Submitted:
27 June 2025
Posted:
30 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics and Treatment Data
3.2. Treatment Outcomes
3.2.1. Emergency Department Visits and Hospitalizations
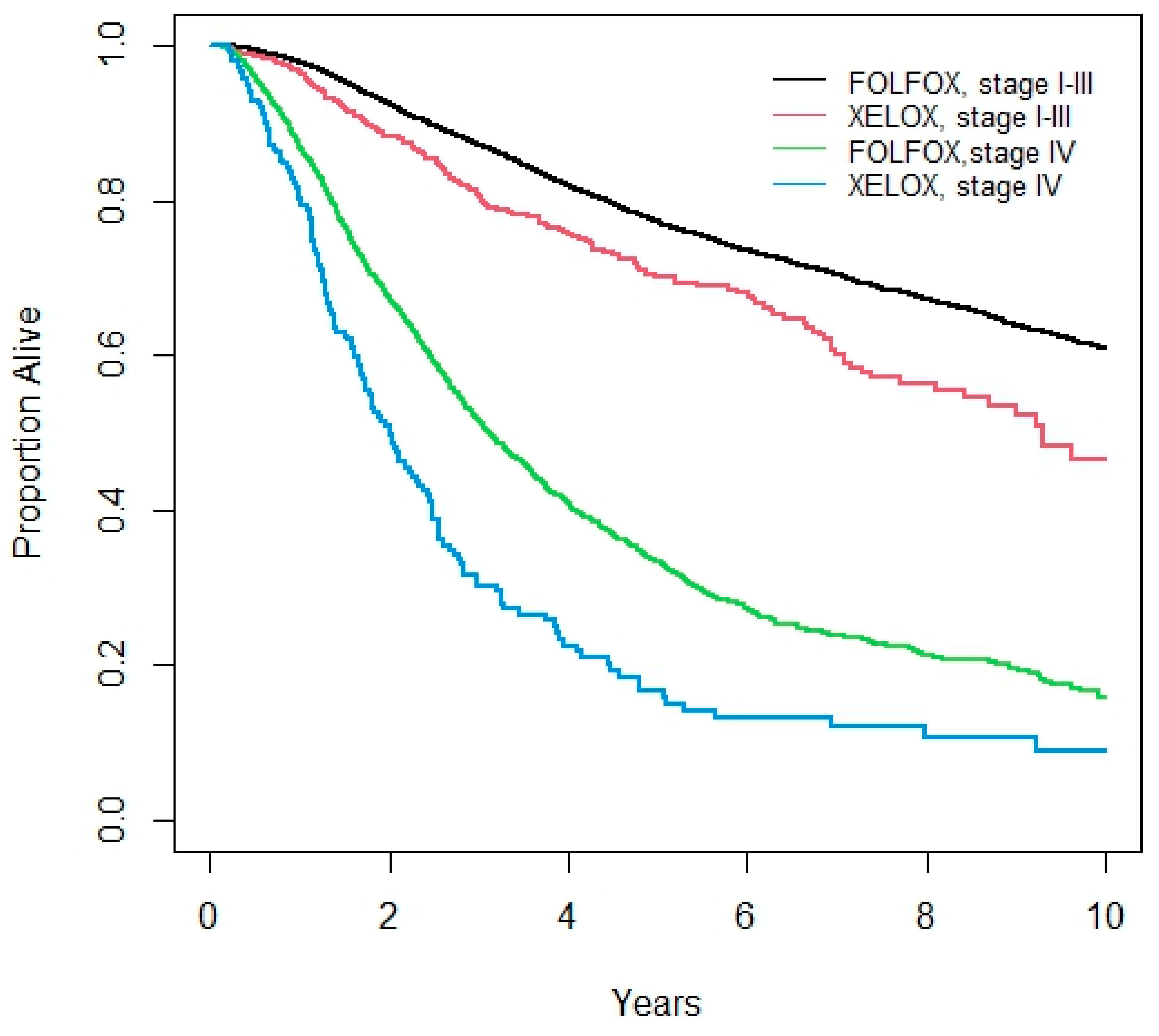
3.3. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix I – Data Definitions
- ●
- Cancer diagnosis: colon or rectal cancer diagnosis by ICD-10 codes C18, C19, C20
- ○
- ICD C18.0-C18.9 (colon), C19.9 (rectosigmoid junction), or C20.9 (rectum)
- ●
- Cancer diagnosis date = date of first OHIP billing date for colon or rectal cancer plus an ‘any time’ positive Ontario Cancer Registry recording
- ●
- Cancer stage: I to IV per the Ontario Cancer Registry
- ●
- First Chemotherapy Date = First date of administration within 365 days of diagnosis.
