Submitted:
16 June 2025
Posted:
18 June 2025
You are already at the latest version
Abstract
Keywords:
Introduction
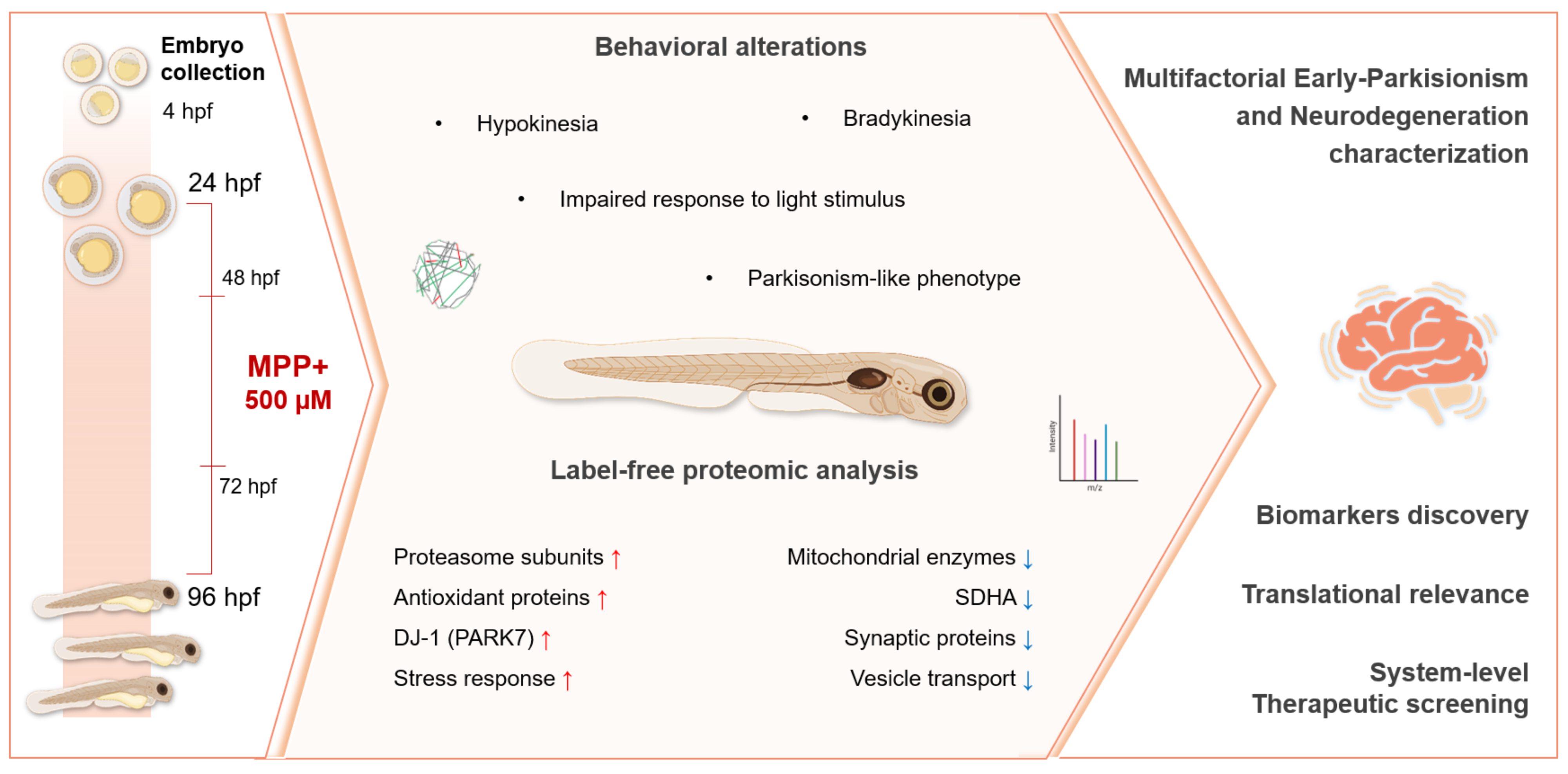
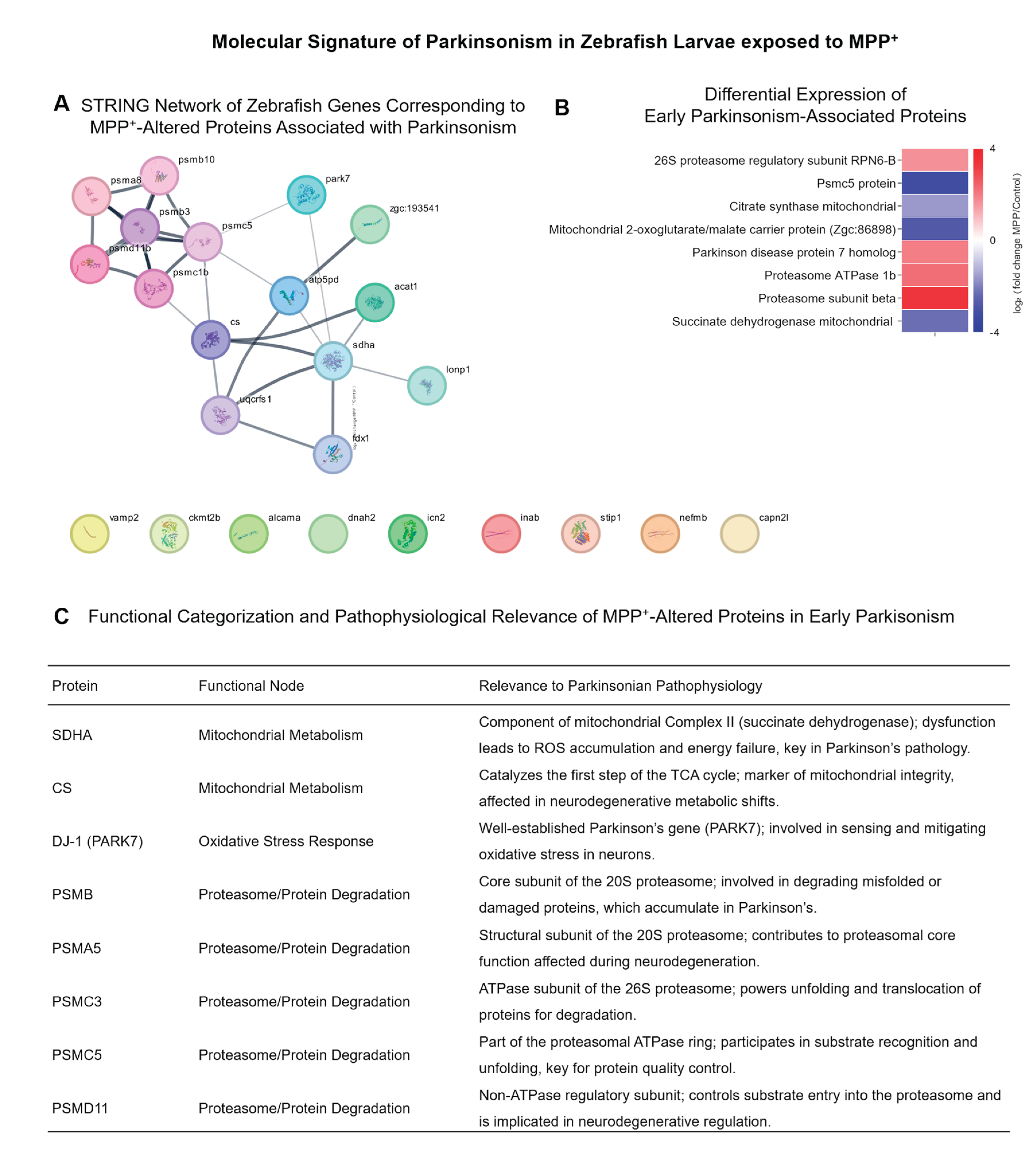
Results
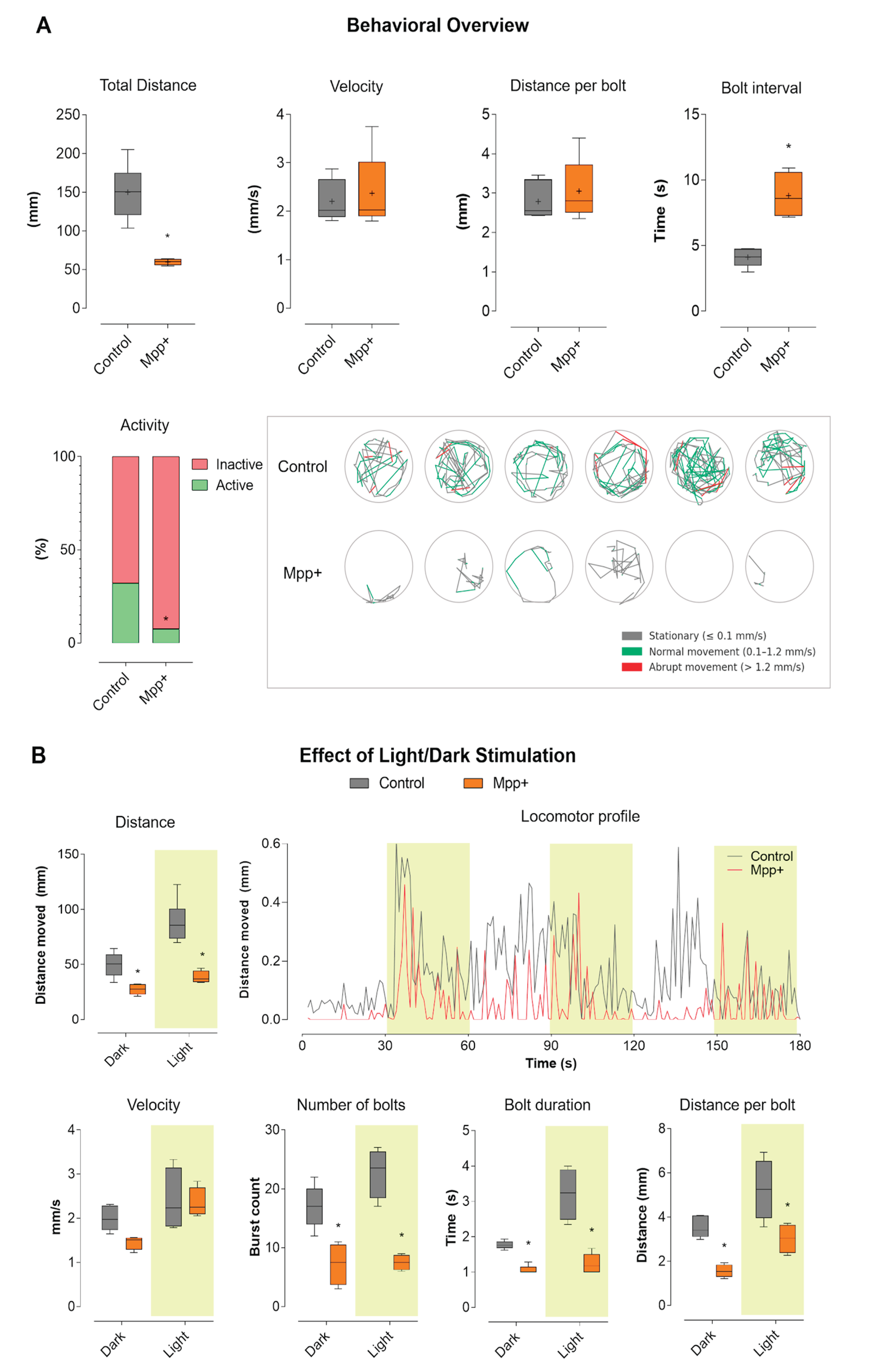
Evaluation of Locomotor Behavior Reveals Parkinsonian Motor Features in MPP⁺-Exposed Zebrafish Larvae
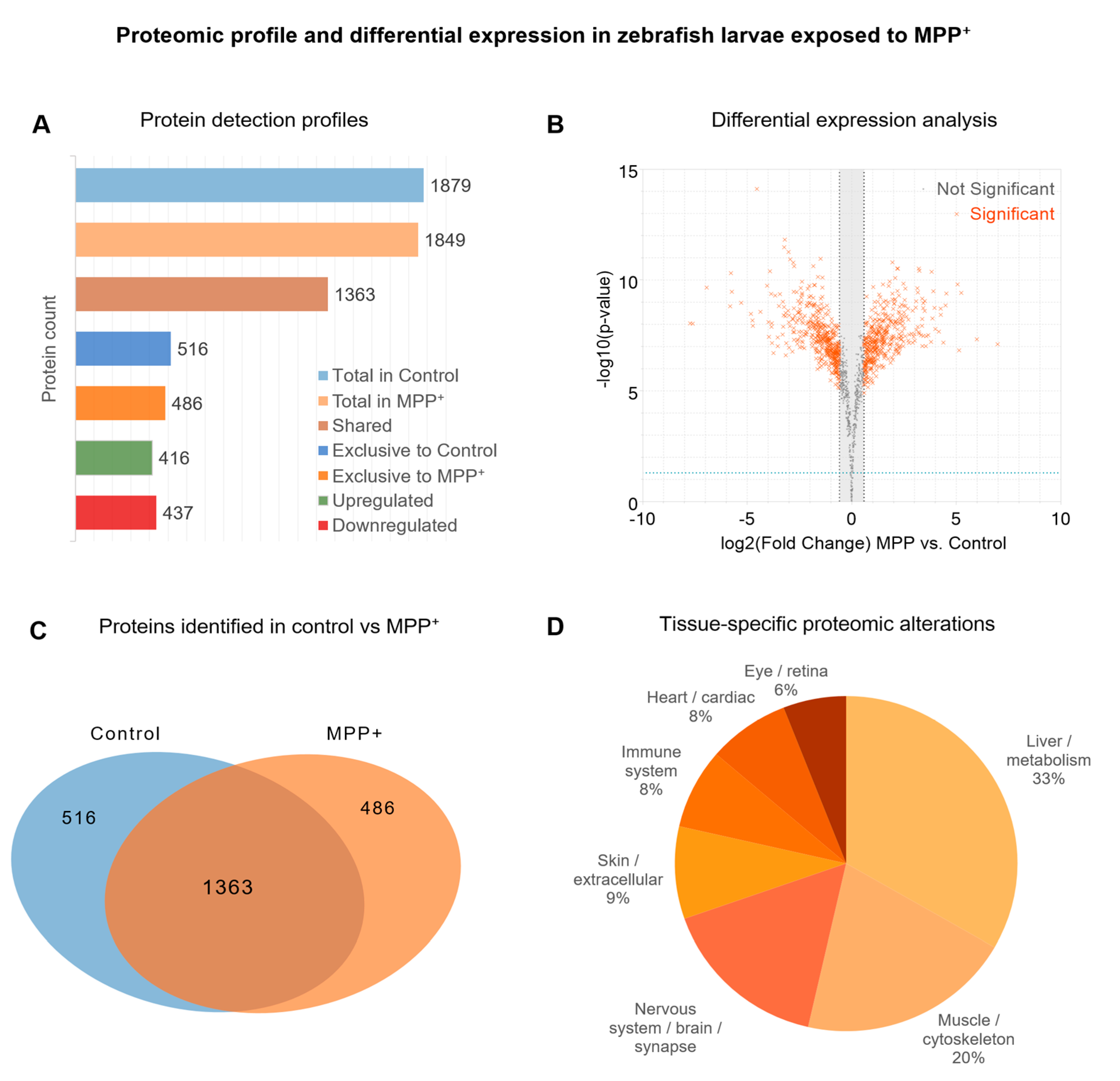
MPP⁺ Exposure Elicits Broad Proteomic Dysregulation in Zebrafish Larvae
Top Differentially Expressed Proteins Involved in Neurodegeneration-Related Processes
Coordinated Dysregulation of Parkinsonism-Associated Proteins
Discussion
Conclusions
Materials and Methods
Zebrafish Maintenance and Embryo Collection
Early Neurotoxic Exposure Protocol Using MPP⁺
Behavioral Analysis
Sample Preparation for Proteomic Analysis
Protein Quantification
Mass Spectrometry Analysis
Proteomic Data Analysis and Bioinformatics
Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| MPP⁺ | 1-Methyl-4-phenylpyridinium |
| DA | Dopaminergic |
| CNS | Central nervous system |
| DJ-1 | Parkinsonism-associated deglycase (also known as PARK7) |
| SDHA | Succinate dehydrogenase complex flavoprotein subunit A |
| PPI | Protein–protein interaction |
| MS | Mass spectrometry |
| LC–MS/MS | Liquid chromatography–tandem mass spectrometry |
| GO | Gene Ontology |
| FDR | False discovery rate |
| log₂FC | Log₂ fold change |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ATP | Adenosine triphosphate |
| ROS | Reactive oxygen species |
| PBS | Phosphate-buffered saline |
| dpf | Days post-fertilization |
| hpf | Hours post-fertilization |
| DEPs | Differentially expressed proteins |
| TCA cycle | Tricarboxylic acid cycle |
References
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Rink, E.; Wullimann, M.F. The Teleostean (Zebrafish) Dopaminergic System Ascending to the Subpallium (Striatum) Is Located in the Basal Diencephalon (Posterior Tuberculum). Brain Res. 2001, 889, 316–330. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.L.; Fetcho, J.R. Relationship of Tyrosine Hydroxylase and Serotonin Immunoreactivity to Sensorimotor Circuitry in Larval Zebrafish. J. Comp. Neurol. 2004, 480, 57–71. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef]
- Maleski, A.L.A.; Rosa, J.G.S.; Bernardo, J.T.G.; Astray, R.M.; Walker, C.I.B.; Lopes-Ferreira, M.; Lima, C. Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Mearns, D.S.; Donovan, J.C.; Larsch, J.; Helmbrecht, T.O.; Kölsch, Y.; Laurell, E.; Kawakami, K.; dal Maschio, M.; Baier, H. Neural Circuitry for Stimulus Selection in the Zebrafish Visual System. Neuron 2021, 109, 805–822.e6. [Google Scholar] [CrossRef]
