Submitted:
30 June 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Population
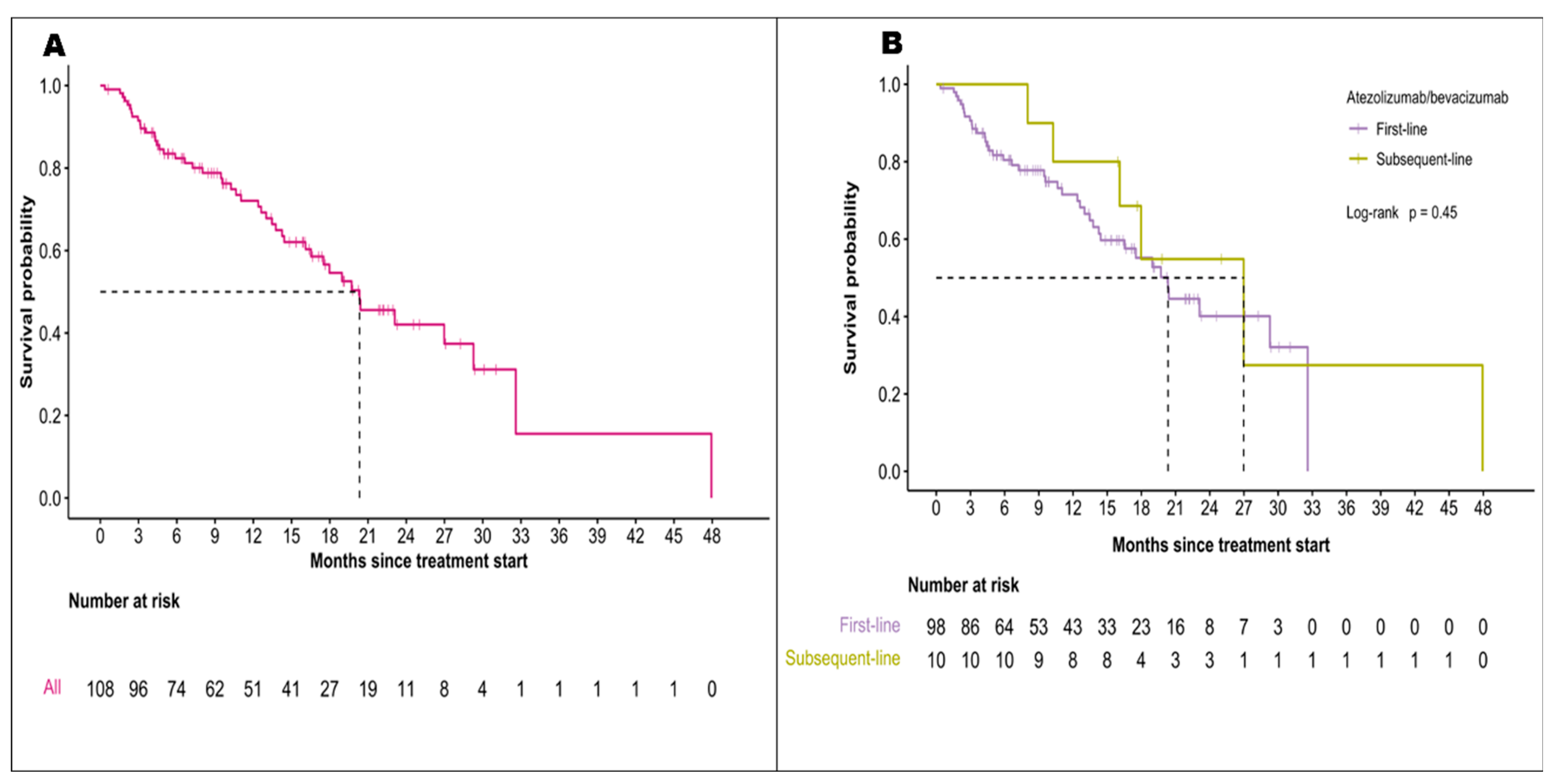
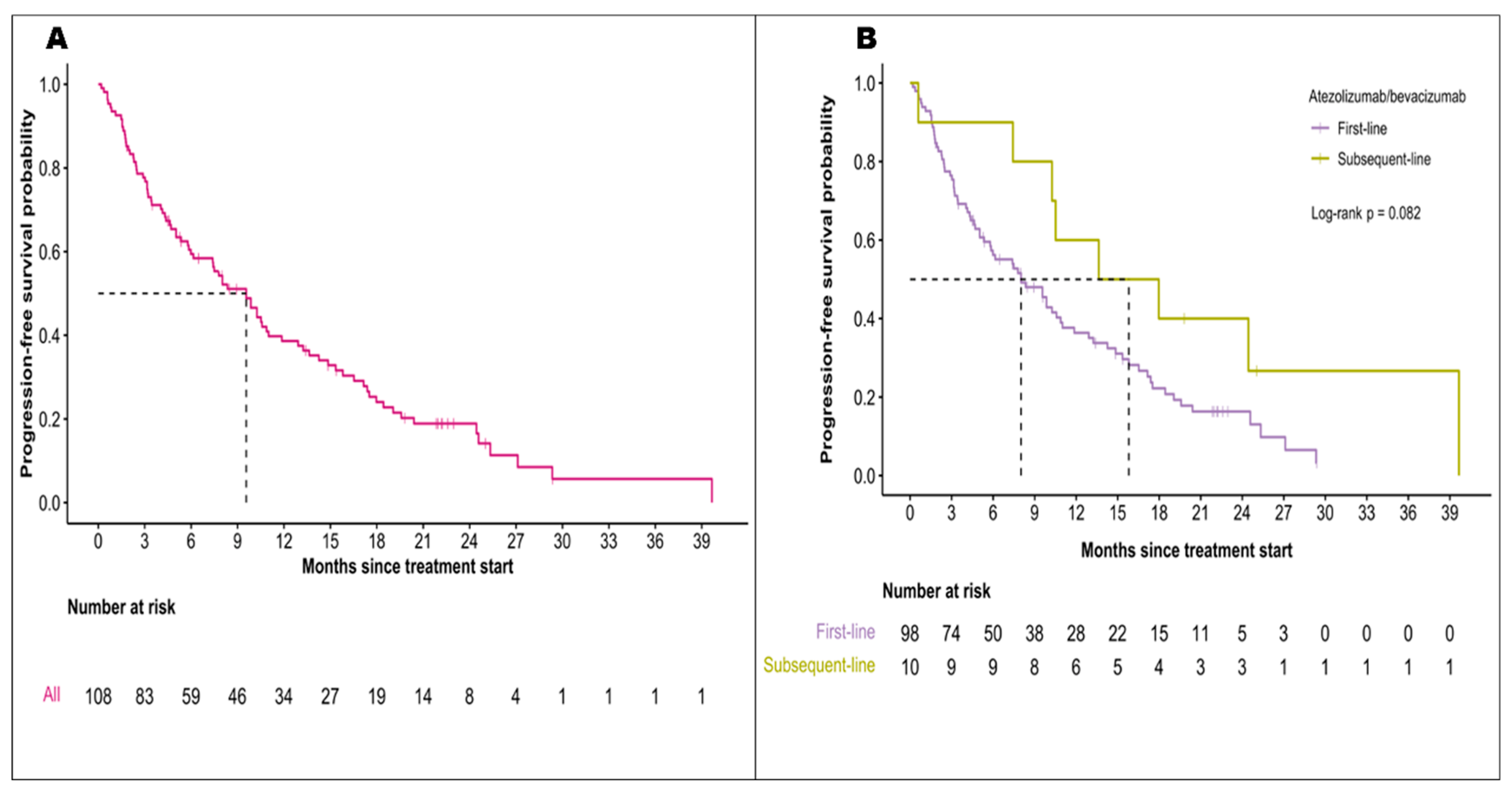
3.2. Effectiveness of Atezolizumab and Bevacizumab
3.3. EGD Utilization and Esophagogastric Varices
3.4. Bleeding Events during A+B Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Zhao, R.; Ma, H.; Zuo, S. Efficacy and Safety of Atezolizumab plus Bevacizumab Treatment for Advanced Hepatocellular Carcinoma in the Real World: A Single-Arm Meta-Analysis. BMC Cancer 2023, 23, 635. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rich, N.E.; Yopp, A.C.; Singal, A.G.; Murphy, C.C. Hepatocellular Carcinoma Incidence Is Decreasing Among Younger Adults in the United States. Clinical Gastroenterology and Hepatology 2020, 18, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, R.C.; Pestana, R.C.; Miyamura, B.V.; Kaseb, A.O. Hepatocellular Carcinoma Immunotherapy. Annu. Rev. Med. 2022, 73, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clinical Cancer Research 2019, 25, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. The Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Bentley, G.; Britten, C.D.; Amado, R.; Busuttil, R.W. Targeting Vascular Endothelial Growth Factor with the Monoclonal Antibody Bevacizumab Inhibits Human Hepatocellular Carcinoma Cells Growing in an Orthotopic Mouse Model. Liver International 2009, 29, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.-L.; Frenel, J.-S.; Raimbourg, J.; Gilabert, M. Current Options and Future Possibilities for the Systemic Treatment of Hepatocellular Carcinoma. Hepatic Oncology 2019, 6, HEP11. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J Hepatol 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.-C.; Roudot-Thoraval, F. The Burden of Liver Disease in Europe: A Review of Available Epidemiological Data. Journal of Hepatology 2013, 58, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Trauner, M.; Peck-Radosavljevic, M.; Sieghart, W. Cancer and Liver Cirrhosis: Implications on Prognosis and Management. ESMO Open 2016, 1, e000042. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a Bevacizumab Biosimilar (IBI305) versus Sorafenib in Unresectable Hepatocellular Carcinoma (ORIENT-32): A Randomised, Open-Label, Phase 2–3 Study. The Lancet Oncology 2021, 22, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E.; Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; et al. Updated Treatment Recommendations for Hepatocellular Carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Annals of Oncology 2021, 32, 801–805. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2023. [Google Scholar] [CrossRef]
- Su, G.L.; Altayar, O.; O’Shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Bureau, C.; Stefanescu, H.; Augustin, S.; Ney, M.; Blasco, H.; Procopet, B.; Bosch, J.; Genesca, J.; Berzigotti, A.; et al. Noninvasive Tools and Risk of Clinically Significant Portal Hypertension and Varices in Compensated Cirrhosis: The “Anticipate” Study. Hepatology 2016, 64, 2173–2184. [Google Scholar] [CrossRef]
- Pons, M.; Augustin, S.; Scheiner, B.; Guillaume, M.; Rosselli, M.; Rodrigues, S.G.; Stefanescu, H.; Ma, M.M.; Mandorfer, M.; Mergeay-Fabre, M.; et al. Noninvasive Diagnosis of Portal Hypertension in Patients With Compensated Advanced Chronic Liver Disease. Am J Gastroenterol 2021, 116, 723–732. [Google Scholar] [CrossRef]
- Villanueva, C.; Albillos, A.; Genescà, J.; Garcia-Pagan, J.C.; Calleja, J.L.; Aracil, C.; Bañares, R.; Morillas, R.M.; Poca, M.; Peñas, B.; et al. β Blockers to Prevent Decompensation of Cirrhosis in Patients with Clinically Significant Portal Hypertension (PREDESCI): A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. The Lancet 2019, 393, 1597–1608. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Abraldes, J.G. Nonselective Beta-Blockers in Compensated Cirrhosis: Preventing Variceal Hemorrhage or Preventing Decompensation? Gastroenterology 2021, 161, 770–773. [Google Scholar] [CrossRef]
- D’Alessio, A.; Fulgenzi, C.A.M.; Nishida, N.; Schönlein, M.; Von Felden, J.; Schulze, K.; Wege, H.; Gaillard, V.E.; Saeed, A.; Wietharn, B.; et al. Preliminary Evidence of Safety and Tolerability of Atezolizumab plus Bevacizumab in Patients with Hepatocellular Carcinoma and Child-Pugh A and B Cirrhosis: A Real-world Study. Hepatology 2022, 76, 1000–1012. [Google Scholar] [CrossRef]
