Submitted:
04 July 2025
Posted:
04 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Study Area and Data Sources
Length-Frequency Data
Estimation of Growth Parameters and Natural Mortality
Length-Converted Catch Curve (LCCC)
CMSY++
3. Results
3.1. Length-Based Assessment
3.2. Catch-Based Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Al Abdessalaam, T.Z. Length-frequency data for five commercially important species from Abu Dhabi (June 2000–June 2001). In Beech, M., Ed. The Fish Fauna of Abu Dhabi Emirate; Environmental Agency–Abu Dhabi: Abu Dhabi, United Arab Emirates, 2002; pp. 166–167. [Google Scholar]
- Al Hameli, S.; Al Dhaheri, H.; Al Zaabi, S.; Al Mazrouei, A.; Al Shehhi, M.; Al Suwaidi, K. Assessing threats to marine biodiversity in Abu Dhabi. Endanger. Species Res. 2024, 53, 89–95. [Google Scholar] [CrossRef]
- Al Ansi, M.A.; Abdel Moati, M.A.R.; Al Ansari, I.S. Causes of fish mortality along the Qatari waters (Arabian Gulf). Int. J. Environ. Stud. 2002, 59(1), 59–71. [Google Scholar] [CrossRef]
- AlMusallami, M.; Dimech, M.; Francis, F.; Hamza, W.; Henderson, A.C.; Muzaffar, S.B.; Scarcella, G.; Alhashmi, A.E.A.; Al Marzooqi, M.H.A.; Pinello, D. Updated life history traits of narrow-barred Spanish mackerel, Scomberomorus commerson (Lacépède, 1800) of UAE waters in the southeastern Arabian Peninsula. Aquaculture, Fish and Fisheries. 2025, 10, 59. [Google Scholar] [CrossRef]
- AlMusallami, M.; Dimech, M.; Francis, F.; Hamza, W.; Henderson, A.C.; Muzaffar, S.B.; Scarcella, G.; Demirel, N.; Pinello, D. The stock status of narrow-barred Spanish mackerel, Scomberomorus commerson (Lacépède, 1800) by length-based assessment in the southern Arabian Gulf. Front. Mar. Sci. 2025, 11, 1492238. [Google Scholar] [CrossRef]
- Andrade, H.A.; Mesnildrey, L.; Fernandes, C.C.M.; Moura, D. Data-limited stock assessment methods in tropical multispecies fisheries: Challenges and opportunities. Front. Mar. Sci. 2021, 8, 661002. [Google Scholar] [CrossRef]
- Andriamalala, G.; Gardner, C.J. Laying the foundations for an effective co-management system: The role of community social capital; Blue Ventures Conservation Report: Antananarivo, Madagascar, 2010. [Google Scholar]
