1. Introduction
Pancreatin is a complex of digestive enzymes extracted primarily from the pancreas of cattle or pigs, playing a critical role in the hydrolysis of carbohydrates, proteins, and lipids within the digestive tract [
1,
2]. Due to its potent enzymatic activity, pancreatin is extensively utilized in pharmaceutical formulations to support digestion and in the food industry for processing complex biomolecules [
3,
4,
5,
6]. Its key enzymatic constituents—amylase, protease, and lipase—specifically catalyze the breakdown of starch, proteins, and dietary fats, respectively [
7,
8,
9].
For effective application, pancreatin enzymes must retain both catalytic activity and structural stability throughout production, storage, and use [
10,
11,
12,
13,
14]. However, CM techniques often subject the material to thermal and mechanical stresses, potentially causing enzyme denaturation, aggregation, or partial inactivation. This can reduce enzymatic potency, thereby limiting the shelf life and functional efficacy of the product [
15,
16,
17,
18,
19,
20]. Consequently, an important challenge in pancreatin processing is developing extraction methods that preserve enzymatic functionality and stability [
21,
22,
23,
24].
UAM emerges as a promising non-thermal technique, utilizing high-frequency sound waves to disrupt cellular structures and enhance the release of intracellular compounds [
25]. Compared to traditional agitation methods, UAM offers several advantages, including shorter processing times, lower energy consumption, and better preservation of sensitive biomolecules [
26,
27,
28]. Although ultrasound has been shown to improve extraction efficiency of various bioactives, such as plant metabolites and proteins, its effects on the functional properties and thermal stability of animal-derived enzymes—specifically those in bovine pancreatin—remain insufficiently characterized [
29,
30,
31,
32,
33,
34].
This study aims to fill this knowledge gap by comparing conventional mixing CM and UAM methods in terms of their impact on pancreatin’s enzymatic activity. We evaluate AA and PA over a broad pH range (5.5–8.0) and temperature spectrum (10–50 °C), reflecting physiological and industrial conditions. Our objective is to identify the extraction approach that better preserves enzymatic functionality and to determine conditions yielding maximal activity. The findings are expected to advance the development of efficient, scalable, and thermally stable pancreatin formulations for pharmaceutical, food, and biotechnological applications.
2. Results and Discussion
2.1. Effect of pH and Temperature on AA
2.1.1. Temperature
The AA of pancreatin extracted from bovine pancreas was determined according to GOST 34440-2018 [
35] using a UV–Vis spectrophotometer (Agilent Technologies, USA) equipped with Cary WinUV software, operating over a spectral range of 190–1100 nm. To assess the thermal sensitivity of the enzyme, AA was evaluated across a temperature gradient (10–50 °C) at a fixed pH of 6.0, using pancreatin preparations obtained via both CM and UAM.
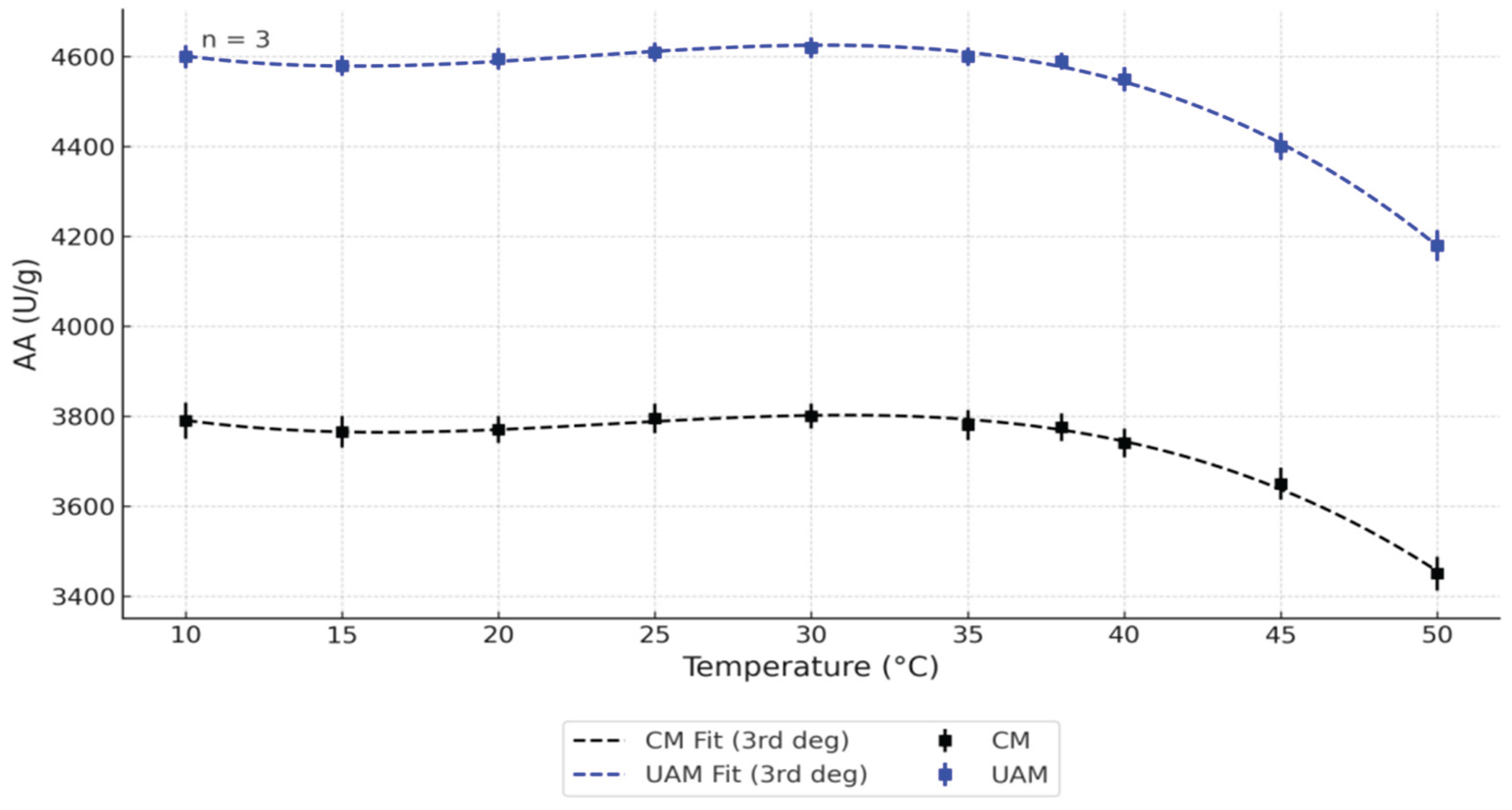
Temperature was selected as a critical variable, given its direct influence on enzymatic stability and activity, particularly in relation to the thermostability of pancreatic enzymes. All measurements, data processing, and result interpretations were carried out by qualified specialists from the Kazakh Research Institute of Processing and Food Industry (KazRIPFI, Kazakhstan), who have extensive experience in enzymatic assay methodology. The results of this analysis are shown in
Figure 1.