- Fulgenzi, C.A.M.; Cheon, J.; D’Alessio, A.; Nishida, N.; Ang, C.; Marron, T.U.; Wu, L.; Saeed, A.; Wietharn, B.; Cammarota, A.; et al. Reproducible Safety and Efficacy of Atezolizumab plus Bevacizumab for HCC in Clinical Practice: Results of the AB-Real Study. European Journal of Cancer 2022, 175, 204–213. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Rimassa, L.; et al. Atezolizumab plus Bevacizumab versus Lenvatinib for Unresectable Hepatocellular Carcinoma: A Large Real-Life Worldwide Population. European Journal of Cancer 2023, 180, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Tevethia, H.; Kumar, K.; Premkumar, M.; Muttaiah, M.D.; Hiraoka, A.; Hatanaka, T.; Tada, T.; Kumada, T.; Kakizaki, S.; et al. Effectiveness and Safety of Atezolizumab-Bevacizumab in Patients with Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. eClinicalMedicine 2023, 63, 102179. [Google Scholar] [CrossRef] [PubMed]
- Rimini, M.; Rimassa, L.; Ueshima, K.; Burgio, V.; Shigeo, S.; Tada, T.; Suda, G.; Yoo, C.; Cheon, J.; Pinato, D.J.; et al. Atezolizumab plus Bevacizumab versus Lenvatinib or Sorafenib in Non-Viral Unresectable Hepatocellular Carcinoma: An International Propensity Score Matching Analysis. ESMO Open 2022, 7, 100591. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Lu, S.-N.; Chen, Y.-Y.; Kee, K.-M.; Yen, Y.-H.; Hung, C.-H.; Hu, T.-H.; Chen, C.-H.; Wang, J.-H. Real-World Lenvatinib Versus Sorafenib in Patients With Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis. Front Oncol 2021, 11, 737767. [Google Scholar] [CrossRef] [PubMed]
- Pomej, K.; Balcar, L.; Shmanko, K.; Welland, S.; Himmelsbach, V.; Scheiner, B.; Mahyera, A.; Mozayani, B.; Trauner, M.; Finkelmeier, F.; et al. Clinical Characteristics and Outcome of Patients with Combined Hepatocellular-Cholangiocarcinoma—a European Multicenter Cohort. ESMO Open 2023, 8, 100783. [Google Scholar] [CrossRef]
- Rodrigues, S.G.; Mendoza, Y.P.; Bosch, J. Beta-Blockers in Cirrhosis: Evidence-Based Indications and Limitations. JHEP Rep 2020, 2, 100063. [Google Scholar] [CrossRef]