- Bahri, T.; Vasconcellos, M.; Welch, D.J.; Johnson, J.; Perry, R.I.; Ma, X.; Sharma, R. (Eds.) Adaptive Management of Fisheries in Response to Climate Change; FAO Fisheries and Aquaculture Technical Paper No. 667; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Bailey, M.; Mulyasari, F.; Viswanathan, K.K. Law, practice, and fisheries performance: Exploring relationships in the Indonesian context. Mar. Policy 2022, 143, 105111. [Google Scholar] [CrossRef]
- Barange, M.; Bahri, T.; Beveridge, M.C.M.; Cochrane, K.L.; Funge-Smith, S.; Poulain, F. (Eds.) Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options; FAO Fisheries and Aquaculture Technical Paper No. 627; FAO: Rome, Italy, 2018. [Google Scholar]
- Bento, R.; Burt, J.A.; González, J.; Feary, D.A.; Priest, M.A.; Bauman, A.G. Oyster beds in the United Arab Emirates: Important fishing grounds in need of protection. Mar. Pollut. Bull. 2022, 182, 113992. [Google Scholar] [CrossRef]
- Blegvad, H. 1944. Fishes of the Iranian Gulf. Einar Munksgaard: Copenhagen.Burt, J.A. The environmental costs of coastal urbanization in the Arabian Gulf. City 2014, 18, 760–770. [Google Scholar] [CrossRef]
- Burt, J.A.; Paparella, F. The marine environment of the Emirates. In A Natural History of the Emirates; Khan, M., Mallon, D.P., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 95–117. [Google Scholar] [CrossRef]
- Cochrane, K.L. A Fishery Manager’s Guidebook: Management Measures and Their Application; FAO: Rome, Italy, 2002. [Google Scholar]
- Dankel, D.J.; Skagen, D.W.; Ulltang, Ø. Fisheries management in practice: A review of 13 commercially important fish stocks. Reviews in Fish Biology and Fisheries 2008, 18(2), 201–233. [Google Scholar] [CrossRef]
- EAD. Abu Dhabi Fisheries & Aquaculture Bulletin: Fisheries and Aquaculture Production in the Emirate of Abu Dhabi – 2019; Environment Agency – Abu Dhabi: Abu Dhabi, UAE, 2020. [Google Scholar]
- EAD. Abu Dhabi Fisheries & Aquaculture Bulletin: Fisheries and Aquaculture Production in the Emirate of Abu Dhabi – 2022; Environment Agency – Abu Dhabi: Abu Dhabi, UAE, 2022. [Google Scholar]
- EAD. The State of Abu Dhabi Fisheries and Aquaculture 2023 – Balancing Science, Conservation, and Impactful Decisions; Environment Agency – Abu Dhabi: Abu Dhabi, UAE, 2023. [Google Scholar]
