Submitted:
06 June 2025
Posted:
06 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
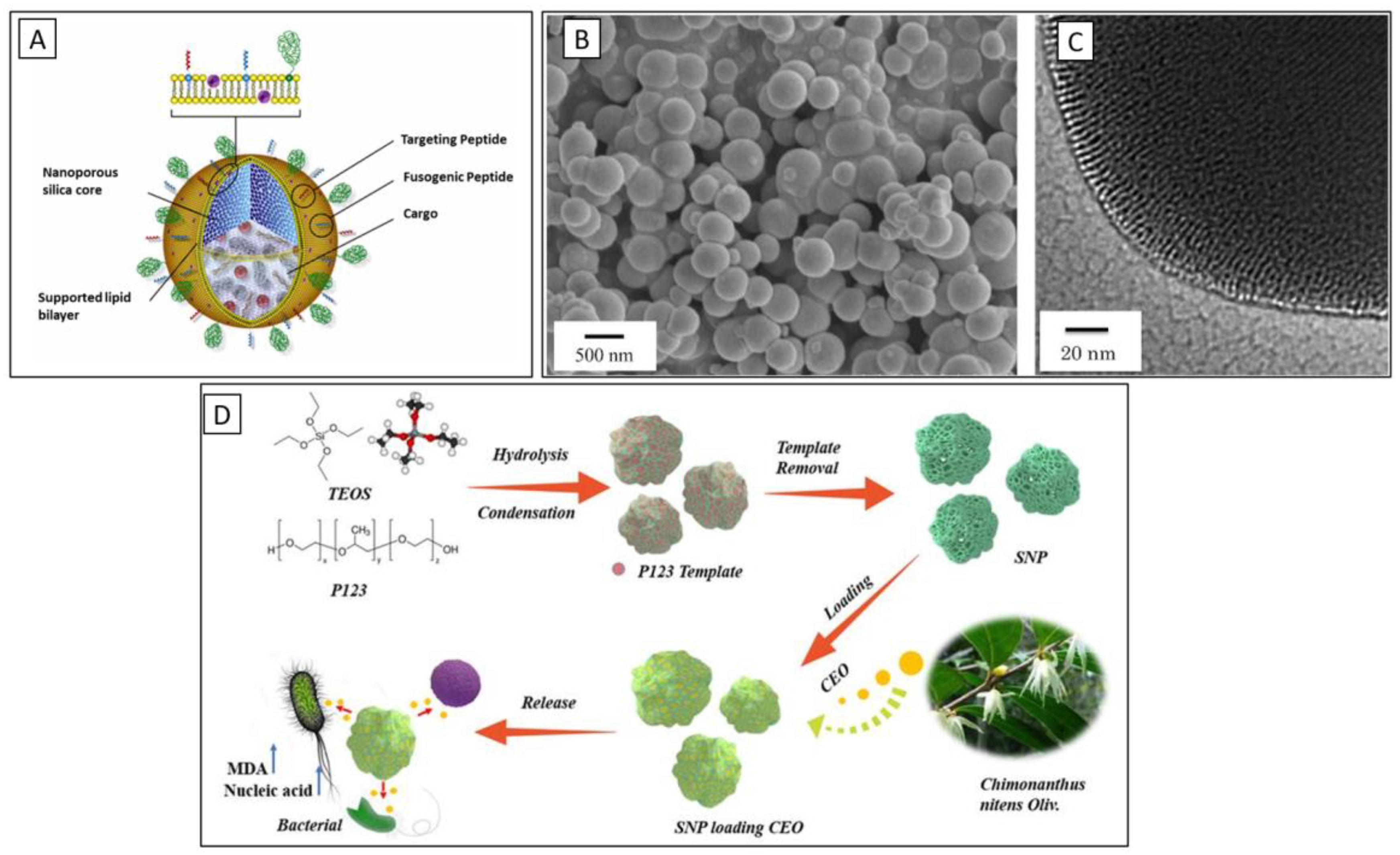
2. Properties of Nanoporous Silica
2.1. High Surface Area and Porosity
2.2. Mechanical Strength
2.3. Biocompatibility
3. Impact on Mechanical Properties of Prosthetic Materials
3.1. Strength Enhancement
3.2. Wear Resistance
3.3. Flexural Strength
3.4. Fatigue Resistance
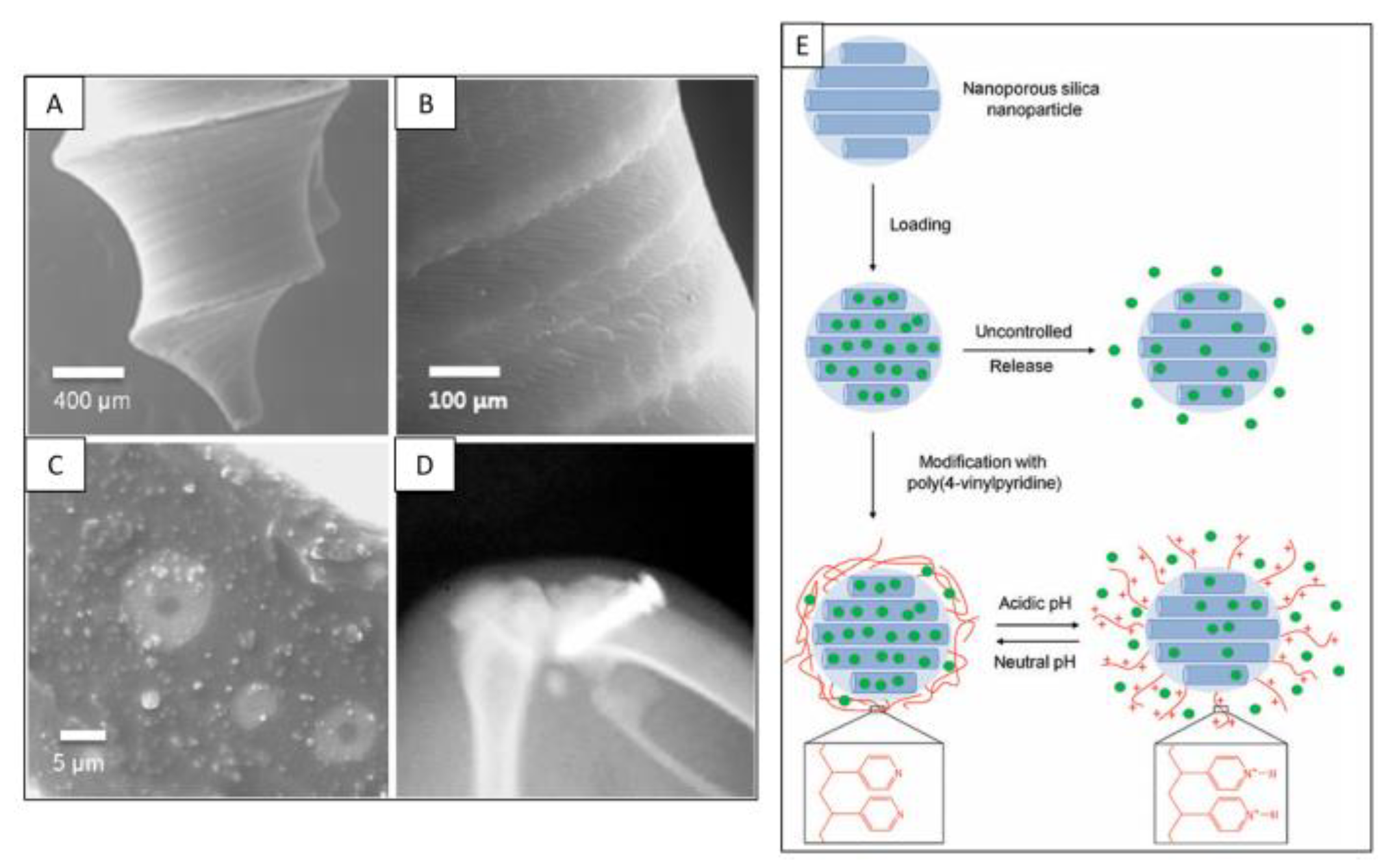
4. Biocompatibility of Nanoporous Silica
