Submitted:
24 June 2025
Posted:
25 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Materials
Synthesis of Mn-Doped Ag₂S and Ag₂S Nanoparticles
Characterization of Nanoparticles
Cell Culture and Cytotoxicity Assays
3. Results
3.1. Characterization of Mn-Doped Ag₂S Nanoparticles
3.1.1. Morphological Analysis
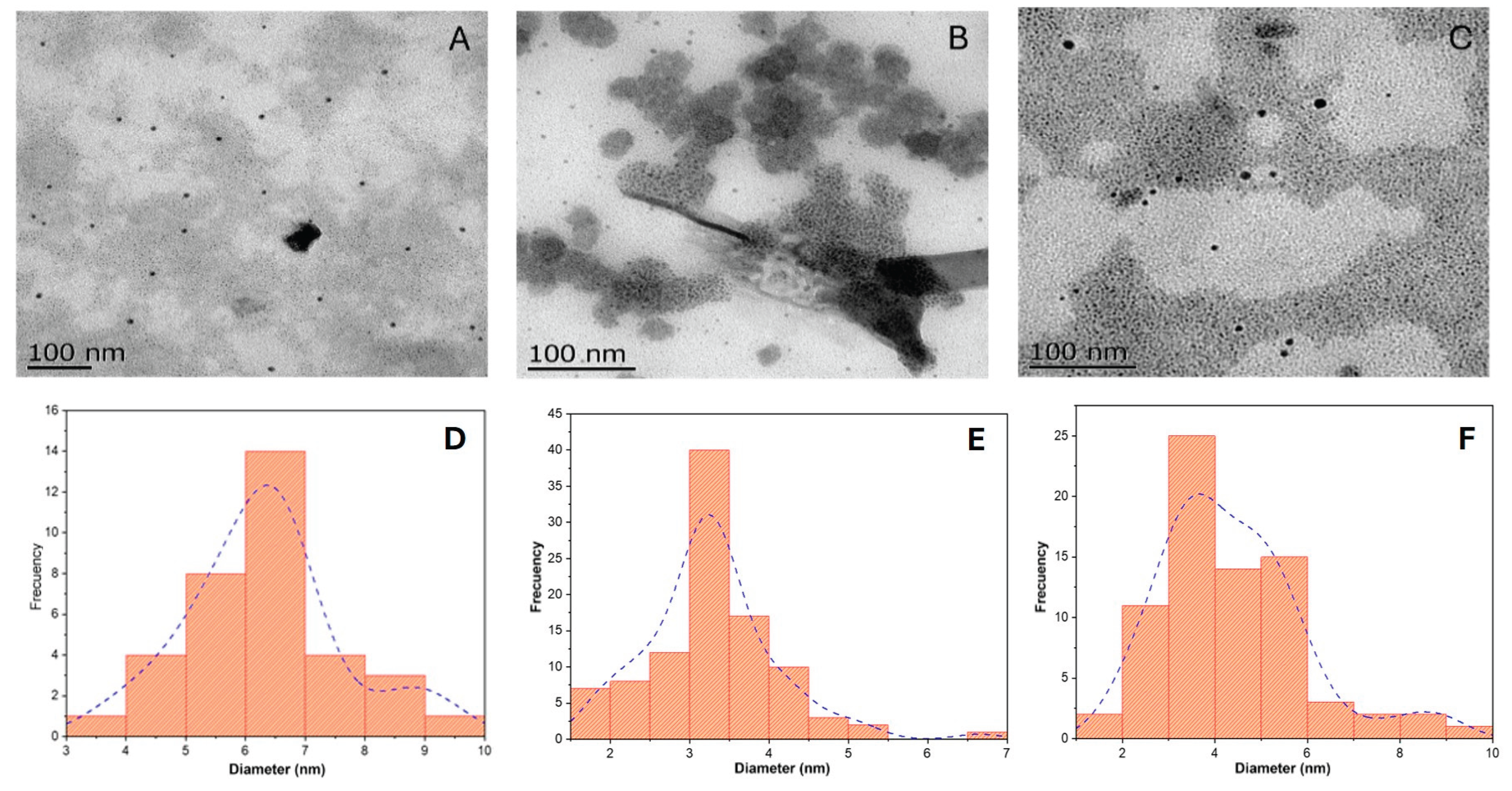
3.1.1.1. Transmission Electron Microscopy (TEM)