- EAD. The State of Abu Dhabi Fisheries and Aquaculture 2024 – Balancing Science, Conservation, and Impactful Decisions; Environment Agency – Abu Dhabi: Abu Dhabi, UAE, 2024. [Google Scholar]
- Food and Agriculture Organization (FAO). The ecosystem approach to fisheries; FAO Fisheries Technical Paper No. 4; FAO: Rome, Italy, 2003. [Google Scholar]
- Fathelrahman, E.; Siddig, K.; Al Qaydi, S.; Muhammad, S.; Tasbih Ullah, R.U. Options for maintaining fishery production in the United Arab Emirates due to climate change adaptation strategies. Emirates Journal of Food and Agriculture 2018, 30, 17–28. [Google Scholar] [CrossRef]
- Ferreira, C.; Amorim, P.; Afonso, P.; Menezes, G.M. Assessing data-poor stocks with length-based indicators: The Azores case. Fisheries Research 2021, 239, 105915. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Palomares, M.L.; Tsikliras, A.C.; Dimarchopoulou, D.; Touloumis, K.; Demirel, N.; Vianna, G.; Scarcella, G. New developments in the analysis of catch time series as the basis for fish stock assessments: The CMSY++ method. Acta Ichthyologica et Piscatoria 2023, 53, 173–189. [Google Scholar] [CrossRef]
- Froese, R.; Demirel, N.; Coro, G.; Winker, H. User Guide for CMSY++; GEOMAR: Kiel, Germany, 2021; p. 17. [Google Scholar]
- Froese, R.; Demirel, N.; Coro, G.; Kleisner, K.M.; Winker, H. Estimating stock status from relative abundance and resilience. ICES Journal of Marine Science 2019, 76, 944–956. [Google Scholar] [CrossRef]
- Froese, R.; Demirel, N.; Coro, G.; Zeller, D.; Kleisner, K.; Winker, H. Estimating fisheries reference points from catch and resilience. Fish and Fisheries 2017, 18, 506–526. [Google Scholar] [CrossRef]
- Garcia, S.M.; Ye, Y.; Rice, J.; Charles, A. (Eds.) Rebuilding of Marine Fisheries. Part 1: Global Review; FAO Fisheries and Aquaculture Technical Paper No. 630/1; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar] [CrossRef]
- Gilman, E.; Koehn, J.Z.; Dunn, D.C.; Essington, T. Advancing ecosystem-based fisheries management with data-limited stocks. Fish Fish. 2023, 24(2), 233–251. [Google Scholar] [CrossRef]