4.1. Cell Response
4.2. Inflammatory Response
4.3. Long-Term Stability
4.4. Interaction with Biological Fluids
5. Role of Nanoporous Silica in Drug Delivery Systems
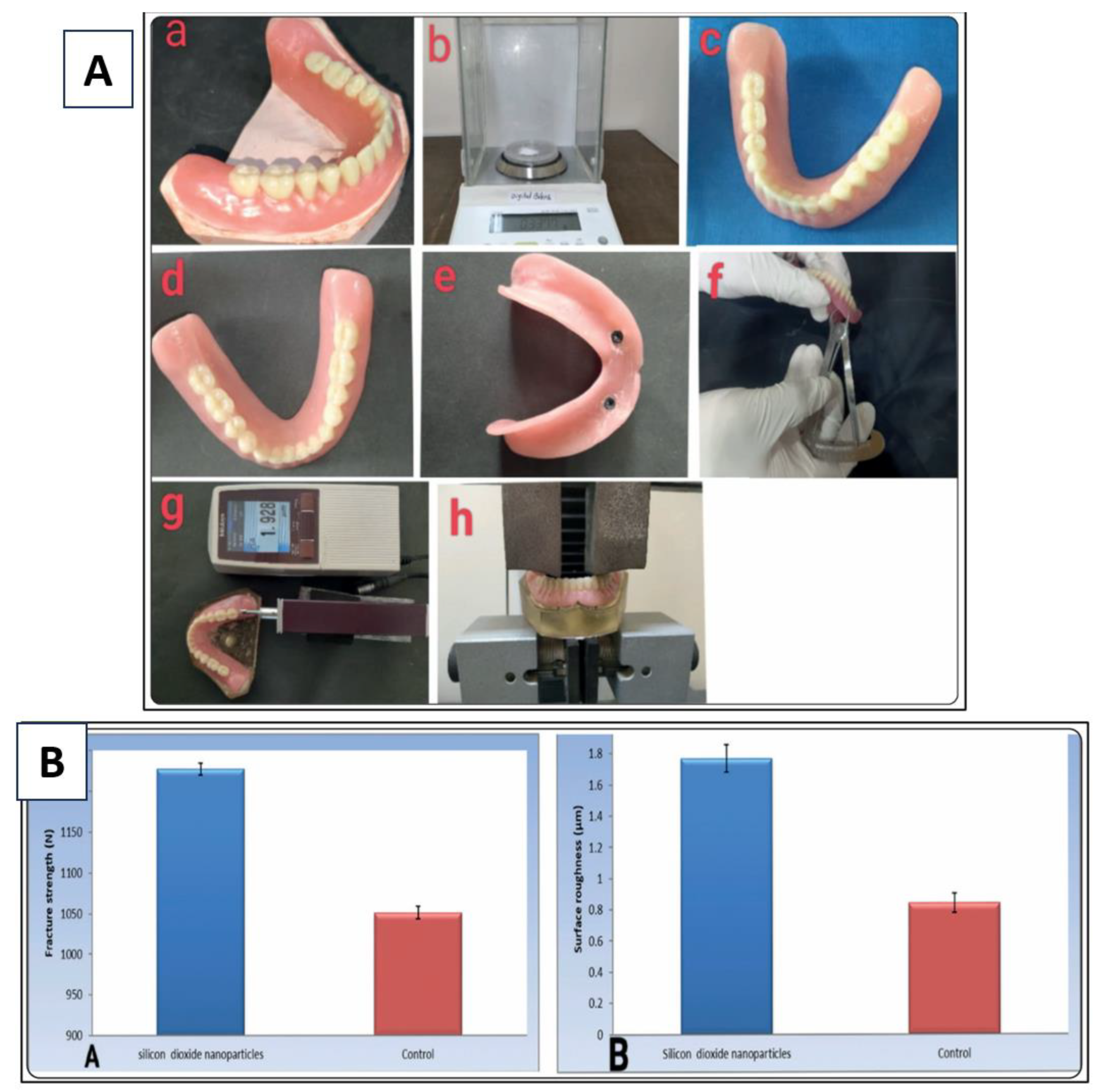
6. Applications of Nanoporous Silica in Prosthetic Dentistry
6.1. Dental Implants
6.2. Crowns and Bridges
6.3. Dentures
6.4. Orthodontic Applications
6.5. Bone Grafting Materials
6.6. Delivery of Growth Factors
6.7. Localized Pain Management
7. Future Directions