- McDowell, H.R.; Chuah, C.S.; Tripathi, D.; Stanley, A.J.; Forrest, E.H.; Hayes, P.C. Carvedilol Is Associated with Improved Survival in Patients with Cirrhosis: A Long-term Follow-up Study. Aliment Pharmacol Ther 2021, 53, 531–539. [Google Scholar] [CrossRef]
- Yang, T.-C.; Chen, W.-C.; Hou, M.-C.; Chen, P.-H.; Lee, P.-C.; Chang, C.-Y.; Lu, H.-S.; Chen, Y.-J.; Hsu, S.-J.; Huang, H.-C.; et al. Endoscopic Variceal Ligation versus Propranolol for the Primary Prevention of Oesophageal Variceal Bleeding in Patients with Hepatocellular Carcinoma: An Open-Label, Two-Centre, Randomised Controlled Trial. Gut, 2023; gutjnl-2023-330419. [Google Scholar] [CrossRef]
- Stafylidou, M.; Paschos, P.; Katsoula, A.; Malandris, K.; Ioakim, K.; Bekiari, E.; Haidich, A.-B.; Akriviadis, E.; Tsapas, A. Performance of Baveno VI and Expanded Baveno VI Criteria for Excluding High-Risk Varices in Patients With Chronic Liver Diseases: A Systematic Review and Meta-Analysis. Clinical Gastroenterology and Hepatology 2019, 17, 1744–1755.e11. [Google Scholar] [CrossRef]
- Wu, C.W.-K.; Lui, R.N.-S.; Wong, V.W.-S.; Yam, T.-F.; Yip, T.C.-F.; Liu, K.; Lai, J.C.-T.; Tse, Y.-K.; Mok, T.S.-K.; Chan, H.L.-Y.; et al. Baveno VII Criteria Is an Accurate Risk Stratification Tool to Predict High-Risk Varices Requiring Intervention and Hepatic Events in Patients with Advanced Hepatocellular Carcinoma. Cancers (Basel) 2023, 15, 2480. [Google Scholar] [CrossRef]
- Allaire, M.; Campion, B.; Demory, A.; Larrey, E.; Wagner, M.; Rudler, M.; Roux, C.; Blaise, L.; Carrie, N.G.; Thabut, D. Baveno VI and VII Criteria Are Not Suitable for Screening for Large Varices or Clinically Significant Portal Hypertension in Patients with Hepatocellular Carcinoma. Aliment Pharmacol Ther 2023, 58, 346–356. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII – Renewing Consensus in Portal Hypertension. Journal of Hepatology 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Characteristic | Total (n=112) |
|---|---|
| Median Age (IQR), years | 66 (24-85) |
| Sex | |
| Male Female |
97 (87%) 15 (13%) |
| Ethnicity | |
| East-Asian | 27 (24%) |
| Other | 85 (76%) |
| Province | |
| Alberta | 60 (54%) |
| Ontario | 48 (43%) |
| Manitoba | 3 (3%) |
| Unknown | 1 |
| ECOG performance score | |
| 0 | 53 (47%) |
| 1 | 54 (48%) |
| 2 | 4 (4%) |