- Gower, H.; Hadj-Hammou, J.; Burt, J.A. The evolution of coral reef monitoring in eastern Arabia: Trends, gaps, and opportunities for the ROPME Sea Area. Front. Mar. Sci. 2025, 12, 1578377. [Google Scholar] [CrossRef]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Hartmann, S.A.; Francis, F.; Al Shamsi, A.T. Evaluation of the Management Effectiveness of a Stock Rebuilding Strategy for Key Target Species in the Trap Fishery of Abu Dhabi; Environment Agency – Abu Dhabi: Abu Dhabi, UAE, 2009; Technical Report; 31p. [Google Scholar]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Francis, F. Population biology and assessment of Scomberomorus commerson in the southern Arabian Gulf. Fish. Res. 2005, 73(1–2), 65–76. [Google Scholar]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A.T. Biology and stock assessment of the sparid Rhabdosargus sarba in the United Arab Emirates. J. Appl. Ichthyol. 2004, 20(1), 66–71. [Google Scholar]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A.T. Preliminary assessment of the biology and fishery for the narrow-barred Spanish mackerel, Scomberomorus commerson (Lacépède, 1800), in the Southern Arabian Gulf. Fish. Res. 2005, 76, 277–290. [Google Scholar] [CrossRef]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A.T. Population biology and assessment of the orange-spotted grouper, Epinephelus coioides (Hamilton, 1822), in the Southern Arabian Gulf. Fish. Res. 2005, 74, 55–68. [Google Scholar] [CrossRef]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A.T. Population biology and assessment of the white-spotted spinefoot, Siganus canaliculatus (Park, 1797), in the Southern Arabian Gulf. J. Appl. Ichthyol. 2007, 23, 53–59. [Google Scholar] [CrossRef]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Francis, F. Population biology and assessment of representatives of the family Carangidae: Carangoides bajad and Gnathanodon speciosus (Forsskål, 1775), in the Southern Arabian Gulf. Fish. Res. 2004, 69, 331–341. [Google Scholar]
- Greboval, D. Report and Documentation of the International Workshop on Factors Contributing to Unsustainability and Overexploitation in Fisheries; FAO Fisheries Report, No. 672; FAO: Rome, Italy, 2002. [Google Scholar]
