1. Introduction
Microplastics (MPs) have become a well-known pollutant in the environment, particularly due to increasing production of plastic waste. The most common definition of MPs is that they are plastic particles less than 5 mm in size [
1]. MPs have been found in a variety of foods, including seafood, such as fish, shellfish, seaweed, packed meat, chicken gizzard, crop, fruits and vegetables, seaweeds, salt, honey, sugar and beverages, such as beer, nonalcoholic beverages, milk, as well as in tap and bottled water [
2,
3,
4,
5,
6]. While studies on the presence of MPs in livestock are still lacking, MPs have been found in chicken gizzards because of trophic transfer [
7]. The main sources of MPs in food are food processing and packaging [
8,
9,
10,
11].
Possible exposure routes of MPs to humans are ingestion, inhalation, and dermal penetration, where ingestion is considered as the primary route. Exposure levels of MPs per capita per day was estimated to be 553 particles (184 ng/capita/day) and 883 particles (583 ng/capita/day) for children and adults, respectively [
12,
13]. Several studies have reported presence of microplastics in almost all human stool samples [
14,
15,
16] including children [
17] and infant feces [
18]. Moreover, microplastics were also found in the human stomach from 26 cadavers [
19] as well as in human colons from surgery samples [
20,
21]. Although MPs have long been considered inert, there is evidence of the impact of MPs on health. In the digestive tract, MPs have been linked to gut dysbiosis, inflammation, intestinal obstruction and mechanical damage, oxidative stress, and gut barrier destruction [
22]
. Due to their large surface area, MPs can interact and bind with different molecules present in the environment, including biomolecules. The mechanism of binding of biopolymers is proposed to be multilayered, with a formation of soft and hard coronae [
23]. However, there are only two studies investigating digestion of biomolecules in the presence of MPs on a molecular level. Tan et al. [
24] showed that lipid digestion is reduced in the presence of MPs (size range from 50 nm to 50 µm) due to decreased lipid bioavailability, as well as the binding of lipase, resulting in its reduced activity through conformational changes. In another study, it has been found that pepsin, the main protease in the stomach, can bind to polystyrene MPs (10 µm) leading to a decrease in its activity, and that the presence of MPs influences pepsin activity and milk protein digestion by pepsin [
25].
Food allergens are antigens triggering an allergic response by IgE binding after initial sensitization, and one of the main characteristics of proteins triggering an allergic response
via gastrointestinal tract is resistance to gastrointestinal digestion [
26]. Although the relationship between MPs and food allergies is yet not fully understood, MPs might contribute by binding to food allergens and inducing structural changes, modifying the digestibility of these allergens, increasing intestinal permeability, promoting an inflammatory gut environment, and causing intestinal dysbiosis [
27]. Furthermore, a recent study has shown that the ’protein coronae’ formed by MPs and the
Platanus acerifolia pollen allergen Pla a3 caused more damage to A549 cells than Pla a3 alone, due to an increase in oxidative stress and cytokine production [
28]. However, publications demonstrating the effect of MPs on digestion of food allergens are still scarce [
25,
29].
The α-Gal syndrome (AGS) represents a severe form of delayed allergy to mammalian meat and is an increasingly recognized public health issue. AGS manifests as a delayed early-phase allergic response, typically occurring 2–6 hours post-consumption, triggered by IgE antibodies targeting the oligosaccharide galactose-α-1,3-galactose present in glycoproteins and glycolipids of mammalian meat (such as pork, beef, or lamb) or dairy products [
30].
Trypsin is the main protease present in the small intestine with a well-understood mechanism of action. It is a serine protease that specifically cleaves peptide chains at the carbonyl end of positively charged amino acid groups, such as lysine and arginine. There are several studies investigating effects of ingested polystyrene (PS) and polyvinyl chloride (PVC) MPs on trypsin activity in marine and freshwater organisms, where decreased [
31,
32,
33] or increased trypsin activity [
34,
35] was observed. Stimulation of pancreatic enzymes activity by the presence of MPs in the gastrointestinal tract (GIT) was attributed to compensatory secretory response to improve digestion and absorption [
35]. However, there is only one study showing direct effects of MPs on trypsin activity. Liu et al. [
36] investigated adsorption of trypsin on PVC MPs (0.8 µm in size), showing that this interaction affected trypsin structure with limited influence on its activity. Currently there is no literature data on direct effects of PET and PP MPs on the structure and function of trypsin, an important intestinal digestive enzyme.
This study aimed to investigate effects of the presence of two types of MPs, polypropylene (PP, 117 and 326 μm,) and polyethylene terephthalate (PET, 55-63 μm), at a concentration of 20 mg/mL, often found in food and water [
4], on trypsin structure and activity, as well as on
in vitro digestibility of proteins and allergens from beef meat extract by trypsin. Adsorption of trypsin on to the surface of MPs was examined by analysis of adsorption isotherms and formation of trypsin hard and soft coronae. The influence of trypsin adsorption on its secondary and tertiary structure was revealed by CD spectrometry, while effects on activity were determined using a small synthetic substrate, Nα-Benzoyl-DL-arginine 4-nitroanilide hydrochloride (BAPNA).
In vitro digestibility of bovine meat extract (BME) sarcoplasmic proteins, and their soft and hard corona formation on the surface of MPs during digestion, were analyzed by SDS PAGE. Additionally, digestibility and soft and hard corona formation of α-Gal-carrying meat allergens were analyzed by immunoblot detection of the α-Gal moiety. This study provides further insight into MP interactions with GIT enzyme and the impact that this interplay can have on protein digestion, including food allergens.
3. Results
3.1. Binding Analysis of Trypsin to MPs
Binding of trypsin to MPs was analyzed by adsorption isotherms and demonstrated that the equilibration time of trypsin and PET is 1 h (
Figure S7), hence, this time point was further used for the determination of adsorption parameters (
Table 1) by fitting the obtained data into four binding isotherms (
Figure S8). Even during mixing, reliable equilibration could not be obtained due to floating of PP MPs in aqueous media. Thus, only adsorption onto PET MPs was analyzed.
Non-linear fitting of binding isotherms gave the best results for PET MPs with the highest R2. The affinity of trypsin for PET was relatively high with an adsorption capacity of 4.16 mg/g and 7.15 mg/g according to Langmuir and GAB models, respectively. Values obtained after fitting data into the Freundlich isotherm imply that the trypsin-PET mixture is a heterogeneous system most likely due to multilayer adsorptions, and that trypsin is favorably adsorbed to PET (1 < n < 10). The RL value in the Langmuir isotherm equilibrium parameter is between 0 and 1, also implying favorable adsorption, but its low value indicates that adsorption leans towards the irreversible. The relatively high Khard G in GAB isotherm, which is the adsorption constant for the hard corona, also indicates strong binding in the hard corona and low desorption. These results demonstrate that trypsin binds to the surface of PET MPs with a particularly strong binding in the hard corona.
