Submitted:
12 May 2025
Posted:
13 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Experimental Techniques
2.3.1. Characterization of Wood Fibers
2.3.2. Characterization of the Composites
3. Results and Discussion
3.1. Characterization of Wood Fibers
3.1.1. Chemical and Microstructural Characterization of Wood Fibers
3.1.2. Thermal Characterization of Wood Fibers
3.2. Characterizaion of the Composites
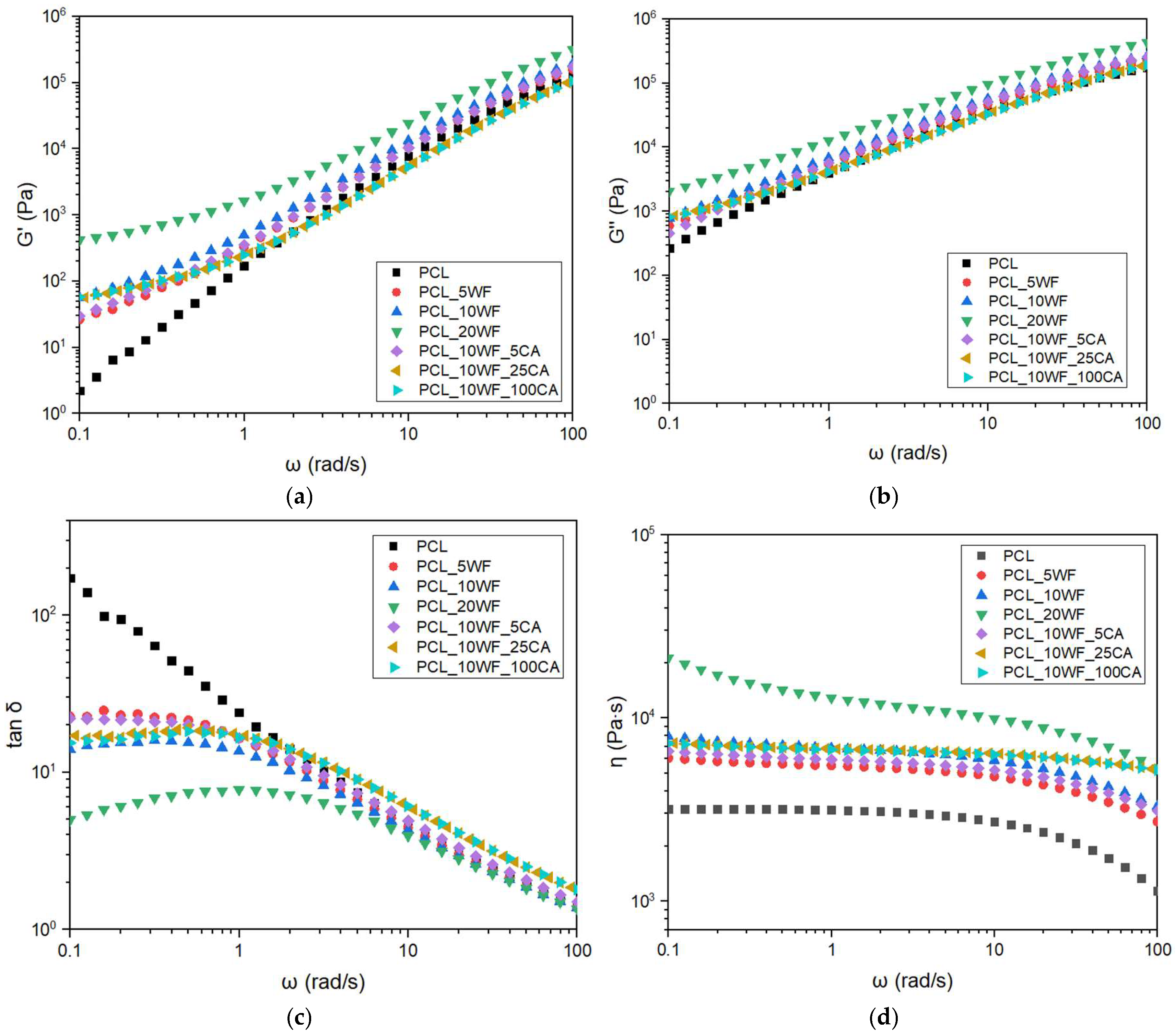
3.2.1. Rheological Characterization of the Composites
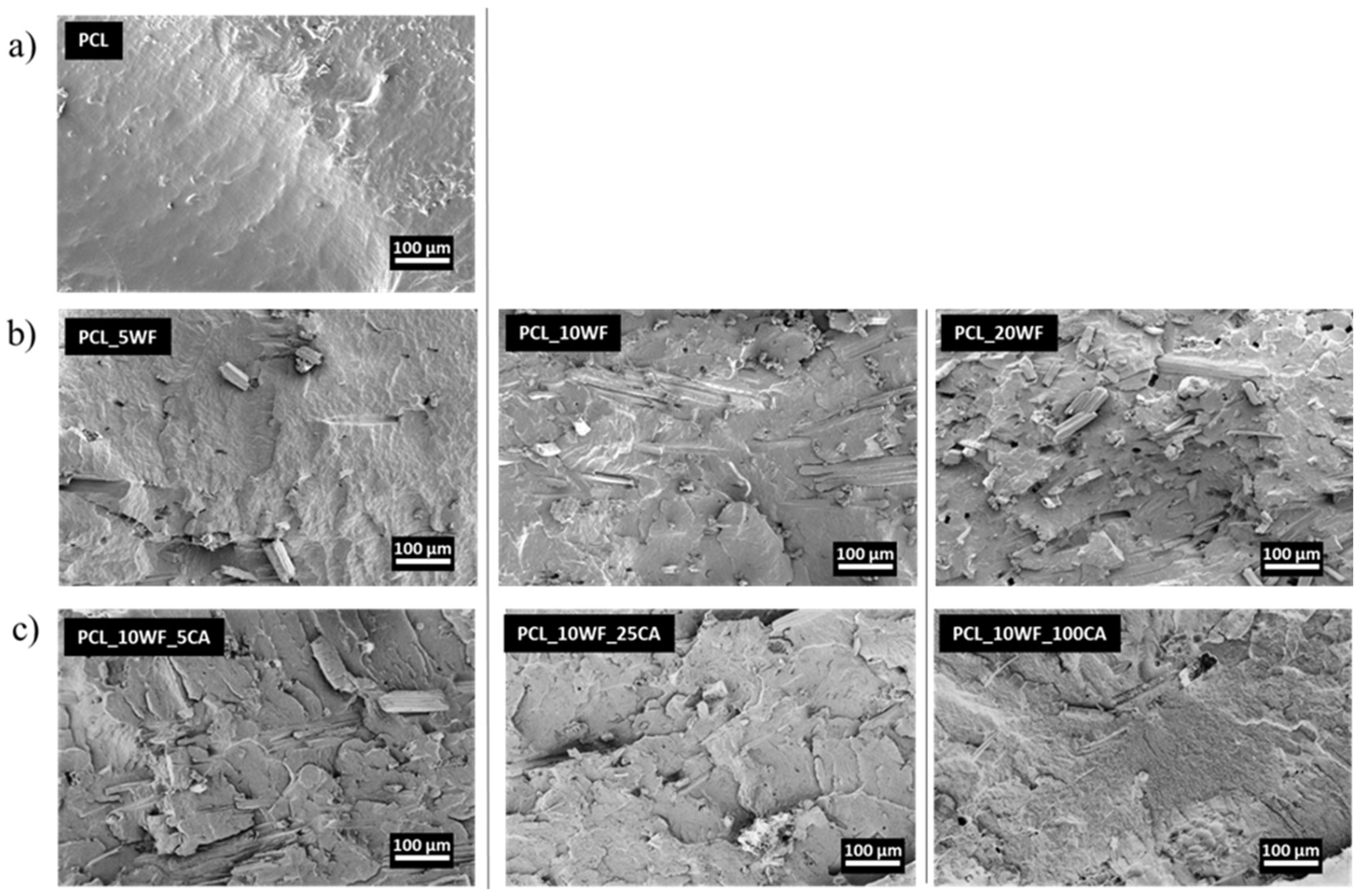
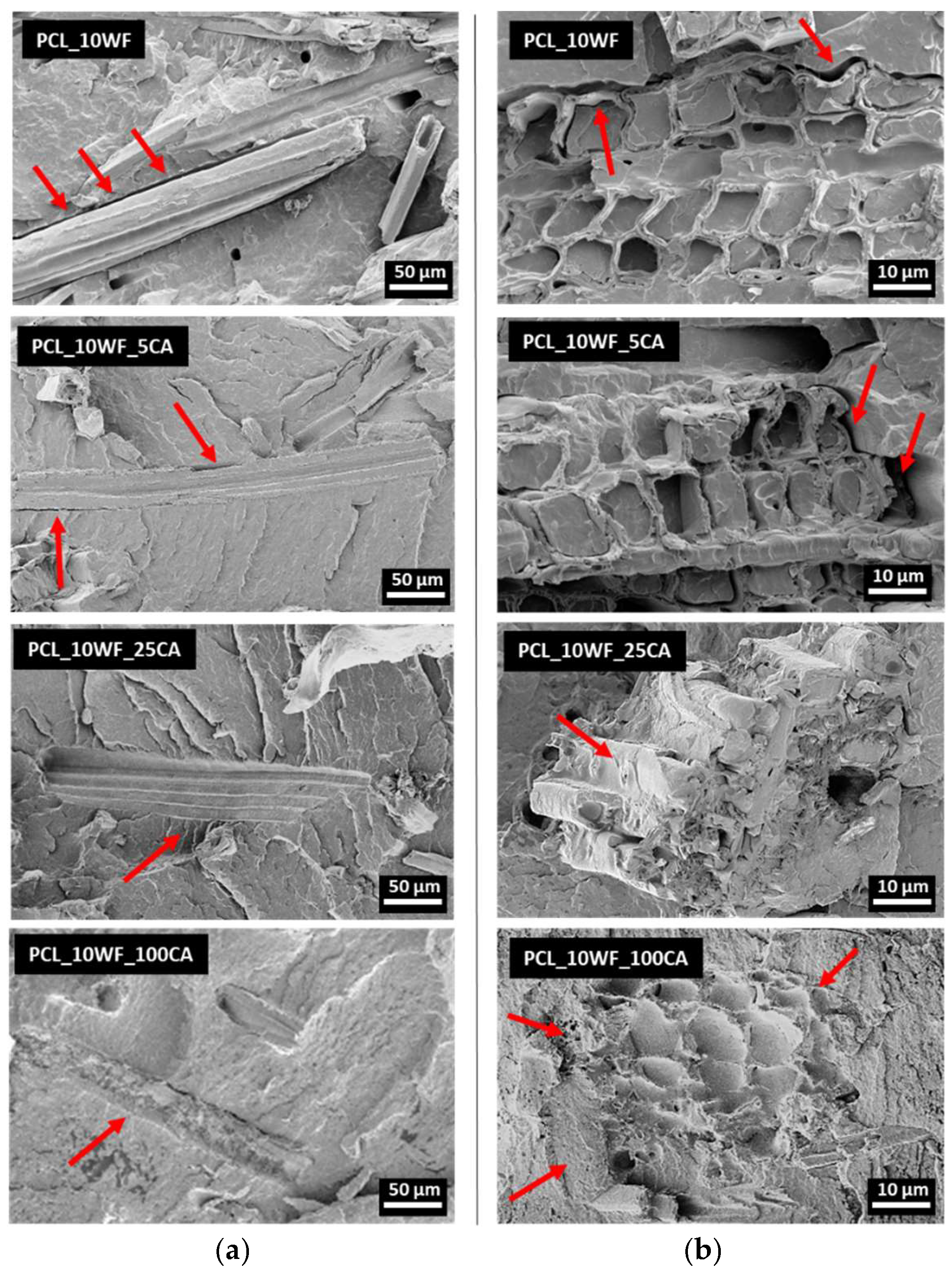
3.2.2. Morphological Characterization of Composites
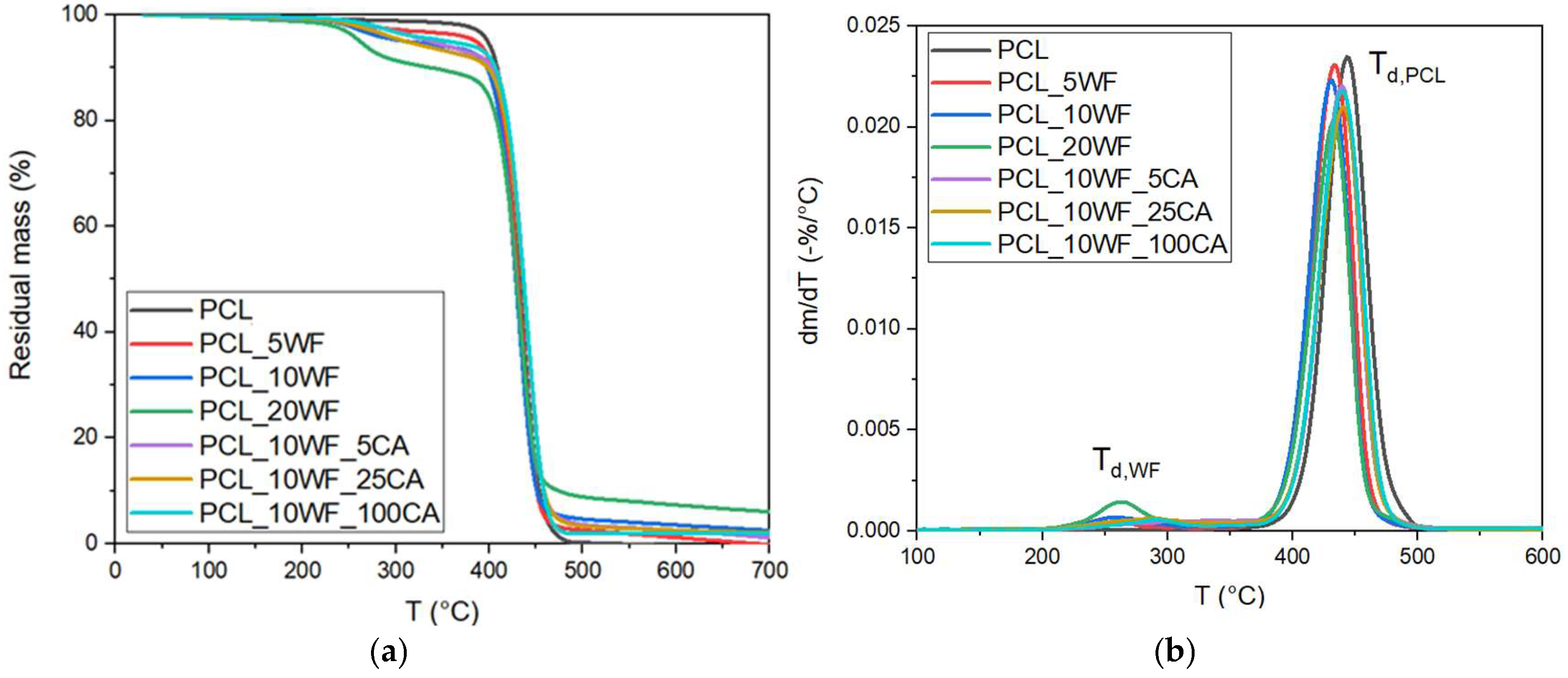
3.2.3. Thermal Characterization of the Composites
3.2.4. Mechanical Characterization of Composites
3.2.5. Water Uptake and Contact Angle Mesurements
4. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Conflicts of Interest
References
- Ncube, L.; Ude, A.; Ogunmuyiwa, E.; Zulkifli, R.; Beas, I. An overview of plastic waste generation and management in food packaging industries. Recycling 2021, 12, 12–37. [Google Scholar] [CrossRef]
- Muhib, M.; Uddin, M.; Rahman, M.; Malafaia, G. Occurrence of microplastics in tap and bottled water, and food packaging: A narrative review on current knowledge. Science of The Total Environment 2023, 865, 161274–161285. [Google Scholar] [CrossRef]
- Tas, C.; Unal, H. Thermally buffering polyethylene/halloysite/phase change material nanocomposite packaging films for cold storage of foods. Journal of Food Engineering 2021, 292, 110351–110359. [Google Scholar] [CrossRef]