In previous studies, the thermal behavior of α-amylase varied depending on the source organism. For example, Dutta et al. [
36] reported that α-amylase extracted from the freshwater zooplankton
Heliodiaptomus viduus exhibited optimal catalytic activity at 30 °C and pH 6.0. Notably, the enzyme retained substantial activity up to 70 °C, remaining stable for 2 h at 60 °C and 1 h at 70 °C. In contrast, Fadeel et al. [
37] found that α-amylase secretion was significantly inhibited at low temperatures (≤15 °C), with an optimal secretion range between 19 °C and 21 °C. Furthermore, Elmansy et al. [
38] identified 45 °C as the optimal temperature for α-amylase production by thermo-halophilic bacterial strains.
In the present study,
Figure 1 illustrates the temperature-dependent changes in AA of bovine pancreatin obtained via CM and UAM across the 10–50 °C range. UAM consistently resulted in significantly higher AA values compared to CM at all tested temperatures. Peak enzymatic activity (~4600 U/g) was observed in UAM-treated samples between 30 and 35 °C, while CM-derived pancreatin achieved a lower maximum (~3800 U/g) within the same interval. Above 38 °C, both methods exhibited a progressive decline in AA, likely due to enzyme denaturation. The data trends were well fitted by third-degree polynomial regression models (
R² > 0.95), supporting the reliability of the observed patterns. Error bars indicate standard deviations from triplicate measurements (
n = 3). These findings provide strong evidence that UAM enhances not only the yield but also the thermal stability of α-amylase, highlighting its potential for producing robust enzyme preparations from bovine pancreatic tissue.
The outcomes of the two-way ANOVA (
Table 1) clearly indicate that both temperature and extraction method had a statistically significant effect on AA, with p-values of 1.00 × 10⁻²⁶ and 1.21 × 10⁻⁵², respectively. In contrast, the interaction between these two factors was not significant (
p = 0.099), suggesting that temperature and method influenced AA independently rather than interactively. These results confirm that the application of UAM consistently enhances enzyme activity across the tested temperature range.
To further dissect the differences between experimental conditions, a post hoc Tukey’s Honestly Significant Difference (HSD) test was performed. This analysis revealed multiple significant pairwise differences (p < 0.05) between extraction method–temperature combinations. Notably, samples obtained via UAM displayed significantly higher AA than those obtained by CM at nearly all tested temperatures (10–50 °C). The most pronounced differences—up to 20%—were recorded in the mid-temperature range (34–38 °C), where enzyme activity reached its peak. These differences remained statistically significant after correction for multiple comparisons (adjusted p < 0.001), reinforcing the conclusion that UAM is a more effective technique for preserving and enhancing the enzymatic activity of pancreatin.
2.1.2. pH and Temperature
The AA was evaluated under varying pH (5.5 to 8.0) and temperature (10–50 °C) conditions. The results for CM and UAM are presented in
Figure 2 (
a,b).
The influence of pH and temperature on AA of pancreatin obtained by CM is shown in
Figure 2a. Across the pH range tested (5.5–8.0), AA remained relatively stable between 10 °C and 38 °C, with activity values fluctuating between approximately 3727 and 3785 U/g. Beyond 38 °C, a gradual decrease in enzymatic activity was observed, dropping to values between 3387 and 3709 U/g at 50 °C. The highest AA for CM samples was observed at pH 6.0 and 38 °C, registering 3785 U/g.
Figure 2b presents corresponding data for pancreatin extracted via UAM. Under the same conditions, UAM samples consistently demonstrated higher AA values than CM, maintaining stable activity between 4587 and 4606 U/g up to 38 °C. At temperatures above 38 °C, a progressive decline was recorded, with AA decreasing to approximately 4100–4500 U/g at 50 °C. The peak AA for UAM pancreatin was also observed at pH 6.0 and 38 °C, reaching 4606 U/g. Overall, these results highlight the enhanced efficacy of UAM in preserving AA across a range of physiologically relevant pH and temperature conditions, underscoring its potential advantage over CM.