7.1. Research and Development
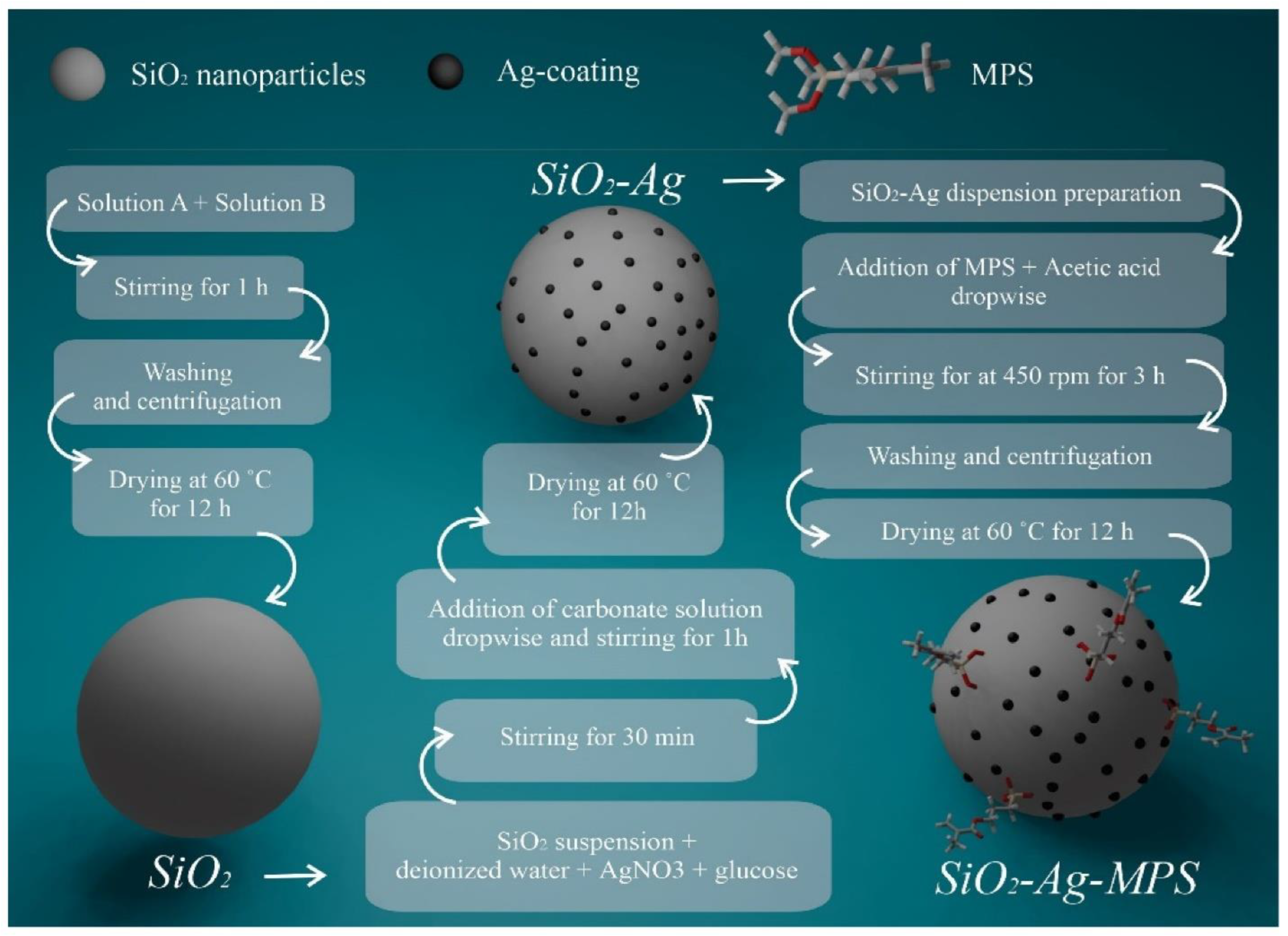
7.2. Clinical Trials
7.3. Integration with Digital Dentistry
7.4. Sustainability and Environmental Considerations
7.5. Educational Initiatives
8. Conclusions
References
- S. Safaee, H. Murata, The effect of incorporating nano-porous silica particles on the viscoelastic behavior of a commercial tissue conditioner and its long-lasting drug-release characteristics. Japan Prosthodontic Society 2024, 16, 385. [Google Scholar]
- Safaee, S. , et al., Multi-layered Coatings for Improving Implant Performance of Titanium. Ceramics International, 2025.
- Timpe, N. , et al., Nanoporous silica nanoparticles with spherical and anisotropic shape as fillers in dental composite materials. BioNanoMaterials 2014, 15, 89–99. [Google Scholar] [CrossRef]
- Jose, A. , et al. Porous inorganic nanomaterials: Their evolution towards hierarchical porous nanostructures. in Micro.