- Antonopoulos, I.; Faraca, G.; Tonini, D. Recycling of post-consumer plastic packaging waste in the EU: Recovery rates, material flows, and barriers. Waste Management 2021, 126, 694–705. [Google Scholar] [CrossRef]
- Simonini, L.; Sorze, A.; Maddalena, L.; Carosio, F.; Dorigato, A. Mechanical reprocessing of polyurethane and phenolic foams to increase the sustainability of thermal insulation materials. Polymer Testing 2024, 8. [Google Scholar] [CrossRef]
- Souza, V.; Pires, J.; Rodrigues, C.; Coelhoso, I.; Fernando, A. Chitosan composites in packaging industry - current trends and future challenges. Polymers 2020, 11, 417–487. [Google Scholar] [CrossRef]
- Amara, C.; El Mahdi, A.; Medimagh, R.; Khwaldia, K. Nanocellulose-based composites for packaging applications. Current Opinion in Green and Sustainable Chemistry 2021, 31, 100512–100527. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, E.; Xu, G.; Naik, N.; Murugadoss, V.; Ma, M.; Guo, Z.; Shi, Z. Overview of renewable polysaccharide-based composites for biodegradable food packaging applications. Green Chemistry 2022, 24, 480–492. [Google Scholar] [CrossRef]
- Stark, N.; Matuana, L. Trends in sustainable biobased packaging materials: A mini review. Materials today sustainability 2021, 15, 100084–100093. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocolloids 2021, 113, 106530–106539. [Google Scholar] [CrossRef]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly (ε-caprolactone): A potential polymer for biodegradable food packaging applications. Packaging