- da Cunha e Silva, F.A.; Silva, B.R. da; Barros, L.R. de; Beraldo-Neto, E.; Maleski, A.L.A.; Alberto-Silva, C. Snake Venom Peptide Fractions from Bothrops Jararaca and Daboia Siamensis Exhibit Differential Neuroprotective Effects in Oxidative Stress-Induced Zebrafish Models. Pharmaceuticals 2025, 18. [Google Scholar] [CrossRef]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional Characterisation of the Maturation of the Blood-Brain Barrier in Larval Zebrafish. PLoS One 2013, 8, e77548. [Google Scholar] [CrossRef]
- Doyle, J.M.; Croll, R.P. A Critical Review of Zebrafish Models of Parkinson’s Disease. Front. Pharmacol. 2022, 13, 835827. [Google Scholar] [CrossRef]
- Beppi, C.; Straumann, D.; Bögli, S.Y. A Model-Based Quantification of Startle Reflex Habituation in Larval Zebrafish. Sci. Rep. 2021, 11, 846. [Google Scholar] [CrossRef]
- Guo, Y.L.; Duan, W.J.; Lu, D.H.; Ma, X.H.; Li, X.X.; Li, Z.; Bi, W.; Kurihara, H.; Liu, H.Z.; Li, Y.F.; et al. Autophagy-Dependent Removal of α-Synuclein: A Novel Mechanism of GM1 Ganglioside Neuroprotection against Parkinson’s Disease. Acta Pharmacol. Sin. 2021, 42, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.; Þorsteinsson, H.; Maier, V.H.; Karlsson, K.Æ. Multi-Parameter Behavioral Phenotyping of the MPP+ Model of Parkinson’s Disease in Zebrafish. Front. Behav. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Nonnekes, J.; Post, B.; Tetrud, J.W.; Langston, J.W.; Bloem, B.R. MPTP-Induced Parkinsonism: An Historical Case Series. Lancet Neurol. 2018, 17, 300–301. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V.; Naini, A.B.; Jakowec, M.; Petzinger, G.; Miller, R.; Akram, M. The Parkinsonian Toxin 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP): A Technical Review of Its Utility and Safety. J. Neurochem. 2001, 76, 1265–1274. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Salach, J.I.; Singer, T.P. Uptake of the Neurotoxin 1-Methyl-4-Phenylpyridine (MPP+) by Mitochondria and Its Relation to the Inhibition of the Mitochondrial Oxidation of NAD+-Linked Substrates by MPP+. Biochem. Biophys. Res. Commun. 1986, 134, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, W.J.; Vyas, I.; Heikkila, R.E. Inhibition of NADH-Linked Oxidation in Brain Mitochondria by 1-Methyl-4-Phenyl-Pyridine, a Metabolite of the Neurotoxin, 1-Methyl-4-Phenyl-1,2,5,6-Tetrahydropyridine. Life Sci. 1985, 36, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Korzh, V.; Strahle, U. Zebrafish Embryos Are Susceptible to the Dopaminergic Neurotoxin MPTP. Eur. J. Neurosci. 2005, 21, 1758–1762. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass-Spectrometric Exploration of Proteome Structure and Function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef]
