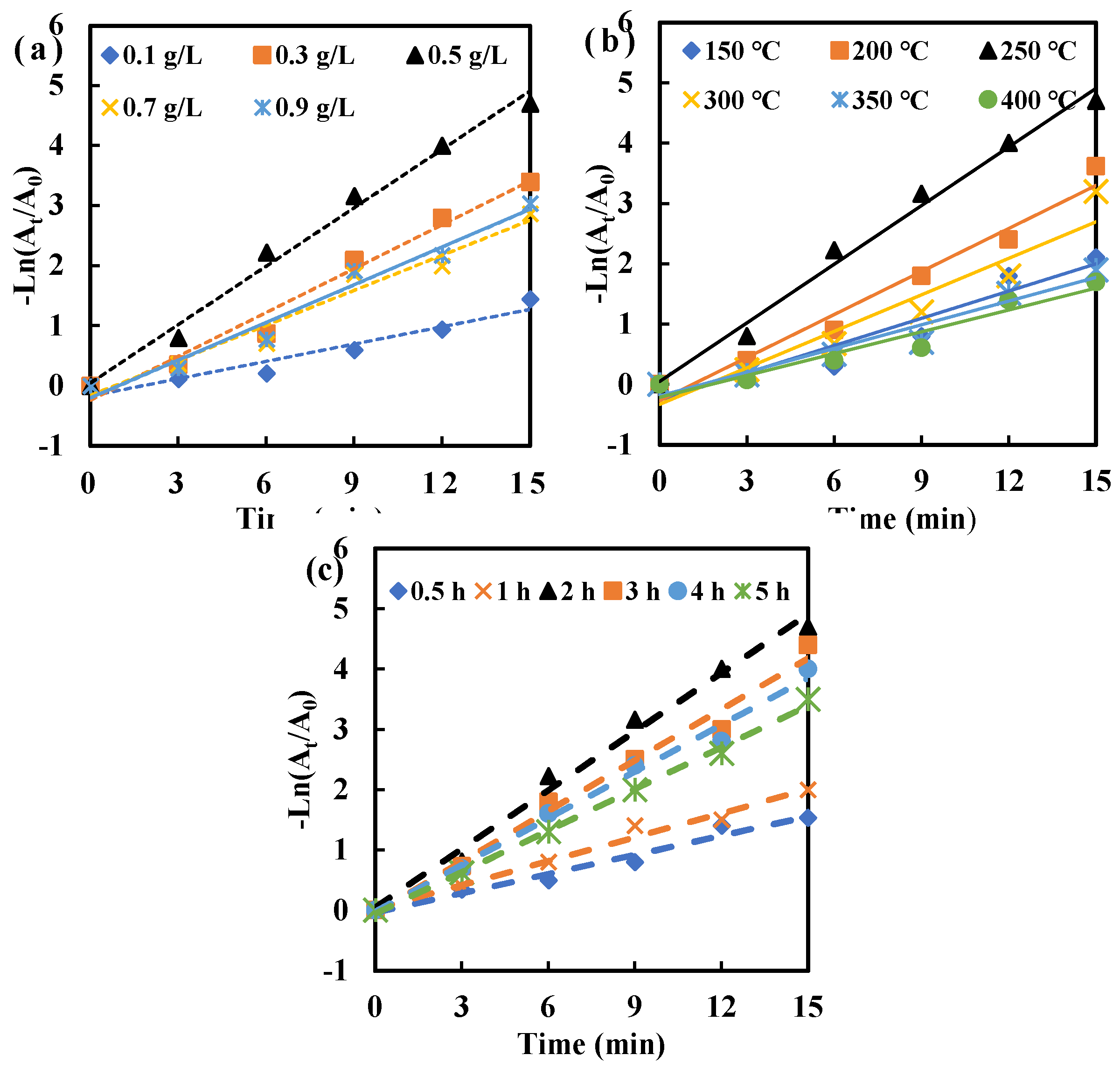
2.6. Antimicrobial Kinetics
2.7. Catalytic Performance of AgNPs/MCFA for Degradation of MO
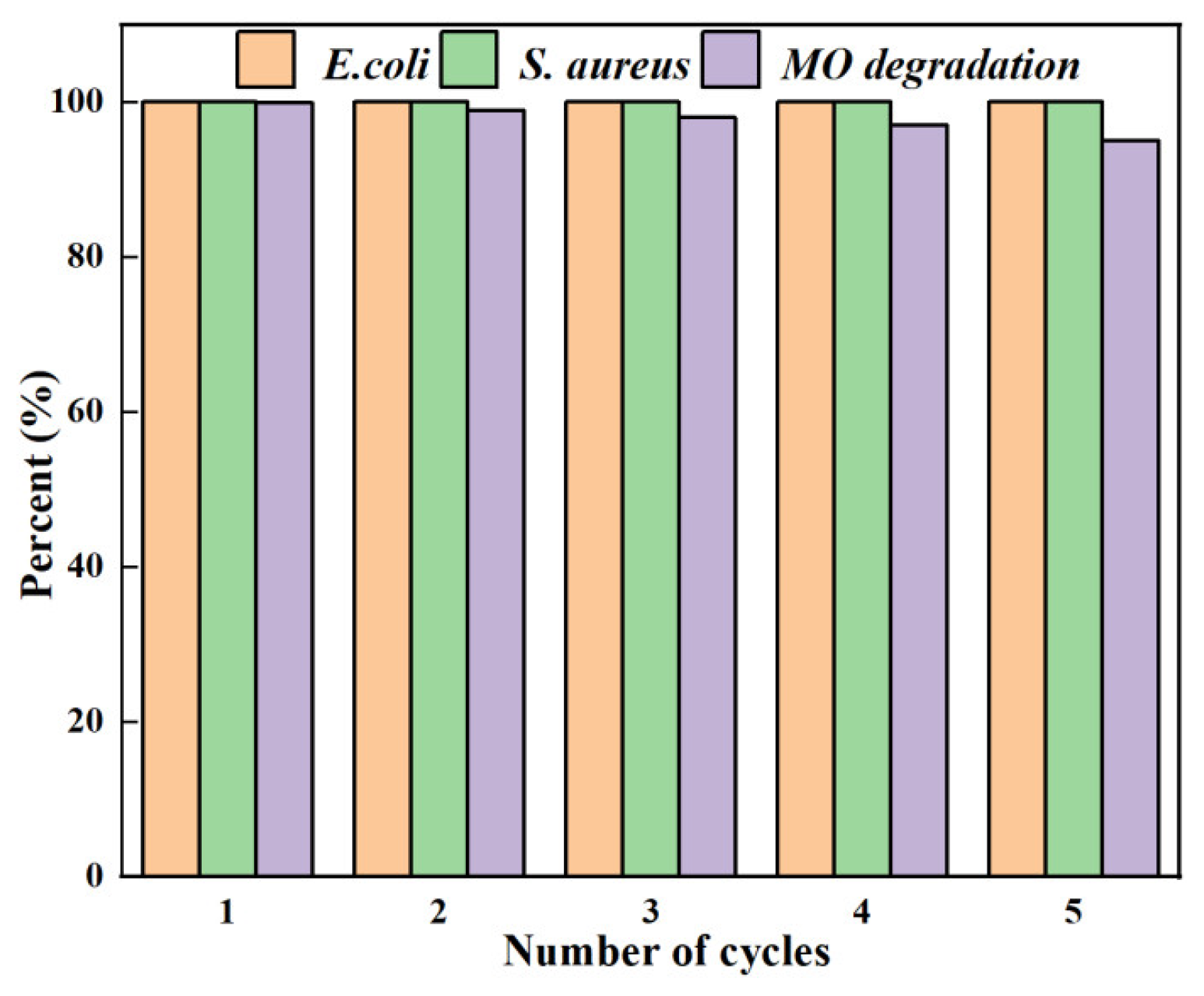
3.8. Reusability of AgNPs/MCFA
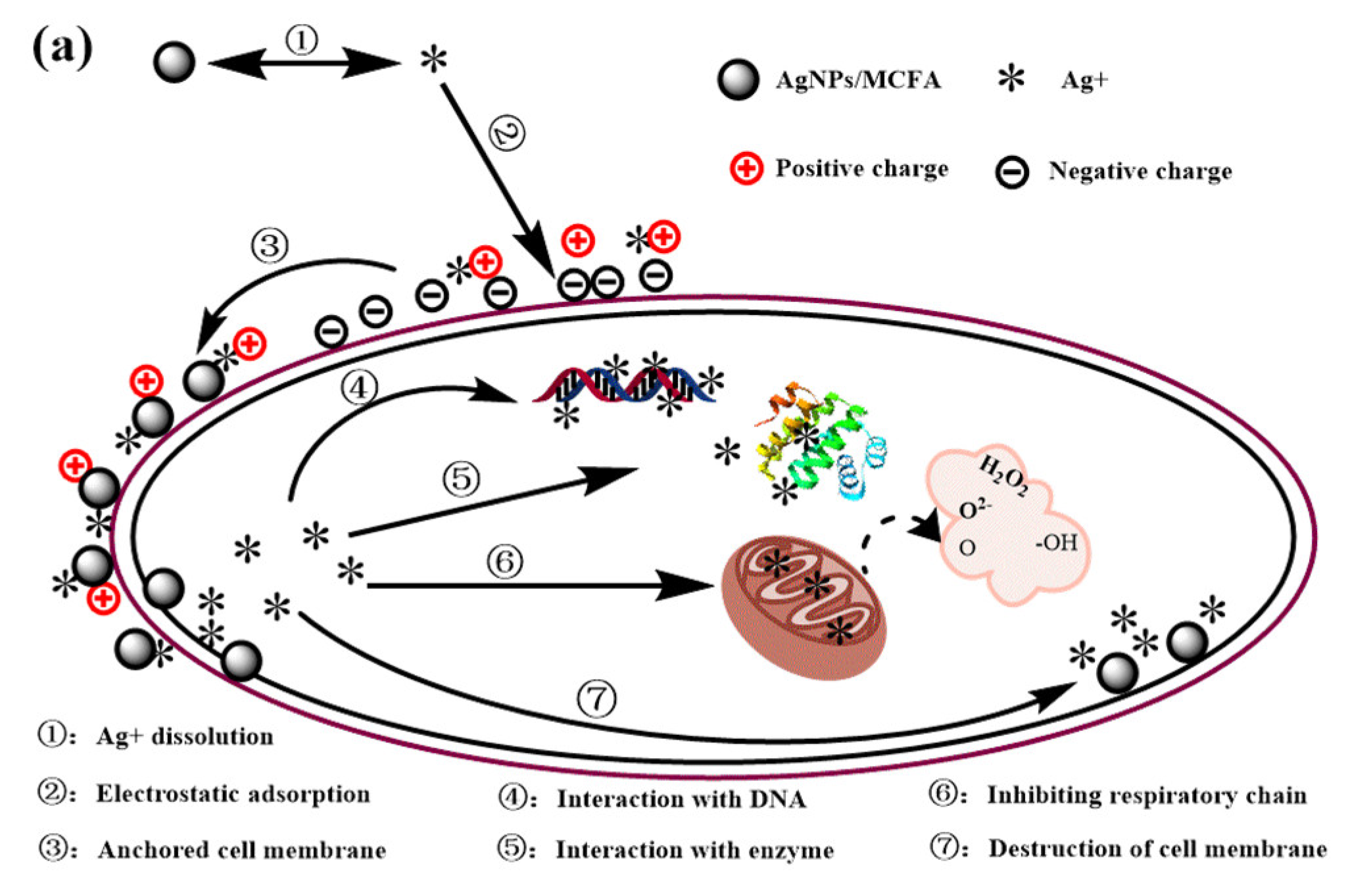
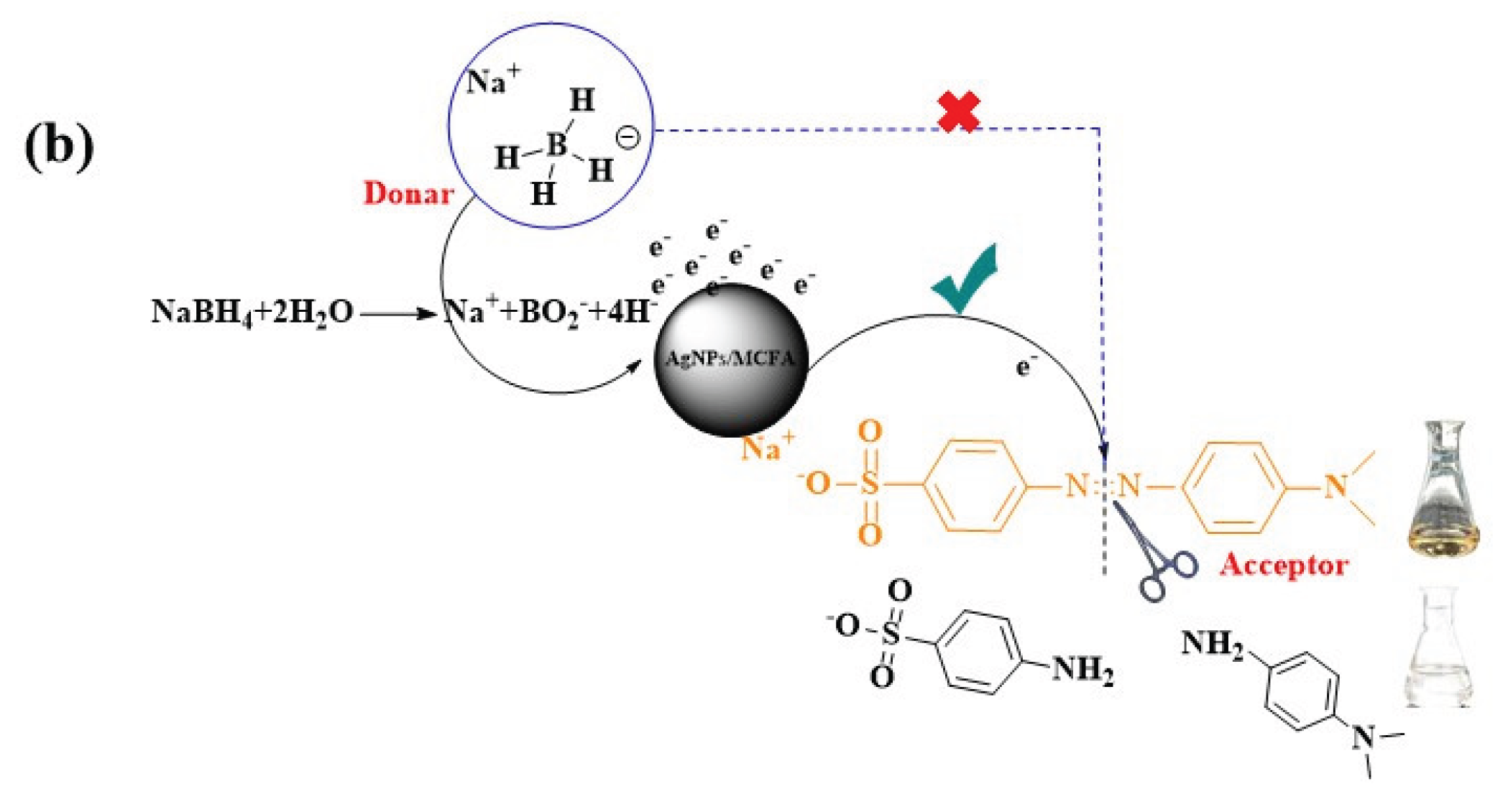
3.9. Proposed Antibacterial and Degradation Mechanism of AgNPs/MCFA
3. Materials and Methods
3.1. Chemicals and Bacteria
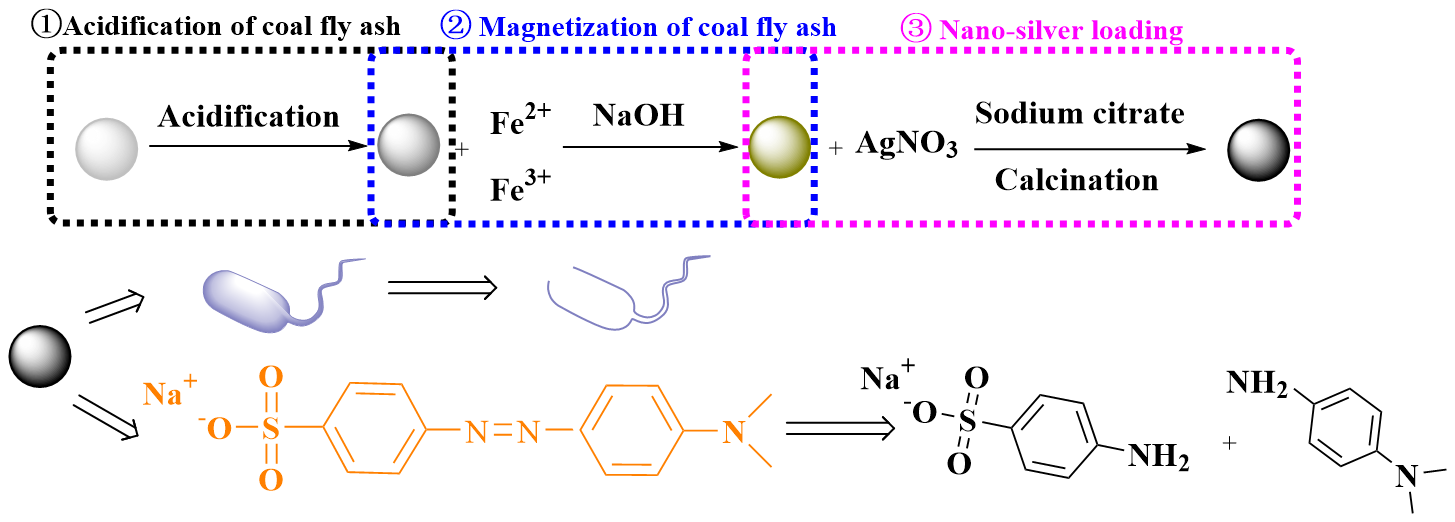
3.2. Preparation of Composite Materials
3.2.1. Magnetization of CFA
3.2.2. Preparation of AgNPs/MCFA
3.3. Characterization of Materials
3.4. Determination of the Antibacterial Activity of AgNPs/MCFA
3.4.1. Size of the Antibacterial Zone
3.4.2. Minimum Inhibitory Concentration (MIC)
3.4.3. Nucleic Acid, Protein Leakage and Electrical Conductivity
3.4.4. Antimicrobial Kinetics
3.5. The Catalytic Efficiency of AgNPs/MCFA
3.6. Reusability of AgNPs/MCFA
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCFA | magnetized coal fly ash |
| AgNPs | silver nanoparticles |
| MO | methyl orange |
| CFA | coal fly ash |
| MIC | minimum inhibitory concentration |
| ROS | reactive oxygen species |
References
- Ma, H.D.; Yu, L.J.; Yang, L.M.; Yao, Y.J.; Shen, G.D.; Wang, Y.Z.; Li, B.; Meng, J.G.; Miao, M.H.; Zhi, C. Graphene oxide composites for dye removal in textile, printing and dyeing wastewaters: a review. Environ. Chem. Lett. 2025, 23, 165–193. [Google Scholar] [CrossRef]
