1. Introduction
Variants in the X-linked cyclin-dependent kinase-like 5 (
CDKL5) gene are associated with a rare neurodevelopmental disorder known as CDKL5 deficiency disorder (CDD) [
1]. The
CDKL5 gene encodes a serine/threonine kinase that is abundantly expressed in both the developing and adult brain [
2], and plays a crucial role in regulating key processes essential for proper neural network formation [
3,
4]. The loss of CDKL5 function leads to a complex clinical phenotype, characterized by early infantile-onset, drug-resistant epilepsy, followed by severe neurodevelopmental impairment. Associated neurological features include hypotonia, intellectual and motor disabilities, autistic-like traits, and cortical visual impairment, as well as other comorbidities such as gastrointestinal issues and sleep disturbances.
Over the past several years, multiple constitutive
Cdkl5 knockout (KO) mouse models have been generated by permanently inactivating
Cdkl5 in all cells and tissues throughout development [
5,
6,
7,
8]. These animal models, which exhibit constitutive Cdkl5 deficiency, recapitulate most of the phenotypic features of the disorder, including severe impairments in hippocampal-dependent learning and memory, autistic-like behaviors, altered social interactions, visual and respiratory deficits, and motor stereotypies [
5,
6,
8,
9,
10,
11,
12,
13]. Neuroanatomical analyses have shown that these defects are associated with altered neuronal architecture, characterized by impairments in dendritic arborization, synaptogenesis, and connectivity of pyramidal neurons in both the cortical [
14,
15,
16,
17] and hippocampal regions [
6,
9,
10,
18,
19,
20] of
Cdkl5 KO mice. Additionally, the absence of Cdkl5 leads to decreased survival of CA1 pyramidal neurons in the hippocampus of these animal models [
9,
21,
22], as well as the presence of a generalized neuroinflammatory process [
23,
24].
Although most studies on
Cdkl5 KO mice have primarily focused on the adult brain phenotype that results from constitutive inactivation, a recent comparative analysis of brain defects across developmental stages in a
Cdkl5 KO mouse model has shown that many structural alterations emerge early in postnatal development and are largely maintained throughout later stages of brain maturation [
17]. These findings suggest that CDKL5 function is crucial for early postnatal developmental processes that underlie brain maturation. However, using a genetic approach to selectively ablate
Cdkl5 expression in adult mice, Terzic and colleagues demonstrated that postdevelopmental deletion of
Cdkl5 leads to behavioral and neuroanatomical defects similar to those observed in germline knockout mice [
25], suggesting a crucial functional role of CDKL5 also in the mature brain.
Since Cdkl5 is expressed in both glutamatergic and GABAergic neurons [
2,
26,
27], conditional knockout (cKO) models have been developed to selectively delete
Cdkl5 in forebrain excitatory [
6,
7,
27,
28] or inhibitory neurons [
6,
27], in order to identify the specific cell type in which Cdkl5 deficiency drives pathological phenotypes. These studies highlight the critical role of CDKL5 in glutamatergic neurons, where it serves as a key regulator of circuit development and synaptic activity. Notably, prenatal deletion of
Cdkl5 in forebrain excitatory neurons leads to both morphological and functional changes in hippocampal and cortical neurons, impairing synaptic activity, hippocampal-dependent memory, and cortical visual responses [
7,
28].
Although conditional knockout (cKO) mouse models have been pivotal in advancing our understanding of the primary role of Cdkl5 in glutamatergic neurons during brain development, the selective deletion of
Cdkl5 in these neurons within the mature brain has yet to be investigated. Understanding how the absence of CDKL5 in the adult brain impacts the brain phenotypes is crucial in order to predict the effectiveness of future therapies aimed at restoring CDKL5 expression in CDD patients. To investigate the role of CDKL5 in the adult brain, we used a recently developed cKO mouse model that allows for inducible deletion of
Cdkl5 expression in forebrain glutamatergic neurons [
26,
29]. Our findings demonstrate that the loss of
Cdkl5 in excitatory neurons of the adult brain leads to neuroanatomical alterations, including a reduction in excitatory synapse density, impaired dendritic spine maturation, and decreased neuronal survival. These structural changes were accompanied by increased microglial activation, indicative of localized neuroinflammation. Behaviorally,
Cdkl5 cKO mice exhibited impairments in hippocampus-dependent learning and memory, reduced sociability, motor coordination deficits, and autistic-like features. Collectively, these results highlight that CDKL5 plays a critical postdevelopmental role in glutamatergic neurons, maintaining synaptic architecture, neuronal viability, and functional integrity of forebrain circuits essential for normal cognitive and social behaviors.
3. Discussion
This study provides compelling evidence for the essential role of CDKL5 in the adult brain, showing that its selective deletion in glutamatergic neurons of the forebrain, even after the completion of brain development, is sufficient to induce neuroanatomical, synaptic, and behavioral alterations that are characteristic of the CDKL5 deficiency disorder (CDD) phenotype. These findings reinforce the hypothesis that CDKL5 is not only critical during early neurodevelopment but also plays a key role in maintaining neuronal function and the structural integrity of brain circuits throughout adulthood. Previous studies in constitutive
Cdkl5 KO mouse models have highlighted the importance of CDKL5 during early developmental stages, with animals displaying profound synaptic deficits, impaired neuronal maturation, and a wide spectrum of behavioral abnormalities that resemble core features of CDD [
5,
6,
8,
9,
10,
14,
15,
16,
17,
18,
19,
20,
39]. However, it remained unclear whether these phenotypes were exclusively the result of early developmental dysfunction or whether CDKL5 continued to exert essential functions in the mature brain. By employing a conditional and neuron-specific deletion strategy, our study clearly demonstrates that the postdevelopmental loss of CDKL5 in forebrain excitatory neurons is sufficient to recapitulate many of the structural and behavioral deficits observed in constitutive models. This suggests that the function of CDKL5 extends beyond neurodevelopment and remains crucial for synaptic maintenance and the regulation of complex behaviors in adulthood.
In particular, our data show that postnatal loss of
Cdkl5 in excitatory neurons leads to a reduction in glutamatergic synapse density in both the cortex and hippocampus, without significant changes in the inhibitory synaptic component. The presence of mature spines is essential for the establishment of excitatory synaptic contacts. Therefore, the reduction in the number of excitatory terminals observed in
Cdkl5flox/Y(Cre⁺) mice is consistent with the decreased number of mature spines. Notably, Ricciardi et al. [
40], using in utero electroporation of shRNA targeting
Cdkl5, similarly reported a decrease in VGLUT1 terminal density in cortical neurons, supporting our findings. However, other studies in the visual cortex [
15], and our own recent observations in the somatosensory cortex [
17], have reported an increase in VGLUT1 puncta, which appears contradictory and warrants further discussion. This discrepancy could reflect differences in developmental stages, or the timing and method of
Cdkl5 deletion, suggesting a complex and possibly region-specific role of CDKL5 in synapse regulation. Importantly, our model specifically targets
Cdkl5 deletion in excitatory neurons postnatally, whereas many of the contrasting studies use constitutive
Cdkl5 KO mice that lack CDKL5 in all cell types throughout development. This difference may explain some of the divergent results, as the absence of CDKL5 in non-excitatory cells (e.g., inhibitory neurons) in constitutive KOs could influence synaptic connectivity in ways that are not captured by excitatory neuron-specific deletion.
As previously reported in a conditional
Cdkl5 KO model in which selective deletion in excitatory neurons is induced during early cortical development (embryonic day 9.5–10.5; [
28]), we observed an increased density of immature dendritic spines accompanied by a marked reduction in mature spines. These findings further support the critical role of CDKL5 in regulating synaptic maturation and stability in the adult brain. Additionally, in
Cdkl5flox/Y(Cre⁺) mice, overall spine density on CA1 pyramidal neurons was increased compared to controls. However, a higher proportion of these spines exhibited a filopodia-like morphology, characteristic of immature spines. This shift in the balance between immature and mature spines, favoring the former, is also consistent with observations from constitutive
Cdkl5 KO models [
18,
33,
39,
40], further supporting the idea that CDKL5 function in excitatory neurons is essential to maintain synaptic integrity and confirming that synaptic abnormalities are not exclusively the result of disrupted neurodevelopment but can also arise following postnatal loss of CDKL5 function.
Our results showed that in
Cdkl5flox/Y(Cre
+) mice, the ratio between glutamatergic and GABAergic terminals in both the cortex and hippocampus was reduced, primarily due to a decrease in excitatory terminals. This altered balance, with its shift toward excessive inhibition, may impair neuronal firing and disrupt proper signal processing. Such an inhibitory dominance has previously been hypothesized in constitutive
Cdkl5 KO mouse models, in which an increase in inhibitory terminals was reported, associated with impaired long-term potentiation (LTP) [
41,
42].