2.2. Effect of pH and Temperature on PA
2.2.1. Temperature
PA serves as a critical measure of the functional potential of enzyme preparations, particularly those enriched with pancreatic proteases such as trypsin and chymotrypsin. This parameter reflects the enzymes’ capacity to hydrolyze protein substrates into peptides and free amino acids, a process essential in diverse fields including food processing, biotechnology, and pharmaceutical manufacturing. Precise evaluation of PA allows for assessment of enzymatic strength, stability, and suitability for targeted industrial applications. Furthermore, understanding how environmental factors like pH and temperature influence PA is crucial for optimizing extraction protocols and maintaining the quality and consistency of enzyme products [
39,
40,
41,
42].
In this study, PA was determined following the standardized method outlined in GOST 34443-2018 “Enzyme Preparations for the Food Industry. Method for Determining Proteolytic Activity” [
43]. The experimental results are summarized in
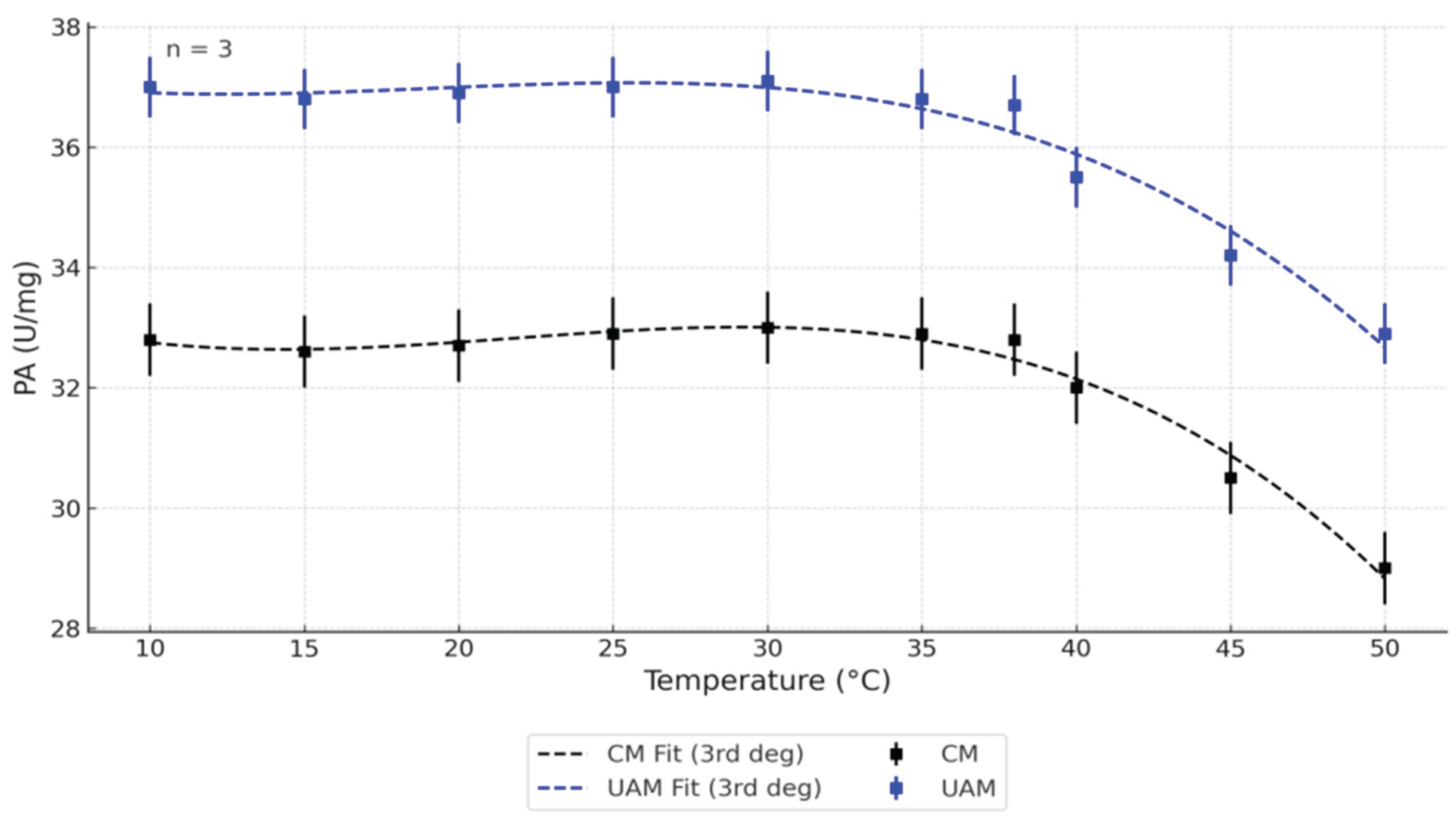
Figure 3.
Majeed et al. [
44] reported that protease derived from
Bacillus subtilis BSP exhibited maximal catalytic activity at 50 °C and pH 8.0. The enzyme retained approximately 73% of its activity after 1 hour at 70 °C, although exposure to 80 °C led to an 84% reduction. Similarly, Suleiman et al. [
45] demonstrated that
Geobacillus thermoglucosidasius SKF4 showed optimal growth and protease synthesis between 60–65 °C, with peak activity at 60 °C. In another study, Sarker et al. [
46] found that protease from a halo-tolerant strain of
Bacillus subtilis Rand maintained full activity after 30 minutes at 37–55 °C and retained 80% activity following incubation at 60 °C.