- ●
- Chemotherapy regimens:
- ○
- FOLFOX = 5-fluorouracil administered within 365 days of diagnosis with oxaliplatin prescribed at any point during the treatment period (since oxaliplatin is only used in combination with a fluoropyrimidine)
- ○
- CAPOX = a prescription for capecitabine is filled with oxaliplatin infused within 7 days.
- ●
- Curative (adjuvant) vs. Palliative vs. Relapse chemotherapy definitions:
- ○
- Curative (adjuvant) chemotherapy = a 5-FU, capecitabine, FOLFOX, or CAPOX regimen begun within 4 months after a curative surgical procedure and having stage II or III colorectal cancer.
- ○
- Relapse chemotherapy = prior receipt of “curative chemotherapy” according to the above definition, followed by another, different, colorectal cancer chemotherapy regimen starting at least 12 months after the surgical date.
- ○
- Palliative chemotherapy = colorectal chemotherapy regimen given for Stage IV disease by registry.
- ○
- Number of cycles = Number of oxaliplatin doses given prior to Day 200 post-operatively (in Stage II-III patients) or over patient’s lifetime (in Stage IV patients).
- ●
- Hospitalization during treatment = hospitalization for any reason from first date of a chemotherapy regimen to 30 days after last dose of a chemotherapy regimen
- ●
- Death - Y/N. Age at death or last follow-up. Last follow-up is otherwise defined as the date of the last OHIP billing or hospital discharge for the individual.
- ○
- Death during chemotherapy = death from first date of a chemotherapy regimen to 30 days after last dose of that same chemotherapy regimen
References
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; De Gramont, A. Improved Overall Survival with Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef]
- André, T.; Meyerhardt, J.; Iveson, T.; Sobrero, A.; Yoshino, T.; Souglakos, I.; Grothey, A.; Niedzwiecki, D.; Saunders, M.; Labianca, R.; Yamanaka, T.; Boukovinas, I.; Vernerey, D.; Meyers, J.; Harkin, A.; Torri, V.; Oki, E.; Georgoulias, V.; Taieb, J.; Shields, A.; Shi, Q. Effect of Duration of Adjuvant Chemotherapy for Patients with Stage III Colon Cancer (IDEA Collaboration): Final Results from a Prospective, Pooled Analysis of Six Randomised, Phase 3 Trials. Lancet Oncol. 2020, 21, 1620–1629. [Google Scholar] [CrossRef]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; Papamichael, D.; Le Bail, N.; Louvet, C.; Hendler, D.; de Braud, F.; Wilson, C.; Morvan, F.; Bonetti, A. Leucovorin and Fluorouracil With or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef]
- Bertoglio, S.; Faccini, B.; Lalli, L.; Cafiero, F.; Bruzzi, P. Peripherally Inserted Central Catheters (PICCs) in Cancer Patients under Chemotherapy: A Prospective Study on the Incidence of Complications and Overall Failures. J. Surg. Oncol. 2016, 113, 708–714. [Google Scholar] [CrossRef]