3.1.2. Chemical Composition
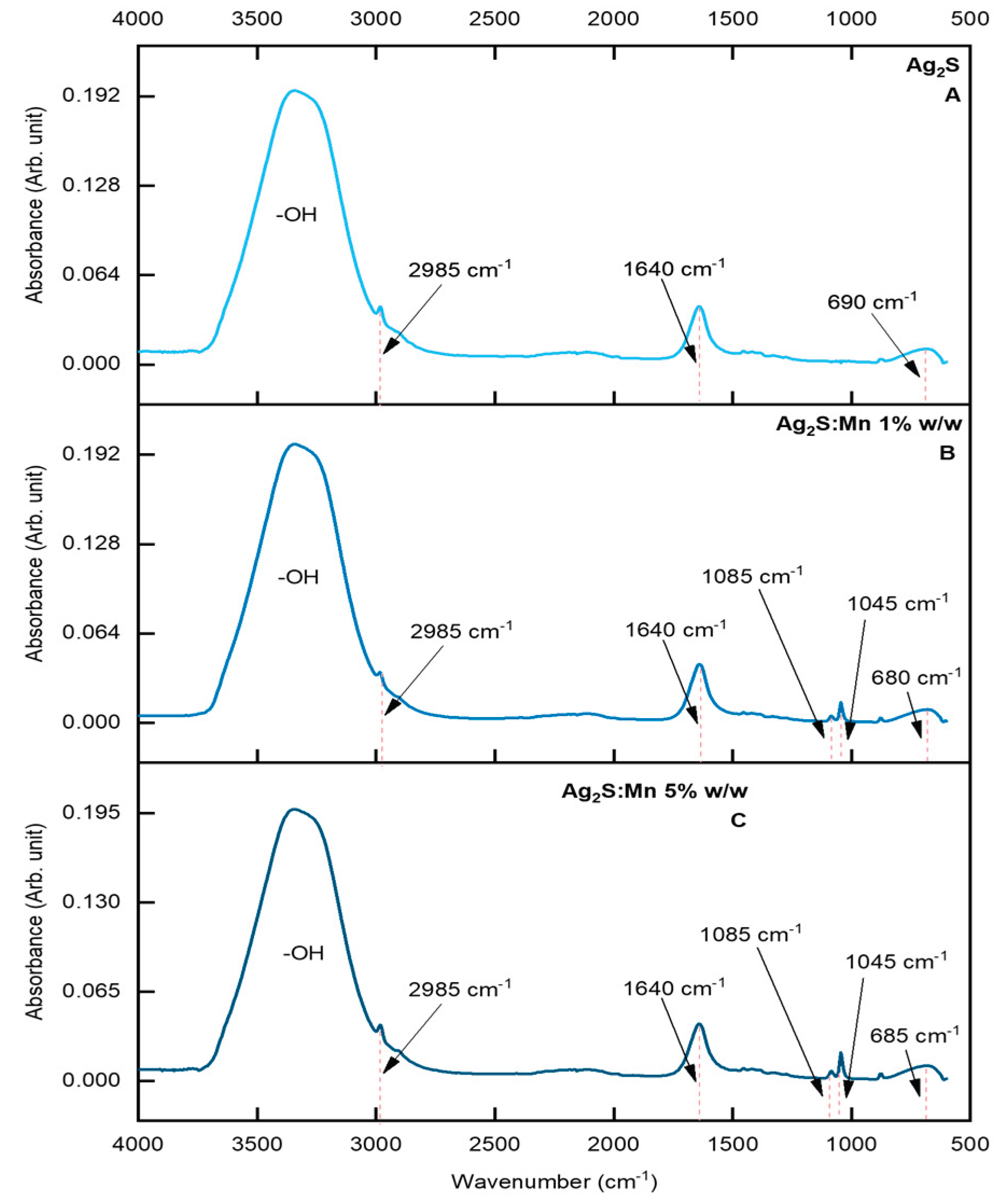
3.1.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
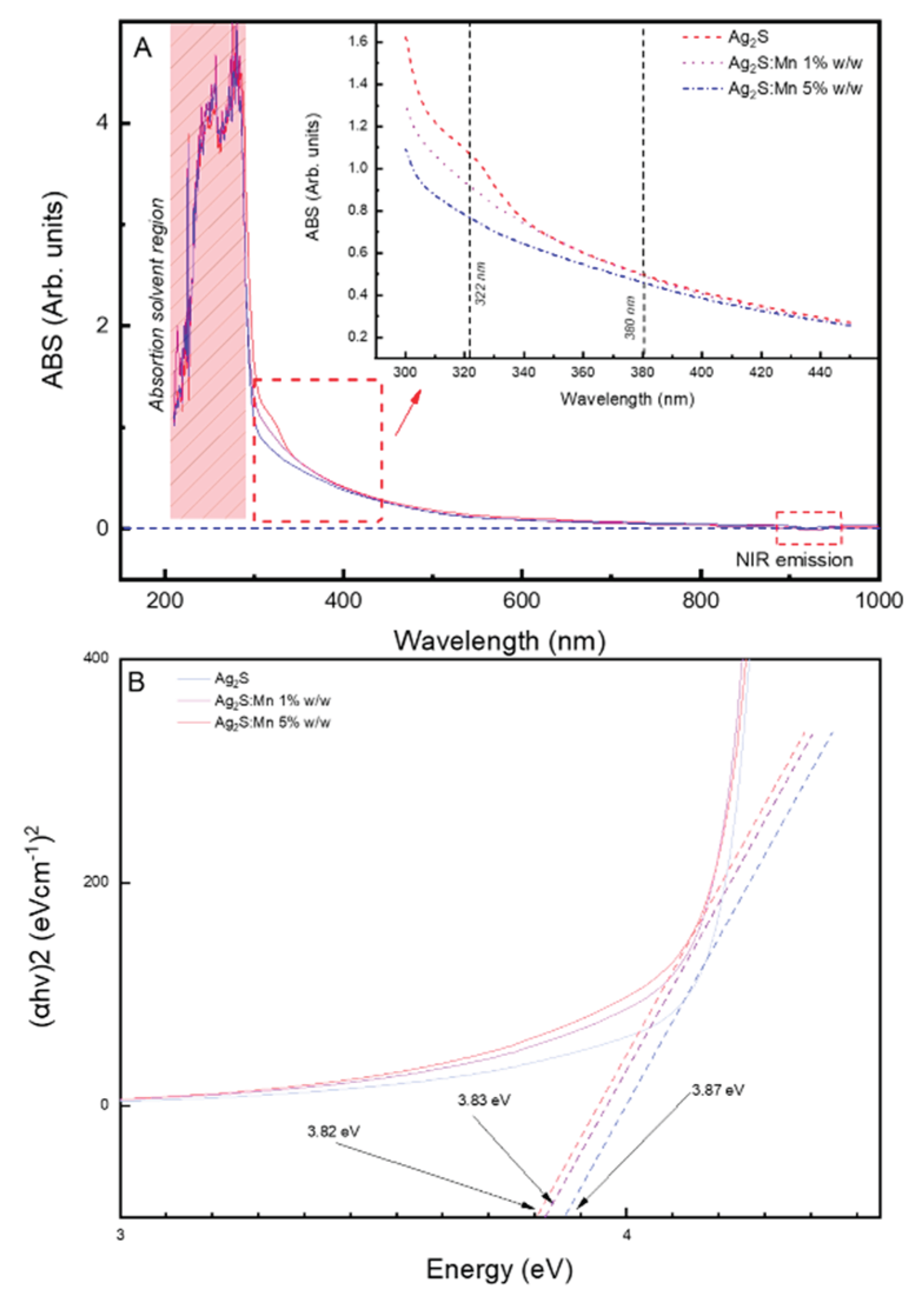
3.1.2.2. Ultraviolet-visible Spectroscopy (UV-vis)
3.1.3. Elemental Analysis
3.1.3.1. X-ray Fluorescence (XRF)
3.2. Cytotoxic Effects on Breast Cancer Cell Lines
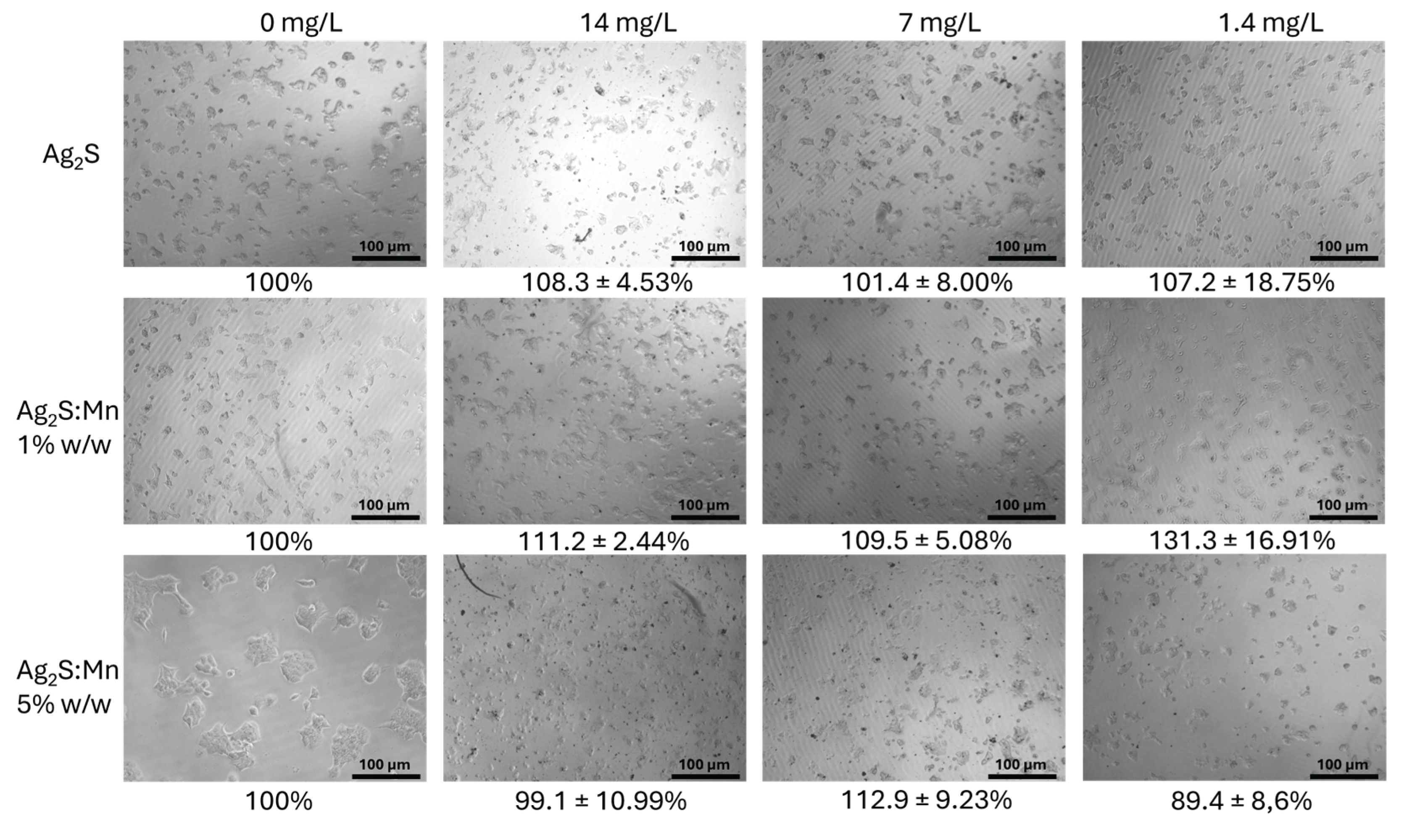
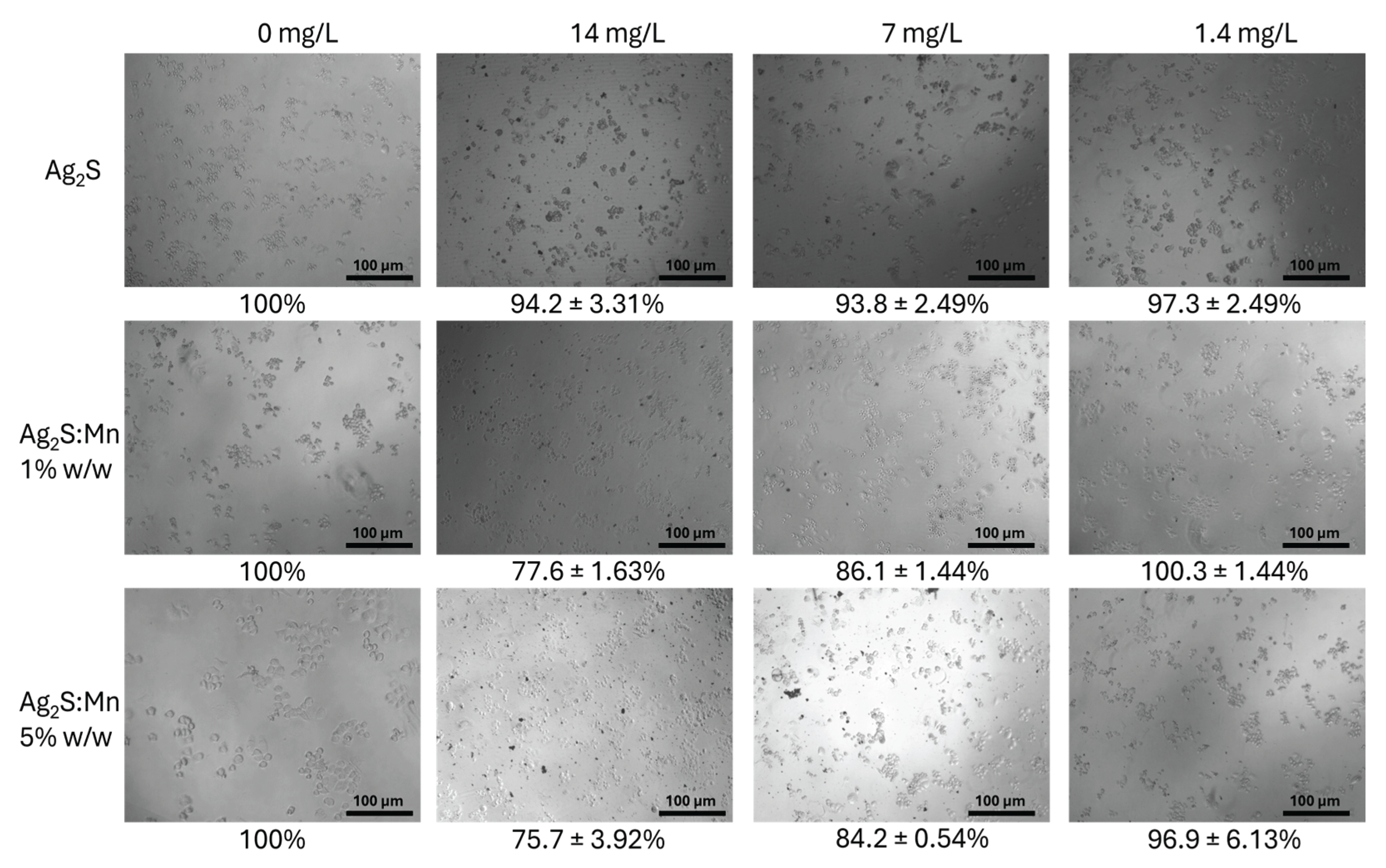