Technology and Science 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Diken, M.; Kocer Kizilduman, B.; Doğan, S.; Doğan, M. Antibacterial and antioxidant phenolic compounds loaded PCL biocomposites for active food packaging application. Journal of Applied Polymer Science 2022, 139, 52423–52438. [Google Scholar] [CrossRef]
- Amini, E.; Valls, C.; Roncero, M. Promising nanocomposites for food packaging based on cellulose–PCL films reinforced by using ZnO nanoparticles in an ionic liquid. Industrial Crops and Products 2023, 193, 116246–116257. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Mendieta, J.; Ortega-Toro, R. In-depth study from gluten/PCL-based food packaging films obtained under reactive extrusion conditions using chrome octanoate as a potential food grade catalyst. Food Hydrocolloids 2021, 111, 106255–106270. [Google Scholar] [CrossRef]
- Gürler, N.; Pekdemir, M.; Torğut, G.; Kök, M. Binary PCL–waste photopolymer blends for biodegradable food packaging applications. Journal of Molecular Structure 2023, 1279, 134990–134997. [Google Scholar] [CrossRef]
- Khan, S.; Dhakal, H.; Saifullah, A.; Z, Z. Improved Mechanical and Thermal Properties of Date Palm Microfiber-Reinforced PCL Biocomposites for Rigid Packaging. Molecules 2025, 30, 857–886. [Google Scholar] [CrossRef] [PubMed]
- Simonini, L.; Canale, R.; Mahmood, H.; Dorigato, A.; Pegoretti, A. Multifunctional epoxy/carbon composites with a fully reparable interface. Polymer Composites 2024, 45, 2558–1568. [Google Scholar] [CrossRef]