- Li, K.W.; Ganz, A.B.; Smit, A.B. Proteomics of Neurodegenerative Diseases: Analysis of Human Post-mortem Brain. J. Neurochem. 2018, 151, 435. [Google Scholar] [CrossRef]
- Kalia, L. V; Lang, A.E. Parkinson’s Disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloemd, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinsons. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. Non-Motor Symptoms of Parkinson’s Disease: Diagnosis and Management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-Motor Features of Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The Scientific and Clinical Basis for the Treatment of Parkinson Disease (2009). Neurology 2009, 72. [Google Scholar] [CrossRef]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an Animal Model for Drug Discovery in Parkinson’s Disease and Other Movement Disorders: A Systematic Review. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lev, N.; Ickowicz, D.; Melamed, E.; Offen, D. Oxidative Insults Induce DJ-1 Upregulation and Redistribution: Implications for Neuroprotection. Neurotoxicology 2008, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Patil, D.; Patil, V.; BaRI, S.; Surana, S.; Patil, P. Animal Models for Parkinson's Disease. CNS Neurol. Disord. - Drug Targets 2014, 13, 1580–1594. [Google Scholar] [CrossRef]
- Tremblay, L. Pathophysiology of Parkinson’s Disease: An Update. Bull. Acad. Natl. Med. 2010, 194, 1321–1331. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Maleski, A.L.A.; Rosa, J.G.S.; Bernardo, J.T.G.; Astray, R.M.; Walker, C.I.B.; Lopes-Ferreira, M.; Lima, C. Recapitulation of Retinal Damage in Zebrafish Larvae Infected with Zika Virus. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, V.; Torkko, V.; Sundvik, M.; Reenilä, I.; Khrustalyov, D.; Kaslin, J.; Panula, P. MPTP and MPP+ Target Specific Aminergic Cell Populations in Larval Zebrafish. J. Neurochem. 2009, 108, 719–731. [Google Scholar] [CrossRef]
- McKinley, E.T.; Baranowski, T.C.; Blavo, D.O.; Cato, C.; Doan, T.N.; Rubinstein, A.L. Neuroprotection of MPTP-Induced Toxicity in Zebrafish Dopaminergic Neurons. Mol. Brain Res. 2005, 141, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Haobam, R.; Sindhu, K.M.; Chandra, G.; Mohanakumar, K.P. Swim-Test as a Function of Motor Impairment in MPTP Model of Parkinson’s Disease: A Comparative Study in Two Mouse Strains. Behav. Brain Res. 2005, 163, 159–167. [Google Scholar] [CrossRef]