- Schmoll, H. J.; Tabernero, J.; Maroun, J.; De Braud, F.; Price, T.; Van Cutsem, E.; Hill, M.; Hoersch, S.; Rittweger, K.; Haller, D. G. Capecitabine plus Oxaliplatin Compared with Fluorouracil/Folinic Acid as Adjuvant Therapy for Stage III Colon Cancer: Final Results of the NO16968 Randomized Controlled Phase III Trial. J. Clin. Oncol. 2015, 33, 3733–3740. [Google Scholar] [CrossRef]
- Cassidy, J.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Rittweger, K.; Gilberg, F.; Saltz, L. XELOX vs FOLFOX-4 as First-Line Therapy for Metastatic Colorectal Cancer: NO16966 Updated Results. Br. J. Cancer 2011, 105, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M. L.; Cox, J. V; Butts, C.; Navarro, M.; Bang, Y.-J.; Goel, R.; Gollins, S.; Siu, L. L.; Laguerre, S.; Cunningham, D. Capecitabine plus Oxaliplatin (XELOX) versus 5-Fluorouracil/Folinic Acid plus Oxaliplatin (FOLFOX-4) as Second-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Noninferiority Study. Ann. Oncol. 2008, 19, 1720–1726. [Google Scholar] [CrossRef]
- Baqai, T. I.; Haider, G.; Abro, N. A.; Rani, B.; Kumari, R.; Anzar, J.; Abbas, K. Efficacy and Safety of Xelox in Comparison with Folfox in Metastatic Colorectal Cancer. Pakistan J. Med. Heal. Sci. 2022, 16, 833–835. [Google Scholar] [CrossRef]
- Scheithauer, W.; Cassidy, J.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.; Clarke, S.; Diaz-Rubio, E.; Garrison, L. A Comparison of Medical Resource Use for 4 Chemotherapy Regimens as First-Line Treatment for Metastatic Colorectal Cancer (MCRC): XELOX vs. FOLFOX4 ± Bevacizumab (A). J. Clin. Oncol. 2007, 25. [Google Scholar] [CrossRef]
- Grothey, A.; Sobrero, A. F.; Shields, A. F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; Meyerhardt, J. A.; Vernerey, D.; Yamanaka, T.; Boukovinas, I.; Meyers, J. P.; Renfro, L. A.; Niedzwiecki, D.; Watanabe, T.; Torri, V.; Saunders, M.; Sargent, D. J.; Andre, T.; Iveson, T. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef]
- Yu, I. S.; Pereira, A. A. L.; Lee, M.; Korphaisarn, K.; Marshall, J.; Segelov, E.; O’Callaghan, C.; Lim, H. J.; Kopetz, S.; Loree, J. M. Medical Oncologists’ Perspectives on How the Results of the IDEA Collaboration Impact the Adjuvant Treatment of Stage III Colon Cancer. Oncologist 2020, 25, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Satram-Hoang, S.; Lee, L.; Yu, S.; Guduru, S. R.; Gunuganti, A. R.; Reyes, C.; McKenna, E. Comparative Effectiveness of Chemotherapy in Elderly Patients with Metastatic Colorectal Cancer. J. Gastrointest. Cancer 2013, 44, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sha, A.; Abadi, S.; Gill, S. Utilization of Capecitabine plus Oxaliplatin and 5-Fluorouracil/Folinic Acid plus Oxaliplatin in the Adjuvant Treatment of Stage IIB and Stage III Colon Cancer: A Multi-Centre, Retrospective, Chart Review Study. J. Oncol. Pharm. Pract. 2018, 24, 501–506. [Google Scholar] [CrossRef]