3.2. Soft and Hard Corona Formation by Trypsin onto the Surface of MPs
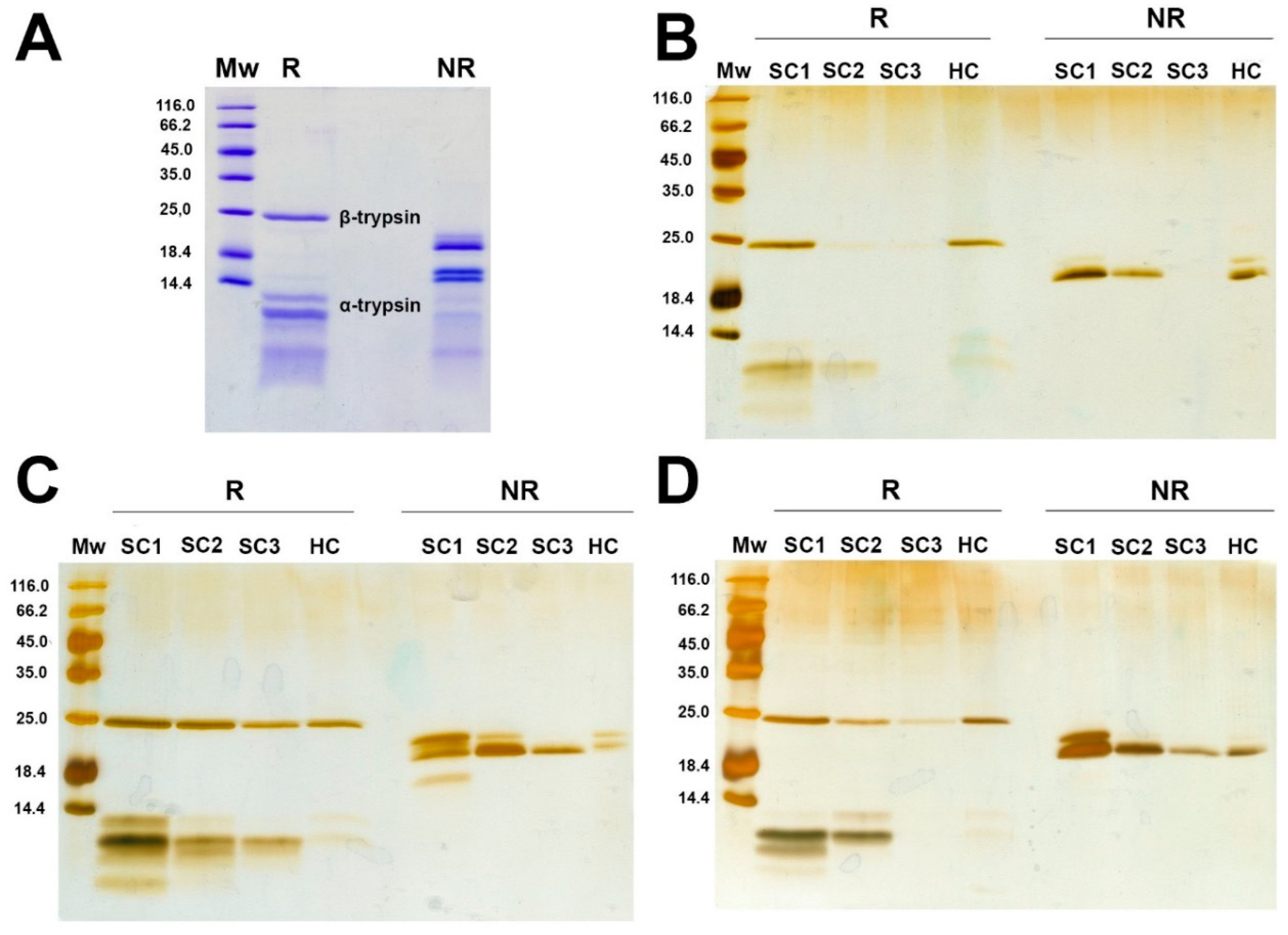
To investigate the formation of trypsin hard (HC) and soft coronae (SC) on PET and PP MPs, MPs were separated after 4 h incubation with trypsin, followed by soft corona isolation. Soft corona washings and the hard corona components were analyzed by reducing and nonreducing SDS PAGE (
Figure 1). The commercial trypsin preparation used in this study shows several bands under both conditions, originating from trypsin proteoforms (
Figure 1A). Trypsin exists in several proteoforms, and the commercial preparation of bovine trypsin contains about 60-70 % β-trypsin, 15-20 % α-trypsin, 5-10 % ψ- trypsin and the remaining are minor isoforms, including γ-, δ-, and ξ-isoforms [
44]. β-trypsin is a single intact polypeptide chain, and its autolysis generates two polypeptide α-trypsin chains. Further, intra-chain autolysis results in pseudotrypsin (ψ-trypsin) with three chains interconnected by disulfide bonds [
45].
Trypsin forms both SC and HC on the surface of both PET and PP MPs (
Figures 1B, C and D). The profiles of trypsin proteoforms from the SC were similar to that of the native trypsin preparation, with α- and β-trypsin dominating, suggesting that almost all proteoforms are bound in the SC.
The specific surface area of the smaller PET MPs (55 μm) per MPs mass was more than one order of magnitude higher than for PP MPs (117 and 326 μm), hence the PET MP samples exhibited a higher surface area available for binding compared to PP MPs. Despite this, trypsin adsorbed in higher quantities to the more hydrophobic PP surface, which is in accordance to the general experimental finding that in most cases the affinity of proteins to surfaces increases with increased surface hydrophobicity [
46]. Also, adsorption was slightly higher for sPP with a higher surface per mass ratio, than for lPP. Moreover, in the SC of sPP there was a higher ratio of autolyzed proteoforms (α-, γ-,δ-, ξ-trypsin)/β-trypsin than in the SC of PET MPs, suggesting higher affinity of autolyzed proteoforms for more hydrophobic MPs.
Figure 1.
Reducing and non-reducing SDS-PAGE analysis of trypsin preparation on 16% PAA gel (A) and soft and hard corona obtained after 4h incubation of trypsin with PET (B), sPP (C) and lPP MPs (D) on 14% PAA gels. Mw – Molecular weight markers, R – reducing conditions, NR – non-reducing conditions, SC – soft corona, HC – hard corona.
Figure 1.
Reducing and non-reducing SDS-PAGE analysis of trypsin preparation on 16% PAA gel (A) and soft and hard corona obtained after 4h incubation of trypsin with PET (B), sPP (C) and lPP MPs (D) on 14% PAA gels. Mw – Molecular weight markers, R – reducing conditions, NR – non-reducing conditions, SC – soft corona, HC – hard corona.
3.3. Structural Analysis of Trypsin After Incubation with MPs
Further, we investigated the effects of protein adsorption to the MP surface on the secondary and tertiary structure of trypsin. After incubating trypsin up to 4 h with MPs, near (
Figure 2) and far (
Figure 3) UV CD spectra of separated bulk and soft corona trypsin solutions were recorded.