- Kalueff, A. V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Naskar, A.; Prabhakar, V.; Singh, R.; Dutta, D.; Mohanakumar, K.P. Melatonin Enhances L-DOPA Therapeutic Effects, Helps to Reduce Its Dose, and Protects Dopaminergic Neurons in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonism in Mice. J. Pineal Res. 2015, 58, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science (80-. ). 2010, 327, 348–351. [Google Scholar] [CrossRef]
- Rink, E.; Wullimann, M.F. Development of the Catecholaminergic System in the Early Zebrafish Brain: An Immunohistochemical Study. Brain Res. Dev. Brain Res. 2002, 137, 89–100. [Google Scholar] [CrossRef]
- McKinley, E.T.; Baranowski, T.C.; Blavo, D.O.; Cato, C.; Doan, T.N.; Rubinstein, A.L. Neuroprotection of MPTP-Induced Toxicity in Zebrafish Dopaminergic Neurons. Brain Res. Mol. Brain Res. 2005, 141, 128–137. [Google Scholar] [CrossRef]
- Meredith, G.E.; Sonsalla, P.K.; Chesselet, M.F. Animal Models of Parkinson’s Disease Progression. Acta Neuropathol. 2008, 115, 385–398. [Google Scholar] [CrossRef]
- Cooper, J.F.; Van Raamsdonk, J.M. Modeling Parkinson’s Disease in C. Elegans. J. Parkinsons. Dis. 2018, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Coulom, H.; Birman, S. Chronic Exposure to Rotenone Models Sporadic Parkinson’s Disease in Drosophila Melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef]
- Bretaud, S.; Lee, S.; Guo, S. Sensitivity of Zebrafish to Environmental Toxins Implicated in Parkinson’s Disease. Neurotoxicol. Teratol. 2004, 26, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Salach, J.I.; Singer, T.P. Uptake of the Neurotoxin 1-Methyl-4-Phenylpyridine (MPP+) by Mitochondria and Its Relation to the Inhibition of the Mitochondrial Oxidation of NAD+-Linked Substrates by MPP+. Biochem. Biophys. Res. Commun. 1986, 134, 743–748. [Google Scholar] [CrossRef]
- Schapira, A.H. V Mitochondrial Dysfunction in Parkinson’s Disease. Cell Death Differ. 2007, 14, 1261–1266. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Jenner, P. Etiology and Pathogenesis of Parkinson’s Disease. Mov. Disord. 2011, 26, 1049–1055. [Google Scholar] [CrossRef]