In the present study, the effect of temperature on PA of bovine pancreatin was evaluated using both CM and UAM across a temperature range of 10–50 °C (
Figure 3). The results clearly show that pancreatin obtained via UAM exhibited consistently higher PA values compared to CM at all tested temperatures. The highest PA in UAM samples was recorded between 30–35 °C, after which a gradual decline was observed with increasing temperature, likely due to partial thermal inactivation of proteases. In contrast, CM samples showed a lower peak and more pronounced loss of activity at elevated temperatures.
Third-degree polynomial regression models (R² > 0.95) accurately described the temperature-dependent PA trends for both extraction methods. Standard deviations (n = 3) are shown as error bars. These findings align with previous results for α-amylase activity and confirm that UAM enhances both the efficiency and thermal stability of proteolytic enzyme extraction from bovine pancreatic tissue.
To assess the influence of temperature and extraction method on PA, a two-way analysis of variance (ANOVA) was conducted. As shown in
Table 2, both temperature (p = 2.30 × 10⁻¹⁶) and extraction method (p = 9.52 × 10⁻²⁸) had statistically significant effects on PA. However, the interaction between these two factors was not significant (p = 0.85), indicating that temperature and method influenced PA independently. The highest activity was observed in the 34–38 °C range for both extraction methods, though UAM consistently produced greater PA values across the full temperature spectrum. At elevated temperatures (≥40 °C), a marked reduction in PA was observed, likely reflecting partial thermal denaturation of the enzyme complex. These results suggest that UAM is more effective than CM in both preserving and enhancing PA, with its advantage remaining stable under varying thermal conditions.
To further explore differences among groups, Tukey’s Honestly Significant Difference (HSD) post hoc test was applied following ANOVA. The analysis revealed that UAM samples exhibited significantly higher PA values than CM samples at nearly all tested temperatures (adjusted p < 0.01). The most notable differences were recorded between 34 °C and 38 °C, where UAM-treated samples demonstrated PA levels approximately 10–15% higher than those of CM. Although overall activity declined at temperatures above 40 °C for both methods, UAM continued to provide significantly higher PA, underscoring its consistent and robust effect on enzyme recovery. These findings validate UAM as a superior extraction approach for maintaining proteolytic enzyme functionality under thermal stress.
2.2.2. pH and Temperature
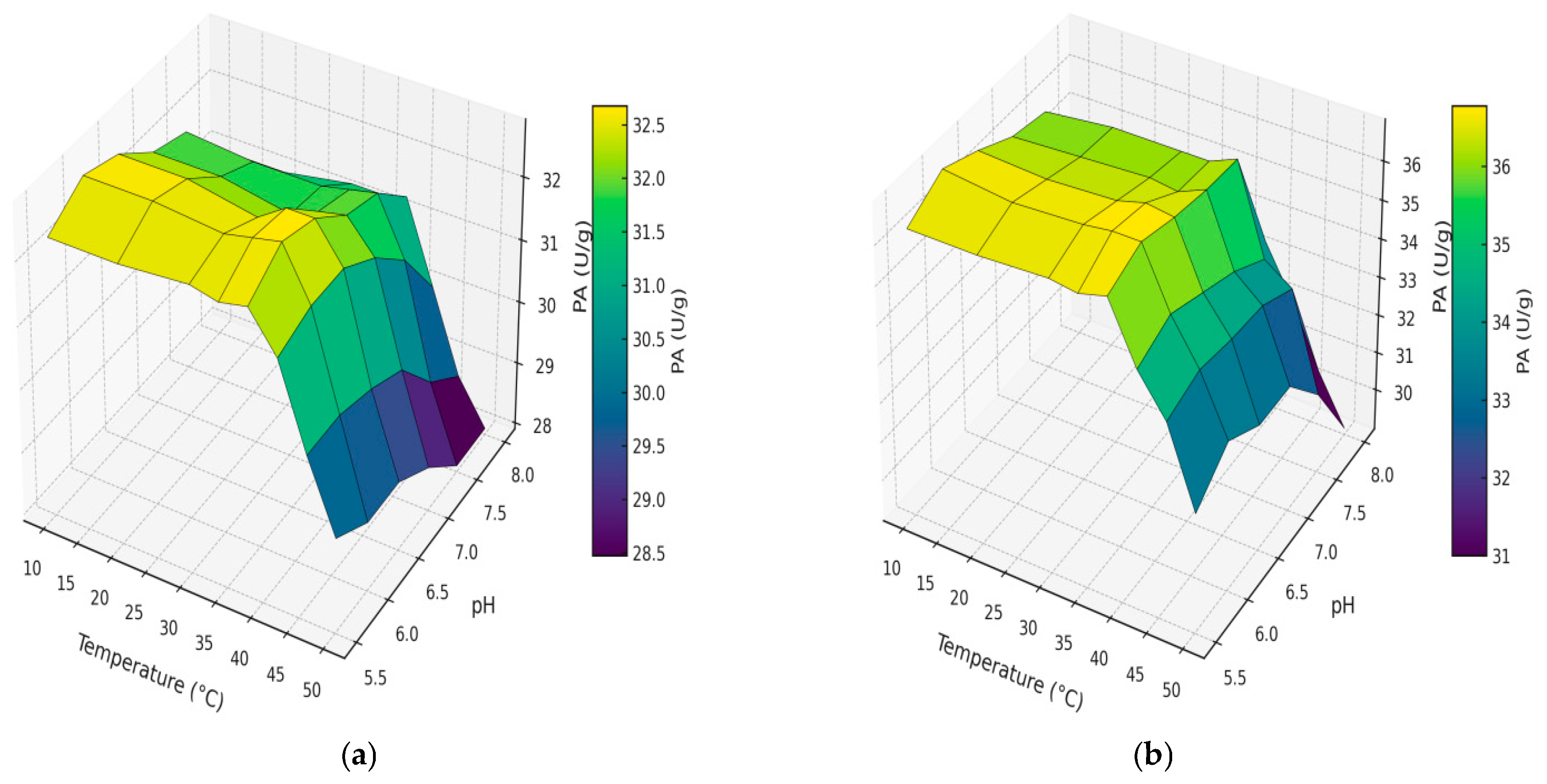
The 3D surface plots (
Figure 4) illustrate the dependence of enzymatic activity on temperature and pH for pancreatin samples obtained using CM and UAM.