- Hoolihan, J.P.; Anandh, P.; van Herwerden, L. Mitochondrial DNA analyses of narrow-barred Spanish mackerel (Scomberomorus commerson) suggest a single genetic stock in the ROPME Sea area (Arabian Gulf, Gulf of Oman, and Arabian Sea). ICES J. Mar. Sci. 2006, 63(6), 1066–1074. [Google Scholar] [CrossRef]
- Jabado, R.W.; Spaet, J.L.Y. Elasmobranch fisheries in the Arabian Seas Region: Characteristics, trade, and management. Fish Fish. 2017, 18, 1096–1118. [Google Scholar] [CrossRef]
- Khatun, M.A.; Islam, M.M.; Viswanathan, K.K. Legal recognition and fisheries performance in small-scale coastal fisheries of Bangladesh. Marine Policy 2022, 141, 105079. [Google Scholar] [CrossRef]
- Lyon, W.; Tilney, R.; Kennedy. Fisheries Resources Assessment Survey of the United Arab Emirates Waters of the Arabian Gulf and Gulf of Oman 2023; Final Report; OFNZ for Environment Agency – Abu Dhabi: Abu Dhabi, UAE, 2024. [Google Scholar]
- Mesnildrey, L.; Okemwa, E.N.; Maina, G.W.; Dutoit, L. Performance and sensitivity of length-based indicators to gear selectivity: A case study of a tropical shrimp fishery. Frontiers in Marine Science 2023, 10, 1143836. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Mangi, S. Gear restrictions create conservation and fisheries trade-offs: A multi-species, length-based modelling study. Fish and Fisheries 2021, 22, 1624–1638. [Google Scholar] [CrossRef]
- OECD. Rebuilding Fisheries: The Way Forward; OECD Publishing: Paris, France, 2012. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. Journal du Conseil International pour l’Exploration de la Mer 1980, 39(2), 175–192. [Google Scholar] [CrossRef]
- Pinello, D.; Gee, J.; Dimech, M. Handbook for Fisheries Socio-Economic Sample Survey: Principles and Practice; FAO Fisheries and Aquaculture Technical Paper No. 613; FAO: Rome, Italy, 2017. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Kim, D.-N.; Zhang, C.-I. Rebuilding overfished stocks in data-limited situations: A Yellow Sea case study. Fisheries Research 2023, 262, 106625. [Google Scholar] [CrossRef]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment. Part 1: Manual, 2nd rev. ed.; FAO Fisheries Technical Paper No. 306/1, Rev. 2; FAO: Rome, Italy, 1998. [Google Scholar]