From a phenotypic perspective,
Cdkl5flox/Y(Cre
+) adult mice display a phenotype that is strikingly similar to that of germline knockouts, including deficits in motor coordination, repetitive and stereotyped behaviors, reduced sociability, and impaired spatial memory. Despite the absence of spontaneous seizures in this model, in contrast with previous reports [
26,
29], these mice exhibit clear behavioral abnormalities that align with core symptoms of CDKL5 deficiency disorder (CDD).
Our findings from postnatal deletion of Cdkl5 in forebrain glutamatergic neurons reveal a behavioral phenotype that partially overlaps with previously reported studies in [
7]. Importantly, unlike the milder phenotype reported in the Nex-Cre conditional knockout model [
7], which showed relatively selective impairments in hippocampal-dependent memory and modest behavioral alterations, our inducible deletion leads to a more severe and broader spectrum of behavioral deficits. These include pronounced motor coordination impairments and marked social interaction deficits that exceed those observed in the Nex-Cre model. Differently from GABAergic neuron-specific knockouts [
6], all conditional models targeting forebrain excitatory neurons, whether during brain development (
Emx1::Cre [
6]
; Nex-Cre [
7]) or in adulthood (present study,
Camk2a-iCre), consistently display hindlimb clasping behavior. This indicates that loss of
Cdkl5 in excitatory neurons is sufficient to induce this motor phenotype regardless of the timing of deletion, and further supports the notion that this abnormality is specifically linked to excitatory, rather than inhibitory, neuron dysfunction. All conditional models maintained normal body weight and viability [
6,
7], paralleling the general health parameters observed in our
Cdkl5flox/Y(Cre
+) mice.
In contrast, hyperactivity in the open field test and stereotypic jumping behavior, hallmark phenotypes consistently observed in constitutive
Cdkl5 knockout mice [
20,
33,
39,
43], were absent in our
Cdkl5flox/Y(Cre
+) animals. Similarly, some sensory functions, such as olfaction and mechanical pain perception, remain preserved, likely because the selective deletion of CDKL5 in central glutamatergic neurons spares peripheral sensory neurons and primary sensory pathways. This suggests that CDKL5’s critical role in the adult brain is focused on cortical and hippocampal circuits involved in cognition, memory, and social behavior, rather than on peripheral or primary sensory processing. Indeed,
Cdkl5 is expressed in nociceptive dorsal root ganglia (DRG) neurons [
35], further supporting the idea that peripheral CDKL5 expression contributes to pain processing pathways.
Together, these findings emphasize that the timing and cell-type specificity of Cdkl5 deletion critically influence the spectrum and severity of behavioral phenotypes. While postnatal deletion in forebrain excitatory neurons is sufficient to reproduce core features of CDD, such as motor deficits, social impairments, and cognitive dysfunction, it does not fully recapitulate the broader phenotype observed in constitutive knockouts. This highlights the pivotal role of excitatory neurons in mediating key aspects of the disorder, while also suggesting that additional features may require Cdkl5 loss in other neuronal populations and/or during early developmental stages.
The presence of microglial activation in the brains of
Cdkl5flox/Y(Cre⁺) mice suggests a potential neuroinflammatory response that is secondary to neuronal dysfunction, a phenomenon previously reported in constitutive
Cdkl5 knockout models [
23,
24]. Notably, the increase in microglial cells is restricted to the cortex and absent in the hippocampus, indicating a region-specific neuroinflammatory response that may reflect differential vulnerability or the engagement of compensatory mechanisms across brain regions. Since microglial activation is often associated with epilepsy, and considering that this model has shown epileptic activity in other studies [
26,
29], the elevated microglial cell number may point to underlying neuronal hyperactivity that is not readily detectable without electrophysiological assessment. However, this microgliosis, observed six weeks after
Cdkl5 deletion, does not appear to worsen over time, suggesting it represents an early and transient response to functional alterations in neuronal circuits rather than a progressive neuroinflammatory process. This pattern implies that microglial activation in this model may reflect a homeostatic adaptation to altered neuronal activity rather than an ongoing degenerative process. Supporting this, the reduced number of neurons observed in the hippocampal CA1 field of
Cdkl5flox/Y(Cre⁺) mice compared to controls also shows no deterioration over time.
At present, we do not have a clear explanation for why we do not observe epilepsy or increased mortality in this model, unlike in previous studies [
26,
29]. Since the genetic background of the mice and the timing of
Cdkl5 deletion closely match previously published conditions, we can hypothesize that environmental and husbandry factors, such as animal management, diet, and environmental stressors, may influence the onset of epileptic seizures and overall survival. This lack of reproducibility has also been reported in other murine models of epilepsy [
44], and it nonetheless underscores the limitations of this phenotype in the mouse models of CDD.
4. Materials and Methods
4.1. Animals
All experiments were conducted on male C57BL/6J mice carrying a floxed allele of the
Cdkl5 gene (
Cdkl5flox/Y) either with or without the Camk2a-CreERT2 transgene.
Cdkl5flox/Y; Camk2a-CreERT2 (referred to as
Cdkl5flox/Y(Cre⁺)) mice were generated by crossing
Cdkl5flox/flox females [
5] with Camk2a-CreERT2 males [
45]. Male
Cdkl5flox/Y littermates lacking the Camk2a-CreERT2 transgene (
Cdkl5flox/Y (Cre⁻)) were used as controls. Postnatal day zero (P0) was designated as the day of birth, and 24 h later mice were considered as one-day-old animals (P1). After weaning (P21–23), mice were housed three to five per cage under a 12-h light/dark cycle in a temperature- and humidity-controlled environment, with food and water provided ad libitum. Animal health and well-being were monitored by the veterinary service. All efforts were made to minimize animal suffering and to keep the number of animals used to a minimum. Experiments were carried out on a total of 48 mice (
Cdkl5flox/Y(Cre⁻), n = 18;
Cdkl5flox/Y(Cre⁺), n = 30).
4.2. Tamoxifen Treatment
Starting at three months of age, all mice were treated with tamoxifen (Sigma-Aldrich, Saint Louis, MO, USA) dissolved in corn oil (Sigma-Aldrich, Saint Louis, MO, USA) at a concentration of 20 mg/ml. Tamoxifen was administered via intraperitoneal injections at a dose of 100 mg/kg once daily for five consecutive days.
4.3. Behavioral Assays
Mice were subjected to a battery of behavioral tests, arranged to minimize the effect of one test on the evaluation of the next. To further reduce inter-test interference, a recovery period of at least 24 h was allowed between each test. All behavioral experiments and subsequent analyses were conducted blind to genotype. Prior to each session, mice were habituated to the testing room for at least 1 h, and all tests were performed at the same time of day.
4.3.1. Marble Burying
The marble burying test was performed by placing mice individually in transparent polycarbonate cages (40 × 25 × 15 cm) filled with 6 cm of clean wood chip bedding. Each mouse was first habituated to the testing cage (without marbles) for 5 min. After the habituation period, the mouse was gently removed, and twenty glass marbles (14.3 mm in diameter) were arranged in a 4 × 5 grid on the surface of the bedding. The mouse was then reintroduced into the same cage and left undisturbed for 30 min. The number of marbles that were at least two-thirds buried at the end of the trial was counted.
4.3.2. Hind-Limb Clasping
Mice were suspended by their tail for 2 min, and their behavior was video-recorded. A clasping event is defined by the retraction of the hind-limbs toward the abdomen and midline. From the video recordings, two parameters were analyzed: (1) the total duration of hind-limb clasping exhibited by each mouse during the 2-min suspension and (2) the percentage of mice showing clasping for at least 3 s during the trial. Evaluation from video recordings was performed by an experimenter blinded to genotype.
4.3.3. Accelerating Rotarod Assay
Motor coordination was assessed using an accelerating rotarod apparatus (Ugo Basile, Gemonio, Italy). Prior to testing, mice were briefly trained on the rotarod at a constant speed of 5 rpm for 30 s. Testing was then performed at an accelerating linear speed (5-35 rpm within 270 s + 30 s max speed). Four testing trials with an intertrial interval of 1 h were performed. The latency to fall from the rotating rod and the number of passive rotations (rotations in which the mouse does not perform any coordinated movement but is passively transported by the rotating apparatus) were recorded for each trial.
4.3.4. Open Field
Mice were individually placed in the center of a square open field arena (50 × 50 cm), and their behavior was recorded for 20 min using a video camera positioned above the center of the arena. Distinct features of locomotor activity, including total distance traveled, average locomotion velocity, and the time spent in the central and peripheral zones were scored using EthoVision15XT software (Noldus Information Technology, Wageningen, The Netherlands). The open field arena was cleaned with 70% ethanol between test subjects.