- Kankala, R.K. , et al., Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Advanced materials 2020, 32, 1907035. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P. , et al., Sol-Gel processing of silica nanoparticles and their applications. Advances in colloid and interface science 2014, 214, 17–37. [Google Scholar] [CrossRef]
- Zhao, X. , et al. , Templating methods for preparation of porous structures. Journal of Materials Chemistry 2006, 16, 637–648. [Google Scholar]
- Yang, G. and S.-J. Park, Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef]
- SEHRAWAT¹, S. and M. AHUJA, ANISHA'ARYAN BOORA¹, BHAVNA ROHILLA¹. Nanomaterials: Synthesis and Applications 2024, 174.
- Hoskeri, J. , Mesoporous Materials for Tissue Engineering Applications. Materials Research Foundations. 173.
- Wang, S. , et al., Nanoporosity significantly enhances the biological performance of engineered glass tissue scaffolds. Tissue Engineering Part A 2013, 19, 1632–1640. [Google Scholar] [CrossRef]
- Zhang, C. , et al., Dental implants loaded with bioactive agents promote osseointegration in osteoporosis: a review. Frontiers in Bioengineering and Biotechnology 2021, 9, 591796. [Google Scholar]
- Chen, L. , et al., Silicon-containing nanomedicine and biomaterials: materials chemistry, multi-dimensional design, and biomedical application. Chemical Society Reviews 2024, 53, 1167–1315. [Google Scholar] [CrossRef]
- Yang, X.-Y. , et al., Hierarchically porous materials: synthesis strategies and structure design. Chemical Society Reviews 2017, 46, 481–558. [Google Scholar] [CrossRef]
- Bružauskaitė, I. , et al., Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Rimsza, J. and J. Du, Structural and mechanical properties of nanoporous silica. Journal of the American Ceramic Society 2014, 97, 772–781. [Google Scholar] [CrossRef]
- Atai, M., A. Pahlavan, and N. Moin, Nano-porous thermally sintered nano silica as novel fillers for dental composites. Dental materials 2012, 28, 133–145. [Google Scholar] [CrossRef]
- Hata, K. , et al., Dental poly (methyl methacrylate)-based resin containing a nanoporous silica filler. Journal of functional biomaterials 2022, 13, 32. [Google Scholar] [CrossRef]
- Aminoroaya, A. , et al., Mesoporous silica aerogel reinforced dental composite: Effects of microstructure and surface modification. Journal of the Mechanical Behavior of Biomedical Materials 2022, 125, 104947. [Google Scholar] [CrossRef]
- Abid Althaqafi, K. , et al., Properties of a model self-healing microcapsule-based dental composite reinforced with silica nanoparticles. Journal of functional biomaterials 2022, 13, 19. [Google Scholar] [CrossRef]
- Inzunza, D. , et al., Synthesis of nanostructured porous silica coatings on titanium and their cell adhesive and osteogenic differentiation properties. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2014, 102, 37–48. [Google Scholar] [CrossRef]
- Vandamme, K. , et al., Implant functionalization with mesoporous silica: A promising antibacterial strategy, but does such an implant osseointegrate? Clinical and Experimental Dental Research 2021, 7, 502–511. [Google Scholar] [CrossRef]
- Wang, X. , et al., Nano-porous silica aerogels as promising biomaterials for oral drug delivery of paclitaxel. Journal of Biomedical Nanotechnology 2019, 15, 1532–1545. [Google Scholar] [CrossRef]
- Shahbazi, M.-A. , et al., The mechanisms of surface chemistry effects of mesoporous silicon nanoparticles on immunotoxicity and biocompatibility. Biomaterials 2013, 34, 7776–7789. [Google Scholar] [CrossRef]
- Rana, D. , et al., Surface functionalization of nanobiomaterials for application in stem cell culture, tissue engineering, and regenerative medicine. Biotechnology progress 2016, 32, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Anil, S. , et al. , Dental implant surface enhancement and osseointegration. Implant dentistry-a rapidly evolving practice 2011, 2011, 82–108. [Google Scholar]
- Attik, N. , et al., Mesoporous silica fillers and resin composition effect on dental composites cytocompatibility. Dental Materials 2017, 33, 166–174. [Google Scholar] [CrossRef]
- Topouzi, M. , et al. , Reinforcement of a PMMA resin for interim fixed prostheses with silica nanoparticles. Journal of the mechanical behavior of biomedical materials 2017, 69, 213–222. [Google Scholar]
- Su, C. , et al., Enhancement of mechanical behavior of FRP composites modified by silica nanoparticles. Construction and Building Materials 2020, 262, 120769. [Google Scholar] [CrossRef]
- Yang, Q. , et al., Characterization of mesoporous silica nanoparticle composites at low filler content. Journal of Composite Materials 2016, 50, 715–722. [Google Scholar] [CrossRef]
- Fonseca, R.B. , et al., Effect of short glass fiber/filler particle proportion on flexural and diametral tensile strength of a novel fiber-reinforced composite. Journal of prosthodontic research 2016, 60, 47–53. [Google Scholar] [CrossRef]
- Luo, J., J. J. Lannutti, and R.R. Seghi, Effect of filler porosity on the abrasion resistance of nanoporous silica gel/polymer composites. Dental Materials 1998, 14, 29–36. [Google Scholar] [CrossRef]
- Merola, M. and S. Affatato, Materials for hip prostheses: a review of wear and loading considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef]
- Alfaer, A.S. , et al., Wear Patterns in Modern Dental Materials Used in Extensive Tooth Replacement Treatments. 2025.