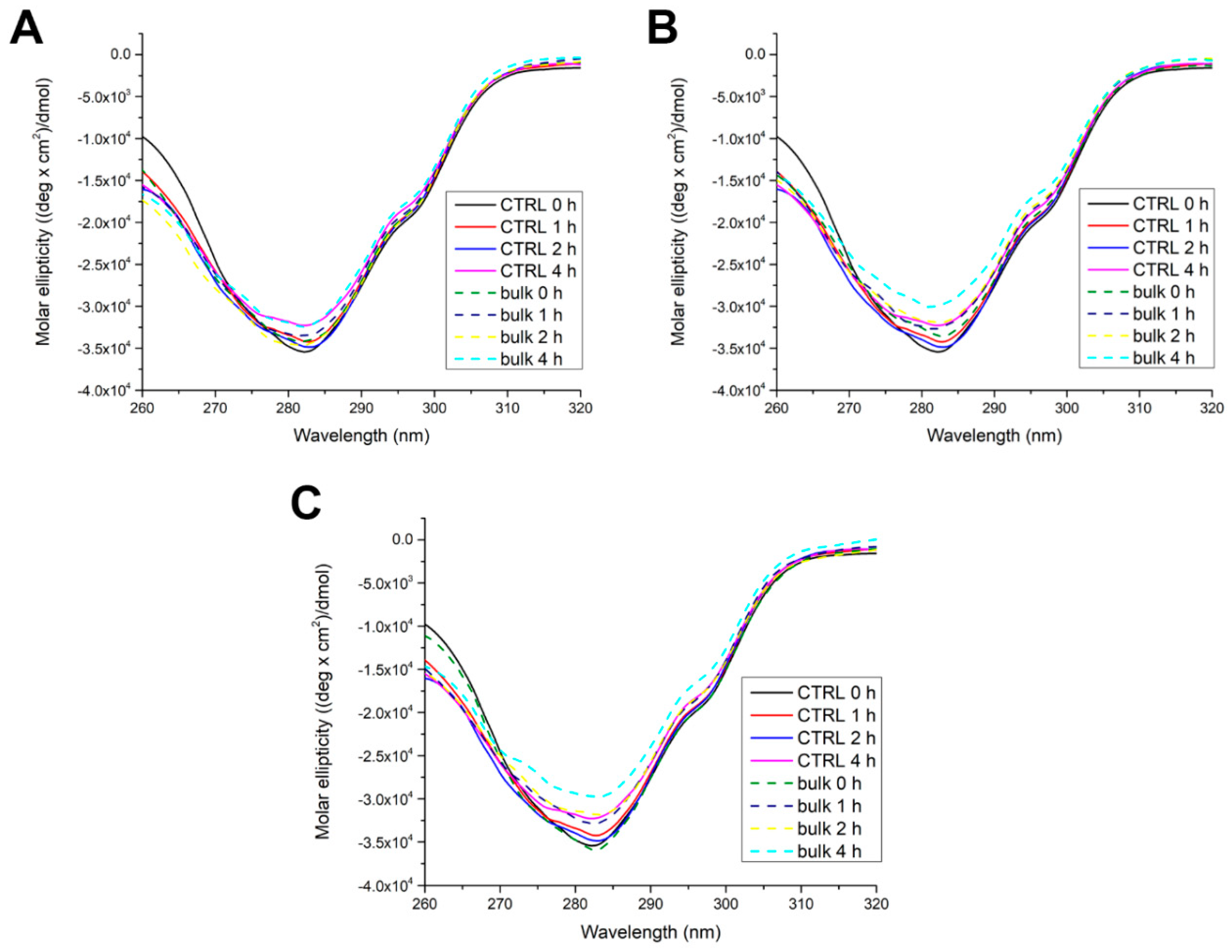
Figure 2.
Near CD spectra of bulk trypsin after incubation without (CTRL - control) or with PET (A), sPP (B), and lPP MPs (C).
Figure 2.
Near CD spectra of bulk trypsin after incubation without (CTRL - control) or with PET (A), sPP (B), and lPP MPs (C).
In the near CD spectra of native trypsin (control 0h), a minimum at around 282 nm could be observed, originating from 8 Tyr and 4 Trp. Over time, the signal gradually decreased and was lowest after 4 h. This suggests that trypsin’s tertiary structure becomes slightly looser. In the presence of PET, trypsin’s tertiary structure behaves similarly to the control, having the highest flexibility after 4 h at the level of the control. The presence of sPP and lPP induced a gradual decrease in signal over time but the CD signal after 2 h was at the level of the control after 4h. These results imply that PP MPs of both sizes induced only a slight loosening of trypsin’s tertiary structure.
Far UV CD spectra of trypsin show one larger minimum at 208 nm and a smaller minimum at 222 nm, which is typical for porcine trypsin [
47], indicating dominant β-sheet secondary structures (
Section S3., Figure S10). The far UV CD signal of control trypsin decreased over time.
Calculation of secondary structure content provides deeper insight into secondary structure changes (
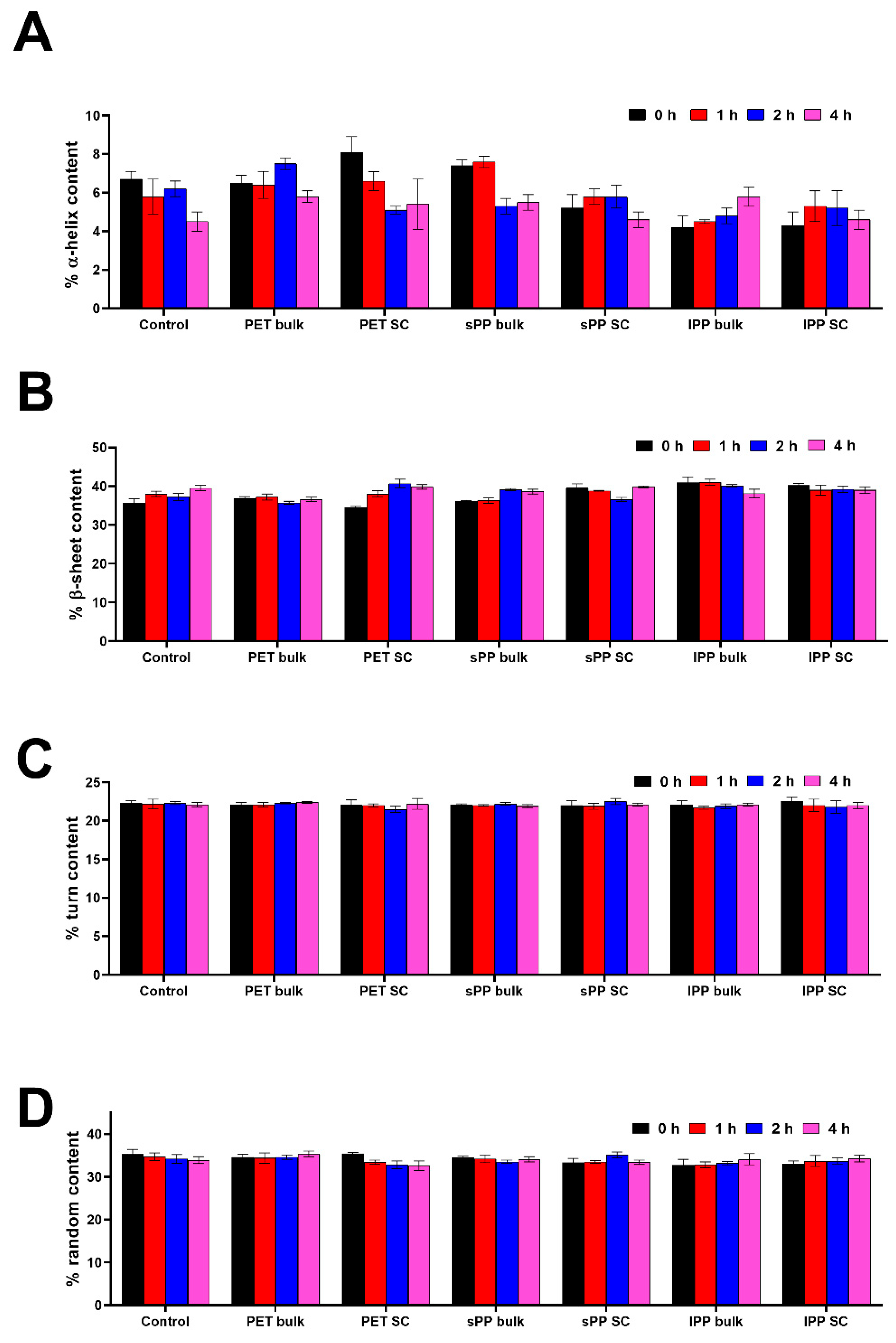
Figure 3, Table S4 in Section S5.). In comparison to the control, in the sPP SC there is a slight decrease in α-helix content, and it is more pronounced for the lPP SC, while in the PET SC it was slightly increased. In bulk solutions, however, the α-helix content in PET and sPP samples was similar or higher than in the control. In the lPP sample, it was already lower after 5 min of incubation. In all SCs, the β-sheet content was slightly increased and especially pronounced for lPP SC. In bulk solutions, β-sheet content in PET and sPP samples was similar to the control, and in lPP sample it was higher. These results show that lPP has mostly an opposite effect on both α-helix and β-sheet content in comparison to PET and sPP. These results suggests that lPP induced a slight α to β transition in both bulk and SC, whereas sPP induced it only in the SC. There were no differences in turn content. Random content in sPP bulk and PET bulk was similar to the control, while it was slightly lower in all SC and lPP bulk samples, suggesting that in all SC there was a slight increase in ordered structures.