| 3 | 1 (1%) |
| Child-Pugh score | |
| A | 99 (91%) |
| B | 10 (9%) |
| Unknown | 3 |
| Albumin-bilirubin (ALBI) grade | |
| 1 | 50 (45%) |
| 2 | 60 (54%) |
| 3 | 1 (1%) |
| Unknown | 1 |
| Barcelona Clinic Liver Cancer (BCLC) stage | |
| A | 7 (6%) |
| B C |
26 (23%) 79 (71%) |
| Liver disease etiology | |
| Hepatitis C Hepatitis B Alcohol use |
37 (35%) 28 (26%) 25 (23%) |
| NASH Other |
17 (16%) 5 |
| Cirrhosis | |
| Yes | 75 (67%) |
| No | 37 (33%) |
| Liver resection | 29 (28%) |
| Prior locoregional therapy | |
| Yes | 57(72%) |
| No Unknown |
22 (28%) 33 |
| Type of locoregional therapy | |
| Radiofrequency Ablation (RFA) | 33 (29%) |
| Stereotactic Body Radiation Therapy (SBRT) | 17 (15%) |
| y-90 Radioembolization | 6 (5.4%) |
| Transarterial embolization (TAE) | 4 (3.6%) |
| Transarterial chemoembolization (TACE) | 29 (26%) |
| 1 | 11 (38%) |
| 2 | 14 (48%) |
| 3 | 4 (14%) |
| Tumor histology | |
| HCC | 73 (65%) |
| Mixed HCC-CCA | 3 (3%) |
| No biopsy/Unknown | 36 (32%) |
| Size of largest lesion, cm | |
| Median (IQR) Unknown |
3.8 (0.0-18.0) 16 |
| Tumor distribution | |
| Unilobar | 55 (50%) |
| Bilobar Unknown |
55 (50%) 2 |
| Macrovascular invasion | |
| Yes | 41 (37%) |
| No | 70 (63%) |
| Unknown | 1 |
| Presence of lymph node or extrahepatic metastasis | |
| Lymph node involvement Extrahepatic metastasis |
31 (28%) 41 (37%) |
| Completed EGD within 6 months | |
| Yes No |
79 (71%) 33 (29%) |
| Reasons for no EGD No radiological evidence of cirrhosis or varices Limited EGD availability Patient refusal (with EGD within 7 months) Unknown Varices |
4 (12%) 2 (6%) 1(3%) 26 (79%) |
| Detected on EGD | 32 (41%) |
| Required intervention before A+B No previous EGD/Unknown |
15 (19%) 34 |
| Baseline AFP level, µg/L | |
| Median (IQR) | 47.0 (1.0-86,709) |
| Unknown | 2 |
| Baseline platelet count, x109/L | |
| Median (IQR) Unknown |
177.0 (49.0- 574.0) 1 |
| Reasons for A+B discontinuation | |
| Disease progression Patient choice Toxicities Death |
30 (37%) 21 (26%) 15 (18.5%) 15 (18.5%) |
| Characteristic | Total (n = 112) |
|---|---|
| First-line regimen (n=112) | |
| A+B | 101(90%) |
| Lenvatinib | 6 (5%) |
| Sorafenib | 4 (4%) |
| Chemotherapy | 1 (1%) |