4.3.5. Three-Chambers
Social interaction was assessed using an open-topped plastic apparatus divided into three chambers (20 × 40 × 22 cm) by two transparent walls, each equipped with a retractable doorway to allow access between compartments. The experimental mouse was placed in the center of the middle chamber and allowed to explore the whole apparatus for two trials of 5 min each. On the first trial (habituation phase) the stimulus cages were empty, and the experimental mouse was left undisturbed to explore the apparatus and habituate to the testing environment. At the end of this trial, the experimental mouse was confined to the central chamber using two transparent Plexiglas doors. On the second trial (sociability phase), a stimulus mouse (an age-matched unfamiliar C57BL/6 J male mouse) was introduced into one of the stimulus cages, while a novel object (a plastic cylinder filled with clean wood chip bedding) was introduced in the other one. The position of the social stimulus and of the object was counterbalanced between genotypes. The apparatus and the stimulus cages were cleaned with 70% ethanol at the end of each second trial. The total time spent in each chamber was automatically scored using EthoVision15XT software (Noldus Information Technology, Wageningen, The Netherlands).
4.3.6. Barnes Maze
Spatial memory acquisition and retention were evaluated using the Barnes maze test. The apparatus consisted of a circular platform (1 m in diameter) elevated 60 cm above the floor, with twenty evenly spaced holes (each 5 cm in diameter) along the perimeter (Ugo Basile, Gemonio, Italy). An escape box was placed under one of the holes. Mice were trained to locate the escape box over three consecutive days (three trials per day, maximum duration of 3 min per trial), with a 30-min intertrial interval. At the beginning of each trial, mice were placed in the center of the platform under a dark cylindrical chamber for 10 s; the chamber was then lifted to start the trial. Mice that initially failed to locate the escape box within 3 min were gently guided to the target by the experimenter. To assess spatial memory retention, a 90 s probe trial was performed 24 h after the last trial, during which the escape box was removed. Latency to enter the escape box during training sessions, latency to locate the former location of the escape box (i.e., the target zone) during the probe trial, and the total time spent in the target zone were automatically scored using EthoVision XT 15 software (Noldus Information Technology, Wageningen, The Netherlands).
4.3.7. Passive Avoidance
For the passive avoidance test, a memory task involving contributions from both the hippocampus and amygdala, the apparatus consisted of a tilting-floor box (47 x 18 x 26 cm) divided into two compartments (illuminated and dark) by a sliding door, and a control unit incorporating a shock generator (Ugo Basile, Gemonio, Italy). On day 1, mice were placed in the illuminated compartment, and upon entering the dark compartment, they received a brief mild foot shock (0.4 mA for 3 s). Mice were removed from the apparatus 15 s after the shock was delivered. After a 24 h retention period, mice were returned to the illuminated compartment, and the latency to re-enter the dark compartment was scored, up to a maximum of 360 s. The two compartments were cleaned with 70% ethanol between the testing of each subject.
4.3.8. Buried Food Test
The buried food test assesses olfactory function based on the animal’s ability to detect volatile odors and its natural tendency to use olfactory cues for foraging. The testing protocol consisted of three days and included an odor familiarization phase on day 1, food deprivation on day 2, and testing on day 3. On day 1, mice were placed in a clean cage with a piece of raisin (identical to the one used during the testing day) and left overnight. On day 2, cages were inspected to confirm raisin consumption, ensuring that the bait was highly palatable. Mice were then food-deprived for 24 h. On day 3, mice were individually placed in a clean cage containing 4 cm of bedding and allowed to acclimate for 5 min to reduce the interference of novel environment exploration during the test. A piece of raisin was then buried beneath 1 cm of bedding in a randomly selected corner of the cage. Mice were reintroduced into the cage, and the latency to retrieve the raisin using their front paws was measured, up to a maximum of 900 s.
4.3.8. Von Frey Filament Test
Mechanical allodynia was quantified by measuring the hind paw withdrawal response to von Frey filament stimulation (Ugo Basile, Gemonio, Italy). Mice were acclimated for 1 hour in acrylic cages (12 × 20 × 17 cm) with a wire-grid floor (5 mm²). A single, unbending von Frey filament was applied perpendicularly to the plantar surface of each hind paw with gradually increasing force, and stimulation was stopped upon observation of a withdrawal response. Latency to paw withdrawal and the force (in grams) required to elicit the response were calculated as the average of three measurements taken from each hind paw.
4.4. Histological and Immunohistochemistry Procedures
Animals were anesthetized with isoflurane (2% in pure oxygen) and sacrificed through cervical dislocation. Brains were quickly removed and cut along the midline. Left hemispheres were Golgi-stained or quickly frozen and used for Western blot analyses. Right hemispheres were fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) for 48 h, kept in 15-20% sucrose for an additional 24 h, frozen with dry ice, and stored at - 80°C.
4.4.1. Immunofluorescence Staining
Right hemispheres were cut using a freezing microtome (Microm GmbH, Walldorf, Germany) into 30-μm-thick coronal sections, which were serially collected in 96-well plates containing a solution composed of 30% glycerol, 30% ethylene glycol, 0.02% sodium azide in 0.1 M PBS. One out of every eight free-floating sections from the hippocampal formation was incubated overnight at 4°C with one of the following primary antibodies: rabbit polyclonal anti-AIF-1 antibody (1:300; Thermo Fisher Scientific, Waltham, MA, USA), rabbit polyclonal anti-VGAT antibody (1:300; Synaptic Systems, Göttingen, Germany), or goat polyclonal anti- VGLUT1 antibody (1:300; Synaptic Systems, Göttingen, Germany). The following day, the sections were incubated for 2 h at room temperature with one of the following secondary antibodies: Alexa Fluor 555-conjugated anti-rabbit IgG antibody (1:200; Thermo Fisher Scientific, Waltham, MA, USA), or Alexa Fluor 555-conjugated anti-goat IgG antibody (1:200; Thermo Fisher Scientific, Waltham, MA, USA). Sections were then mounted with DAPI (40,6-diamidino-2-phenylindole)-Fluoromount-G® (SouthernBiotech, Birmingham, AL, USA).
4.4.2. Golgi Staining
Left hemispheres were Golgi-stained using the FD Rapid Golgi StainTM Kit (FD Neuro Technologies, Columbia, MD, USA), as previously described [
46]. Hemispheres were cut using a cryostat (Histo-Line Laboratories, Pantigliate, Italy) into 100-µm-thick coronal sections, which were directly mounted onto Superfrost
® Plus microscope slides (Thermo Fisher Scientific, Waltham, MA, USA) and air-dried at room temperature for 1 day. After drying, sections were rinsed with distilled water, stained in the developing solution of FD Rapid Golgi StainTM Kit (FD NeuroTechnologies, Columbia, MD, USA), and coverslipped with DPX mounting medium (Sigma-Aldrich, Saint Louis, MO, USA).
4.4.3. In Situ Hybridization (ISH)
Right hemispheres were cut using a cryostat into 15-μm-thick sagittal sections which were serially collected onto Superfrost
® Plus microscope slides (Thermo Fisher Scientific, Waltham, MA, USA). The in situ hybridization (ISH) for
Cdkl5 mRNA was performed using the BaseScope™ technology (Bio-Techne, Minneapolis, MN, USA), following the manufacturer’s protocol. A 1ZZ probe targeting exon 4 of the
CDKL5 transcript was used, as previously described [
47].
4.5. Image Acquisition and Measurements
Fluorescence images of brain sections processed for AIF-1 immunofluorescence or for Cdkl5 mRNA detection via BaseScope™ were acquired using a Nikon Eclipse TE 2000-S fluorescence microscope equipped with a DS-Qi2 digital SLR camera (Nikon Instruments Inc., Tokyo, Japan). Fluorescence images of sections immunostained for VGAT or VGLUT1 were acquired using a Nikon Ti-E fluorescence microscope connected to an A1R confocal system (Nikon Instruments Inc., Tokyo, Japan). Bright-field images of Golgi-stained brain sections were acquired using a light microscope (Leica Microsystems, Shinjuku City, Tokyo, Japan) equipped with a motorized stage and focus control system, and a color digital camera (Coolsnap-Pro, Media Cybernetics, Rockville, MD, USA).
4.5.1. Quantification of VGAT and VGLUT1 Immunoreactive Puncta
Three to four sections per animal were analyzed. For each section, three images were acquired from layers II–III of the somatosensory cortex and from the stratum oriens of the hippocampus using a 60× oil immersion objective lens (1.32 NA; zoom factor = 8). The density of individual puncta exhibiting VGAT or VGLUT1 immunoreactivity was manually quantified using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA), as previously described [
17]. The number of immunoreactive puncta was expressed per µm
2.