Figure 4 illustrates how PA in bovine pancreatin responds to combined variations in pH and temperature for both extraction methods. In both cases, PA remained relatively stable across the pH range tested at temperatures between 10 °C and 38 °C. However, a noticeable decline in enzymatic activity was observed at higher temperatures (42–50 °C), particularly under alkaline conditions. For CM (
Figure 4a), the highest activity was recorded between pH 6.0 and 6.5, and at temperatures ranging from 30 °C to 38 °C. Beyond 42 °C, enzyme activity dropped significantly, likely due to partial thermal denaturation of the protease complex. In contrast, samples obtained using UAM (
Figure 4b) consistently exhibited higher PA across all temperature and pH combinations. The highest value (~36.9 U/g) was observed at pH 6.0 and 38 °C. Importantly, even at elevated temperatures (46–50 °C), UAM-derived pancreatin maintained substantial activity levels, indicating improved thermal resistance. These results further confirm the beneficial effect of ultrasound in stabilizing proteolytic enzymes during extraction. This improvement may be attributed to more efficient enzyme release, reduced aggregation, and greater preservation of native protein structure under processing conditions.
As summarized in
Table 3, both extraction approaches—CM and UAM —demonstrated peak enzymatic activities at pH 6.0 and 38 °C. These conditions can thus be considered optimal for maximizing the functional performance of bovine pancreatin, irrespective of the extraction strategy. However, the efficiency of enzyme recovery differed markedly between methods. Under identical conditions, the AA obtained via UAM reached 4606 U/g, compared to 3785 U/g for CM. Similarly, PA increased from 32.8 U/g in CM samples to 36.9 U/g in UAM samples. These results underscore the superior performance of UAM in enhancing both amylolytic and proteolytic functions of pancreatin. The improvement is likely associated with more efficient disruption of tissue structures, better enzyme release, and enhanced preservation of native enzyme conformation during processing. Overall, UAM appears to be a more effective and robust extraction method, particularly in applications where high enzymatic activity and thermal stability are critical for product functionality.
3. Materials and Methods
3.1. Materials and Chemicals
Fresh bovine pancreases (n = 27; total mass 10.0 kg) were obtained from a certified meat supplier, S-Meat LLP (Almaty Region, Kazakhstan). Upon collection, the tissues were immediately stored in a portable automotive freezer (XMSJ, China) at a controlled temperature of −5 to −7 °C to preserve enzymatic integrity prior to extraction. All analytical-grade reagents and chemicals used in this study, including buffers and substrates for enzymatic assays, were supplied by Laborpharma LLP (Almaty, Kazakhstan).
3.2. Sample Preparation
Proteolytic enzyme preparations were obtained using a CM, adapted from the procedure described in patent [
47]. Freshly minced bovine pancreas (10 kg) was homogenized with 15 L of distilled water containing 75 mL of glacial acetic acid. The mixture was stirred continuously at 8–12 °C for 4 h, after which the liquid phase was separated from the solid tissue residue.
The remaining solid material was subjected to a second extraction step using an additional 5 L of the same acetic acid solution. After 30 min of mixing, the extract was again collected. The combined extracts were enriched with 0.5 mol of calcium chloride (CaCl₂) and supplemented with pancreatin at a concentration of 0.1 g/L. The pH of the mixture was adjusted to 8.1 using a 20% NaOH solution, and the enzymatic activation was carried out over 24 h at 0–5 °C.
Following activation, the pH was reduced to 6.0 using 5 N hydrochloric acid. Insoluble materials were removed by centrifugation using a separator. Enzymes were precipitated from the resulting supernatant with acetone, dried in a vacuum oven at 30–35 °C, and then finely ground and sieved to obtain a uniform powder.
For UAM, the above protocol was modified by incorporating an ultrasonic homogenization step. A Sonopuls HD 2200 ultrasonic processor (Bandelin, Germany) operating at 200 W was applied in a pulsed mode (1 s on / 1 s off), enhancing cell disruption and accelerating enzyme release from the tissue matrix.
3.3. Thawing Loss
After thawing at 2 °C for 24 h, each sample was gently blotted with filter paper to remove surface moisture. The sample weight was recorded before (m1) and after (m2) thawing. Thawing loss (%) was calculated using the following formula:
3.4. Determination of AA
The AA of the pancreatin samples was determined in accordance with GOST 34440-2018 “Enzyme preparations for the food industry. Methods for determination of amylolytic activity” [
35]. The method is based on the enzymatic hydrolysis of soluble starch into dextrins of varying molecular weight by the amylolytic enzyme complex, under standardized conditions.
The hydrolysis reaction was conducted over 10 min using 6.0 units of enzymatic activity under controlled pH and temperature conditions. One unit of AA was defined as the amount of enzyme required to hydrolyze 1 g of soluble starch into dextrins, corresponding to 30–50% degradation of the initial starch mass. The final enzymatic activity was expressed in units per gram of dry enzyme preparation (AA/g).