- Simonini, L.; Kakkonen, M.; Dsouza, R.; Kanerva, M.; Mahmood, H.; Dorigato, A.; Pegoretti, A. Tailoring the interfacial properties of glass fiber-epoxy microcomposites through the development of a self-healing poly (ϵ-caprolactone) coating. Composites Science and Technology 2024, 261, 110991–111007. [Google Scholar] [CrossRef]
- Simonini, L.; Mahmood, H.; Dorigato, A.; Pegoretti, A. Evaluation of self-healing capability of a polycaprolactone interphase in epoxy/glass composites. Composites Part A: Applied Science and Manufacturing 2023, 169, 107539–107548. [Google Scholar] [CrossRef]
- Dorigato, A.; Rigotti, D.; Pegoretti, A. Novel poly(caprolactone)/epoxy blends by additive manufacturing. Materials 2020, 13, 819–826. [Google Scholar] [CrossRef]
- Cescato, R.; Rigotti, D.; Mahmood, H.; Dorigato, A.; Pegoretti, A. Thermal mending of electroactive carbon/epoxy laminates using a porous poly(ε-caprolactone) electrospun mesh. Polymers 2021, 13, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Physical Sciences Reviews 2023, 8, 4391–4414. [Google Scholar] [CrossRef]
- Luna, C.; Siqueira, D.; Ferreira, E.; Araujo, E.; Wellen, R. Effect of injection parameters on the thermal, mechanical and thermomechanical properties of polycaprolactone (PCL). Journal of Elastomers and Plastics 2021, 53, 1045–1062. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Progress in Polymer Science 2021, 101395–101435. [Google Scholar] [CrossRef]