4.5.2. Dendritic Spine Number and Morphology
In Golgi-stained sections, dendritic spines located in the basal dendrites of hippocampal pyramidal neurons were captured with a 100× oil immersion objective lens (1.4 NA) and manually counted using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA). For each mouse, 10-12 dendritic segments of 10 μm each were analyzed, and spine density was expressed as the number of spines per 10 μm of dendrite. Based on their morphology, dendritic spines can be classified into two categories reflecting their maturation state: immature spines (filopodium-like, thin, and stubby-shaped) and mature spines (mushroom- and cup-shaped). The number of spines in each category was quantified and expressed as a percentage.
4.5.3. Cell Density
Starting from 20× magnification images, the number of AIF-1-positive cells in the hippocampus and somatosensory cortex was manually counted using the point tool in Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA), and cell density was expressed as AIF-1-positive cells/mm². Similarly, starting from 40× magnification images, the density of DAPI-positive nuclei in the CA1 field of the hippocampus was manually counted and expressed as cells/mm².
4.6. Western Blotting
Tissue samples were homogenized in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton-X100, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with 1 mM PMSF and 1% protease and phosphatase inhibitor cocktail (Sigma-Aldrich, Saint Louis, MO, USA). Protein concentration was determined using the Bradford method [
48]. Equal amounts of protein (50 μg) were separated by electrophoresis on a 4-12% Mini-PROTEAN
® TGX™ Gel (Bio-Rad, Hercules, CA, USA) and transferred to a Hybond ECL nitrocellulose membrane (GE Healthcare, Chicago, IL, USA). The following primary antibodies were used: sheep polyclonal anti-CDKL5 (1:1000; University of Dundee, Dundee, UK); rabbit polyclonal anti-P-EB2 (S222; 1:1000; Covalab, Bron, France); rabbit polyclonal anti-EB2 (1:1000; Abcam, Cambridge, UK); rabbit polyclonal anti-GAPDH (1:5000; Sigma-Aldrich, Saint Louis, MO, USA). The following secondary antibodies were used: HRP-conjugated anti-sheep IgG antibody (1:5000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA); HRP-conjugated anti-rabbit IgG antibody (1:5000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Densitometric analysis of digitized Western blot images was performed using ChemiDocTM MP Imaging System equipped with Image Lab Touch Software (Bio-Rad, Hercules, CA, USA), which automatically highlights saturated pixels in red. Images acquired with exposure times that generated protein signals out of a linear range were not considered for quantification.
4.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0.1 (GraphPad Software, Boston, MA, USA). Data are presented as means ± standard error of the mean (SEM). Statistical significance was assessed using Student’s t-test (or Welch’s t-test in case of unequal variances), or two-way analysis of variance (ANOVA) followed by Fisher’s LSD post hoc test for parametric data, or Mann-Whitney for non-parametric data, as specified in the figure legends. A p-value < 0.05 was considered statistically significant.
Figure 1.
Postnatal Cdkl5 deletion in forebrain glutamatergic neurons of Cdkl5flox/Y(Cre⁺) mice. (A) Schematic representation of the experimental design. Three-month-old conditional knockout (cKO) hemizygous male Cdkl5flox/Y(Cre⁺) mice received intraperitoneal (i.p.) injections of tamoxifen (100 mg/kg) for five consecutive days to activate Cre recombinase and induce Cdkl5 gene deletion. Cdkl5flox/Y(Cre⁻) littermates were used as controls. Animals were divided into three groups and sacrificed at different time points (4, 6, and 20 weeks after the start of tamoxifen treatment). (B) Western blot analysis of Cdkl5 protein expression in tamoxifen-treated mice. Protein extracts from the somatosensory cortex (Ctx), hippocampus (Hp), and cerebellum (Cb) were analyzed in Cdkl5flox/Y(Cre⁻) (n = 3) and Cdkl5flox/Y(Cre⁺) (n = 3) mice. The histogram on the right shows Cdkl5 levels normalized to GAPDH and expressed as a percentage relative to controls. Representative immunoblots for Cdkl5 and GAPDH from two animals per group are shown on the left. (C) Western blot analysis of phosphorylated EB2 (phospho-EB2) and total EB2. Protein extracts from the cortex (Ctx), hippocampus (Hp), and cerebellum (Cb) were analyzed as in panel (B). The histogram on the right shows phospho-EB2 levels normalized to total EB2 and expressed relative to controls. Representative immunoblots from two animals per group are shown on the left. Values in panels (B,C) are presented as means ± SEM. *p < 0.05, ***p < 0.001 (two-tailed Student’s t-test). (D) Representative fluorescence images of cortical and hippocampal sections from one animal per group processed by in situ hybridization (ISH) for Cdkl5 mRNA (red). Scale bar = 50 µm. (E) Body weight (in grams) of Cdkl5flox/Y(Cre⁻) (n = 15-18) and Cdkl5flox/Y(Cre⁺) (n = 23-26) mice measured weekly over a five-week period following tamoxifen treatment, and of an additional cohort of Cdkl5flox/Y(Cre⁻) (n = 15) and Cdkl5flox/Y(Cre⁺) (n = 10) mice assessed 12 weeks after tamoxifen administration. Data are shown as mean ± SEM. *p < 0.05, Fisher’s LSD post hoc test following two-way ANOVA. Abbreviations: Ctx = somatosensory cortex; Hp = hippocampus; Cb = cerebellum; CA1 = hippocampal CA1 region.
Figure 1.
Postnatal Cdkl5 deletion in forebrain glutamatergic neurons of Cdkl5flox/Y(Cre⁺) mice. (A) Schematic representation of the experimental design. Three-month-old conditional knockout (cKO) hemizygous male Cdkl5flox/Y(Cre⁺) mice received intraperitoneal (i.p.) injections of tamoxifen (100 mg/kg) for five consecutive days to activate Cre recombinase and induce Cdkl5 gene deletion. Cdkl5flox/Y(Cre⁻) littermates were used as controls. Animals were divided into three groups and sacrificed at different time points (4, 6, and 20 weeks after the start of tamoxifen treatment). (B) Western blot analysis of Cdkl5 protein expression in tamoxifen-treated mice. Protein extracts from the somatosensory cortex (Ctx), hippocampus (Hp), and cerebellum (Cb) were analyzed in Cdkl5flox/Y(Cre⁻) (n = 3) and Cdkl5flox/Y(Cre⁺) (n = 3) mice. The histogram on the right shows Cdkl5 levels normalized to GAPDH and expressed as a percentage relative to controls. Representative immunoblots for Cdkl5 and GAPDH from two animals per group are shown on the left. (C) Western blot analysis of phosphorylated EB2 (phospho-EB2) and total EB2. Protein extracts from the cortex (Ctx), hippocampus (Hp), and cerebellum (Cb) were analyzed as in panel (B). The histogram on the right shows phospho-EB2 levels normalized to total EB2 and expressed relative to controls. Representative immunoblots from two animals per group are shown on the left. Values in panels (B,C) are presented as means ± SEM. *p < 0.05, ***p < 0.001 (two-tailed Student’s t-test). (D) Representative fluorescence images of cortical and hippocampal sections from one animal per group processed by in situ hybridization (ISH) for Cdkl5 mRNA (red). Scale bar = 50 µm. (E) Body weight (in grams) of Cdkl5flox/Y(Cre⁻) (n = 15-18) and Cdkl5flox/Y(Cre⁺) (n = 23-26) mice measured weekly over a five-week period following tamoxifen treatment, and of an additional cohort of Cdkl5flox/Y(Cre⁻) (n = 15) and Cdkl5flox/Y(Cre⁺) (n = 10) mice assessed 12 weeks after tamoxifen administration. Data are shown as mean ± SEM. *p < 0.05, Fisher’s LSD post hoc test following two-way ANOVA. Abbreviations: Ctx = somatosensory cortex; Hp = hippocampus; Cb = cerebellum; CA1 = hippocampal CA1 region.