Figure 3.
Content of secondary structures (%) in trypsin fractions (bulk and soft corona) after its incubation without (control) or with PET and PP MPs – α-helix (A), β-sheet (B), turn (C) and random (D) (n=4).
Figure 3.
Content of secondary structures (%) in trypsin fractions (bulk and soft corona) after its incubation without (control) or with PET and PP MPs – α-helix (A), β-sheet (B), turn (C) and random (D) (n=4).
3.4. Influence of MPs on the Activity of Trypsin
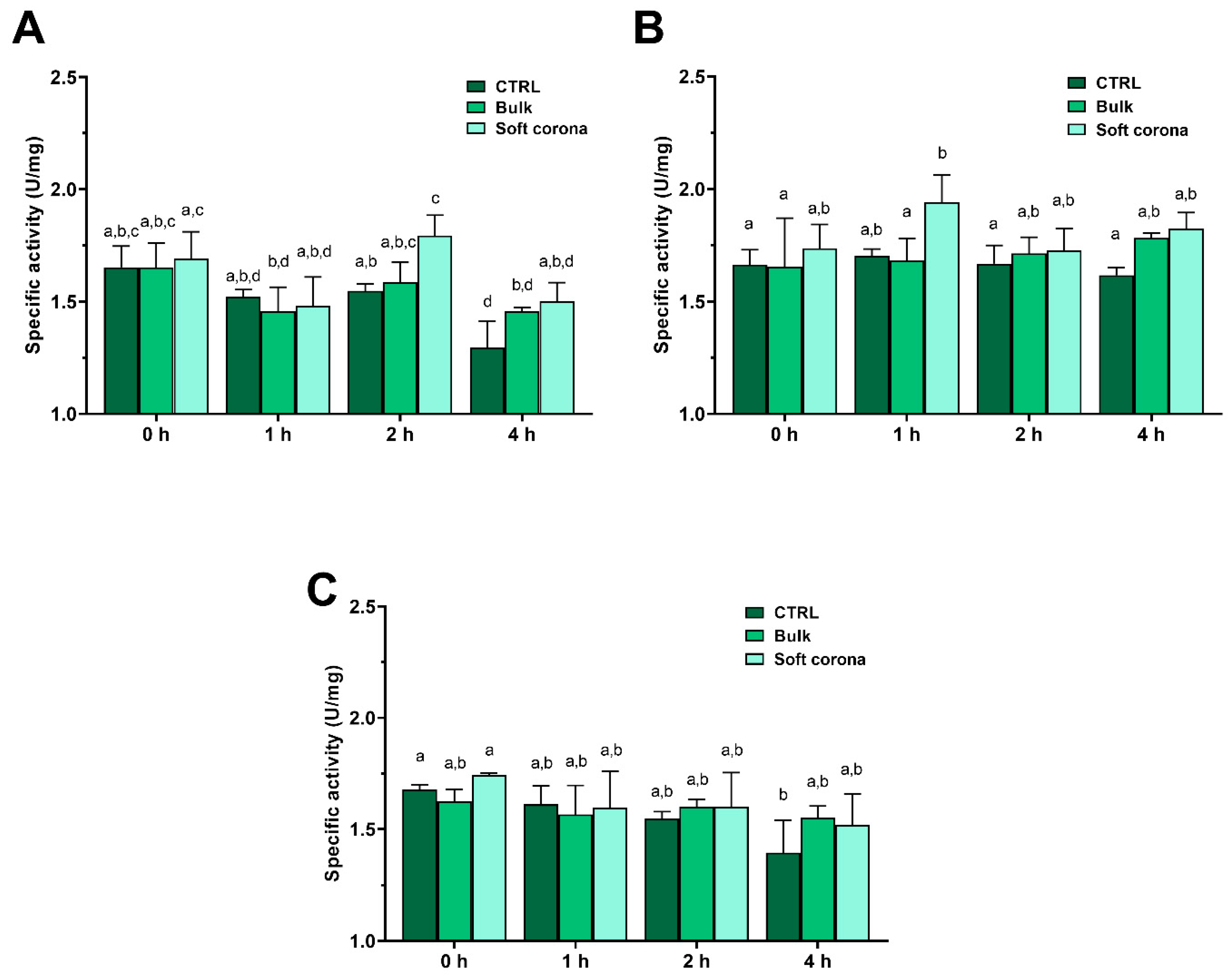
As adsorption-induced changes of enzyme structure are likely to affect enzymatic activity, we explored the extent to which trypsin adsorption to MPs influenced activity in bulk solution and in the adsorbed fractions (SC and HC). Trypsin was incubated with MPs for up to 4 hours. After separation of bulk solution and isolation of SC, trypsin activity was determined in bulk solution, the SC and HC corona.
3.4.1. Influence of MPs on the Activity of Bulk and Soft Corona Trypsin
In two out of three repeated positive controls (incubation of trypsin without addition of MPs), the trypsin specific activity decreased over time (
Figure 4). This can be attributed to autolysis at low Ca
2+ concentration (0.6 mM), which is insufficient to stabilize the structure and prevent autolysis [
48]. Following incubation with PET, there was a statistically significant increase in activity (p<0.05) in the SC relative to the positive control after 2 h of incubation. Similarly, trypsin incubation with sPP demonstrated a statistically significant (p<0.05) increase in activity in the SC after 1 h relative to the positive control after 0, 2 and 4 h, and bulk trypsin after 0 and 1 h. Following incubation with lPP, however, this difference was only statistically significant (p<0.05) between the positive control after 4 h of incubation and at the 0 h time point. Compared to the control, presence of MPs tended to preserve trypsin activity in bulk solution and the SC after 4 h of incubation.