- Song, J.T.; Chen, S.F.; Lyu, R.; Zhang, D.H.; Hong, J.P. Facile synthesis of hyperbranched magnetic nanospheres for highly efficient removal of methyl orange. New J. Chem. 2024, 48, 9945–9953. [Google Scholar] [CrossRef]
- Dai, J.Z.; Wu, Y.H.; Yao, Y.H.; Zhang, B. ZnO/TiO2 photocatalysts for degradation of methyl orange by low-power irradiation. Sci. Progress 2025, 108, 1–25. [Google Scholar] [CrossRef]
- Xue, S.H.; Lin, P.Y.; Pang, Y.X.; Li, Z.X.; Zhou, M.S.; Qiu, X.Q.; Lou, H.M. A composite of AgNPs and lignin porous microspheres via in-situ reduction of Ag+ and its catalytic performance. Int. J. Biol. Macromol. 2024, 273, 132899. [Google Scholar] [CrossRef]
- Fatima, I.; Ajmal, M.; Naseem, A.; Ali, A.; Javed, F.; Hashmi, M.A.; Mahmood, K.; Ahmad, M.; Ullah, F.; Ahmad, Z. Fabrication of efficient and easily recyclable silver nanoparticles-anionic polymer hydrogel composite catalyst for rapid degradation of water pollutants. J. Appl. Polym. Sci. 2025, 142, e56841. [Google Scholar] [CrossRef]
- Ghasemi, M.; Govahi, M.; Litkohi, H.R. Green synthesis of silver nanoparticles (AgNPs) and chitosan-coated silver nanoparticles (CS-AgNPs) using Ferula gummosa Boiss gum extract: A green nano drug for potential applications in medicine. Int. J. Biol. Macromol. 2025, 291, 138619. [Google Scholar] [CrossRef]
- Lu, F.F.; Liu, Y.X.; Dai, Y.X.; Zhang, G.X.; Tong, Y.N. Preparation of nanosilver/polymer composites and evaluation of their antimicrobial and antitumor effect. RSC Adv. 2025, 15, 6357–6369. [Google Scholar] [CrossRef]