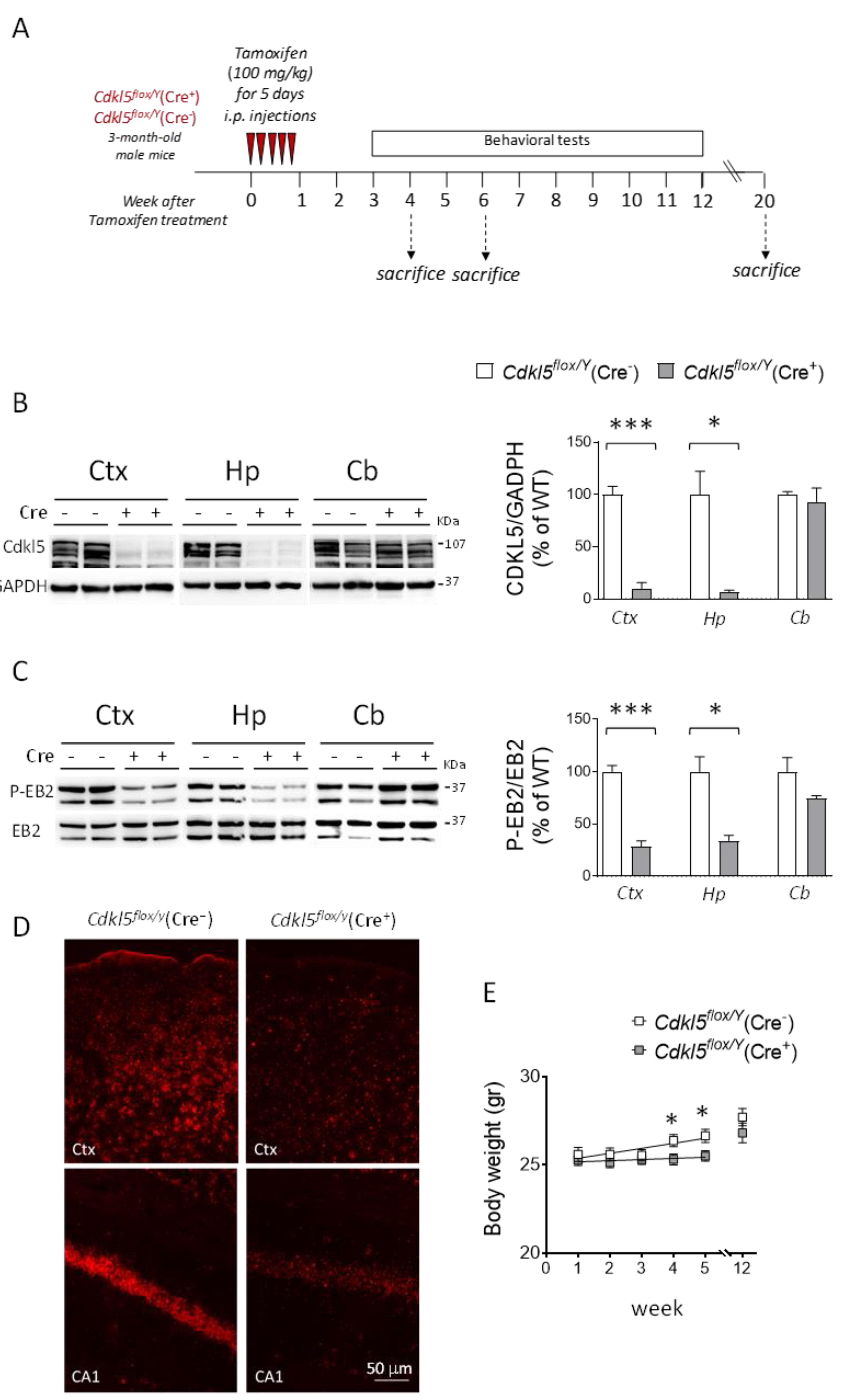
Figure 2.
Effect of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on motor control and autistic-like behaviors in Cdkl5flox/Y(Cre⁺) mice. (A) Accelerating rotarod test performed over four consecutive trials with 1-hour inter-trial intervals. The graph on the left shows the number of passive rotations per trial (i.e., instances in which the mouse remains immobile and is passively carried by the rotating rod). The graph on the right reports the average number of passive rotations across trials in tamoxifen-treated Cdkl5flox/Y(Cre⁻) (n = 17) and Cdkl5flox/Y(Cre⁺) (n = 26) mice. (B) Assessment of repetitive and stereotyped behaviors using the marble-burying test. The number of marbles buried by Cdkl5flox/Y(Cre⁻) (n = 18) and Cdkl5flox/Y (Cre⁺) (n = 26) mice is shown. (C) Hind-limb clasping behavior: total duration of clasping behavior over a 2-minute observation period (left) and percentage of mice displaying clasping (right) in tamoxifen-treated Cdkl5flox/Y(Cre⁻) (n = 16) and Cdkl5flox/Y(Cre⁺) (n = 24) mice. (D) Locomotor activity assessed in a 20-minute open field test. The graphs show average locomotion velocity (left) and total distance traveled (right) in tamoxifen-treated Cdkl5flox/Y(Cre⁻) (n = 8) and Cdkl5flox/Y(Cre⁺) (n = 20) mice. (E) Anxiety-like behavior in the open field test: time spent in the border (left) and center (right) zones of the arena during the same 20-minute session as in panel (D). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01. Unpaired two-tailed Student’s t-test for data in (E); Mann-Whitney test for data in (A right graph, B, and C); Fisher’s LSD post hoc test following two-way ANOVA for (A left graph and D).
Figure 2.
Effect of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on motor control and autistic-like behaviors in Cdkl5flox/Y(Cre⁺) mice. (A) Accelerating rotarod test performed over four consecutive trials with 1-hour inter-trial intervals. The graph on the left shows the number of passive rotations per trial (i.e., instances in which the mouse remains immobile and is passively carried by the rotating rod). The graph on the right reports the average number of passive rotations across trials in tamoxifen-treated Cdkl5flox/Y(Cre⁻) (n = 17) and Cdkl5flox/Y(Cre⁺) (n = 26) mice. (B) Assessment of repetitive and stereotyped behaviors using the marble-burying test. The number of marbles buried by Cdkl5flox/Y(Cre⁻) (n = 18) and Cdkl5flox/Y (Cre⁺) (n = 26) mice is shown. (C) Hind-limb clasping behavior: total duration of clasping behavior over a 2-minute observation period (left) and percentage of mice displaying clasping (right) in tamoxifen-treated Cdkl5flox/Y(Cre⁻) (n = 16) and Cdkl5flox/Y(Cre⁺) (n = 24) mice. (D) Locomotor activity assessed in a 20-minute open field test. The graphs show average locomotion velocity (left) and total distance traveled (right) in tamoxifen-treated Cdkl5flox/Y(Cre⁻) (n = 8) and Cdkl5flox/Y(Cre⁺) (n = 20) mice. (E) Anxiety-like behavior in the open field test: time spent in the border (left) and center (right) zones of the arena during the same 20-minute session as in panel (D). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01. Unpaired two-tailed Student’s t-test for data in (E); Mann-Whitney test for data in (A right graph, B, and C); Fisher’s LSD post hoc test following two-way ANOVA for (A left graph and D).
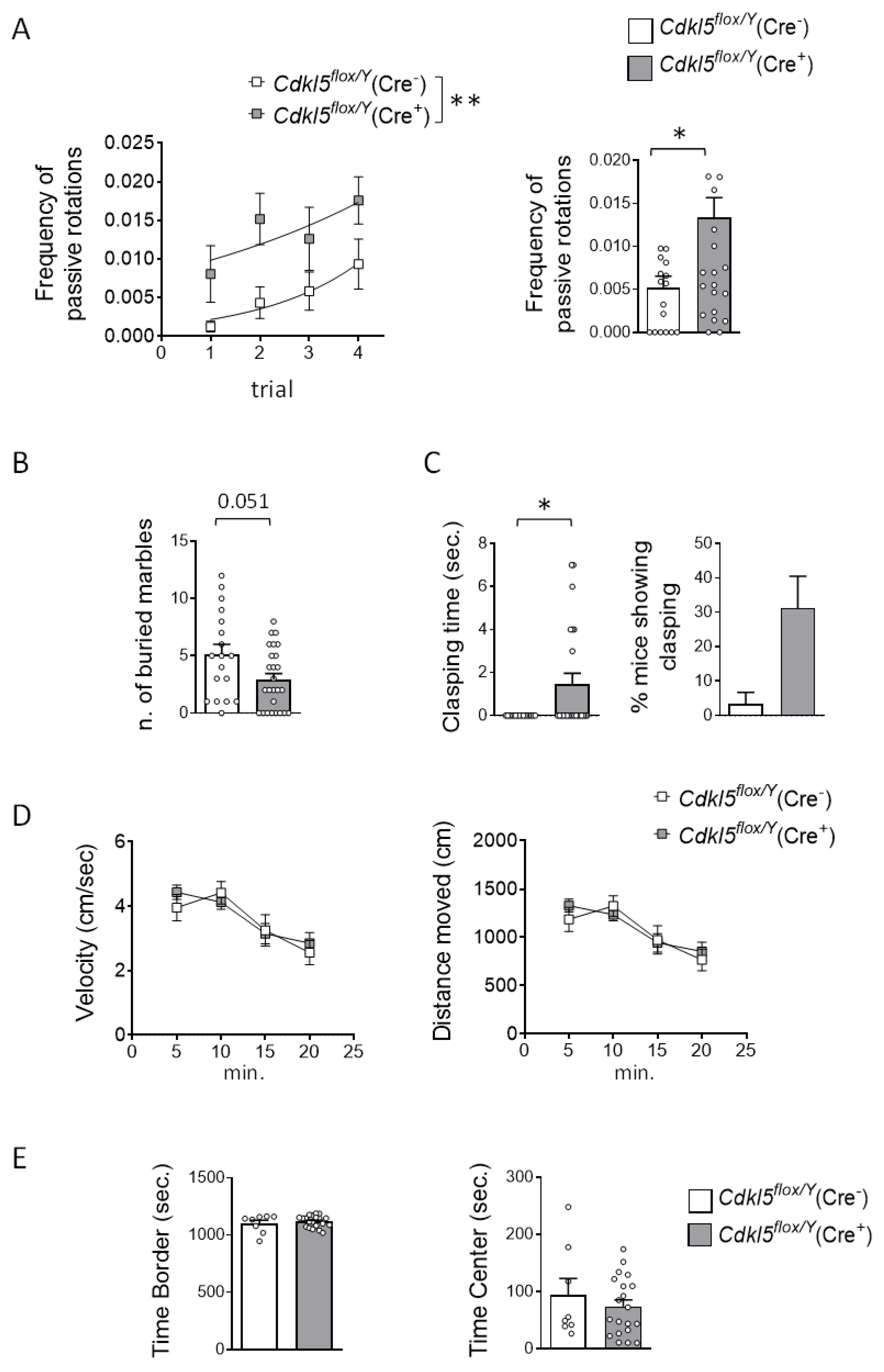
Figure 3.