The extent of starch hydrolysis was assessed by a colorimetric method, based on the reduction in iodine staining intensity, which reflects the decrease in the concentration of unhydrolyzed starch. Measurements were performed using a Cary 60 UV–Vis spectrophotometer (Agilent Technologies, USA) equipped with Cary WinUV software, operating in the spectral range of 190–1100 nm.
Reagents used in the assay included soluble starch, sodium acetate trihydrate, acetic acid, disodium phosphate, monopotassium phosphate, hydrochloric acid, and crystalline iodine. The thermostability of the AA was subsequently evaluated and expressed as enzyme units per gram, using the following equation:
where
and
are empirical coefficients obtained from regression analysis of hydrolyzed starch mass versus enzyme mass per hour of enzymatic action;
— degree of starch hydrolysis;
— enzyme sample mass used in the analysis, g (adjusted for dilution);
— density of the enzyme preparation (for liquid form), g/cm³.
Calculations were performed with one decimal precision and then rounded to the nearest whole number, as the AA values exceeded 100 U/g. Each reported result represents the average of two independent measurements carried out under repeatability conditions. The measurements met the established acceptability criteria, with the relative error maintained within ±7%, ensuring the reliability and consistency of the data.
3.5. Determination of PA
PA was assessed following the procedure outlined in GOST 34443-2018, “Enzyme Preparations for the Food Industry. Method for Determining Proteolytic Activity” [
36]. This method is based on the enzymatic hydrolysis of bovine hemoglobin, a natural protein substrate, under different pH conditions (acidic pH 3.0, mildly acidic pH 5.3, neutral pH 7.0, and alkaline pH 9.0). The enzyme preparation catalyzes the breakdown of hemoglobin into smaller peptides and free amino acids. The reaction was stopped by precipitating the remaining intact protein with trichloroacetic acid (TCA), and the resulting peptides and amino acids were quantified.
One unit of PA corresponds to the amount of enzyme that releases peptides equivalent to 1 µmol of tyrosine per minute at 30 °C (where 1 µmol tyrosine equals 0.181 mg). Enzyme activity was expressed as units of PA per gram of sample (U/g).
The concentration of hydrolyzed protein was determined by reacting the free amino groups with Folin’s reagent, producing a blue complex whose absorbance was measured at 670 nm using a colorimeter.
The reagents used in this assay included lyophilized bovine hemoglobin, tyrosine, Folin’s reagent, trichloroacetic acid, hydrochloric acid, orthophosphoric acid, glacial acetic acid, boric acid, urea, sodium carbonate, and sodium hydroxide.
PA values were calculated using the following formula:
where
— optical density; 4 — dilution factor after TCA addition;
— tyrosine equivalent corresponding to the optical density of 1 µmol tyrosine (determined from calibration curve);
— hydrolysis time, min;
— mass of the enzyme preparation used for analysis (based on 1 cm³ working solution), g;
— density of the enzyme preparation, g/cm³.
Calculations were carried out to two decimal places and rounded to one decimal place, as the PA was below 100 PA/g. The final result was reported as the arithmetic mean of two parallel measurements under repeatability conditions.
3.6. pH and Color Measurement
The pH of the samples was measured following a modified version of the method described previously [
48]. Briefly, a 10 g aliquot of each sample was homogenized with 20 mL of distilled water using a digital homogenizer (S-10, Stegler, China) at 1000 rpm for 15 seconds. The pH of the resulting homogenate was then recorded using a calibrated pH meter (F20-Std-Kit, Mettler-Toledo, Shanghai, China).
3.7. Statistical Analysis
All experiments were performed in triplicate, and the results are expressed as mean ±SD. Statistical significance of the main effects (p < 0.05) was assessed by ANOVA. Post hoc multiple comparisons were conducted using Tukey’s HSD test with adjusted p-values.
4. Conclusions
This study provides new insights into the influence of extraction methods on the enzymatic functionality of bovine pancreatin, specifically α-amylase and protease, under variable pH and temperature conditions. The novelty of this work lies in the application of ultrasound-assisted extraction (UAM) to animal-derived enzyme systems and in the systematic comparison of its effectiveness with conventional mixing (CM) using standardized industrial protocols. Our results demonstrate that UAM significantly improves both the activity and thermal stability of pancreatin enzymes compared to CM. Across a wide temperature range (10–50 °C) and pH spectrum (5.5–8.0), enzymes extracted via UAM consistently exhibited higher activity and greater resistance to denaturation. Notably, optimal enzymatic activity for both α-amylase and protease was achieved at pH 6.0 and 38 °C, with UAM-extracted samples maintaining superior performance even at elevated temperatures up to 50 °C. These findings suggest that ultrasound enhances enzyme release and protects structural integrity during extraction, making it a promising strategy for the production of robust, high-activity enzyme preparations. From a technological standpoint, this study is among the first to evaluate the impact of UAM on the functional resilience of bovine pancreatin across physiologically and industrially relevant conditions. The results contribute to the growing body of knowledge supporting ultrasound-based bioprocessing as an efficient and scalable alternative to traditional methods. Given the high sensitivity of digestive enzymes to extraction conditions, the use of UAM could be especially valuable for food and pharmaceutical applications where enzyme stability is critical.
Future research should further explore the molecular mechanisms underlying the stabilizing effects of ultrasound, as well as optimize acoustic parameters to maximize enzyme yield and activity. Overall, this study establishes a solid foundation for integrating ultrasound technology into the industrial production of pancreatin and other biologically active protein complexes.