- Heo, J.Y.; Park, J.H.; Kim, S.J.; Seo, K.S.; Han, J.S.; Lee, S.H.; Kim, J.M.; Park, J. Il; Park, S.K.; Lim, K.; et al. DJ-1 Null Dopaminergic Neuronal Cells Exhibit Defects in Mitochondrial Function and Structure: Involvement of Mitochondrial Complex i Assembly. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to Dopaminergic Neurons by Oxidative Stress in Parkinson’s Disease (Review). Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Chen, J.; Li, Q.; Huo, L.; Wang, Y.; Wang, H.; Du, J. Pharmacological Modulation of Nrf2/HO-1 Signaling Pathway as a Therapeutic Target of Parkinson’s Disease. Front. Pharmacol. 2021, 12, 757161. [Google Scholar] [CrossRef]
- Bao, B.; Zhang, M.Q.; Chen, Z.Y.; Wu, X.B.; Xia, Z.B.; Chai, J.Y.; Yin, X.P. Sulforaphane Prevents PC12 Cells from Oxidative Damage via the Nrf2 Pathway. Mol. Med. Rep. 2019, 19, 4890. [Google Scholar] [CrossRef]
- Rafique, H.; Hu, X.; Ren, T.; Dong, R.; Aadil, R.M.; Zou, L.; Sharif, M.K.; Li, L. Characterization and Exploration of the Neuroprotective Potential of Oat-Protein-Derived Peptides in PC12 Cells and Scopolamine-Treated Zebrafish. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- McNaught, K.S.P.; Belizaire, R.; Isacson, O.; Jenner, P.; Olanow, C.W. Altered Proteasomal Function in Sporadic Parkinson’s Disease. Exp. Neurol. 2003, 179, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Bender, A.; García-Arumí, E.; Melià, M.J.; Bové, J.; Laub, C.; Klopstock, T.; Elstner, M.; Mounsey, R.B.; Teismann, P.; et al. Accumulation of Mitochondrial DNA Deletions within Dopaminergic Neurons Triggers Neuroprotective Mechanisms. Brain 2013, 136, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, Š.; Sonninen, T.M.; Wojciechowski, S.; Goldsteins, G.; Koistinaho, J. Dysfunction of Cellular Proteostasis in Parkinson’s Disease. Front. Neurosci. 2019, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. A Critical Evaluation of Current Staging of α-Synuclein Pathology in Lewy Body Disorders. Biochim. Biophys. Acta - Mol. Basis Dis. 2009, 1792, 730–740. [Google Scholar] [CrossRef]
- Bi, M.; Du, X.; Jiao, Q.; Chen, X.; Jiang, H. Expanding the Role of Proteasome Homeostasis in Parkinson’s Disease: Beyond Protein Breakdown. Cell Death Dis. 2021 122 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, J.; Li, X.; Zhuang, J. Intersection of the Ubiquitin–Proteasome System with Oxidative Stress in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Betarbet, R.; Canet-Aviles, R.M.; Sherer, T.B.; Mastroberardino, P.G.; McLendon, C.; Kim, J.H.; Lund, S.; Na, H.M.; Taylor, G.; Bence, N.F.; et al. Intersecting Pathways to Neurodegeneration in Parkinson’s Disease: Effects of the Pesticide Rotenone on DJ-1, α-Synuclein, and the Ubiquitin-Proteasome System. Neurobiol. Dis. 2006, 22, 404–420. [Google Scholar] [CrossRef]