- Dybka-Stępień, K.; Antolak, H.; Kmiotek, M.; Piechota, D.; Koziróg, A. Disposable food packaging and serving materials - Trends and biodegradability. Polymers 2021, 19, 3606–3644. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, E.; França, D.; Morais, D.; Rosa, M.; Morais, J.; Araújo, E.; Wellen, R. Processing and properties of PCL/cotton linter compounds. Materials Research 2017, 20, 317–325. [Google Scholar] [CrossRef]
- Rytlewski, P.; Stepczyńska, M.; Moraczewski, K.; Malinowski, R.; Jagodziński, B.; Żenkiewicz, M. Mechanical properties and biodegradability of flax fiber-reinforced composite of polylactide and polycaprolactone. Polimery 2018, 63, 603–610. [Google Scholar] [CrossRef]
- Dhakal, H.; Ismail, S.; Zhang, Z.; Barber, A.; Welsh, E.; Maigret, J.; Beaugrand, J. Development of sustainable biodegradable lignocellulosic hemp fiber/polycaprolactone biocomposites for light weight applications. Composites Part A: Applied Science and Manufacturing 2018, 113, 350–358. [Google Scholar] [CrossRef]
- Ilyas, R.; Zuhri, M.; Norrrahim, M.; Misenan, M.; Jenol, M.; Samsudin, S.; Nurazzi, N.; Asyraf, M.; Supian, A.; Bangar, S.; R, N. Natural fiber-reinforced polycaprolactone green and hybrid biocomposites for various advanced applications. Polymers 2022, 14, 182–210. [Google Scholar] [CrossRef]
- Jian, B.; Mohrmann, S.; Li, H.; Li, Y.; Ashraf, M.; Zhou, J.; Zheng, X. A review on flexural properties of wood-plastic composites. Polymers 2022, 14, 3942–3961. [Google Scholar] [CrossRef]
- Cintra, S.; Braga, N.; Morgado, G.; Montanheiro, T.; Marini, J.; Passador, F.; Montagna, L. Development of new biodegradable composites materials from polycaprolactone and wood flour. Wood Material Science and Engineering 2022, 17, 586–597. [Google Scholar] [CrossRef]