- Loree, J. M.; Sha, A.; Soleimani, M.; Kennecke, H. F.; Ho, M. Y.; Cheung, W. Y.; Mulder, K. E.; Abadi, S.; Spratlin, J. L.; Gill, S. Survival Impact of CAPOX Versus FOLFOX in the Adjuvant Treatment of Stage III Colon Cancer. Clin. Colorectal Cancer 2018, 17, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Loree, J. M.; Mulder, K. E.; Ghosh, S.; Spratlin, J. L. Retrospective Comparison of CAPOX and FOLFOX Dose Intensity, Toxicity, and Clinical Outcomes in the Treatment of Metastatic Colon Cancer. J. Gastrointest. Cancer 2014, 45, 154–160. [Google Scholar] [CrossRef]
- Yoon, R.; Wilkinson, K.; Gabriel, G.; Kadaan, N.; Roberts, T.; Lim, S.; Asghari, R.; Lee, C. S.; Chua, W.; Ng, W. Real-World Tolerance and Outcomes of Oxaliplatin-Based Adjuvant Chemotherapy for Stage III Colon Cancer—Does Dose Intensity Matter? Asia. Pac. J. Clin. Oncol. n/a. [CrossRef]
- Grewal, K.; Calzavara, A.; McLeod, S. L.; Eskander, A.; Savage, D. W.; Thompson, C.; Borgundvaag, B.; Ovens, H.; Cheskes, S.; de Wit, K.; Irish, J.; Krzyzanowska, M. K.; Walsh, R.; Mohindra, R.; Thiruganasambandamoorthy, V.; Sutradhar, R. Emergency Department Use before Cancer Diagnosis in Ontario, Canada: A Population-Based Study. CMAJ 2024, 196, E1252–E1261. [Google Scholar] [CrossRef] [PubMed]
- Moe, J.; Wang, E. Y.; McGregor, M. J.; Schull, M. J.; Dong, K.; Holroyd, B. R.; Hohl, C. M.; Grafstein, E.; O’Sullivan, F.; Trimble, J.; McGrail, K. M. People Who Make Frequent Emergency Department Visits Based on Persistence of Frequent Use in Ontario and Alberta: A Retrospective Cohort Study. C. open 2022, 10, E220–E231. [Google Scholar] [CrossRef]
- Pilleron, S.; Withrow, D. R.; Nicholson, B. D.; Morris, E. J. A. Age-Related Differences in Colon and Rectal Cancer Survival by Stage, Histology, and Tumour Site: An Analysis of United States SEER-18 Data. Cancer Epidemiol. 2023, 84, 102363. [Google Scholar] [CrossRef]
- van Eeghen, E. E.; Bakker, S. D.; van Bochove, A.; Loffeld, R. J. L. F. Impact of Age and Comorbidity on Survival in Colorectal Cancer. J. Gastrointest. Oncol. 2015, 6, 605–612. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.-S.; Pan, S.-X.; Cai, X.-Q.; Pan, Q.-C. Urban vs. Rural: Colorectal Cancer Survival and Prognostic Disparities from 2000 to 2019. Front. public Heal. 2024, 12, 1319977. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.; Boeckx, N.; Van Camp, G.; De Beeck, K. O.; Fransen, E.; Calay, F.; Van Damme, N.; Peeters, M. Comparing Survival in Left-Sided and Right-Sided Colorectal Carcinoma: A Belgian Population-Based Study. Ann. Oncol. 2018, 29, v98. [Google Scholar] [CrossRef]