3.2.1. Cell Viability Assessment
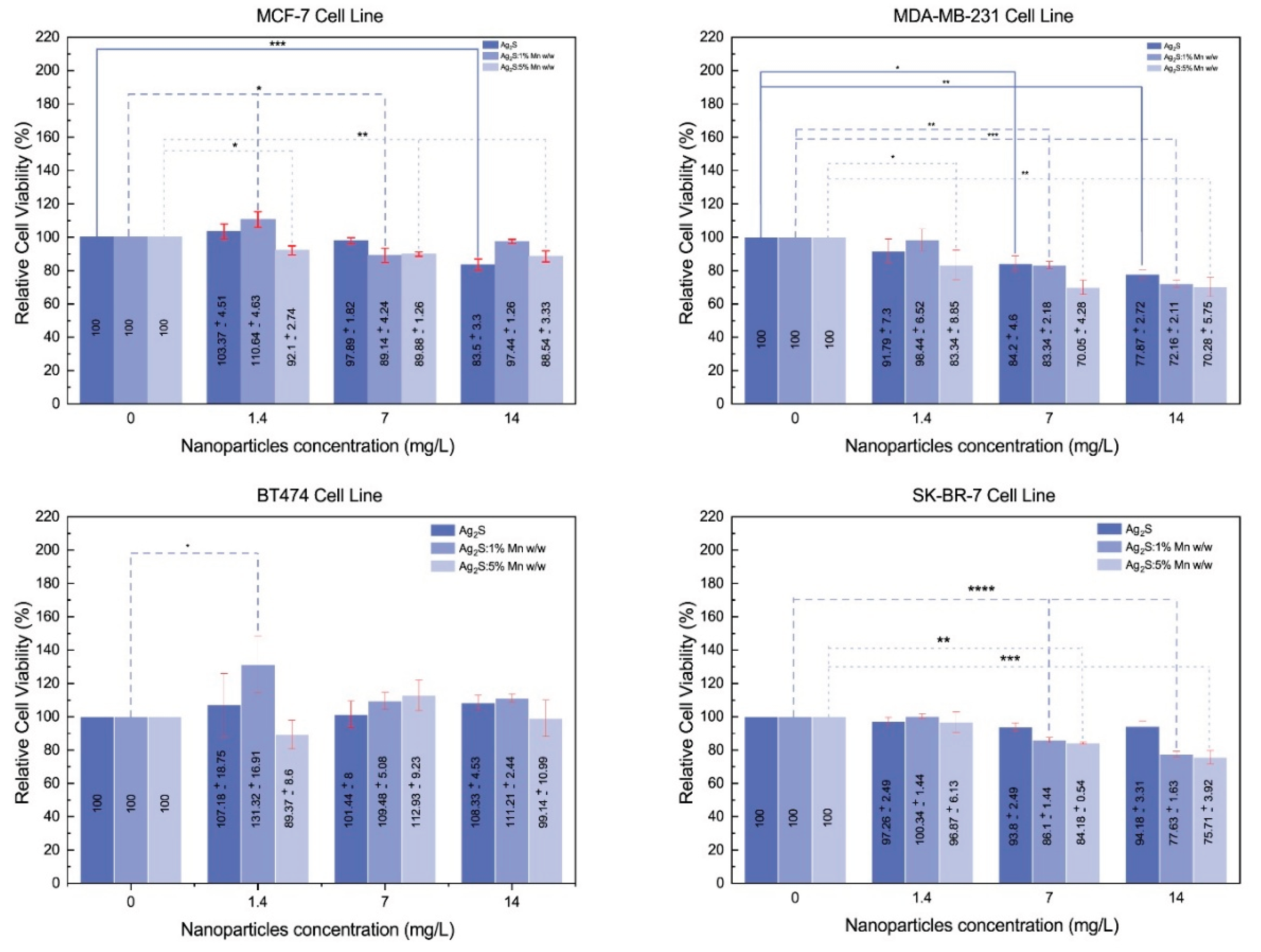
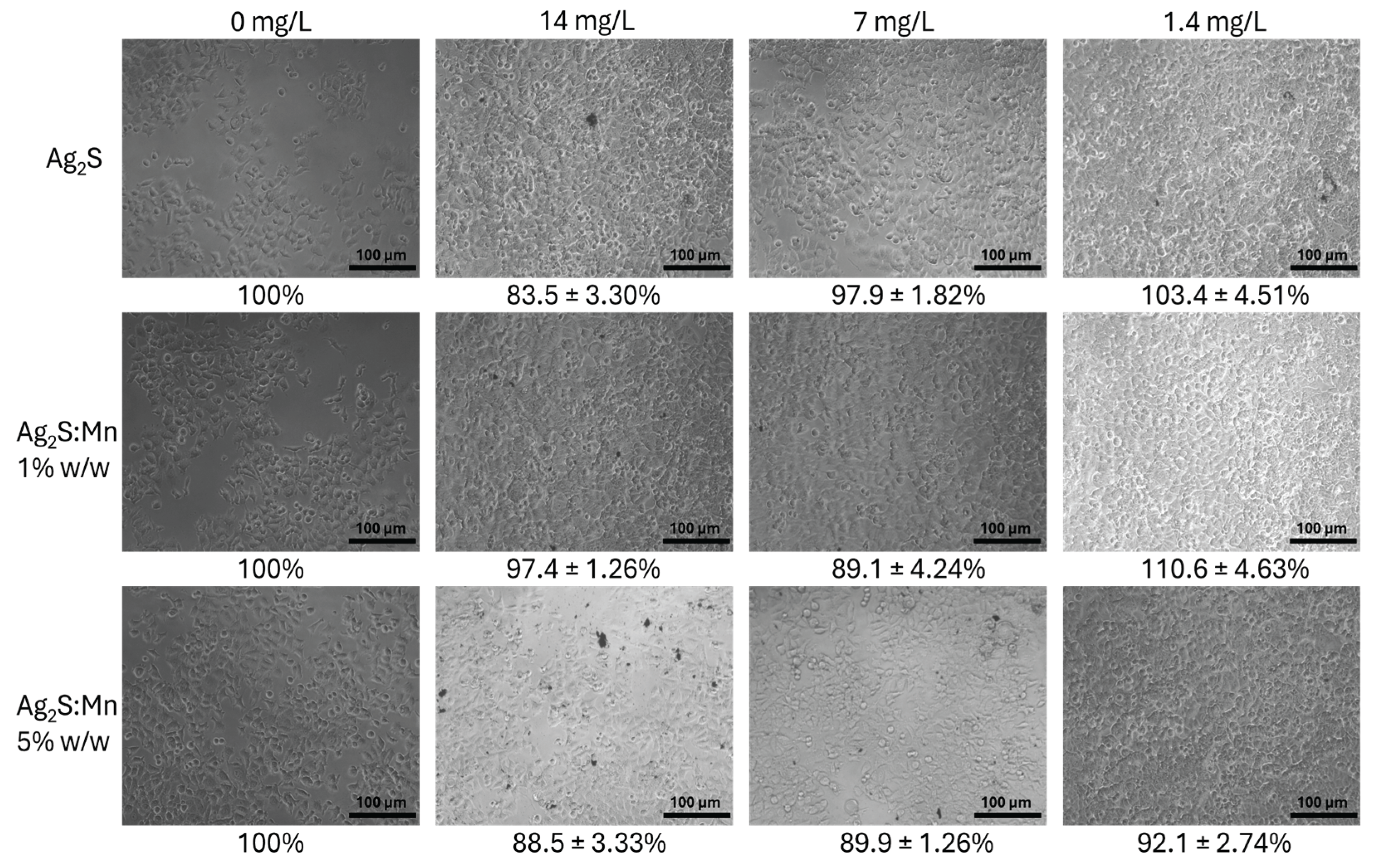
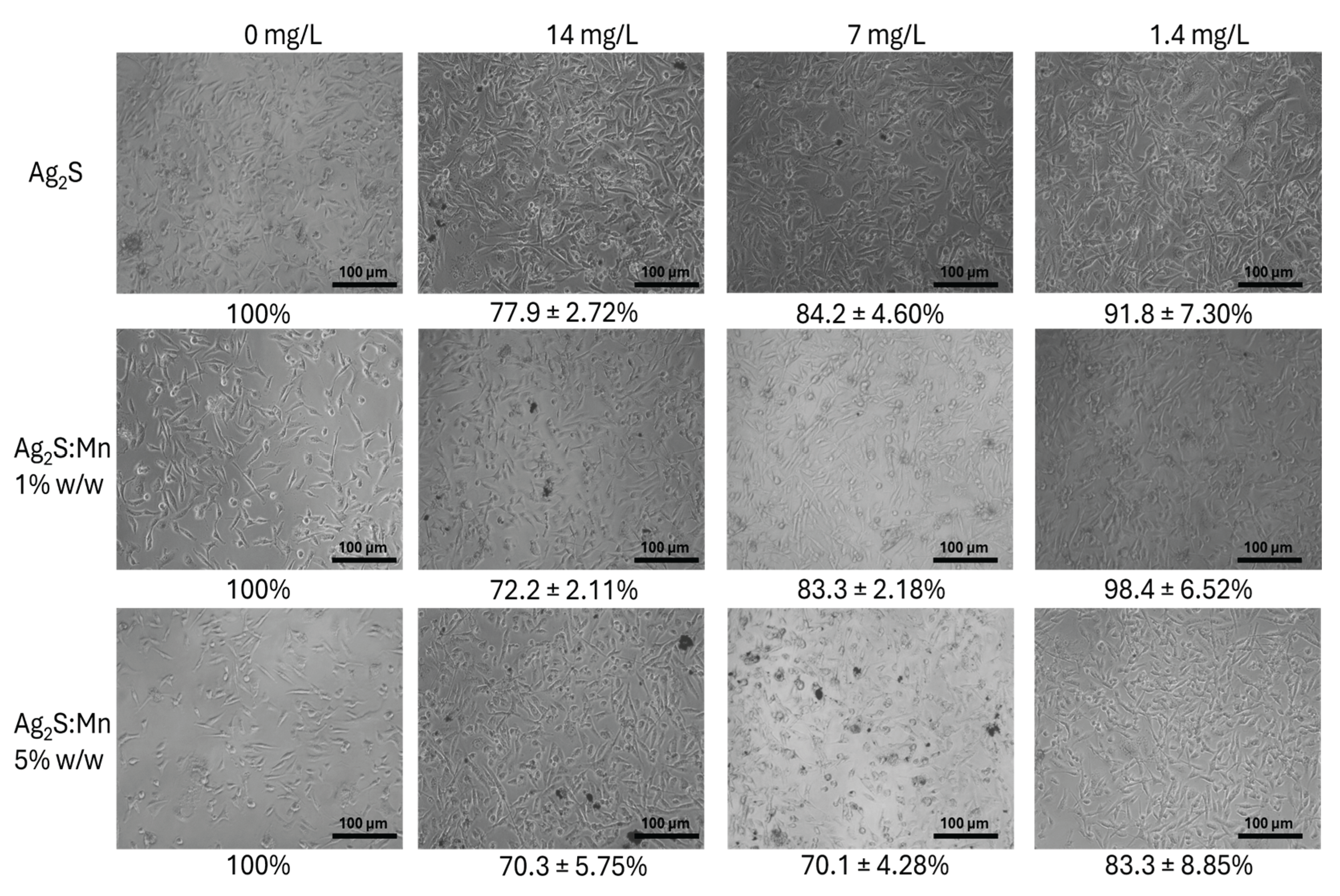
3.2.1.1. MTT Assay Results
3.2.1.2. MTT assay statistical results
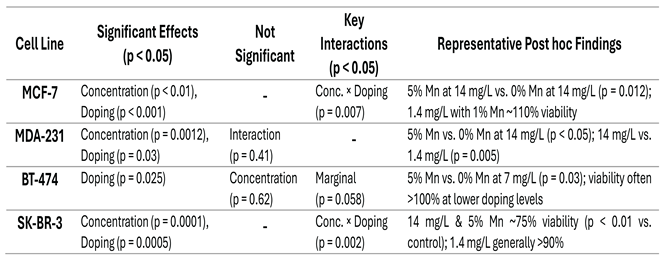 |
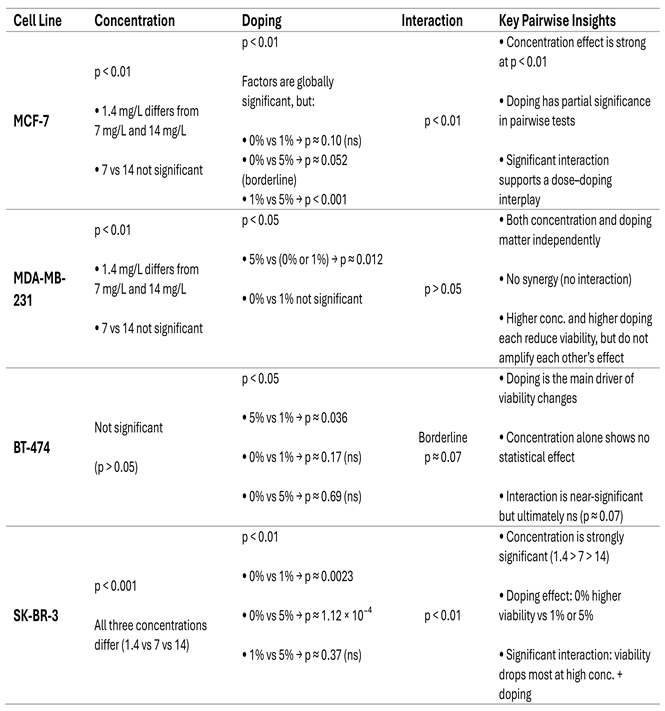 |
3.2.1.3. Dose-Dependent Effects
3.2.2. Comparative Analysis Across Cell Lines
3.2.2.1. Differential Sensitivity