3.4.2. Activity of Trypsin in the Hard Corona
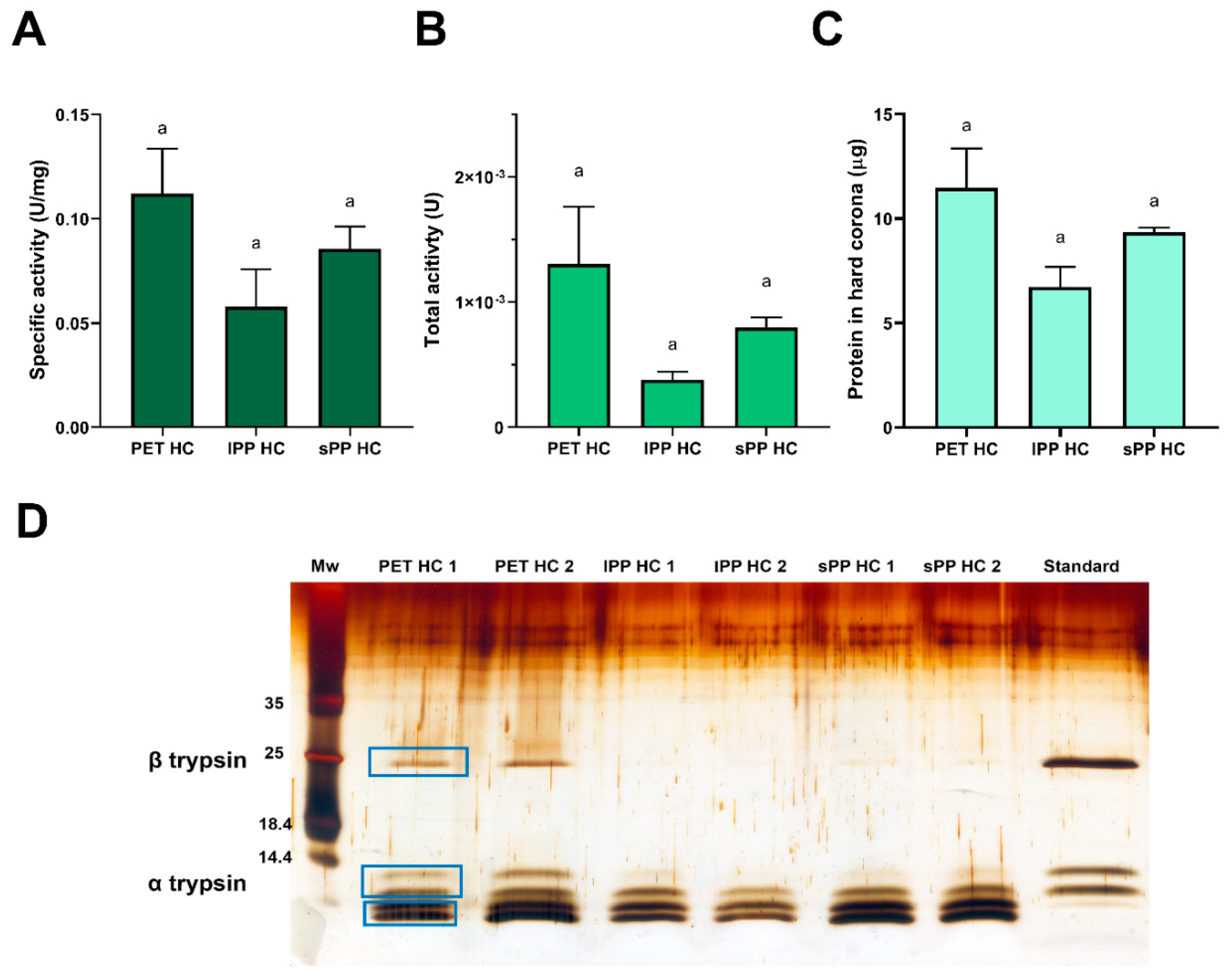
After trypsin incubation with MPs for 1 h and the subsequent separation of bulk solution and soft corona, trypsin specific activity in the HC was determined. Moreover, the protein content in the HC after trypsin activity determination was semi-quantified by reducing SDS PAGE analysis, followed by densitometry.
The specific activity of trypsin in the HC after 1 h of incubation with MPs was an order of magnitude lower than the activity in the bulk or soft corona (
Figure 5A). Similar results have been obtained before for trypsin that was irreversibly bound to polystyrene nanoparticles as a result of changes in both secondary and tertiary structure [
49]. There is a trend towards a higher total trypsin activity in the PET HC compared to the lPP HC and sPP HC, although not statistically significant (
Figure 5B). This is due to the tendency for binding of a higher amount of protein in HC of PET than in sPP and lPP HC (
Figure 5C), as well as due to a higher amount of more active α and β trypsin isoforms in PET HC, in comparison to both PP HC (
Figures 5D). After 1 h of MP incubation with trypsin, the amount of protein bound in the HC was as follows: PET > sPP > lPP, in accordance with the available MP surface area for trypsin binding (
Figure 5C and
5D). As stated previously, a 1 h incubation of trypsin with PET MPs was enough to reach the adsorption equilibrium (
Figure S6), However, this time seems to be longer for PP MPs, according to hard corona profiles obtained after a 4 h incubation (
Figure 1) After 1 h of incubation, there is a tendency for a twofold increase in the ratio of specific activity in the SC and HC for sPP and lPP MPs compared to PET MPs (24 and 26 vs 12.5, respectively). This demonstrates that the equilibrium between SC and HC was not reached after 1 h for PP MPs, resulting in lower amounts of trypsin in the PP hard corona, particularly of its active β and α proteoforms. Compared to the starting trypsin preparation, where the β and α-proteoforms are predominantly present, in the hard coronae there is a noticeable depletion of β and α-proteoforms and increase in peptides below the 14.4 kDa marker, most likely other less active autolytic proteoforms such as ψ-, γ- and δ-trypsin (
Figure 5D). It is hypothesized, that β-trypsin desorbs during incubation with BAPNA, while only traces of autolyzed proteoforms were desorbed due to their higher affinity to MPs. β-trypsin has a more compact hydrophobic core than α-trypsin [
50], and it seems that gradual loosening of this compact hydrophobic core with autolysis extent enables higher affinity of autolyzed proteoforms towards the hydrophobic surface of MPs in comparison to β-trypsin (
Section S2) (
Figure 5D).
The content of trypsin in the HC represents only about 0.5% of total trypsin (0.57% for PET, 0.34% for lPP and 0.47% for sPP). This suggests that, taking into account total trypsin, the presence of MPs, even at the relatively high concentration of 20 mg/mL, exerted only a negligible effect on trypsin activity overall.
Figure 5.
Trypsin specific activity (A), total activity (B), estimated amount of protein (C) and protein profile of trypsin (D) bound in hard corona after 1 h of trypsin incubation with MPs (n=2). Protein profile of trypsin was analyzed using SDS PAGE under reducing conditions after silver staining. The amount of protein in hard corona was estimated by densitometry of reducing SDS PAGE analysis. For the trypsin standard a preparation of 0.66 μg was applied.
Figure 5.
Trypsin specific activity (A), total activity (B), estimated amount of protein (C) and protein profile of trypsin (D) bound in hard corona after 1 h of trypsin incubation with MPs (n=2). Protein profile of trypsin was analyzed using SDS PAGE under reducing conditions after silver staining. The amount of protein in hard corona was estimated by densitometry of reducing SDS PAGE analysis. For the trypsin standard a preparation of 0.66 μg was applied.