- Stamatopoulos, C. Sample-Based Fishery Surveys: A Technical Handbook; FAO Fisheries Technical Paper No. 425; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; 132p. [Google Scholar]
- Swan, J.; Greboval, D. Report and Documentation of the International Workshop on the Implementation of International Fisheries Instruments and Factors of Unsustainability and Overexploitation in Fisheries; FAO Fisheries Report, No. 700; FAO: Rome, Italy, 2003. [Google Scholar]
- Swan, J.; Greboval, D. Overcoming Factors of Unsustainability and Overexploitation in Fisheries: Selected Papers on Issues and Approaches; FAO Fisheries Report No. 782; FAO: Rome, Italy, 2005. [Google Scholar]
- Venema, S.C. Fishery resources in the North Arabian Sea and adjacent waters. Deep Sea Research Part A: Oceanographic Research Papers 1984, 31(6–8), 1001–1018. [Google Scholar] [CrossRef]
- Viswanathan, K.K.; Zakaria, Z.; Nurhakim, S.; Bailey, M. Adaptive co-management and legal pluralism in Indonesian small-scale fisheries. Marine Policy 2020, 118, 104001. [Google Scholar] [CrossRef]
- Wabnitz, C.C.C.; Lam, V.W.Y.; Reygondeau, G.; Teh, L.C.L.; Al-Abdulrazzak, D.; Khalfallah, M.; et al. Climate change impacts on marine biodiversity, fisheries and society in the Arabian Gulf. PLoS ONE 2018, 13(5), e0194537. [Google Scholar] [CrossRef] [PubMed]
- Wetherall, J. A. ; Polovina, J. J.; Ralston, S. Estimating growth and mortality in steady-state fish stocks from length-frequency data. ICLARM Conf. Proc. 1987, 13, 53–74. [Google Scholar]
- Zhou, S.; Punt, A.E.; Smith, A.D.M.; Richardson, A.J.; Haddon, M.; Dichmont, C.M.; Fulton, E.A. Identifying spawner biomass per-recruit reference points from life-history parameters. Fish and Fisheries 2020, 21(4), 760–773. [Google Scholar] [CrossRef]
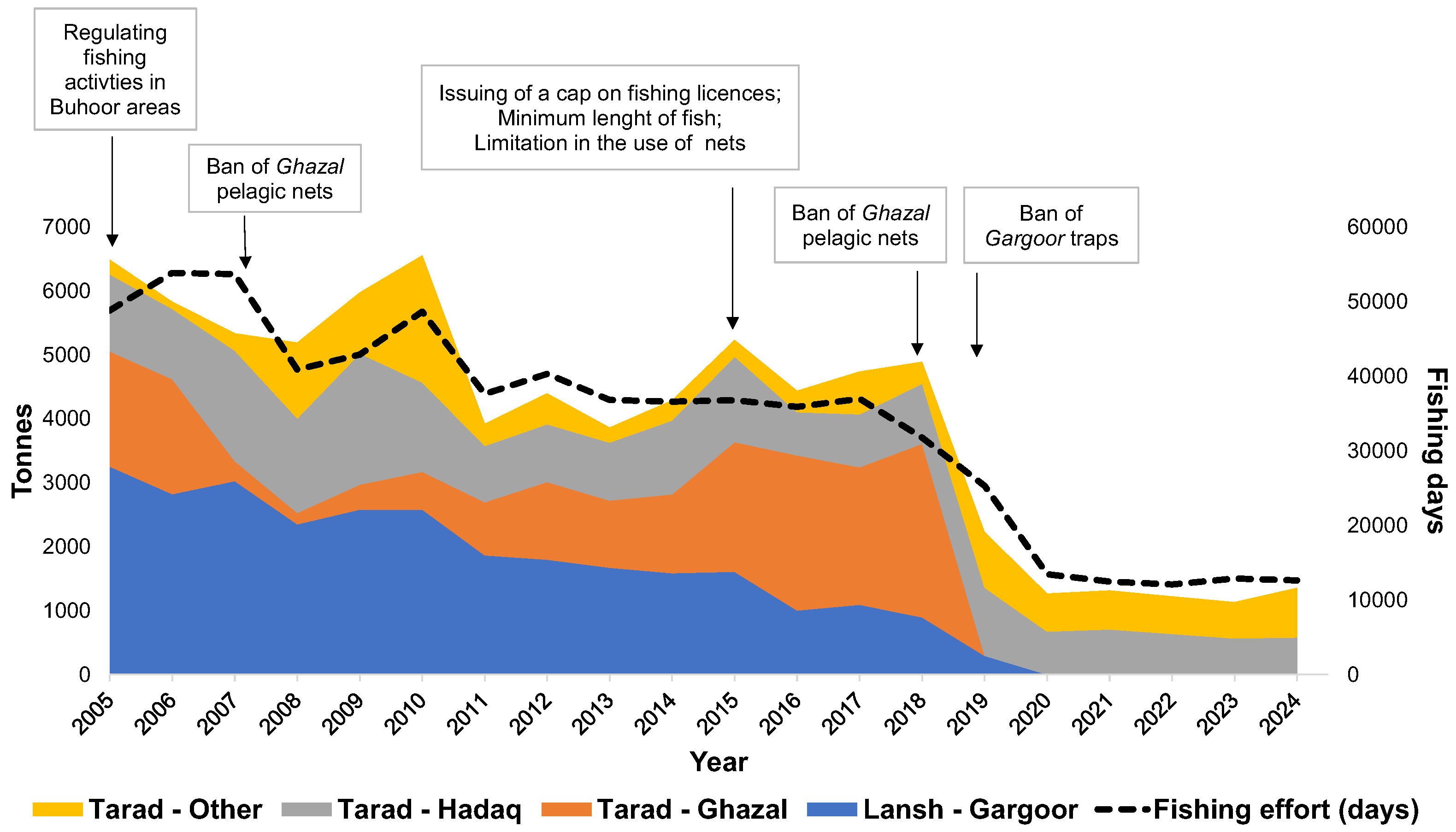
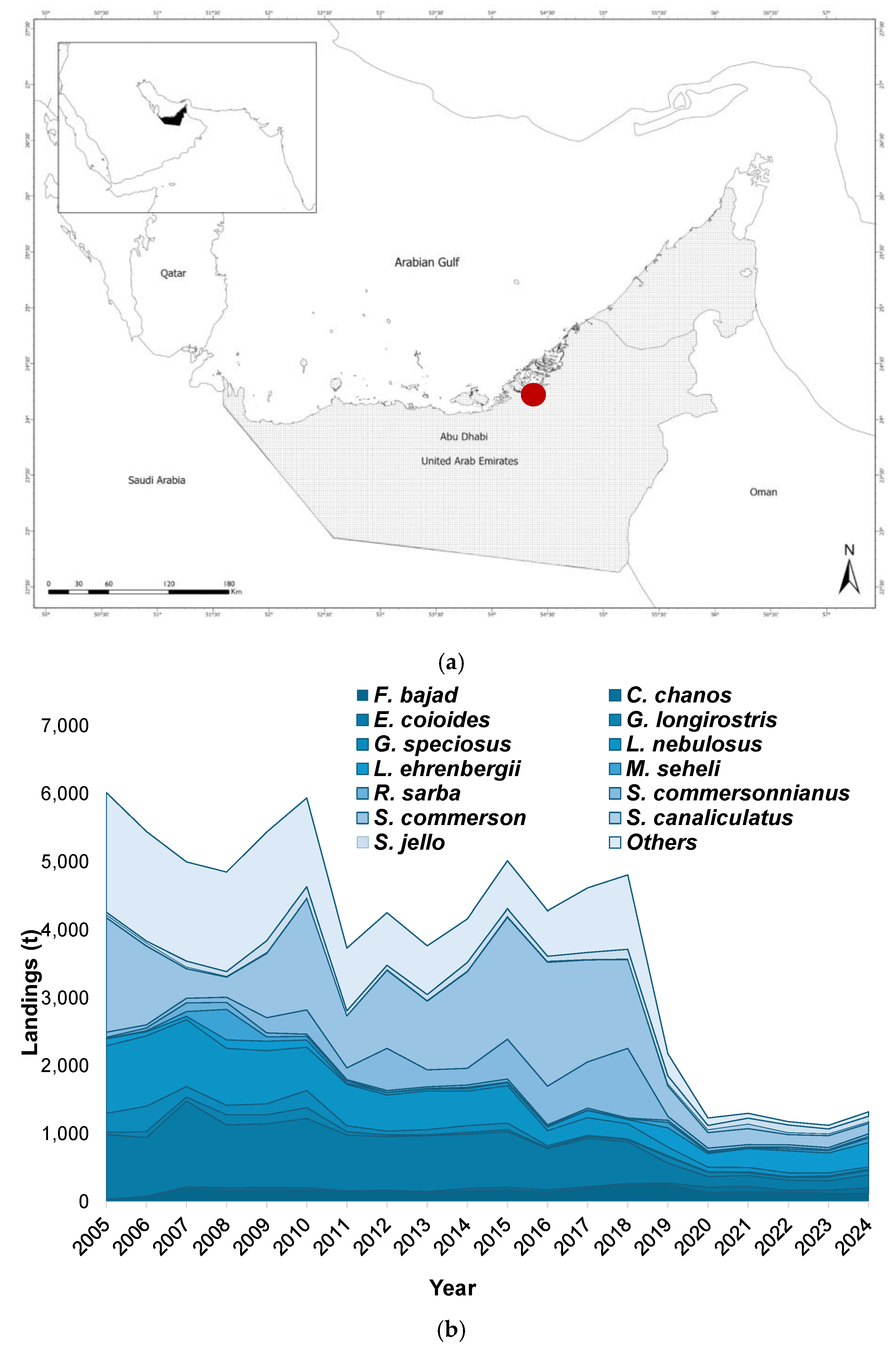
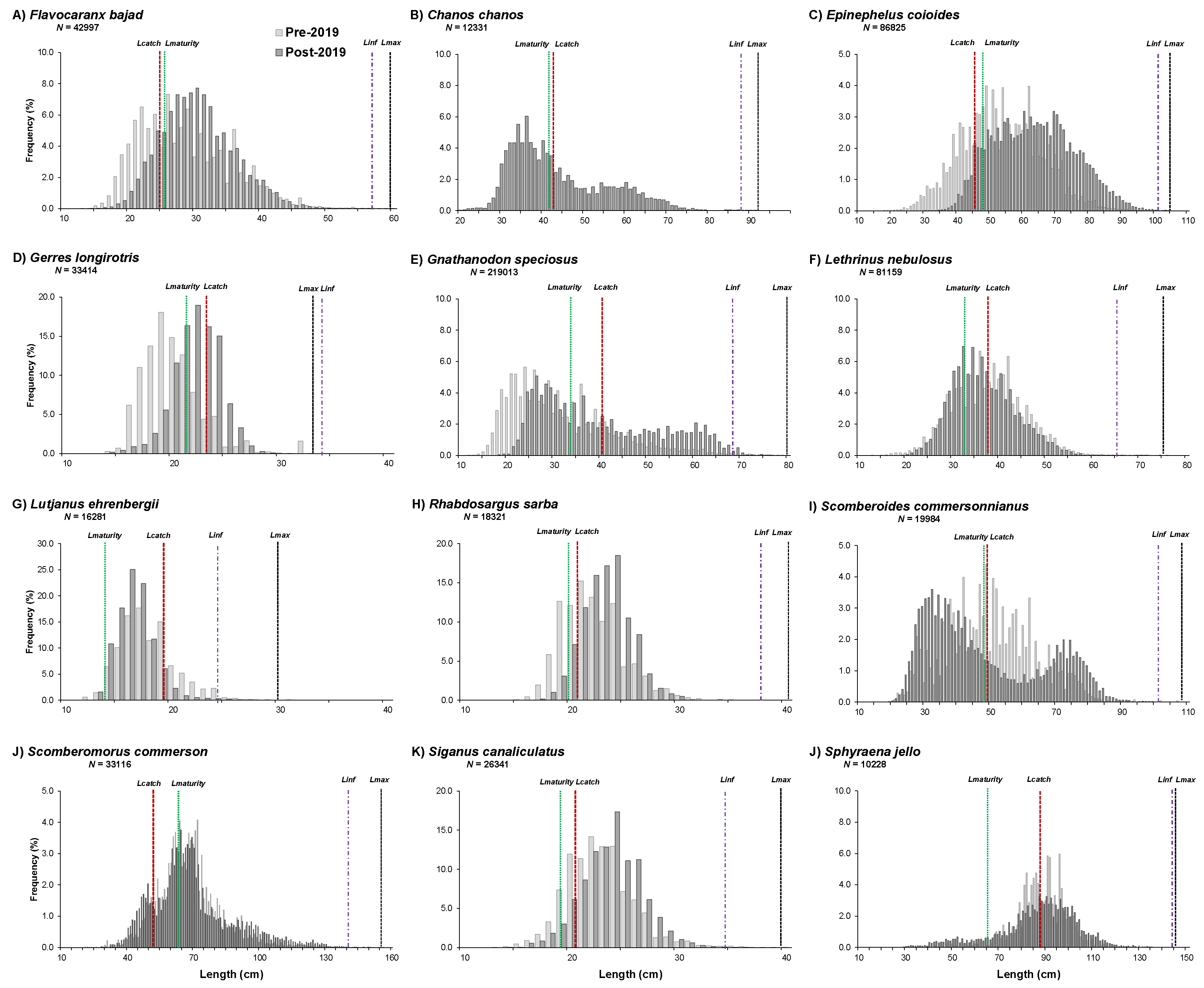
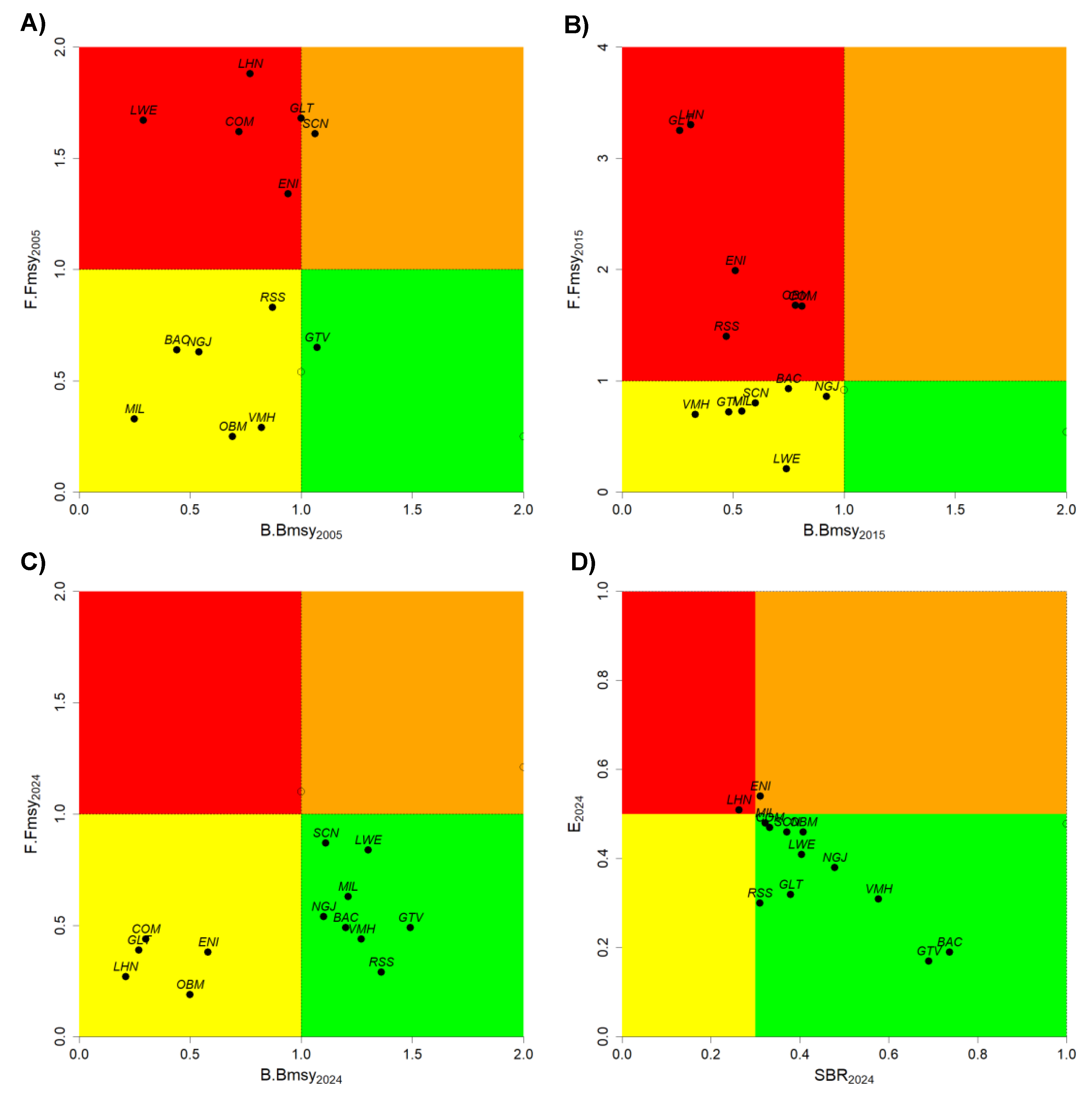
| Scientific Name | English name | FAO Code | Group | CPUE | Length Frequency | FT < 2019 | FT > 2019 | Definition |
|---|---|---|---|---|---|---|---|---|
| Flavocaranx bajad | Orangespotted trevally | NGJ | P | 2005-2024 | 2001-204; 2011-2015; 2020; 2022-2024 | G & H | H & D | Handline |