- Singh, J.; Perumal, V.; Singh, U.; Tripathi, D.K.; Sharma, S. Green synthesis of silver nanoparticles from bark extract of Terminalia arjuna and their application as next generation antibacterial agents. Curr. Nanosci. 2022, 18, 743–757. [Google Scholar] [CrossRef]
- Korkmaz, N.; Ceylan, Y.; İmamoğlu, R.; Kısa, D.; Şen, F.; Karadağ, A. Eco-friendly biogenic silver nanoparticles: synthesis, characterization and biological applications. Int. J. Environ. Sci. Te. 2024, 22, 3707–3716. [Google Scholar] [CrossRef]
- Eslam, I.E.; Seleem, E.G.; Moustafa, M.Z.; Abdelaleem, H.A. Characterization of biosynthesized silver nanoparticles by Haplophyllum tuberculatum plant extract under microwave irradiation and detecting their antibacterial activity against some wastewater microbes. Desalin Water Treat. 2020, 195, 275–285. [Google Scholar] [CrossRef]
- Zheng, L.Q.; Yu, X.D.; Xu, J.J.; Chen, H.Y. Reversible catalysis for the reaction between methyl orange and NaBH4 by silver nanoparticles. Chem. Commun., 2015, 51, 1050–1053. [Google Scholar] [CrossRef]
- Kebir-Medjhouda, A.Z.; Abdelkrim, S.; Zahraoui, M.; Mokhtar, A.; Maloufi, M.; Belkadi, A.; Djelad, A.; Belarbi, H.; Boukoussa, B.; Hasnaoui, M.A.; Sassi, M. Preparation and characterization of silver nanoparticles-magadiite materials. Application to reduction of toxic organic dyes. Silicon 2023, 15, 3767–3781. [Google Scholar] [CrossRef]
- Gil-Korilis, A.; Cojocaru, M.; Berzosa, M.; Gamazo, C.; Andrade, N.J.; Ciuffi, K.J. Comparison of antibacterial activity and cytotoxicity of silver nanoparticles and silver-loaded montmorillonite and saponite. Appl. Clay Sci. 2023, 240, 106968. [Google Scholar] [CrossRef]