3.5. Influence of MPs on In Vitro Digestion of Meat Extract Proteins by Trypsin
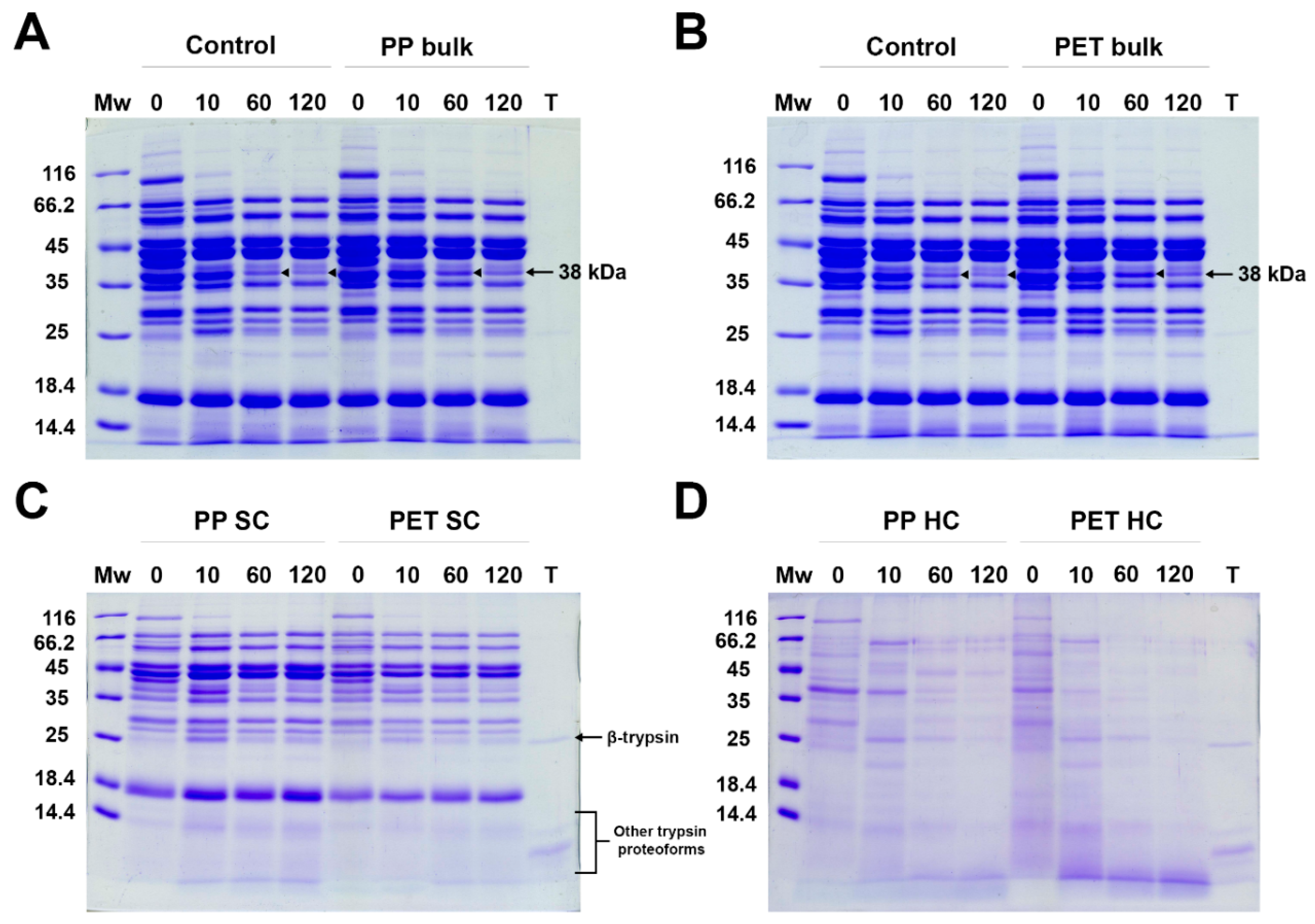
Beef meat extract (BME), containing mostly sarcoplasmic proteins, was subjected to in vitro trypsin digestion in SIF with and without the presence of lPP and PET MPs. After digestion, the bulk solution was separated for all time points, and the SC was isolated. Bulk solutions, SC, and HC were analyzed by reducing SDS PAGE.
In general, incubation with PET and lPP MPs did not seem to have a substantial effect on the digestion rate of BME proteins with trypsin (
Figure 6A and
B). In all digestion mixtures, proteins around 150, 100, 40, 33 and 30 kDa were rapidly digested after 10 min, generating fragments of about 22 and 25 kDa. After 60 min, several more proteins were digested, including bands at 100, 60 and 38 kD, as well as generating a fragment at 25 kDa. After 120 min, only the band at 38 kDa was further digested in all samples. The intensity of the band at 38 kDa, however, was more intense in the presence of MPs than in the positive control, suggesting that trypsin digestion of proteins within this band was delayed by the presence of both MPs.
At time point zero (0 min), BME proteins were actually in contact with MPs during the 5 min of centrifugation but even this short time was enough to form both a SC and HC by meat proteins (
Figure 6C). At this time point the SC of both MPs was mostly a reflection of the most abundant protein present in the BME. However, after 10 min in the presence of PP, SC protein bands were more intense than at 0 min, demonstrating further adsorption of the most abundant proteins in the SC, including a fragment at 25 kDa generated by digestion. Even though PET MPs have a much larger available surface area, there was no further protein adsorption in the PET SC after 10 min, indicating that BME proteins exhibited a much higher affinity for PP than for PET MPs. Similar to the bulk solution, the SC fraction investigated after 60 min showed bands at 100, 60, 38 kD and 25 kDa are also digested, implying that proteins residing within SC are prone to digestion at similar level as proteins in solution. Although binding of β-trypsin cannot be judged due to protein/proteolytical fragments at 25 kDa, other trypsin proteoforms are poorly adsorbed in the SC.
In contrast to the SC, the 0 min HC time point for both mixtures with PP and PET MPs was dominated by proteins at about 30, 38, 70, 60 and 100 kDa. After 10 min, there were dominant bands at 60 and 38 kDa, as well as a generated fragment at 25 kDa, and these bands were more intense in the PP HC. Additionally, after 60 min and 120 min a higher desorption of proteins from the PET HC than from the PP HC was observed, indicating a higher affinity to the PP surface rather than PET surface. There were abundant short fragments (<5 kDa) in the PET HC, which were faint in the PP HC, due to a much larger available PET surface area. Despite the fact that all proteins found in the SC were almost proportional to their concentration in the bulk solution, the HC composition of tightly bound proteins appeared to be also determined by the protein affinity for the MP surface and the available surface area. Similar to the SC, the trypsin proteoforms seem to be poorly adsorbed in the HC. These results suggest that in competition with about 50 times higher amount of BME proteins, trypsin adsorbs to MPs only in trace amounts, and thus trypsin structure and activity is almost completely preserved. The observed slight decrease in digestibility of proteins in the band of about 38 kDa may be a consequence of MPs affecting the structure of some proteins and their susceptibility to trypsin attack.
Figure 6.
SDS PAGE analysis of BME proteins digestion by trypsin in SIF in the presence of MPs and without MPs (control). A) Digestion of control and bulk solution in the presence of lPP MPs (lPP bulk); B) Digestion of control and bulk solution in the presence of PET MPs; C) Soft coronae (SC) of lPP and PET MPs during BME digestion; D) Hard coronae (HC) of lPP and PET MPs during BME digestion; Mw – molecular weight markers, 0, 10, 60 and 120 – digestion time in min, T – trypsin alone.
Figure 6.
SDS PAGE analysis of BME proteins digestion by trypsin in SIF in the presence of MPs and without MPs (control). A) Digestion of control and bulk solution in the presence of lPP MPs (lPP bulk); B) Digestion of control and bulk solution in the presence of PET MPs; C) Soft coronae (SC) of lPP and PET MPs during BME digestion; D) Hard coronae (HC) of lPP and PET MPs during BME digestion; Mw – molecular weight markers, 0, 10, 60 and 120 – digestion time in min, T – trypsin alone.