- Mirjalili, A., A. Zamanian, and S.M.M. Hadavi, The effect of TiO2 nanotubes reinforcement on the mechanical properties and wear resistance of silica micro-filled dental composites. Journal of Composite Materials 2019, 53, 3217–3228. [Google Scholar] [CrossRef]
- Chen, H. , et al., Micromechanical interlocking-inspired dendritic porous silica-based multimodal resin composites for the tooth restoration. Nano Research 2024, 17, 9065–9077. [Google Scholar] [CrossRef]
- Jin, H.-J., J. Weissmüller, and D. Farkas, Mechanical response of nanoporous metals: A story of size, surface stress, and severed struts. Mrs Bulletin 2018, 43, 35–42. [Google Scholar] [CrossRef]
- Zandinejad, A., M. Atai, and A. Pahlevan, The effect of ceramic and porous fillers on the mechanical properties of experimental dental composites. Dental Materials 2006, 22, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Taha, N., J. Palamara, and H. Messer, Fracture strength and fracture patterns of root filled teeth restored with direct resin restorations. Journal of dentistry 2011, 39, 527–535. [Google Scholar] [CrossRef]
- Zamani, P., A. Jaamialahmadi, and L.F. Da Silva, The influence of GNP and nano-silica additives on fatigue life and crack initiation phase of Al-GFRP bonded lap joints subjected to four-point bending. Composites Part B: Engineering 2021, 207, 108589. [Google Scholar] [CrossRef]
- Zhang, J. , et al. , Antibacterial dental composites with chlorhexidine and mesoporous silica. Journal of dental research 2014, 93, 1283–1289. [Google Scholar]
- Abdelhamid, S.M. , et al., Influence of Reinforcement with Silicon Dioxide Nanoparticles on The Fracture Strength and Surface Roughness of Implant-Retained Mandibular Overdenture: An In Vitro Comparative Study. Egyptian Dental Journal 2024, 70, 1503–1513. [Google Scholar]
- Yang, D.-L. , et al., The properties of dental resin composites reinforced with silica colloidal nanoparticle clusters: Effects of heat treatment and filler composition. Composites Part B: Engineering 2020, 186, 107791. [Google Scholar] [CrossRef]
- Tah, R. , et al., Effect of zirconia silica nanofibers on flexural strength of feldspathic ceramic-An experimental study. Advanced Biomedical Research 2021, 10, 31. [Google Scholar] [CrossRef]
- Rezvani, M.B. , et al., The effect of silica nanoparticles on the mechanical properties of fiber-reinforced composite resins. Journal of Dental Research, Dental Clinics, Dental Prospects.
- Pourhajibagher, M. and A. Bahador, Effects of incorporation of nanoparticles into dental acrylic resins on antimicrobial and physico-mechanical properties: A meta-analysis of in vitro studies. Journal of Oral Biology and Craniofacial Research 2022, 12, 557–568. [Google Scholar] [CrossRef]
- Safaee, S. , et al., Fabrication of bioactive glass coating on pure titanium by sol-dip method: Dental applications. Dental Materials Journal 2021, 40, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Bellino, M.G. , et al., Controlled adhesion and proliferation of a human osteoblastic cell line by tuning the nanoporosity of titania and silica coatings. Biomaterials Science 2013, 1, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Malek, N.A.N.N., N. S. Sani, and A. Taufiq, Cell Adhesion and Proliferation of Silica Nanocomposite, in Biomedical Materials and Biofabrication for Regenerative Medicine. 2025, CRC Press. 166-180.