- Haller, D. G.; Cassidy, J.; Clarke, S. J.; Cunningham, D.; Van Cutsem, E.; Hoff, P. M.; Rothenberg, M. L.; Saltz, L. B.; Schmoll, H.-J.; Allegra, C.; Bertino, J. R.; Douillard, J.-Y.; Gustavsson, B. G.; Milano, G.; O’Connell, M.; Rustum, Y.; Tabernero, J.; Gilberg, F.; Sirzén, F.; Twelves, C. Potential Regional Differences for the Tolerability Profiles of Fluoropyrimidines. J. Clin. Oncol. 2008, 26, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Chan, S. L.; Chan, A. W. H.; Mo, F.; Ma, B. B. Y.; Wong, K. C. W.; Lam, D.; Mok, F. S. T.; Chan, A. T. C.; Mok, T.; Chan, K. C. A. Association Between Serum Folate Level and Toxicity of Capecitabine During Treatment for Colorectal Cancer. Oncologist 2018, 23, 1436–1445. [Google Scholar] [CrossRef]
- Sharma, R.; Rivory, L.; Beale, P.; Ong, S.; Horvath, L.; Clarke, S. J. A Phase II Study of Fixed-Dose Capecitabine and Assessment of Predictors of Toxicity in Patients with Advanced/Metastatic Colorectal Cancer. Br. J. Cancer 2006, 94, 964–968. [Google Scholar] [CrossRef]
- Vincent, M. D.; Breadner, D.; Cripps, M. C.; Jonker, D. J.; Klimo, P.; Biagi, J. J.; Lam, W.; O’Connell, A.; Whiston, F.; Stitt, L.; Welch, S. A. Phase I/II Trial of Dose-Reduced Capecitabine in Elderly Patients with Advanced Colorectal Cancer. Curr. Oncol. 2017, 24, 261–268. [Google Scholar] [CrossRef]
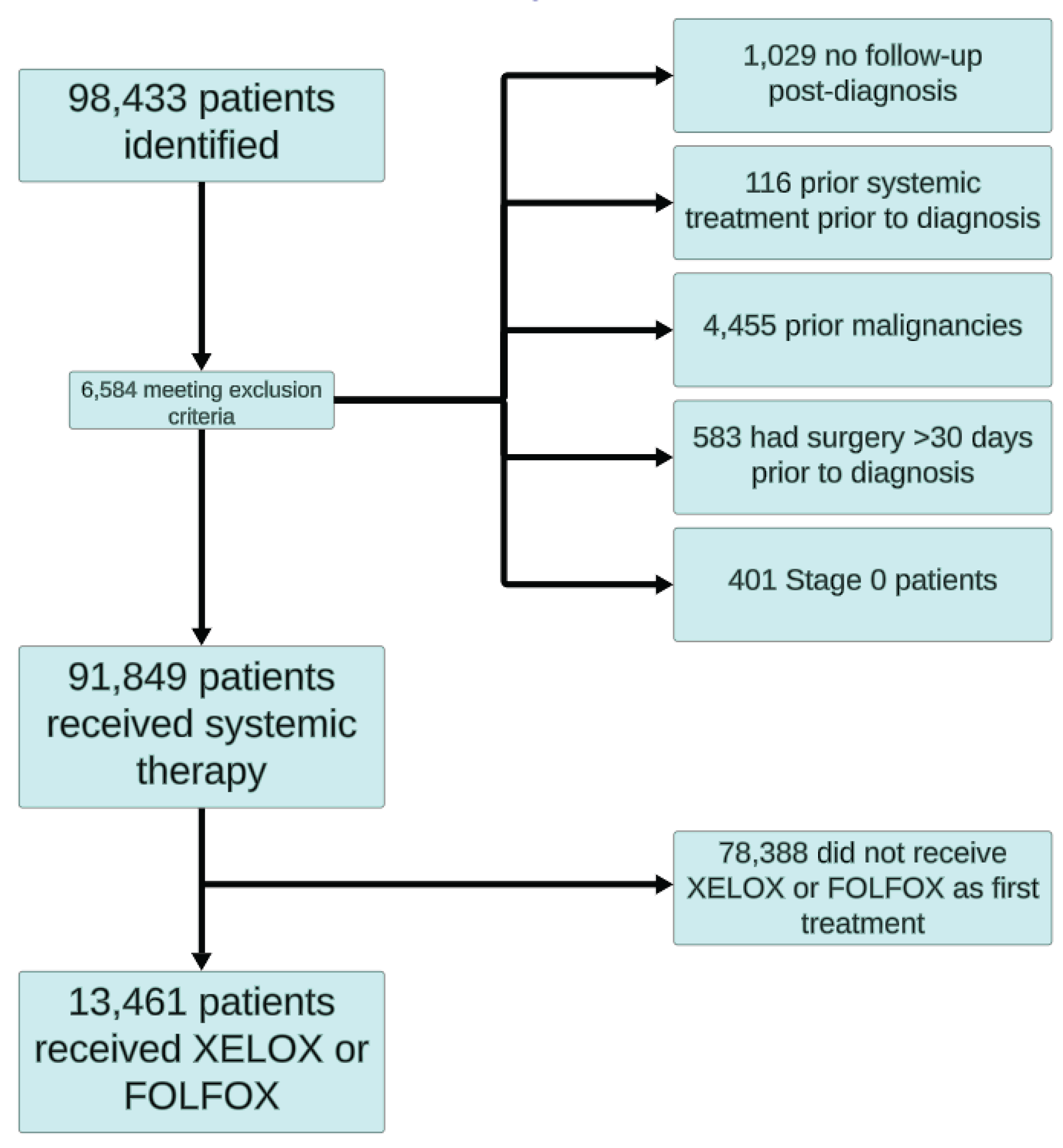
| Patient Characteristics | Stage 1-3 | Stage 4 | |||||
| CAPOX | FOLFOX | P-value | CAPOX | FOLFOX | P-value | ||
| N | 867 | 9722 | 214 | 1803 | |||
| Age Groups | N (%) 18-39 | 31 (3.6) | 395 (4.1) | 0.003 | 7 (3.3) | 107 (5.9) | <0.001 |
| 40-64 | 480 (55.4) | 5760 (59.3) | 100 (46.7) | 1049 (58.2) | |||
| 65-69 | 185 (21.3) | 1812 (18.6) | 39 (18.2) | 280 (15.5) | |||
| 70-74 | 100 (11.5) | 1177 (12.1) | 27 (12.6) | 212 (11.8) | |||
| 75-79 | 49 (5.7) | 468 (4.8) | 25 (11.7) | 121 (6.7) | |||
| 80+ | 22 (2.5) | 110 (1.1) | 16 (7.5) | 34 (1.9) | |||
| Sex | N (%) Male | 495 (57.1) | 5502 (56.6) | 0.802 | 105 (49.1) | 1030 (57.1) | 0.029 |
| Year of Diagnosis | 2007 | 32 (3.7) | 751 (7.7) | <0.001 | 26 (12.2) | 171 (9.5) | 0.303 |
| 2008 | 43 (5.0) | 955 (9.8) | 23 (10.8) | 176 (9.8) | |||
| 2009 | 33 (3.8) | 959 (9.9) | 13 (6.1) | 121 (6.7) | |||
| 2010 | 35 (4.0) | 958 (9.9) | 13 (6.1) | 111 (6.2) | |||
| 2011 | 57 (5.4) | 890 (9.2) | 15 (7.0) | 153 (8.5) | |||
| 2012 | 53 (6.1) | 960 (9.9) | 15 (7.0) | 200 (11.1) | |||
| 2013 | 46 (5.3) | 965 (9.9) | 11 (5.1) | 200 (11.1) | |||
| 2014 | 64 (7.4) | 910 (9.4) | 13 (6.1) | 206 (11.4) | |||
| 2015 | 84 (9.7) | 889 (9.1) | 31 (14.5) | 157 (8.7) | |||
| 2016 | 228 (26.3) | 761 (7.8) | 28 (13.1) | 160 (8.9) | |||