Effects of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on sociability and hippocampal-dependent memory in Cdkl5flox/Y(Cre+) mice. (A,B) Three-Chamber Social Interaction Test. Panel (A) illustrates the schematic of the experimental paradigm. Panel (B) quantifies the cumulative time spent interacting with either an inanimate object or a social partner during the second trial (sociability phase). The test was conducted on the same cohort of tamoxifen-treated Cdkl5flox/Y(Cre−) (n = 10) and Cdkl5flox/Y(Cre+) (n = 10) mice. The asterisk denotes within-subject comparison. Data are presented as mean ± SEM. *p < 0.05, Fisher’s LSD post hoc test following two-way ANOVA. (C-E) Barnes Maze – Spatial Learning and Memory. Spatial learning performance during the 3-day acquisition phase is shown in Panel (C) as the mean latency to find the target hole. Panel (D) presents representative heat maps from the probe trial (day 4), with warmer colors indicating longer dwell times. Panel (E) displays quantitative results from the probe trial, including (left) latency to enter the target zone, (middle) number of errors (incorrect hole entries), and (right) cumulative time spent in the target zone. (F) Passive Avoidance Test. Graph depicts the latency to enter the dark compartment on day 1 (training) and day 2 (retention) for tamoxifen-treated Cdkl5flox/Y(Cre−) and Cdkl5flox/Y(Cre+) mice (n = 10 and 9 per group, respectively). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01. Fisher’s LSD post hoc test following two-way ANOVA (C); unpaired two-tailed Student’s t-test (B within groups, E right graph, and F); Welch’s t-test (E left graph); Mann-Whitney test (E middle graph).
Figure 3.
Effects of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on sociability and hippocampal-dependent memory in Cdkl5flox/Y(Cre+) mice. (A,B) Three-Chamber Social Interaction Test. Panel (A) illustrates the schematic of the experimental paradigm. Panel (B) quantifies the cumulative time spent interacting with either an inanimate object or a social partner during the second trial (sociability phase). The test was conducted on the same cohort of tamoxifen-treated Cdkl5flox/Y(Cre−) (n = 10) and Cdkl5flox/Y(Cre+) (n = 10) mice. The asterisk denotes within-subject comparison. Data are presented as mean ± SEM. *p < 0.05, Fisher’s LSD post hoc test following two-way ANOVA. (C-E) Barnes Maze – Spatial Learning and Memory. Spatial learning performance during the 3-day acquisition phase is shown in Panel (C) as the mean latency to find the target hole. Panel (D) presents representative heat maps from the probe trial (day 4), with warmer colors indicating longer dwell times. Panel (E) displays quantitative results from the probe trial, including (left) latency to enter the target zone, (middle) number of errors (incorrect hole entries), and (right) cumulative time spent in the target zone. (F) Passive Avoidance Test. Graph depicts the latency to enter the dark compartment on day 1 (training) and day 2 (retention) for tamoxifen-treated Cdkl5flox/Y(Cre−) and Cdkl5flox/Y(Cre+) mice (n = 10 and 9 per group, respectively). Data are presented as mean ± SEM. *p < 0.05, **p < 0.01. Fisher’s LSD post hoc test following two-way ANOVA (C); unpaired two-tailed Student’s t-test (B within groups, E right graph, and F); Welch’s t-test (E left graph); Mann-Whitney test (E middle graph).
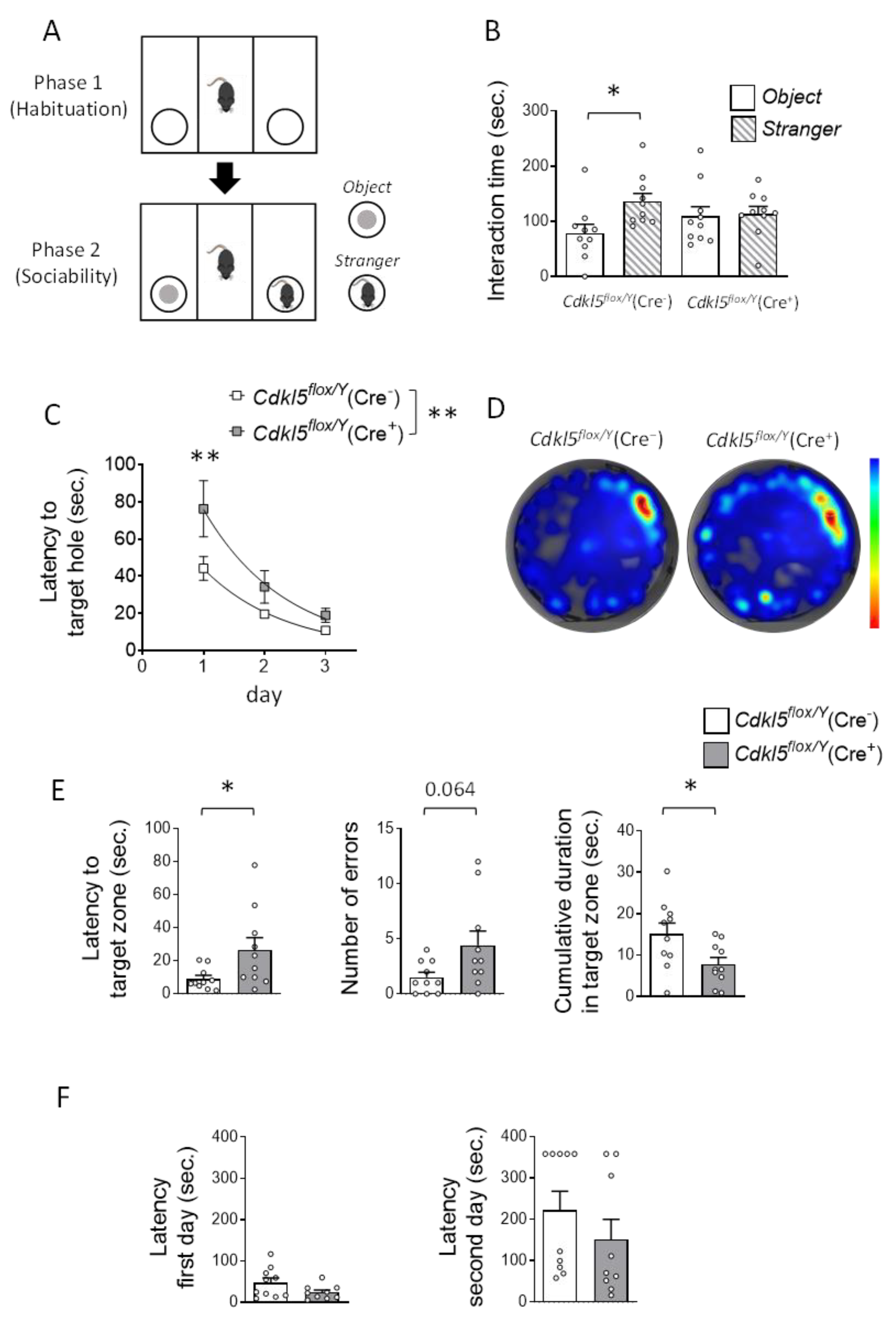
Figure 4.