3.2.3. Time-Dependent Effects
3.2.3.1. Extended Exposure Outcomes
3.3. Correlation Between Nanoparticle Properties and Biological Activity
3.3.1. Role of Mn Doping
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, A., Al Hindawi, A. M.,; Shiltagh, N. M. Role of manganese ion in tuning the structural and optical properties of silver sulfide nanostructures. Chemical Review and Letters 2024, 7, 622–629. [Google Scholar] [CrossRef]
- Al-thoubaity, F. K. (2020). Molecular classification of breast cancer: A retrospective cohort study. Annals of Medicine and Surgery, 49, 44. [CrossRef]
- Arrieta-Sandoval, N., Estrada Rojas, P., Olivas-Armendáriz, I., Valencia Gómez, L. E., Hernández Paz, J. F., Monarrez Cordero, B. E., & Rodríguez González, C. A. (n.d.). Effect of Ag 2 S-BSA nanoparticle size on 3T3 fibroblast cell line cytotoxicity. [CrossRef]
- Chen, H., Li, B., Zhang, M., Sun, K., Wang, Y., Peng, K., Ao, M., Guo, Y., & Gu, Y. (2014). Characterization of tumor-targeting Ag2S quantum dots for cancer imaging and therapy in vivo. Undefined, 6(21), 12580–12590. [CrossRef]
- Gao, J. J., & Swain, S. M. (2018). Luminal A Breast Cancer and Molecular Assays: A Review. The Oncologist, 23(5), 556. [CrossRef]
- He, X., Gao, J., Gambhir, S. S., & Cheng, Z. (2010). Near-infrared fluorescent nanoprobes for cancer molecular imaging: status and challenges. Trends in Molecular Medicine, 16(12), 574–583. [CrossRef]
- Ismail, R. A. , R. H. A. , & A. D. S. (2020). High-responsivity hybrid α-Ag2S/Si photodetector prepared by pulsed laser ablation in liquid. Beilstein Journal of Nanotechnology, 11, 1596–1607.