Author Contributions
Conceptualization, G.K. and U.C.; methodology, U.C.; software, G.K. and A.T.; formal analysis, G.K. and A.T.; investigation, G.K., U.C. and A.T.; resources G.K.; data curation, G.K. and U.C.; writing—original draft preparation, A.T.; writing—review and editing, G.K. and A.T.; supervision, G.K. and U.C.; project administration, , G.K.; funding acquisition, G.K. and U.C.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the project “Development of a highly efficient technology for obtaining pancreatin for the purpose of producing export-oriented products”, scientific and technical program for 2024-2026 “Development of a technology for complex and deep processing of agricultural raw materials for food production, ensuring high quality and safety of manufactured products” BR24892775, funded by the Ministry of Agriculture of the Republic of Kazakhstan.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 13th ed.; Elsevier: Philadelphia, PA, USA, 2016.
- Daniel, R.M.; Danson, M.J.; Eisenthal, R.; Lee, C.K.; Peterson, M.E. The Effect of T emperature on Enzyme Activity: New Insights and Their Implications. Extremophiles 2007, 11, 1–8. [CrossRef]
- Ferraris, R.P. Nutrient Transporters in the Small Intestine: Structure, Function and Regulation. Physiol. Rev. 2001, 81, 125–188. [CrossRef]
- van der Maarel, M.J.E.C.; van der Veen, B.; Uitdehaag, J.C.M.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [CrossRef]
- Treves, S.; Vianello, F.; Arslanoglu, S. Digestion and Absorption of Carbohydrates. In Physiology of the Gastrointestinal Tract, 6th ed.; Johnson, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1065–1082.
- Peterson, M. E.; Eisenthal, R.; Danson, M. J.; Spence, A.; Daniel, R. M. A new intrinsic thermal parameter for enzymes reveals true temperature optima. J. Biol. Chem. 2007, 282, 31243–31248. https://pubmed.ncbi.nlm.nih.gov/14973131/.
- Rawlings, N.D.; Salvesen, G. Handbook of Proteolytic Enzymes, 3rd ed.; Academic Press: London, UK, 2013.
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [CrossRef]
- De Terra, G. P.; De Farias M. V.; Trevisan M. G.; Garcia J. S. Evaluation of pancreatin stability through enzyme activity determination. Acta Pharm. 2016, 66, 101–110. https://pubmed.ncbi.nlm.nih.gov/27383890/.
- Mu, H.; Hoy, C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [CrossRef]
- Reboul, E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients 2013, 5, 3563–3581. [CrossRef]
- Busto, M. D.; Ortega, N.; Pérez-Mateos, M. Thermal inactivation of pancreatic lipase and its protection with additives. Enzyme Microb. Technol. 1999, 25, 527–532. [CrossRef]
- Tessier, A.G.; Dombi, G.W; Bouwman, D. L. Thermostability of purified human pancreatic α-amylase is increased by calcium and human serum albumin. Biochim. Biophys. Acta 1996, 1292, 71–78. https://pubmed.ncbi.nlm.nih.gov/8814357/.
- Šimundić, A.M.; Velić, N.; Marijančević, D. Extraction and Stabilization of Enzymes from Animal Tissues. Food Technol. Biotechnol. 2017, 55, 187–199. [CrossRef]
- Li, Y.; Zhao, D.; Liu, J.; Jiang, Y.; Chen, F. Optimization of Enzyme Extraction from Animal Tissues: Parameters Affecting Yield and Activity. J. Food Process Eng. 2020, 43, e13452. [CrossRef]
- Yu, P.; Pan, X.; Chen, M.; Ma, J.; Xu, B.; Zhao, Y. Ultrasound-assisted enzymatic extraction of soluble dietary fiber from Hericium erinaceus and its in vitro lipid-lowering effect.. Food Chem. 2023,6,100545 . [CrossRef]
- Peng, P.; Yu, H.; Xian, M.; Qu, C.; Guo, Z.; Li, S.; Zhu, Z.; Xiao, J. Preparation of acetylcholinesterase inhibitory peptides from yellowfin tuna pancreas using moderate ultrasound-assisted enzymatic hydrolysis. Mar. Drugs 2023, 21, 75. [CrossRef]
- Zhang, T.; Chen, H.; Wang, Y.; Wang, J. Effects of particle size on the extraction and functional properties of enzymes from animal by-products. Food Bioprod. Process. 2019, 117, 245–252. [CrossRef]
- Kurozawa, L.E.; Morassi, A.G.; Vanzo, A.A.; Park, K.J. Influence of grinding and homogenization techniques on enzyme extraction and quality attributes of food ingredients. LWT–Food Sci. Technol. 2015, 61, 350–357. [CrossRef]
- Xu, K.; Fu, H.; Chen, Q.; Sun, R.; Li, R.; Zhao, X.; Zhou , J.; Wang X. Engineering thermostability of industrial enzymes for enhanced application performance. Int. J. Biol. Macromol. 2025, 291, 139067. [CrossRef]
- Suman, M.; Manzocco, L.; Anese, M.; Nicoli, M.C. Grinding processes and their impact on food quality: a review. Trends Food Sci. Technol. 2011, 22, 585–592. [CrossRef]
- Zhou, Y.; Wang, X.; Li, Z. Thermal stability and enzymatic activity of RNase A in the presence of cationic gemini surfactants. Int. J. Biol. Macromol. 2012, 50, 1151-1157. [CrossRef]
- Abdullahi, N.; Atiku, M. K.; Umar, N. B. The roles of enzyme in food processing – an overview. Fudma J. Sci. 2021, 5, 157–164. [CrossRef]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial proteases and their applications. Front. Microbiol. 2023, 14, 14:1236368. [CrossRef]
- Whitaker, J.R.; Voragen, A.G.J.; Wong, D.W.S. Handbook of Food Enzymology; Marcel Dekker: New York, NY, USA, 2003.