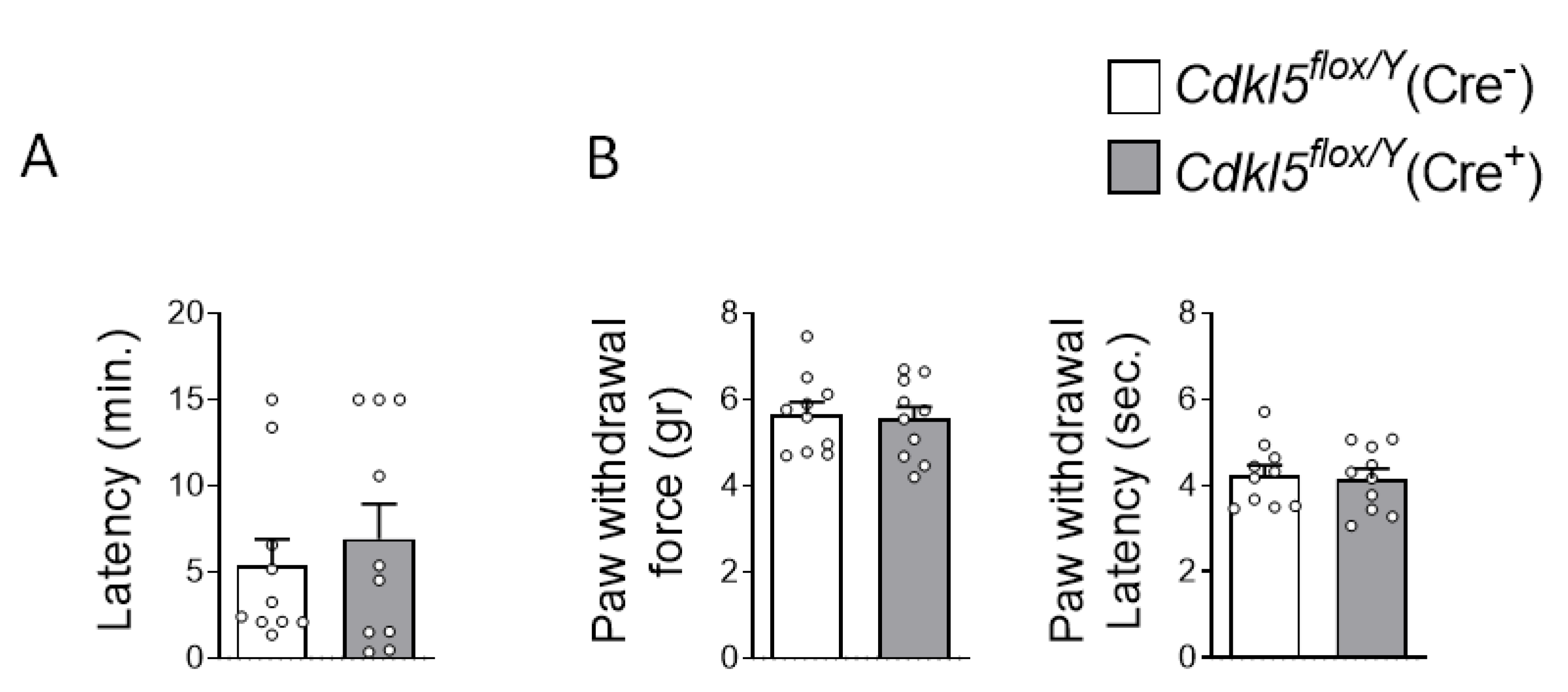
Effects of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on olfactory and pain perception in Cdkl5flox/Y(Cre+) mice. (A) Olfactory function was assessed using the buried food test. The histogram shows the latency to locate the buried raisin in tamoxifen-treated Cdkl5flox/Y(Cre−) (n = 10) and Cdkl5flox/Y(Cre+) (n = 10) mice. (B) Pain sensitivity was evaluated using the Von Frey filament test in the same animals. Mechanical allodynia was quantified by measuring the force (in grams) required to elicit paw withdrawal (left) and the latency of hind paw withdrawal following stimulation (right). Data are presented as mean ± SEM. Statistical analysis: unpaired two-tailed Student’s t-test.
Figure 4.
Effects of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on olfactory and pain perception in Cdkl5flox/Y(Cre+) mice. (A) Olfactory function was assessed using the buried food test. The histogram shows the latency to locate the buried raisin in tamoxifen-treated Cdkl5flox/Y(Cre−) (n = 10) and Cdkl5flox/Y(Cre+) (n = 10) mice. (B) Pain sensitivity was evaluated using the Von Frey filament test in the same animals. Mechanical allodynia was quantified by measuring the force (in grams) required to elicit paw withdrawal (left) and the latency of hind paw withdrawal following stimulation (right). Data are presented as mean ± SEM. Statistical analysis: unpaired two-tailed Student’s t-test.
Figure 5.
Effects of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on neuronal connectivity and dendritic spine maturation in Cdkl5flox/Y(Cre+) mice. (A,B) Quantification of fluorescent puncta per μm² positive for vesicular glutamate transporter 1 (VGLUT1, A) and vesicular GABA transporter (VGAT, B) in the somatosensory cortex (Ctx) and hippocampus (Hp) of Cdkl5flox/Y(Cre−) (n = 4) and Cdkl5flox/Y(Cre+) (n = 4) mice, six weeks after tamoxifen treatment. (C) Representative confocal images showing VGLUT1 (top) and VGAT (bottom) immunostaining in cortical sections from one mouse per group. Scale bar = 3 μm. (D,E) Dendritic spine analysis in CA1 pyramidal neurons of the hippocampus of Cdkl5flox/Y(Cre−) (n = 3) and Cdkl5flox/Y(Cre+) (n = 4) mice, twenty weeks after tamoxifen treatment. Panel (D) shows spine density in basal dendrites; panel (E) shows the proportion of immature and mature spine types relative to the total number of protrusions. (F) Representative Golgi-stained dendrites from one mouse per group. Blue arrows indicate mature spines; red arrows indicate immature spines. Scale bar = 3 μm. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Fisher’s LSD post hoc test following two-way ANOVA (A, B, and E); two-tailed Student’s t-test (D).
Figure 5.
Effects of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on neuronal connectivity and dendritic spine maturation in Cdkl5flox/Y(Cre+) mice. (A,B) Quantification of fluorescent puncta per μm² positive for vesicular glutamate transporter 1 (VGLUT1, A) and vesicular GABA transporter (VGAT, B) in the somatosensory cortex (Ctx) and hippocampus (Hp) of Cdkl5flox/Y(Cre−) (n = 4) and Cdkl5flox/Y(Cre+) (n = 4) mice, six weeks after tamoxifen treatment. (C) Representative confocal images showing VGLUT1 (top) and VGAT (bottom) immunostaining in cortical sections from one mouse per group. Scale bar = 3 μm. (D,E) Dendritic spine analysis in CA1 pyramidal neurons of the hippocampus of Cdkl5flox/Y(Cre−) (n = 3) and Cdkl5flox/Y(Cre+) (n = 4) mice, twenty weeks after tamoxifen treatment. Panel (D) shows spine density in basal dendrites; panel (E) shows the proportion of immature and mature spine types relative to the total number of protrusions. (F) Representative Golgi-stained dendrites from one mouse per group. Blue arrows indicate mature spines; red arrows indicate immature spines. Scale bar = 3 μm. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Fisher’s LSD post hoc test following two-way ANOVA (A, B, and E); two-tailed Student’s t-test (D).