|
Second-line regime (n=39) Lenvatinib |
25 (64%) |
| A+B | 10 (26%) |
| Sorafenib | 2 (5%) |
| Chemotherapy | 2 (5%) |
|
Third-line regime (n=7) Regorafenib |
2 (29%) |
| Lenvatinib | 2 (29%) |
| A+B | 1 (14%) |
| Cabozantinib | 1 (14%) |
| Sorafenib | 1 (14%) |
| A+B prior to TKIs | 101 (90%) |
| TKIs prior to A+B | 11 (10%) |
|
Characteristics |
This study (n=112) |
IMbrave150 [11] (n=336) |
||
| Median follow-up period (95% CI), months | 10.4 (0.4-47.9) | 17.6 (0.1-28.6) | ||
| Median treatment duration | 6.4 (0.2-29.8) | 8.4 (3.5-18.3) | ||
| Median OS (95% CI), months 18-month OS Median OS (patients with bleeding vs without bleeding) |
20.3 (16.5-NR) 55% 20.3 (13.0-NR) vs 19.7 (16.5-NR) p=0.86 |
19.2 (17.0-23.7) 52% |
||
| Median PFS* (95% CI), months Median PFS (patients with bleeding vs without bleeding) |
9.6 (6.1-11.9) 10.3 (5.0-27.1) vs 9.6 (7.4-13.6) p=0.95 |
6.9 (5.7-8.6) | ||
| ORR* | Overall (n=112) |
First-line (n=101) |
Subsequent-line (n=11) |
Overall (n=336) |
| Complete response no. (%) Partial response no. (%) Stable disease no. (%) Progressive disease no. (%) Could not be evaluated/Missing no. |
1(1%) 36 (35.0%) 42 (40.8%) 24 (23.3%) 9 |
0 (0%) 33 (35.1%) 39 (41.5%) 22 (23.4%) 7 |
1 (11.1%) 3 (33.3%) 3 (33.3%) 2 (22.2%) 2 |
25 (8%) 72 (22%) 144 (44%) 63(19%) 22 (7%) |
| EGD within 6 months | ||||
|---|---|---|---|---|
| Characteristic | Total (n = 112) | Yes (n= 79) | No (n= 33) | p-value |
| Cirrhosis | 75 | 51(68%) | 24(32%) | 0.45 |
| Platelet count, x109/L | ||||
| Median (IQR) | 177 (49-574) | 162 (65-574) | 189 (49-486) | 0.17 |
| Albumin, g/L | ||||
| Median (IQR) | 39.0 (24-421) | 39 (26-421) | 39 (24-48) | 0.70 |
| INR | ||||
| Median (IQR) | 1.1 (0.9-1.8) | 1.1(0.9-1.4) | 1.1 (0.9-1.8) | 0.86 |
| Total Bilirubin, µmol/L | ||||
| Median (IQR) | 15 (3-72) | 15 (3-72) | 16 (3-57) | 0.56 |
| Size of largest tumor, cm | ||||
| Mean ± SD | 96 | 6.0 ± 4.3 | 5.7 ± 4.8 | 0.56 |
| Unknown | 16 | |||
| Macrovascular invasion | 41 | 6 (15%) | 35 (85%) | 0.88 |
| Unknown | 1 | |||
| Bleeding events | 17 | 14 (82%) | 3 (18%) | 0.24 |
| GI-specific bleeding | 6 | 5 (83%) | 1 (17%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