| Chanos chanos | Milkfish | MIL | P | 2005-2024 | 2021; 2023-2024 | D | Surrounding net | |
| Epinephelus coioides | Orange-spotted grouper | ENI | D | 2018-2024 | 2001-2024 | G & H | H | Handline |
| Gerres longirostris | Strongspine silver-biddy | GTV | D | 2005-2024 | 2003-2005; 2011; 2013-2015; 2021; 2023-2024 | Hd & D | Hd & D | Surrounding net |
| Gnathanodon speciosus | Golden trevally | GLT | P | 2005-2024 | 2001-2003; 2011; 2013-2014; 2023-2024 | G & H | H | Stationary pound net |
| Lethrinus nebulosus | Spangled emperor | LHN | D | 2018-2024 | 2000-2018; 2020; 2022-2024 | G & H | H | Handline |
| Lutjanus ehrenbergii | Blackspot snapper | LWE | D | 2018-2024 | 2008-2009; 2011-2014; 2017-2018; 2021-2024 | Hd | Hd | Stationary pound net |
| Moolgarda seheli | Bluespot mullet | VMH | D | 2005-2024 | 2024 | D | Surrounding net | |
| Rhabdosargus sarba | Goldlined seabream | RSS | D | 2005-2024 | 2008-2009; 2011; 2018; 2021; 2023 | G & D | D | Stationary pound net |
| Scomberoides commersonnianus | Talang queenfish | OBM | P | 2018-2024 | 2011-2013; 2020; 2022-2024 | H & Hl | H | Handline |
| Scomberomorus commerson | Narrow-barred Spanish mackerel | COM | P | 2018-2024 | 2003-2005; 2011; 2013-2014; 2016-2020; 2022-2024 | H & H | H | Handline |
| Siganus canaliculatus | White-spotted spinefoot | SCN | D | 2005-2024 | 2003-2005; 2011-2014; 2018-2019; 2022-2024 | G & D | D | Surrounding net |
| Sphyraena jello | Pickhandle barracuda | BAC | P | 2005-2024 | 2011; 2020; 2023-2024 | H & Hl | H | Handline |
| Species | Mortality | Growth | Selectivity | Stock indicator | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Z | M | F | L∞ | K | to | Lc | tc | SBR% | E | Status | |
| F. bajad | 0.70 | 0.43 | 0.27 | 57.0 | 0.17 | -0.84 | 25.0 | 2.1 | 47.8 | 0.38 | Sustainably exploited |
| C. chanos | 1.21 | 0.63 | 0.58 | 109.2 | .35 | -0.55 | 42.5 | 3.0 | 32.2 | 0.48 | Sustainably exploited |
| E. coioides | 0.42 | 0.19 | 0.23 | 97.9 | 0.14 | -1.50 | 45.2 | 2.6 | 31.1 | 0.54 | Sustainably exploited |
| G. longirostris | 0.51 | 0.42 | 0.09 | 34.1 | 0.30 | -0.89 | 23.2 | 1.5 | 69.0 | 0.17 | Sustainably exploited |