- Aiken, C.T.; Kaake, R.M.; Wang, X.; Huang, L. Oxidative Stress-Mediated Regulation of Proteasome Complexes. Mol. Cell. Proteomics 2011, 10. [Google Scholar] [CrossRef]
- Salpietro, V.; Malintan, N.T.; Llano-Rivas, I.; Spaeth, C.G.; Efthymiou, S.; Striano, P.; Vandrovcova, J.; Cutrupi, M.C.; Chimenz, R.; David, E.; et al. Mutations in the Neuronal Vesicular SNARE VAMP2 Affect Synaptic Membrane Fusion and Impair Human Neurodevelopment. Am. J. Hum. Genet. 2019, 104, 721–730. [Google Scholar] [CrossRef]
- Sunaga, Y.; Muramatsu, K.; Kosaki, K.; Sugai, K.; Mizuno, T.; Kouno, M.; Tashiro, M. Variant in the Neuronal Vesicular SNARE VAMP2 (Synaptobrevin-2): First Report in Japan. Brain Dev. 2020, 42, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Imbriani, P.; Schirinzi, T.; Meringolo, M.; Mercuri, N.B.; Pisani, A. Centrality of Early Synaptopathy in Parkinson’s Disease. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bazzu, G.; Calia, G.; Puggioni, G.; Spissu, Y.; Rocchitta, G.; Debetto, P.; Grigoletto, J.; Zusso, M.; Migheli, R.; Andrea Serra, P.; et al. α-Synuclein- and MPTP-Generated Rodent Models of Parkinsons Disease and the Study of Extracellular Striatal Dopamine Dynamics: A Microdialysis Approach. CNS Neurol. Disord. - Drug Targets 2012, 9, 482–490. [Google Scholar] [CrossRef]
- Xiong, X.-P.; Dong, C.-F.; Xu, X.; Weng, S.-P.; Liu, Z.-Y.; He, J.-G. Proteomic Analysis of Zebrafish (Danio Rerio) Infected with Infectious Spleen and Kidney Necrosis Virus. Dev. Comp. Immunol. 2011, 35, 431–440. [Google Scholar] [CrossRef]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an Animal Model for Drug Discovery in Parkinson’s Disease and Other Movement Disorders: A Systematic Review. Front. Neurol. 2018, 9, 347. [Google Scholar] [CrossRef]
- Presti-Silva, S.M.; Rodrigues-Ribeiro, L.; Gorshkov, V.; Kjeldsen, F.; Verano-Braga, T.; Pires, R.G.W. Proteomic Analysis of Substantia Nigra Reveals Molecular Insights Into the Neuroprotection Effect of Rosmarinic Acid Treatment in MPTP-Induced Mouse Model of Parkinson’s Disease. Proteomics - Clin. Appl. 2025, 19. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes.; European Union, 2010; pp. 33–79;
- Conselho Nacional de Controle de Experimentação Animal (CONCEA) Capítulo 8—Peixes. In Guia Brasileiro de Produção, Manutenção ou Utilização de Animais em Atividades de Ensino ou Pesquisa Científica; Brasília.