- Altintig, E.; Sarici, B.; Karatas, S. Prepared activated carbon from hazelnut shell where coated nanocomposite with Ag+ used for antibacterial and adsorption properties. Environ. Sci. Pollut. R. 2023, 30, 13671–13687. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, K.; Thankamuniyandi, P.; Kamalesu, S.; Lokhandwala, S.; Lokhandwala, S.; Sakthinathan, S.; Chiu, T.; Karuppiah, C. Green synthesis, characterization and efficient photocatalytic study of hydrothermal-assisted Ag@TiO2 nanocomposites. Inorg. Chem. Commun. 2023, 148, 110362. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible applications of coal fly ash in wastewater treatment. J. Environ. Manage. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.J.; Huang, Y.; Liao, X.L.; Xu, W.F.; Zhang, T. A comprehensive review of toxicity of coal fly ash and its leachate in the ecosystem. Ecotox. Environ. Safe., 2024, 269, 115905. [Google Scholar] [CrossRef]
- Wang, S.Z.; Baxter, L. Comprehensive study of biomass fly ash in concrete: Strength, microscopy, kinetics and durability. Fuel Process. Technol. 2007, 88, 1165–1170. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Application of fly ash as an adsorbent for removal of air and water pollutants. Appl. Sci-Basel 2018, 8, 1116. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhao, L.; Zhou, T.R.; Ma, S.H.; Wang, X.H. Catalytic oxidation activity of NO over mullite-supported amorphous manganese oxide catalyst. Materials 2023, 16, 3821. [Google Scholar] [CrossRef]