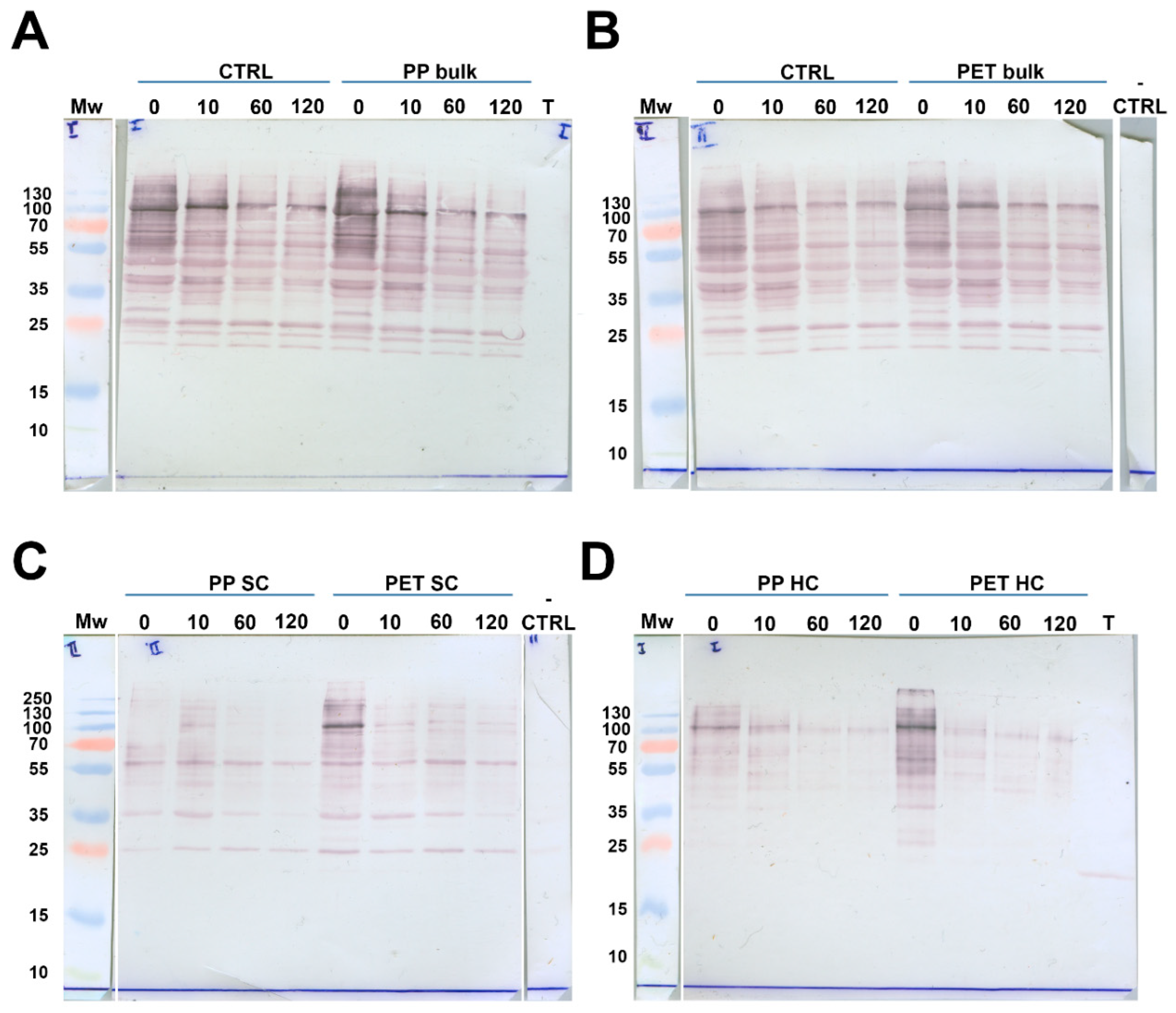
3.6. Influence of MPs on In Vitro Digestion of α-Gal Bearing Meat Allergens from Meat Extract by Trypsin
Bulk solutions, SC, and HC were tested for the presence of proteins containing the α-Gal moiety by immunoblot. Even after 120 min of digestion without MPs (positive control), BME proteins were only partially digested, implying their considerable resistance to trypsin action. The α-Gal –carrying protein (α-GalP) profiles during digestion in the positive control sample were identical to profiles during digestion with both PP and PET MPs, suggesting that the presence of MPs did not have a substantial effect on meat allergens (
Figure 7A and
B).
Similar profiles of α-GalP in bulk solutions and in the SC of both MPs during all digestion times demonstrated that proteins residing within the SC were prone to digestion at similar level as proteins in solution (
Figure 7). This was also observed for BME proteins. α-GalP also quickly formed a SC and HC after only 5 min of contact with the MPs (time point 0 min,
Figures 7C and D). It further showed a higher abundance of α-GalP in the PET SC compared to the PP SC due to the larger available surface area. However, after 10 min the PP SC protein bands were more intense than at 0 min, indicating further adsorption of α-GalP. In the PET SC there was pronounced protein desorption, showing higher α-GalP affinity to PP than to PET, also observed in the SC of total BME proteins.
In the PET HC at 0 min, α-Gal carrying proteins were much more abundant than in the PP HC due to a much larger available surface area. After 10 min, desorption of proteins from the HC of both MPs is observed, but considerably more pronounced from the PET HC. This suggests a lower affinity of α-GalP for the PET than for the PP surface. These results demonstrate that α-Gal carrying proteins bind to both the SC and HC of both MP types, and that these proteins exhibited a higher affinity to PP than to PET, similar to total BME proteins.
4. Discussion
While several recent reviews are covering possible concerns and effects of micro- and nanoplastics on the GIT [
51,
52], studies on direct interactions of food proteins and GIT enzymes with MPs, effects of MPs on the structure and activity of GIT enzymes, as well as the influence of MPs on digestibility of proteins within complex food matrices, particularly food allergens, are still scarce.
Trypsin’s adsorption to PET MPs was analyzed using four isotherm models, demonstrating that trypsin binds to the PET MPs surface with particularly strong and favorable binding in the HC. In this study, the obtained Langmuir constant (10.15 mL mg
-1) and Freundlich constant (4.08 (mg/g)*(mL/mg)
1/n) was several times lower than previously reported by Liu et al. [
36]. For trypsin adsorption to PVC MPs with a size of 0.8 µm (Langmuir: 63.97 mL mg
-1, Freundlich: 49.73 (mg/g)*(mL/mg)
1/n), suggesting a lower trypsin affinity to PET than to the PVC surface. In this study, also a much lower adsorption capacity was obtained than by Liu et al. using the Langmuir model (4.16 vs 51.16 mg/g). This is due to the substantially higher hydrophobicity of PVC over PET, with a two-fold higher LogP(SA)−1 value [
53], as well as a much larger adsorption surface per MPs mass of 0.8 μm-PVC MP in comparison to that one for 55 μm-PET MPs used in this study.
Trypsin and its proteoforms were found in the soft and hard corona of MPs. Both intact β-trypsin and autolyzed forms were present at almost equal amounts in the SC. The quantity of bound protein seems to be higher for more hydrophobic surfaces (PP > PET), despite a much larger available surface area of smaller PET particles. The effect of a larger surface area was seen for PP MPs, where sPP bound more proteins than lPP (sPP > lPP). Higher intensity of protein bands in the SC and HC of lPP MPs, were also found for BME sarcoplasmic proteins and meat allergens, showing their higher affinity for PP MPs. Protein adsorption to PET is mainly based on: I) hydrophobic interactions between long aliphatic side chains and the PET surface, II) π-π interactions between aromatic amino acids and aromatic rings of terephthalate, and III) hydrogen bonds between amino acids and PET oxygens. In contrast, protein interactions with PP are exclusively hydrophobic. PP is the most hydrophobic plastic material with a LogP(SA)
−1 value of about 25 x 10
-3 Å
-2, while PET is much less hydrophobic with a LogP(SA)
-1 around 8 x 10
-3 Å
-2 [
53]. Proteins tend to adhere more strongly to nonpolar than to polar surfaces as a nonpolar environment destabilizes proteins and thus facilitates a conformational rearrangement. This results in strong protein–surface hydrophobic interactions [
54], which explains the observed higher affinity of trypsin to sPP MPs and BME proteins to lPP MPs.
Upon adsorption, PET MPs do not change trypsin’s tertiary structure in bulk solution, while both PP MPs induce only slight loosening. However, trypsin’s secondary structure was more affected upon adsorption, particularly in the SC. All tested MPs slightly induce an increase in the ordered structure of trypsin in the SC and slight rearrangement of its secondary structure, most pronounced for lPP MPs with an α to β transition. Upon adsorption of porcine trypsin to PS NPs X. Li et al. [
55] observed an α to β transition with a decrease in random content. Trypsin adsorption on PVC MPs also resulted in a decrease of random content, but with a β to α transition [
36]. Minor changes of the trypsin structure induced by MPs did not have negative effects on the specific activity in bulk and SC, but in contrast, exhibited slight positive effects with preservation of trypsin’s activity during prolonged incubation.