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinformatics 2017, 18, 529. [Google Scholar] [CrossRef]
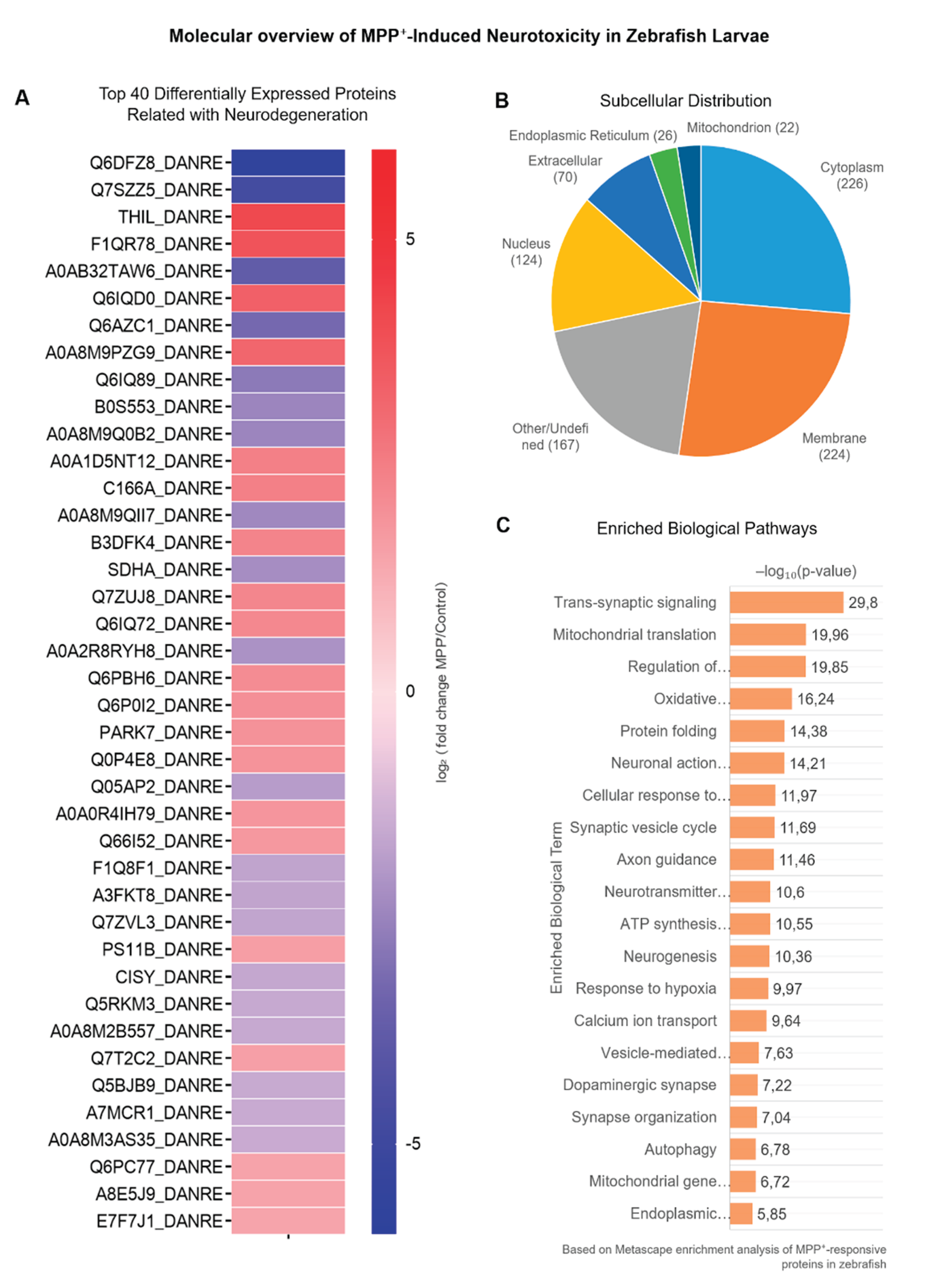
| Canonical Name | Gene Symbol | Protein Name | Log2FC | Reg |
|---|---|---|---|---|
| Q6DFZ8_DANRE | a4fvm2 | Calpain-2 catalytic subunit | -5.777 | ↓ |
| Q7SZZ5_DANRE | a0a8n7uz95 | Vesicle-associated membrane protein 2 | -4.728 | ↓ |
| THIL_DANRE | q7sx97 | Acetyl-CoA acetyltransferase, mitochondrial | 4.168 | ↑ |
| F1QR78_DANRE | q6iqh4 | Proteasome subunit beta | 3.793 | ↑ |
| A0AB32TAW6_DANRE | f1qqn9 | Dimethylglycine dehydrogenase, mitochondrial | -3.745 | ↓ |
| Q6IQD0_DANRE | a0ab32u1m7 | Proteasome subunit beta | 3.372 | ↑ |
| Q6AZC1_DANRE | q6dgv5 | 26S proteasome regulatory subunit 8 | -3.234 | ↓ |
| A0A8M9PZG9_DANRE | q6p6x6 | Vesicle-associated membrane protein-associated protein B/C isoform X1 | 3.207 | ↑ |
| Q6IQ89_DANRE | q6nyj9 | Mitochondrial 2-oxoglutarate/malate carrier protein | -2.773 | ↓ |
| B0S553_DANRE | a8e5e5 | Neurofilament medium chain b | -2.489 | ↓ |
| A0A8M9Q0B2_DANRE | x1wbl9 | Calcium-transporting ATPase | -2.477 | ↓ |
| A0A1D5NT12_DANRE | a0a8m9pmd5 | Dynein axonemal heavy chain 2 isoform X1 | 2.464 | ↑ |
| C166A_DANRE | q6dbz1 | CD166 antigen homolog A | 2.412 | ↑ |
| A0A8M9QII7_DANRE | q7zup4 | Calcium-transporting ATPase | -2.398 | ↓ |
| B3DFK4_DANRE | q4v9b9 | 6.8 kDa mitochondrial proteolipid-like | 2.322 | ↑ |
| SDHA_DANRE | q6nyq7 | Succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial | -2.272 | ↓ |
| Q7ZUJ8_DANRE | a3knn5 | Proteasome subunit beta | 2.26 | ↑ |
| Q6IQ72_DANRE | q7zvg7 | Proteasome | 2.198 | ↑ |
| A0A2R8RYH8_DANRE | q52pj7 | Solute carrier family 25 member 3 | -2.134 | ↓ |
| Q6PBH6_DANRE | a0a0r4iyx1 | Cytochrome b-c1 complex subunit 1, mitochondrial | 2.115 | ↑ |