- Malpani, S.K.; Goyal, D.; Katara, S.; Rani, A. Green, efficient and economical coal fly ash based phosphomolybdic acid catalysts: preparation, characterization and application. Chem. Pap. 2021, 75, 3017–3034. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, J.Y.; Yoon, S.K.; Choi, N.J. Fabrication of low-cost and high-performance coal fly ash nanofibrous membranes via electrospinning for the control of harmful substances. Fuel, 2019, 237, 236–244. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, R.F.; Chang, X.L.; Yan, J.Y.; Gu, Y.J.; Xi, S.; Sun, P.F.; Dong, X.P. Ozone catalysis degradation of sodium acetate via vacancy-driven radical oxidation over Fe-modified fly ash. Water, 2023, 15, 3801. [Google Scholar] [CrossRef]
- Rodwihok, C.; Suwannakeaw, M.; Charoensri, K.; Wongratanaphisan, D.; Woo, S.W.; Kim, H.S. Alkali/zinc-activated fly ash nanocomposites for dye removal and antibacterial applications. Bioresource Technol., 2021, 331, 125060. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, Z.H.; Fan, J.M.; Gu, Z.Y.; Zhang, B.; Yin, S. Ag-TON nanospheres coupled with fly ash cenospheres for wastewater treatment under visible light irradiation. Water Sci. Technol., 2018, 78, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, E.; Subbulekshmi, N.L. Enhanced heterogeneous wet hydrogen peroxide catalytic oxidation performance of fly ash-derived zeolite by CuO incorporation. Sci. Iran., 2017, 24, 1189–1202. [Google Scholar] [CrossRef]