- Jeong, S., Doh, H., & Kim, S. (2020). Colloidal Second Near-Infrared-Emitting Mn-Doped Ag2S Quantum Dots. ChemNanoMat, 6(4), 538–541. [CrossRef]
- Kang, L., Zhang, M., Liu, Z.-H., & Ooi, K. (2007). IR spectra of manganese oxides with either layered or tunnel structures. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 67(3), 864–869. [CrossRef]
- Kim, J., Kim, J., Seo, K. H., Lee, K.-H., Park, Y. H., Lin, C.-H., Lu, Y.-S., Ueno, T., Yap, Y.-S., Wong, F.-Y., Tan, V. K. M., Lim, G.-H., Tan, S.-M., Yeo, W., Liu, Q., Leung, R., Naito, Y., Li, H., Lee, H.-B., … Im, S.-A. (2024). Survival outcomes of young-age female patients with early breast cancer: an international multicenter cohort study. ESMO Open, 9(11), 103732. [CrossRef]
- Levard, C., Hotze, E. M., Colman, B. P., Dale, A. L., Truong, L., Yang, # X Y, Bone, A. J., Gordon, ∥, Brown, E., Tanguay, R. L., Di Giulio, R. T., Bernhardt, E. S., Meyer, J. N., Wiesner, M. R., & Lowry, G. V. (2013). Sulfidation of Silver Nanoparticles: Natural Antidote to Their Toxicity. [CrossRef]
- Niell, B. L., Jochelson, M. S., Amir, T., Brown, A., Adamson, M., Baron, P., Bennett, D. L., Chetlen, A., Dayaratna, S., Freer, P. E., Ivansco, L. K., Klein, K. A., Malak, S. F., Mehta, T. S., Moy, L., Neal, C. H., Newell, M. S., Richman, I. B., Schonberg, M., … Slanetz, P. J. (2024). ACR Appropriateness Criteria® Female Breast Cancer Screening: 2023 Update. Journal of the American College of Radiology, 21(6, Supplement), S126–S143. [CrossRef]
- Opršal, J., Knotek, P., Zickler, G. A., Sigg, L., Schirmer, K., Pouzar, M., & Geppert, M. (2021). Cytotoxicity, Accumulation and Translocation of Silver and Silver Sulfide Nanoparticles in contact with Rainbow Trout Intestinal Cells. Aquatic Toxicology, 237, 105869. [CrossRef]
- Purushothaman, B., & Song, J. M. (2021). Ag2S quantum dot theragnostics. Biomaterials Science, 9(1), 51–69. [CrossRef]
- SalmanOgli, A. (2011). Nanobio applications of quantum dots in cancer: Imaging, sensing, and targeting. In Cancer Nanotechnology (Vol. 2, Issues 1–6, pp. 1–19). BioMed Central. [CrossRef]
- Singh, A., Iyer, A. K., Amiji, M., & Ganta, S. (2013). Multifunctional nanosystems for cancer therapy. Biomaterials for Cancer Therapeutics: Diagnosis, Prevention and Therapy, 387–413. [CrossRef]
- Valenza, C., Trapani, D., Zagami, P., Antonarelli, G., Boscolo Bielo, L., Nicolò, E., Ribeiro, J. M., Guidi, L., Reduzzi, C., Spotti, M., Adamoli, L., Cortès, J., Pistilli, B., Tolaney, S. M., Ueno, N., Layman, R. M., Cristofanilli, M., Carey, L. A., Munzone, E., … Curigliano, G. (2024). Immune checkpoint inhibitors for patients with metastatic triple-negative inflammatory breast cancer (INCORPORATE): An international cohort study. European Journal of Cancer, 213, 115097. [CrossRef]
- Vidya Bhargavi, M., Rao Mudunuru, V., & Kalluri, S. (2017). History of Breast Cancer-A Quick Review. Int J Cur Res Rev. [CrossRef]
- Wu, F.-F., Zhou, Y., Wang, J.-X., Zhuo, Y., Yuan, R., & Chai, Y.-Q. (2017). A novel electrochemiluminescence immunosensor based on Mn doped Ag2S quantum dots probe for laminin detection. Sensors and Actuators B: Chemical, 243, 1067–1074. [CrossRef]
- Zhang, X., Liu, M., Liu, H., & Zhang, S. (2014). Low-toxic Ag2S quantum dots for photoelectrochemical detection glucose and cancer cells. Biosensors and Bioelectronics, 56, 307–312. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