- Nesabi, M. , et al., A novel multi-structural reinforced treatment on Ti implant utilizing a combination of alkali solution and bioactive glass sol. Journal of the Mechanical Behavior of Biomedical Materials 2021, 124, 104837. [Google Scholar] [CrossRef] [PubMed]
- Saikia, J. , et al., Differential protein adsorption and cellular uptake of silica nanoparticles based on size and porosity. ACS applied materials & interfaces 2016, 8, 34820–34832. [Google Scholar]
- Ha, S.-W. , et al., Bioactive effects of silica nanoparticles on bone cells are size, surface, and composition dependent. Acta biomaterialia 2018, 82, 184–196. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S. , et al., Mesoporous silica-based nanoplatforms are theranostic agents for the treatment of inflammatory disorders. Pharmaceutics 2023, 15, 439. [Google Scholar] [CrossRef]
- Chiang, Y.-C. , et al., A mesoporous silica biomaterial for dental biomimetic crystallization. ACS nano 2014, 8, 12502–12513. [Google Scholar] [CrossRef]
- Yu, Y. , et al., Short-term oral administration of mesoporous silica nanoparticles potentially induced colon inflammation in rats through alteration of gut microbiota. International Journal of Nanomedicine 2021, 881–893. [Google Scholar] [CrossRef]
- Pavanello, L. , et al., Physicochemical and biological properties of dental materials and formulations with silica nanoparticles: A narrative review. Dental Materials, 2024.
- Adiga, S.P. , et al. , Nanoporous membranes for medical and biological applications. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2009, 1, 568–581. [Google Scholar]
- Zhu, H., K. Zheng, and A.R. Boccaccini, Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomaterialia 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Wang, G. , et al., Antibacterial peptides-loaded bioactive materials for the treatment of bone infection. Colloids and Surfaces B: Biointerfaces 2023, 225, 113255. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, K.L., T. J. Barnes, and C.A. Prestidge, Surface chemistry of porous silicon and implications for drug encapsulation and delivery applications. Advances in Colloid and Interface Science 2012, 175, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Maderuelo, C., A. Zarzuelo, and J.M. Lanao, Critical factors in the release of drugs from sustained release hydrophilic matrices. Journal of controlled release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Sun, J. , et al., Nanoporous silica-based protocells at multiple scales for designs of life and nanomedicine. Life 2015, 5, 214–229. [Google Scholar] [CrossRef]
- Nakamura, K. , et al., Calcium Charge and Release of Conventional Glass-Ionomer Cement Containing Nanoporous Silica. Materials 2018, 11, 1295. [Google Scholar] [CrossRef]
- Lai, H. , et al., Sponge-liked silica nanoporous particles for sustaining release and long-term antibacterial activity of natural essential oil. Molecules 2023, 28, 594. [Google Scholar] [CrossRef]
- Massa, M.A. , et al., Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Materials Science and Engineering: C 2014, 45, 146–153. [Google Scholar] [CrossRef]
- Wang, L. , et al., Osteoblast/bone-tissue responses to porous surface of polyetheretherketone–nanoporous lithium-doped magnesium silicate blends' integration with polyetheretherketone. International journal of nanomedicine 2019, 4975–4989. [Google Scholar] [CrossRef]
- Safaee, S. , et al., Osseointegration Dynamics: Insights into the Dental Bone-Implant Interface. The Journal of Applied Tissue Engineering.
- Pasha, M. , et al., Ceramic nanomaterials in dental applications. Nanoengineering of Biomaterials 2022, 123–144. [Google Scholar]
- Aati, S. , et al., Silver-loaded mesoporous silica nanoparticles enhanced the mechanical and antimicrobial properties of 3D printed denture base resin. Journal of the mechanical behavior of biomedical materials 2022, 134, 105421. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A. and A.A. Balhaddad, Prospects on tuning bioactive and antimicrobial denture base resin materials: A narrative review. Polymers 2022, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Alkhazaleh, A.M. , Mechanical and chemical analysis of experimental adhesive containing drug-loaded mesoporous silica nanoparticles. 2022, The University of Iowa.