| Q6P0I2_DANRE | q8jhv7 | Proteasome subunit alpha type | 2.005 | ↑ |
| PARK7_DANRE | q6pbx4 | Parkinson disease protein 7 homolog | 1.946 | ↑ |
| Q0P4E8_DANRE | q7zvv5 | Mitochondrial import receptor subunit TOM7 homolog | 1.909 | ↑ |
| Q05AP2_DANRE | a0a0r4ila8 | ATPase, Ca++ transporting, plasma membrane 4 | -1.882 | ↓ |
| A0A0R4IH79_DANRE | psb1 | Lon protease homolog, mitochondrial | 1.827 | ↑ |
| Q66I52_DANRE | q3kr14 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 6, mitochondrial | 1.77 | ↑ |
| F1Q8F1_DANRE | a0ab32tbz3 | Internexin neuronal intermediate filament protein, alpha b | -1.694 | ↓ |
| A3FKT8_DANRE | a0a8m9qll5 | Protein S100 | -1.669 | ↓ |
| Q7ZVL3_DANRE | b6ide6 | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex, mitochondrial | -1.652 | ↓ |
| PS11B_DANRE | f1qvc6 | 26S proteasome non-ATPase regulatory subunit 11B | 1.633 | ↑ |
| CISY_DANRE | e9qg20 | Citrate synthase, mitochondrial | -1.614 | ↓ |
| Q5RKM3_DANRE | q1ecx9 | Stress-induced-phosphoprotein 1 | -1.573 | ↓ |
| A0A8M2B557_DANRE | h9gxn3 | Neuroblast differentiation-associated protein AHNAK | -1.567 | ↓ |
| Q7T2C2_DANRE | q7zus9 | Very long-chain specific acyl-CoA dehydrogenase, mitochondrial | 1.562 | ↑ |
| Q5BJB9_DANRE | q6pc16 | Thioredoxin-dependent peroxide reductase, mitochondrial | -1.548 | ↓ |
| A7MCR1_DANRE | a0a8m9phr9 | Cytochrome b-c1 complex subunit Rieske, mitochondrial | -1.516 | ↓ |
| A0A8M3AS35_DANRE | a0a8m6z4u4 | calcium/calmodulin-dependent protein kinase | -1.503 | ↓ |
| Q6PC77_DANRE | a0a8m2bbs1 | ATP synthase subunit d, mitochondrial | 1.499 | ↑ |
| A8E5J9_DANRE | q7zuj8 | Creatine kinase S-type, mitochondrial | 1.477 | ↑ |
| E7F7J1_DANRE | a0a8m3aqm3 | Adrenodoxin, mitochondrial | 1.468 | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