| 2017 | 202 (23.3) | 724 (7.5) | 26 (12.2) | 148 (8.2) | |||
| Rural | N (%) Yes | 160 (18.5) | 1447 (14.9) | 0.007 | 29 (13.6) | 218 (12.1) | 0.510 |
| Distance to Nearest RCC | Median (IQR) | 15.1 (6.0, 58.1) | 12.6 (5.6, 45.0) | <0.001 | 10.5 (5.1, 34.8) | 10.5 (5.1, 34.8) | 0.393 |
| Charlson Score | n (%) 0 | 204 (23.5) | 2214 (22.8) | 0.794 | 46 (21.5) | 393 (21.8) | 0.511 |
| 1 | 34 (3.9) | 375 (3.9) | 8 (3.7) | 52 (2.9) | |||
| 2+ | 29 (3.3) | 387 (4.0) | 10 (4.7) | 54 (3.0) | |||
| No admission | 600 (69.2) | 6746 (69.4) | 150 (70.1) | 1304 (72.3) | |||
| Hospital Type (for Surgery) | Community | 332 (71.4) | 3388 (74.7) | 86 (64.7) | 648 (71.0) | ||
| Teaching | 102 (21.9) | 992 (21.9) | 47 (35.3) | 241 (26.4) | |||
| Instability Quintile | 1 | 155 (18.1) | 1815 (18.9) | 0.55 | 41 (19.4) | 364 (20.3) | 0.62 |
| 2 | 170 (19.9) | 2038 (21.2) | 37 (17.5) | 356 (19.9) | |||
| 3 | 222 (25.9) | 1975 (20.5) | 41 (19.4) | 306 (17.1) | |||
| 4 | 157 (18.3) | 1868 (19.4) | 45 (21.3) | 372 (20.8) | |||
| 5 | 152 (17.8) | 1935 (20.1) | 47 (22.3) | 394 (22.0) | |||
| Income Quintile | 1 | 153 (17.7) | 1713 (17.7) | 0.595 | 28 (13.1) | 343 (19.1) | 0.104 |
| 2 | 176 (20.4) | 1900 (19.6) | 46 (21.5) | 336 (18.7) | |||
| 3 | 179 (20.7) | 1995 (20.6) | 42 (19.6) | 366 (20.4) | |||
| 4 | 182 (21.0) | 2026 (20.9) | 46 (21.5) | 378 (21.0) | |||
| 5 | 175 (20.2) | 2058 (21.2) | 52 (24.3) | 375 (20.9) | |||
| Deprivation Quintile | 1 | 170 (19.9) | 1979 (20.6) | 0.92 | 55 (26.1) | 384 (21.4) | 0.20 |
| 2 | 183 (21.4) | 1995 (20.7) | 38 (18.0) | 353 (19.7) | |||
| 3 | 180 (21.0) | 1974 (20.5) | 45 (21.3) | 373 (20.8) | |||
| 4 | 166 (19.4) | 1942 (20.2) | 37 (17.5) | 334 (18.6) | |||
| 5 | 157 (18.3) | 1741 (18.1) | 36 (17.1) | 348 (19.4) | |||
| Dependency Quintile | 1 | 121 (14.1) | 1790 (18.6) | <0.001 | 42 (19.9) | 338 (18.9) | 0.58 |
| 2 | 156 (18.2) | 1873 (19.5) | 33 (15.6) | 394 (22.0) | |||
| 3 | 164 (19.2) | 1850 (19.2) | 46 (21.8) | 335 (18.7) | |||
| 4 | 197 (23.0) | 1871 (19.4) | 44 (20.9) | 321 (17.9) | |||
| 5 | 218 (25.5) | 2247 (23.3) | 46 (21.8) | 404 (22.5) | |||
| Ethnicity Quintile | 1 | 225 (26.3) | 2103 (21.8) | <0.001 | 47 (22.3) | 352 (19.6) | 0.22 |
| 2 | 193 (22.6) | 1910 (19.8) | 39 (18.5) | 327 (18.3) | |||
| 3 | 168 (19.6) | 1765 (18.3) | 38 (18.0) | 355 (19.8) | |||
| 4 | 131 (15.3) | 1835 (19.1) | 53 (25.1) | 360 (20.1) | |||
| 5 | 139 (16.2) | 2018 (21.0) | 34 (16.1) | 398 (22.2) | |||
| Category | CAPOX | FOLFOX | P-value | |||
| N | 1081 | 11525 | ||||
| 5-year Overall Survival (%) | Stage I-III | 70.1 (66.6, 75.3) | 77.2 (76.2, 78.1) | <0.001 | ||
| Stage IV | 16.6 (11.0, 23.2) | 33.2 (30.7, 35.6) | <0.001 | |||
| Median Weeks on Treatment (Interquartile Range, Highest Number) | Stage I-III | 15 (6-21), 30 | 20 (14-24), 28 | 0.002 | ||