- Mahmoud, R.; Kotb, N.M.; Gadelhak, Y.; El-Ela, F.I.A.; Shehata, A.Z.; Othman, S.I.; Allam, A.A.; Rudayni, H.A.; Zaher, A. Investigation of ternary Zn-Co-Fe layered double hydroxide as a multifunctional 2D layered adsorbent for moxifloxacin and antifungal disinfection. Sci. Rep., 2024, 14, 806. [Google Scholar] [CrossRef]
- Mohsen, M.; Towan, K.; Marjan, T.; Mohammad, T.Y.; Parnian, T.; Aseman, L. Facile green synthesis of silver nanoparticles using Crocus Haussknechtii Bois bulb extract: Catalytic activity and antibacterial properties. Colloid Interfac. Sci., 2019, 33, 100211. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, M.K.; Liu, Y.; Yao, Y.Z.; Mai, Z.H.; Zheng, H.X.; Song, B.; Fan, B.B.; Wang, H.L.; Lu, H.X. Synthesis of CS/Fe3O4/TiO2@MXene nanocomposite photocatalyst with excellent degradation and bacteriostatic properties by one-step hydrothermal method. Ceram. Int., 2024, 50, 46334–46346. [Google Scholar] [CrossRef]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int., 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Chika, I.; Hritaal, S.; William, G.; Dominique, D.; Md, A.K.B.; Md., S.P.; Zmg, S.J.; Mohammed, M.R.; Faisal, I.C.; Jamal, U. Synthesis and characterization of silver nanoparticles and their promising antimicrobial effects. Chem. Phys. Impact, 2024, 9, 100758. [Google Scholar] [CrossRef]
- Linker, R.; Kenny, A.; Shaviv, A.; Singher, L.; Shmulevch, I. Fourier Transform Infrared-attenuated total reflection nitrate determination of soil pastes using principal component regression, partial least squares, and cross-correlation. Appl. Spectrosc., 