In the HC, specific activity was several times lower than the activity in the SC and bulk solution, most likely because trypsin bound in the HC contains a higher share of less active/inactive trypsin molecules. This is in accordance with trypsin binding with high affinity in HC observed in adsorption isotherms, which is an irreversible process. However, even in the HC, where only a low trypsin fraction was found, there was residual trypsin activity of about 5-10%. As trypsin molecules in the HC make up 0.34-0.57% of total trypsin mass, it can be concluded that MPs have only negligible effects on trypsin activity. Although, trypsin adsorption to 0.8 μm –size PVC MPs significantly affected trypsin’s secondary and tertiary structure, Liu et al. [
36] observed only a slight decrease in its activity in bulk solution at a MP concentration of 0.08 mg/mL. This suggests that even when high affinity trypsin adsorption to MPs induce a significant structural change, trypsin activity is more or less preserved. In contrast to trypsin, our previous study [
25] demonstrated that 10 μm- PS MPs caused structural changes of pepsin in simulated gastric fluid, and at a concentration of 0.012 mg/mL these MPs induced significant reduction of pepsin activity in bulk. Further, 10 μm –sized PS MPs at a concentration of 0.08 mg/mL reduced lipase activity by 23%, with changes in secondary structure [
24]. This implies that when MPs are incubated with GIT enzymes, the preservation of enzyme activity depends on enzyme structure. Interestingly, even when MPs induced some structural change, this may or may not affect total enzyme activity. The current study demonstrates that trypsin activity seems to be less sensitive to MP-induced structural changes than pepsin and lipase.
There are three possible reasons why only slight effects of MPs on trypsin structure and activity were observed in this study. The first reason is the high trypsin concentration applied, relative to the MPs surface area. This could have prevented enzyme spreading upon adsorption and thus, more intensive conformational changes. It has been shown that only adsorption at low protein concentrations allows high spreading and significant conformational changes of adsorbed proteins [
56]. Secondly, in relation to the stability of proteins at solid-liquid interfaces and surface adsorption, proteins are classified as hard and soft, having high and low structural stability, respectively [
57]. α-helices are more compressive than β-sheets and their unfolding requires less energy, which explains why soft proteins have a higher ratio of α-helix to β-sheet than hard proteins [
58]. Similarly to α-chymotrypsin, categorized as a “hard” protein [
59], trypsin is mainly composed of β-sheets, thus having a low tendency for structural alterations upon surface adsorption. Thirdly, Ca
2+ extraction from trypsin by MP surfaces was completely prevented as the uncharged surface of PP and PET MPs does not enable significant Ca
2+ binding and all experiments were performed in the presence of 0.3 or 0.6 mM Ca
2+. In contrast, upon adsorption of α-lactalbumin, whose structure is also stabilized by Ca
2+ ions, PS nanospheres induce its non-native conformation with preserved secondary structure and loss of tertiary structure due to strong binding of Ca
2+ ions on negatively charged PS [
60].
This study demonstrates that during digestion both trypsin and BME proteins (including αGal-carrying allergens), as its substrates, bind to both PP and PET MPs. Further, they are found in the SC and HC of both MP types. However, BME proteins have a higher affinity for the more hydrophobic PP surface compared to PET. On the other hand, despite trypsin and BME proteins adsorption on the MP surface, and the fact that an extremely high MPs concentration (20 mg/mL MPs with 3 mg/mL meat proteins) was used, only slight effects were observed on
in vitro digestibility of BME proteins. Moreover, only the digestibility of BME proteins with masses of about 38 kDa were affected, leading to its slowing down. In addition, MPs did not affect digestion of α-Gal meat allergens. In contrast, our previous study [
25] demonstrated that presence of 10 μm-sized polystyrene MPs have a negative effect on pepsin digestion of cow’s milk proteins even at a MP concentration of 0.3 mg/ml MPs (with 7.5 mg/mL of milk proteins in gastric digestion mixture).
There are two reasons why only slight effects of MPs on total BME protein and α-Gal bearing meat allergens digestibility was observed. Firstly, it was demonstrated that the presence of MPs does not affect the trypsin activity, except for the low trypsin fraction in the HC. Moreover, during digestion, in the presence of almost 50 times higher mass of BME proteins competing with trypsin for adsorption on the plastic surface, the affected trypsin fraction in HC is even lower, thus an effect on the trypsin activity is even less likely. The second reason is that beef meat proteins are mainly (90%) digested during the gastric phase before reaching intestinal digestion [
61]. Based on the α-Gal binding properties, α-Gal allergens from BME are relatively resistant to gastric, and completely resistant to subsequent intestinal digestion [
62]. Hence, it is expected that in this study BME proteins and allergens are only partially digested, and thus it is hard to observe a profound effect of MPs on meat protein digestion.
Tan et al. [
24] reported that PET MPs with comparable size and shape to the ones used in this study, decreased the
in vitro intestinal digestion of lipids by about 10% at concentration 250 times lower than in this study (80 µg/mL). This was due to MPs-induced decrease in enzyme activity and reduced availability of lipid droplets. In this study, during BME digestion tested MPs, even at concentration of 20 mg/mL, did not influence enzyme activity and only slightly influenced protein substrate susceptibility to trypsin action. However, it was demonstrated that the presence of MPs does not decrease the resistance of BME proteins, nor α-Gal allergens, to trypsin. Even in the presence of 6.7 times higher mass of MPs than BME proteins, no increase in their digestibility was observed. Moreover, BME proteins and α-Gal allergens residing within the SC are prone to digestion at similar levels as proteins in solution, suggesting the same level of protein availability to trypsin attack. These results suggest that, although adsorption of BME proteins and α-Gal allergens to MPs surface most likely changes their structure, at least slightly, this structural change is not sufficient to facilitate trypsin attack.
The SC and HC of BME proteins, as well as meat allergens, forms quickly on both particle types within 5 min of contact with MPs (digestion time point 0 min). During digestion however, both the SC and HC behave as a dynamic system, where in addition to protein digestion by trypsin, adsorption and desorption of proteins and their proteolytic products take place. Moreover, this study demonstrated that both the SC and HC are rich in α-Gal allergens, behaving similarly to total BME proteins with their dynamic adsorption and desorption.
In general, allergens are highly abundant proteins in foods, their adsorption on the surface of MPs results in a corona enriched with allergenic proteins as observed in this study. Upon their adsorption, changes in food allergen secondary and tertiary structure induced by MPs might lead to the exposure or disruption of conformational and/or linear epitopes, resulting in altered interaction with IgE and affected food sensitizing and allergenic capacity [
27]. Phue et al. [
63] reported that milk allergens form a protein corona on SiO
2 and TiO
2 nanoparticles, and that particle-mediated alterations in the protein structure could enhance the allergenicity of milk proteins. It can therefore not be excluded that meat allergens residing in the SC, and particularly in the HC, or allergens desorbed to solution, may have altered IgE binding properties and faith during digestion.
This study demonstrated that neither MPs with highly hydrophobic surface (PP), nor MPs with moderately hydrophobic surface (PET) significantly affect trypsin activity. The estimated median steady-state of MPs in the gut is about 300-500 particles/capita (0.8 to 1.6 ng/capita) [
12]. Therefore, it is unlikely that the realistic exposure of humans to MPs in the intestine would have significant effects on BME proteins digestibility by trypsin. Although further investigations will reveal how the presence of MPs affect other intestinal proteases (e.g. chymotrypsin, carboxypeptidase, elastase, and particularly brush border peptidases), it is also unlikely that the presence of realistic MP amounts in the GIT would influence amino acid release and their overall bioavailability. However, due to the adsorption of BME allergens and their IgE-binding epitopes in the SC and HC, the presence of MPs in the GIT may affect their absorption, transportation, and subsequent presentation to the host immune system.