| Stage IV | 22.5 (12-27), 207 | 24 (16-28), 372 | 0.15 | |||
| EDVisits - N (%) | On treatment | 461 (42.7) | 3713 (32.2) | <0.001 | ||
| Within 60 days | 281 (26.0) | 2025 (17.6) | <0.001 | |||
| Hospitalizations - N (%) | On treatment | 435 (40.2) | 3918 (34.0) | <0.001 | ||
| Within 60 days | 164 (15.2) | 1023 (8.9) | <0.001 | |||
| EDVisitsor hospitalization while on-treatment – N (%) | All patients | Within 60 days | 359 (33.2) | 2608 (22.6) | <0.001 | |
| On-treatment | 657 (60.8) | 5870 (50.9) | <0.001 | |||
| Stage I-III | Within 60 days | 283 (32.6) | 2189 (21.9) | <0.001 | ||
| On-treatment | 522 (60.2) | 4688 (48.2) | <0.001 | |||
| Stage IV | Within 60 days | 76 (35.5) | 480 (26.6) | 0.008 | ||
| On-treatment | 135 (63.1) | 1182 (65.6) | 0.49 | |||
| Factor | Comparator | Univariate | Multivariate | ||
| Odds Ratio (95% CI) | P-Value | Odds Ratio (95% CI) | P-Value | ||
| Age Groups | 18-39 | Reference | 0.3 | Reference | 0.354 |
| 40-64 | 0.80 (0.67, 0.94) | 0.84 (0.70, 1.00) | |||
| 65-69 | 0.81 (0.68, 0.98) | 0.86 (0.71, 1.04) | |||
| 70-74 | 0.81 (0.67, 0.99) | 0.91 (0.74, 1.11) | |||
| 75-79 | 0.79 (0.63, 0.99) | 0.91 (0.72, 1.15) | |||
| 80-84 | 0.82 (0.59, 1.16) | 0.84 (0.59, 1.21) | |||
| 85+ | 0.66 (0.28, 1.54) | 0.57 (0.23, 1.42) | |||
| Sex | Male vs Female | 0.99 (0.93, 1.06) | 0.83 | 0.99 (0.93, 1.07) | 0.877 |
| Year of Diagnosis | / year | 1.20 (1.19, 1.22) | <0.001 | 1.21 (1.20, 1.22) | <0.001 |
| Income Quintile | 1 | Reference | 0.16 | Reference | 0.824 |
| 2 | 0.92 (0.82, 1.03) | 0.98 (0.87, 1.10) | |||
| 3 | 0.90 (0.81, 1.01) | 0.95 (0.84, 1.06) | |||
| 4 | 0.91 (0.81, 1.01) | 0.97 (0.87, 1.09) | |||
| 5 | 0.87 (0.78, 0.97) | 0.94 (0.84, 1.06) | |||
| Rural | Yes vs No | 1.23 (1.12, 1.35) | <0.001 | 1.30 (1.17, 1.43) | <0.001 |
| Charlson Score | 0 | Reference | 0.057 | Reference | 0.017 |
| 1 | 0.97 (0.80, 1.17) | 0.86 (0.70, 1.05) | |||
| 2+ | 1.29 (1.07, 1.56) | 1.23 (1.00, 1.50) | |||
| No Admissionⱡ | 1.04 (0.96, 1.13) | 0.94 (0.86, 1.03) | |||
| Site of Primary Lesion | Cecum | Reference | <0.001 | Reference | <0.001 |
| Ascending colon | 0.84 (0.74, 0.96) | 0.87 (0.76, 1.00) | |||
| Hepatic flexure | 0.87 (0.69, 1.10) | 0.97 (0.76, 1.24) | |||
| Transverse colon | 0.99 (0.83, 1.18) | 0.99 (0.82, 1.19) | |||
| Splenic flexure | 0.83 (0.66, 1.04) | 0.85 (0.67, 1.08) | |||
| Descending colon | 0.97 (0.81, 1.16) | 0.97 (0.80, 1.18) | |||
| Sigmoid colon | 0.86 (0.77, 0.96) | 0.90 (0.80, 1.02) | |||
| Overlapping region | 1.87 (0.45, 7.85) | 2.93 (0.67, 12.86) | |||
| Colon NOS | 1.45 (0.77, 2.75) | 1.12 (0.57, 2.21) | |||
| Rectosigmoid junction | 1.29 (1.13, 1.47) | 1.32 (1.14, 1.52) | |||
| Rectum NOS | 2.34 (2.09, 2.63) | 2.45 (2.16, 2.76) | |||