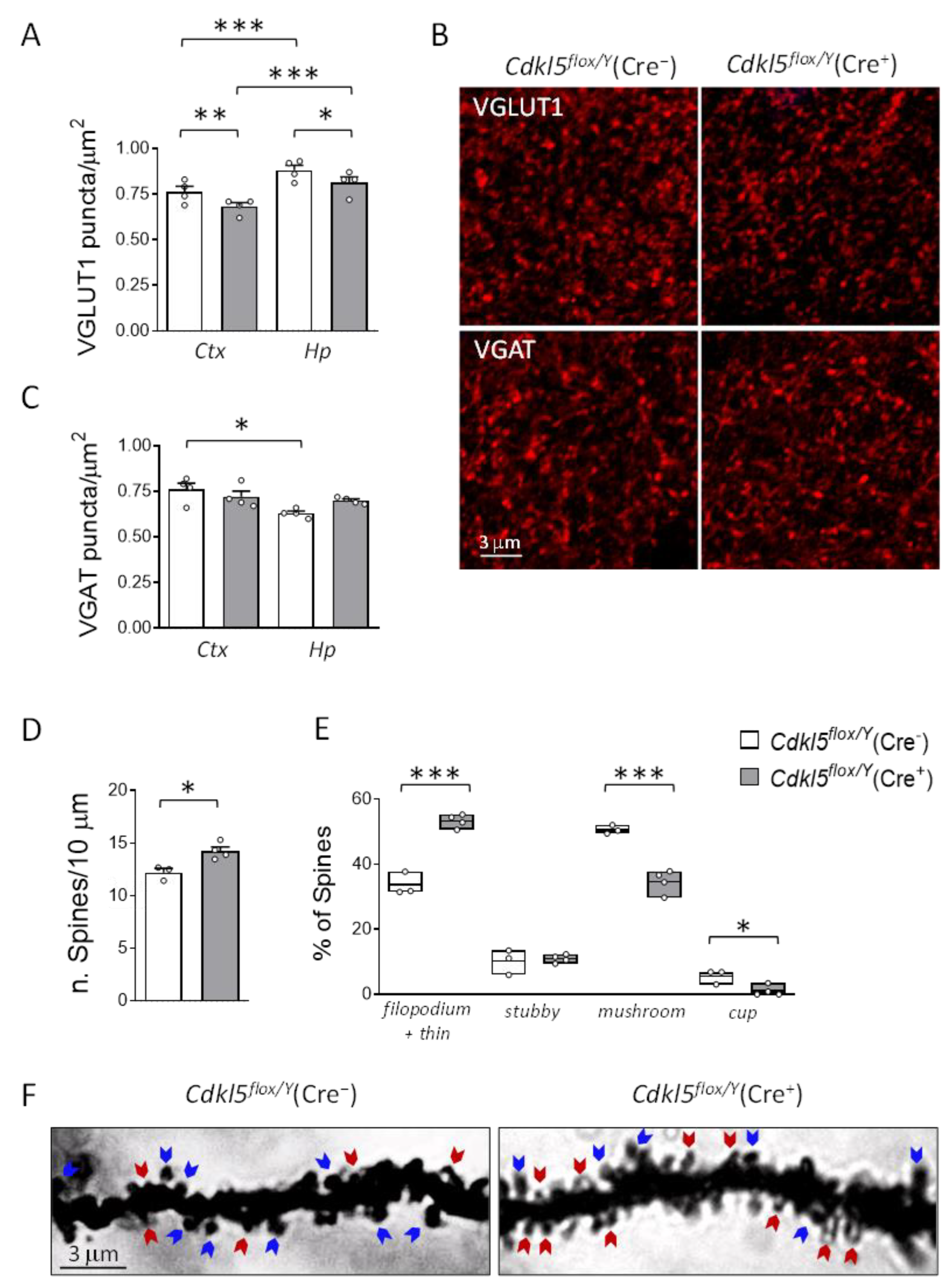
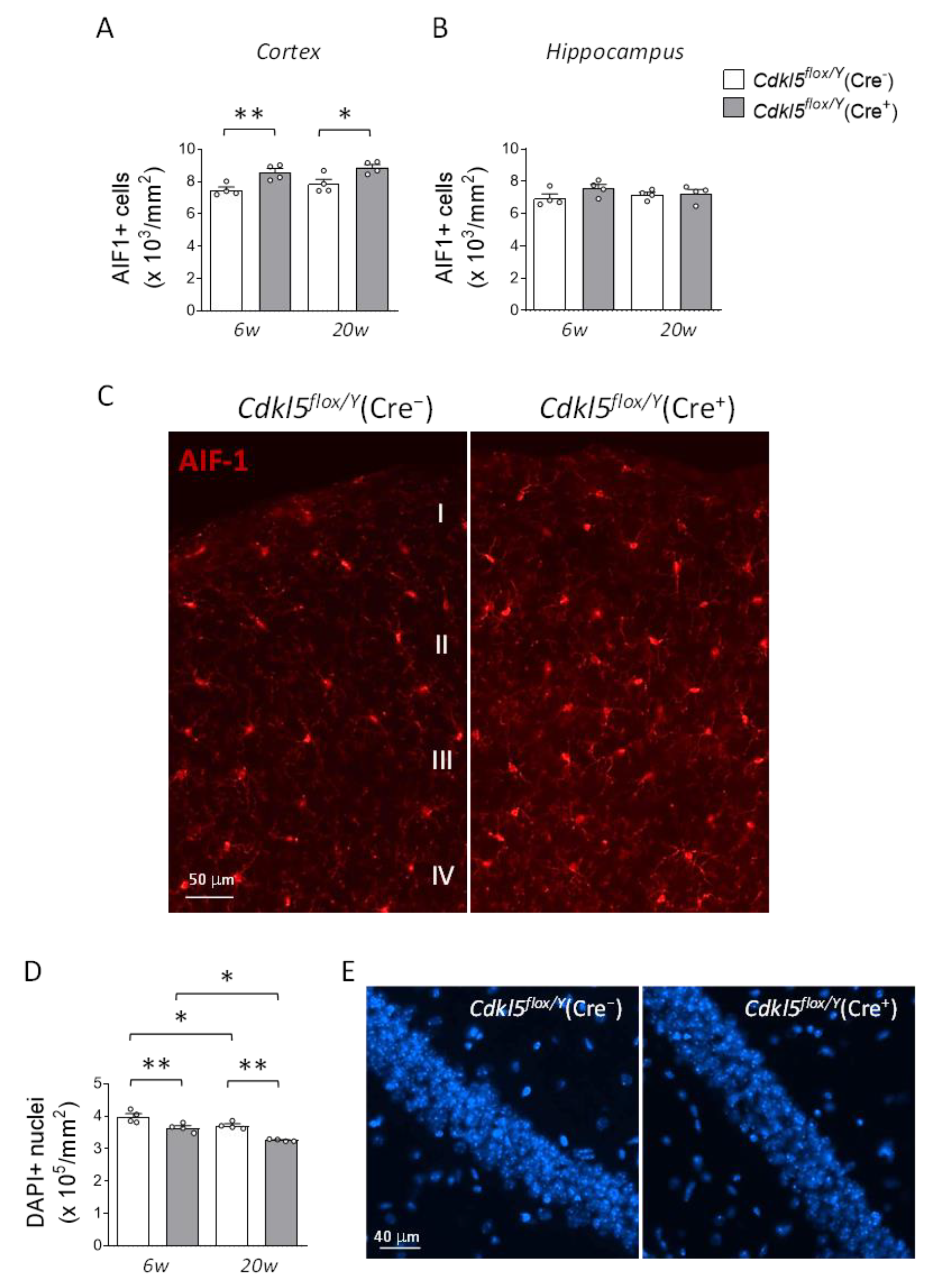
Figure 6.
Effects of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on microglial density and neuronal survival in Cdkl5flox/Y(Cre+) mice. (A,B) Quantification of AIF-1-positive cells in the somatosensory cortex (A) and hippocampus (B) of Cdkl5flox/Y(Cre−) and Cdkl5flox/Y(Cre+) mice (n = 4 per group), at six (6w) and twenty weeks (20w) after tamoxifen treatment. (C) Representative fluorescence images of AIF-1 immunostaining in cortical sections from one mouse per group at six weeks post-treatment. Scale bar = 50 μm. Cortical layers I-IV are indicated. (D) Quantification of DAPI-positive cells in the CA1 region of the hippocampus, from the same animals analyzed in panel (B). (E) Representative DAPI-stained hippocampal sections from one mouse per group, six weeks post-treatment. Scale bar = 40 μm. Data are shown as mean ± SEM. *p < 0.05, **p < 0.01. Fisher’s LSD post hoc test following two-way ANOVA (A, B, and D).
Figure 6.
Effects of postnatal Cdkl5 deletion in forebrain glutamatergic neurons on microglial density and neuronal survival in Cdkl5flox/Y(Cre+) mice. (A,B) Quantification of AIF-1-positive cells in the somatosensory cortex (A) and hippocampus (B) of Cdkl5flox/Y(Cre−) and Cdkl5flox/Y(Cre+) mice (n = 4 per group), at six (6w) and twenty weeks (20w) after tamoxifen treatment. (C) Representative fluorescence images of AIF-1 immunostaining in cortical sections from one mouse per group at six weeks post-treatment. Scale bar = 50 μm. Cortical layers I-IV are indicated. (D) Quantification of DAPI-positive cells in the CA1 region of the hippocampus, from the same animals analyzed in panel (B). (E) Representative DAPI-stained hippocampal sections from one mouse per group, six weeks post-treatment. Scale bar = 40 μm. Data are shown as mean ± SEM. *p < 0.05, **p < 0.01. Fisher’s LSD post hoc test following two-way ANOVA (A, B, and D).