2004, 58, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Uda, M.N.A.; Gopinath, S.C.B.; Hashim, U.; Halim, N.H.; Parmin, N.A.; Uda, M.N.A.; Anbu, P. Production and characterization of silica nanoparticles from fly ash: conversion of agro-waste into resource. Prep. Biochem. Biotech., 2021, 51, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, J.J.; Chang, L.P.; Bao, W.R. Ag modification of SBA-15 and MCM-41 mesoporous materials as sorbents of thiophene. Fuel, 2022, 311, 122537. [Google Scholar] [CrossRef]
- Gao, J.W.; Huang, Y.J.; Wang, S.; Zhu, Z.C.; Song, H.K.; Zhang, Y.Y.; Liu, J.; Qi, S.J.; Zhao, J.Q. Mineral transformation and solidification of heavy metals during co-melting of MSWI fly ash with coal fly ash. Environ. Sci. Pollut. R., 2024, 31, 45793–45807. [Google Scholar] [CrossRef]
- Waldo-Mendoza, M.A.; Rivera-Garcia, N.A.; Robles-Martinez, M.; Mayorga-Colunga, P.C.; Martine-Montejano, R.C.; Perez, E. Effect of the interlayer distribution of ZnO decorated with Ag nanoparticles on the antimicrobial activity of multilayer poly(methyl methacrylate) films. J. Vinyl Addit. Techn., 2024, 30, 1621–1634. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Kontari, E.; Kalantzi, S.; Mamma, D. Green synthesis of silver nanoparticles using the cell-free supernatant of Haematococcus pluvialis culture. Materials 2024, 17, 187. [Google Scholar] [CrossRef]