- Shadjou, N. and M. Hasanzadeh, Silica-based mesoporous nanobiomaterials as promoter of bone regeneration process. Journal of Biomedical Materials Research Part A 2015, 103, 3703–3716. [Google Scholar] [PubMed]
- Casarrubios, L. , et al., Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta biomaterialia 2020, 101, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Satalov, A. , Nanoporous silica nanoparticles and bone morphogenetic protein 2 for bone regeneration. 2017.
- Neumann, A. , et al., BMP2-loaded nanoporous silica nanoparticles promote osteogenic differentiation of human mesenchymal stem cells. Rsc Advances 2013, 3, 24222–24230. [Google Scholar] [CrossRef]
- Zhang, Q. , et al., Porous nanofibrous scaffold incorporated with S1P loaded mesoporous silica nanoparticles and BMP-2 encapsulated PLGA microspheres for enhancing angiogenesis and osteogenesis. Journal of Materials Chemistry B 2018, 6, 6731–6743. [Google Scholar] [CrossRef]
- Wang, H. , et al., An injectable mesoporous silica-based analgesic delivery system prolongs the duration of sciatic nerve block in mice with minimal toxicity. Acta Biomaterialia 2021, 135, 638–649. [Google Scholar] [CrossRef]
- Covarrubias, C. , et al., Osseointegration properties of titanium dental implants modified with a nanostructured coating based on ordered porous silica and bioactive glass nanoparticles. Applied Surface Science 2016, 363, 286–295. [Google Scholar] [CrossRef]
- Fullriede, H. , et al., pH-responsive release of chlorhexidine from modified nanoporous silica nanoparticles for dental applications. BioNanoMaterials 2016, 17, 59–72. [Google Scholar] [CrossRef]
- Rossi, N.R. , et al., Silver-coated silica nanoparticles modified with MPS: potential antimicrobial biomaterials applied in glaze and soft reliner. Polymers 2022, 14, 4306. [Google Scholar] [CrossRef]
| Mechanical Properties | Material System Studied | Silica Details | Key Findings | References |
|---|---|---|---|---|
| Flexural Strength | Dental Resin Composite | Calcined Silica Colloidal Nanoparticle Clusters (CSCNCs) 60 wt% + SCNCs 10 wt% | Flexural strength: 143.5 ± 8.3 MPa (a 14% improvement compared to 70 wt% uncalcined SCNCs). Flexural Modulus: 8.91 ± 0.48 GPa (a 23% improvement). Calcination strengthened the nanoparticle clusters, leading to better reinforcement. | [43] |
| Flexural Strength | Feldspathic Ceramic | Zirconia-Silica Nanofibers | Incorporation of 5 wt% zirconia-silica nanofibers increased mean flexural strength from 141.08 ± 31.27 MPa (control) to 189.07 ± 5.52 MPa. With 7.5 wt%, it reached 196.71 ± 5.25 MPa. | [44] |
| Flexural Strength | Fiber-Reinforced Composite Resin (FRC) | Silica Nanoparticles (5 wt% in matrix resin) | For FRC with 2 fiber bundles, flexural strength increased from 64.2 ± 11.28 MPa (0 wt% silica) to 100.2 ± 18.41 MPa (5 wt% silica). Significant improvement was seen from 0.2 wt% onwards. | [45] |
| Flexural Strength & Hardness | Light Cured Dental Composite Resin | Silica Nanoparticles | 3 wt% silica significantly increased flexural strength (FS) and Vickers hardness (VH). FS (Control): approx. 85.78 MPa; FS (3% SiO₂): approx. 115.96 MPa. VH (Control): approx. 47.8 HV; VH (3% SiO₂): approx. 63.5 HV. | [46] |
| Compressive Strength | Dental Resin Composite | Calcined Silica Colloidal Nanoparticle Clusters (CSCNCs) 60 wt% + SCNCs 10 wt% | Compressive strength reached 260.3 ± 10.5 MPa. The study highlights how specifically engineered silica nanoparticle clusters can significantly bolster resin composites. | [43] |
| Wear Resistance | Light Cured Dental Composite Resin | Silica Nanoparticles (3 wt%) | Wear resistance improved; the increase in surface roughness after a brushing test was significantly less for the 3 wt% silica group (change of 0.14 µm) compared to the control group (change of 0.24 µm). This indicates a ~41.7% reduction in wear-induced surface roughness change. | [46] |
| Fatigue Resistance | Dental Resin Composites | Silica Colloidal Nanoparticle Clusters (SCNCs) | Enhanced static properties (like flexural strength) and improved structural integrity contribute to better fatigue life. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).