- Raut, A. N.; Gedam, P. S.; Dhamole, P. B. Back-extraction of butanol from coacervate phase using Winsor III microemulsion. Process Biochem. 2018, 70, 160-167. [CrossRef]
- Hayes, M. G.; Hurley, M. J.; Larsen, L. B.; Heegard, C. W.; Magboul, A. A. A.; Oliveira J. C.; McSweeney, P. L. H.; Kelly, A. L. Thermal inactivation kinetics of bovine cathepsin D. Journal of Dairy Res. 2001, 68, 267 – 276. [CrossRef]
- Cornish-Bowden, A. Fundamentals of Enzyme Kinetics, 4th ed.; Wiley-Blackwell: Weinheim, Germany, 2012.
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [CrossRef]
- Hussain, M.; Qayum, A.; Zhang, X.; Hao, X,; Liu, L.; Wang Y.; Hussain, K.; Li, X. Improvement in bioactive, functional, structural and digestibility of potato protein and its fraction patatin via ultra-sonication. LWT. 2021, 148, 111747. [CrossRef]
- Babu, A.; John, M.; Liji, M.J.; Maria, E.; Bhaskar, S.J.; Binukmar, B.K.; Sajith, A.M.; Reddy, E.K.; Dileep, K.V.; Sunil, K. Sub-pocket-focused designing of tacrine derivatives as potential acetylcholinesterase inhibitors. Comput. Biol. Med. 2023, 155, 106666. [CrossRef]
- Iqbal, A.; Murtaza, A.; Marszałek, K.; Iqbal, M.A.; Chughtai, M.F.J.; Hu, W.; Barba, F.J.; Bi, J.; Liu, X.; Xu, X. Inactivation and structural changes of polyphenol oxidase in quince (Cydonia oblonga Miller) juice subjected to ultrasonic treatment. J. Sci. Food Agric. 2020, 100, 2065–2073. [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [CrossRef]
- Bankova, L.G.; Barrett, N.A.; Yoshimoto, E.; Ualiyeva, S. Isolation and quantitative evaluation of brush cells from mouse tracheas. J. Vis. Exp. 2019, 148, 10.3791/59496. https://app.jove.com/t/59496/isolation-quantitative-evaluation-brush-cells-from-mouse.
- GOST 34440-2018 «Enzyme preparations for food industry. Methods for the determination of amylase activity. Introduced 01.07.2019. – Moscow: Standartinform, 2018. – 19 p.
- Dutta T.K.; Jana M.; Pahari P.R.; Bhattacharya T. The effect of temperature, pH, and salt on amylase in Heliodiaptomus viduus (Gurney) (Crustacea: Copepoda: Calanoida). Turk J Zool. 2006; 30(2):167–72. https://journals.tubitak.gov.tr/zoology/vol30/iss2/11.
- Fadeel A.; Moll B.A.; Jones R.L. Effect of temperature on the synthesis and secretion of α-amylase in barley aleurone layers. Plant Physiol. 1980; 66(3):466–470. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC440655/.
- Elmansy E.A.; Asker M.S.; El-Kady E.M.; Sholkamy E.N.; Awad M.F. Production and optimization of α-amylase from thermo-halophilic bacteria isolated from different local marine environments. Bull Natl Res Cent. 2018; 42:31. https://bnrc.springeropen.com/articles/10.1186/s42269-018-0033-2.
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [CrossRef]
- Vallés, D.; Furtado, S.; Villadóniga, C.; Cantera, A.M.B. Adsorption onto alumina and stabilization of cysteine proteinases from crude extract of solanum granuloso-leprosum fruits. Process Biochem. 2011, 46, 592–598. [CrossRef]
- Yang, H.; Wei, W.; Liu, S. Monodispersed silica nanoparticles as carrier for co-immobilization of bi-enzyme and its application for glucose biosensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 125, 183–188. [CrossRef]
- Porath, J.; Axén, R. Immobilization of enzymes to agar, agarose, and sephadex support. In Methods in Enzymology; Klaus, M., Ed.; Academic Press: Waltham, MA, USA, 1976; 44, 19–45. [CrossRef]
- GOST 34440-2018 «Enzyme preparations for food industry. Methods for the determination of protease activity. Introduced 01.07.2019. – Moscow: Standartinform, 2018. – 15 p.
- Majeed T.; Lee C.C.; Orts W.J.; Al-Sadi A.M.; Al-Khayri J.M.; Albeshr M.F.; Siddiqui M.H. Characterization of a thermostable protease from Bacillus subtilis BSP strain. BMC Biotechnol. 2024; 24:49.
- Suleiman A.D, Abdul Rahman N., Mohd Yusof H., Mohd Shariff F., Yasid N.A. Effect of cultural conditions on protease production by a thermophilic Geobacillus thermoglucosidasius SKF4 isolated from Sungai Klah Hot Spring Park, Malaysia. Molecules. 2020; 25(11):2609.
- Sarker, P.K., Talukdar, S.A., Deb, P. et al. Optimization and partial characterization of culture conditions for the production of alkaline protease from Bacillus licheniformis P003. SpringerPlus 2013;2:506. https://springerplus.springeropen.com/articles/10.1186/2193-1801-2-506.
- Patent No. 651810. Method for Obtaining Pancreatin. USSR, filed March 15, 1979. In Russian.
- Yuan, D.; Xu, Y.; Kong, B.; Cao, C.; Zhang, F.; Xia, X.; Zhang, H.; Liu, Q.; Zhao, J. Application of seaweed dietary fiber as a potential alternative to phosphates in frankfurters with healthier profiles. Meat Sci. 2023, 196. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).