- Varghese, R.; Almalki, M.A.; Ilavenil, S.; Rebecca, J.; Choi, K.C. Silver nanopaticles synthesized using the seed extract of Trigonella foenum-graecum L. and their antimicrobial mechanism and anticancer properties. Saudi J. Biol. Sci., 2019, 26, 148–154. [Google Scholar] [CrossRef]
- Sulistyani, N.; Nurkhasanah, *!!! REPLACE !!!*; Angelita, L.; Rais, I.R.; Zakaria, Z.A. Role of flavonoid-rich fraction from Persea americana (Mill.) in bacterial leakage of Staphylococcus aureus. Pak. J. Pharm. Sci., 2022, 35, 1805–1811. [Google Scholar] [CrossRef]
- Seku, K.; Hussaini, S.S.; Hussain, M.; Siddiqui, M.A.; Golla, N.; Ravinder, D.; Reddy, G.B. Synthesis of Frankincense gum stabilized AgNPs by microwave irradiation and their catalytic, antioxidant, and antibacterial properties. Physica E: Low-Dimensional Systems & Nanostructures, 2022, 140, 115169. [Google Scholar] [CrossRef]
- Behrens, M.A.; Franzén, A.; Carlert, S.; Skantze, U.; Lindfors, L.; Olsson, U. On the Ostwald ripening of crystalline and amorphous nanoparticles. Soft Matter, 2025, 21, 2349–2354. [Google Scholar] [CrossRef]
- Malik, A.; Nath, M. Synthesis of Ag/ZIF-7 by immobilization of Ag nanoparticles onto ZIF-7 microcrystals: A heterogeneous catalyst for the reduction of nitroaromatic compounds and organic dyes. J. Environ. Chem. Eng., 2020, 8, 104547. [Google Scholar] [CrossRef]
| No. | Concentration (mg·mL-1) | Tested bacteria | |
|---|---|---|---|
| E. coli | S. aureus | ||
| 1 | 64 | – | – |
| 2 | 32 | – | – |
| 3 | 16 | – | – |
| 4 | 8 | – | – |
| 5 | 4 | – | – |
| 6 | 2 | – | – |
| 7 | 1 | – | + |
| 8 | 0.5 | – | + |
| 9 | 0.25 | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