| G. speciosus | 0.89 | 0.60 | 0.29 | 84.5 | 0.28 | -0.45 | 41.4 | 2.0 | 37.9 | 0.32 | Sustainably exploited |
| L. nebulosus | 0.41 | 0.20 | 0.21 | 66.2 | 0.11 | -1.26 | 38.0 | 1.6 | 26.3 | 0.51 | Over-exploited |
| L. ehrenbergii | 0.60 | 0.35 | 0.25 | 24.2 | 0.99 | -0.05 | 19.1 | 1.0 | 40.4 | 0.41 | Sustainably exploited |
| M. seheli | 1.02 | 0.70 | 0.32 | 57.7 | 0.32 | -0.72 | 42.0 | 3.3 | 57.7 | 0.31 | Sustainably exploited |
| R. sarba | 0.70 | 0.30 | 0.30 | 37.8 | 0.60 | -0.30 | 21.7 | 1.1 | 31.0 | 0.46 | Sustainably exploited |
| S. commersonnianus | 0.47 | 0.25 | 0.22 | 142.2 | 0.10 | -1.13 | 46.8 | 2.84 | 40.8 | 0.46 | Sustainably exploited |
| S. commerson | 0.53 | 0.28 | 0.25 | 140.4 | 0.10 | -1.90 | 52.4 | 1.9 | 33.2 | 0.47 | Sustainably exploited |
| S. canaliculatus | 1.00 | 0.54 | 0.46 | 24.8 | 1.00 | -0.10 | 21.0 | 1.7 | 37.1 | 0.46 | Sustainably exploited |
| S. jello | 0.43 | 0.35 | 0.08 | 145.0 | 0.44 | -0.40 | 88.8 | 1.75 | 73.7 | 0.19 | Sustainably exploited |
| Taxon | Max L (t) | L2024 (t) | MSY | L2024/MSY | F2024 | FMSY | F2024/FMSY | B2024 (t) | BMSY (t) | B2024/BMSY |
|---|---|---|---|---|---|---|---|---|---|---|
| F. bajad | 249 | 116 | 203 | 0.57 | 0.10 | 0.19 | 0.54 | 1182 | 1076 | 1.10 |
| C. chanos | 97 | 87 | 86 | 1.00 | 0.10 | 0.17 | 0.63 | 624 | 523 | 1.21 |
| E. coioides | 1264 | 193 | 764 | 0.25 | 0.05 | 0.12 | 0.38 | 3579 | 6189 | 0.58 |
| G. longirostris | 159 | 65 | 79 | 0.82 | 0.16 | 0.32 | 0.49 | 364 | 248 | 1.49 |
| G. speciosus | 373 | 17 | 177 | 0.10 | 0.09 | 0.25 | 0.39 | 192 | 714 | 0.27 |
| L. nebulosus | 1037 | 34 | 693 | 0.05 | 0.03 | 0.11 | 0.27 | 1303 | 6143 | 0.21 |
| L. ehrenbergii | 361 | 362 | 304 | 1.19 | 0.36 | 0.43 | 0.84 | 912 | 706 | 1.30 |
| M. seheli | 449 | 63 | 86 | 0.73 | 0.09 | 0.21 | 0.44 | 522 | 414 | 1.27 |
| R. sarba | 127 | 25 | 54 | 0.47 | 0.07 | 0.23 | 0.29 | 314 | 235 | 1.36 |
| S. commersonnianus | 1023 | 40 | 374 | 0.11 | 0.02 | 0.11 | 0.19 | 1746 | 3448 | 0.50 |
| S. commerson | 1825 | 152 | 1207 | 0.13 | 0.13 | 0.29 | 0.44 | 1269 | 4191 | 0.30 |
| S. canaliculatus | 72 | 28 | 28 | 0.98 | 0.35 | 0.27 | 0.87 | 89 | 105 | 1.11 |
| S. jello | 165 | 78 | 133 | 0.58 | 0.07 | 0.13 | 0.49 | 1197 | 1018 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