| Stage | 1 | Reference | <0.001 | Reference | <0.001 |
| 2 | 0.58 (0.41, 0.80) | 0.62 (0.44, 0.87) | |||
| 3 | 0.47 (0.34, 0.64) | 0.46 (0.33, 0.63) | |||
| 4 | 0.95 (0.68, 1.32) | 0.99 (0.70, 1.39) | |||
| Unknown | 0.27 (0.19, 0.38) | 0.60 (0.42, 0.86) | |||
| 1st Systemic Treatment Received | CAPOX vs FOLFOX | 1.54 (1.36, 1.74) | <0.001 | 1.05 (0.92, 1.20) | 0.466 |
| Factor | Comparator | Univariate | Multivariate | ||
| Odds Ratio (95% CI) | P-Value | Odds Ratio (95% CI) | P-Value | ||
| Age Groups | 18-39 | Reference | <0.001 | Reference | <0.001 |
| 40-64 | 0.94 (0.80, 1.10) | 0.97 (0.83, 1.14) | |||
| 65-69 | 1.09 (0.92, 1.29) | 1.12 (0.94, 1.32) | |||
| 70-74 | 1.46 (1.23, 1.73) | 1.51 (1.27, 1.80) | |||
| 75-79 | 1.99 (1.66, 2.39) | 1.93 (1.60, 2.32) | |||
| 80-84 | 2.69 (2.11, 3.44) | 2.36 (1.84, 3.02) | |||
| 85+ | 4.84 (2.90, 8.09) | 3.93 (2.34, 6.58) | |||
| Sex | Male vs Female | 1.08 (1.02, 1.15) | 0.011 | 1.08 (1.01, 1.15) | 0.016 |
| Year of Diagnosis | / year | 1.05 (1.04, 1.07) | <0.001 | 1.05 (1.04, 1.06) | <0.001 |
| Income Quintile | 1 | Reference | 0.015 | Reference | 0.04 |
| 2 | 1.14 (1.04, 1.26) | 0.97 (0.88, 1.06) | |||
| 3 | 1.09 (0.99, 1.20) | 0.97 (0.88, 1.07) | |||
| 4 | 1.08 (0.98, 1.18) | 0.89 (0.81, 0.98) | |||
| 5 | 0.99 (0.90, 1.09) | 0.89 (0.80, 0.97) | |||
| Rural | Yes vs No | 1.08 (1.00, 1.17) | 0.065 | 1.15 (1.06, 1.24) | 0.001 |
| Charlson Score | 0 | Reference | 0.006 | Reference | 0.041 |
| 1 | 1.20 (1.03, 1.41) | 1.16 (0.99, 1.36) | |||
| 2+ | 1.10 (0.93, 1.28) | 1.09 (0.93, 1.28) | |||
| No Admissionⱡ | 0.96 (0.89, 1.02) | 0.96 (0.90, 1.03) | |||
| Site of Primary Lesion | Cecum | Reference | <0.001 | Reference | <0.001 |
| Ascending colon | 0.95 (0.85, 1.06) | 0.95 (0.85, 1.06) | |||
| Hepatic flexure | 0.95 (0.79, 1.14) | 0.98 (0.82, 1.18) | |||
| Transverse colon | 1.01 (0.88, 1.16) | 0.99 (0.86, 1.14) | |||
| Splenic flexure | 0.86 (0.72, 1.04) | 0.81 (0.67, 0.97) | |||
| Descending colon | 0.72 (0.61, 0.85) | 0.70 (0.60, 0.83) | |||
| Sigmoid colon | 0.74 (0.67, 0.81) | 0.72 (0.65, 0.79) | |||
| Overlapping region | 1.01 (0.38, 2.70) | 1.01 (0.38, 2.69) | |||
| Colon NOS | 1.92 (1.25, 2.97) | 1.22 (0.79, 1.89) | |||
| Rectosigmoid junction | 0.79 (0.70, 0.88) | 0.72 (0.64, 0.81) | |||
| Rectum NOS | 0.72 (0.65, 0.79) | 0.73 (0.66, 0.81) | |||
| Stage | 1 | Reference | <0.001 | Reference | <0.001 |
| 2 | 0.95 (0.66, 1.36) | 0.99 (0.69, 1.42) | |||
| 3 | 1.35 (0.96, 1.91) | 1.36 (0.97, 1.92) | |||
| 4 | 5.50 (3.89, 7.77) | 5.45 (3.85, 7.70) | |||
| Unknown | 1.66 (1.16, 2.37) | 1.99 (1.39, 2.85) | |||
| 1st Systemic Treatment Received | CAPOX vs FOLFOX | 1.69 (1.52, 1.88) | <0.001 | 1.42 (1.27, 1.58) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