- Herrera, N.; Olsen, P.; Berglund, L. Strongly improved mechanical properties of thermoplastic biocomposites by PCL grafting inside holocellulose wood fibers. ACS Sustainable Chemistry & Engineering 2020, 20, 11977–11985. [Google Scholar]
- Lo Re, G.; Spinella, S.; Boujemaoui, A.; Vilaseca, F.; Larsson, P.; Adås, F.; Berglund, L. Poly (ε-caprolactone) biocomposites based on acetylated cellulose fibers and wet compounding for improved mechanical performance. ACS Sustainable Chemistry & Engineering 2018, 6, 6753–6760. [Google Scholar]
- Mohammed, M.; Rahman, R.; Mohammed, A.; Adam, T.; Betar, B.; Osman, A.; Dahham, O. Surface treatment to improve water repellence and compatibility of natural fiber with polymer matrix: Recent advancement. Polymer testing 2022, 115, 107707–107732. [Google Scholar] [CrossRef]
- Mahmood, H.; Simonini, L.; Dorigato, A.; Pegoretti, A. Graphene deposition on glass fibers by triboelectrification. Applied Sciences 2021, 11, 3123–3134. [Google Scholar] [CrossRef]
- Pozueco, S.; Simonini, L.; Mahmood, H.; Rigotti, D.; Kakkonen, M.; Riveiro, A.; Comesaña, R.; Pou, J.; Tanhuanpää, O.; Kanerva, O.; Sarlin, E.; Kallio, P.; Pegoretti, A. Influence of CO2 laser surface treatment of basalt fibers on the mechanical properties of epoxy/basalt composites. Polymer Composites, 2024; in press. [Google Scholar]
- Simonini, L.; Mahmood, H.; Dorigato, A.; Pegoretti, A. Tailoring the physical properties of poly (lactic acid) through the addition of thermoplastic polyurethane and functionalized short carbon fibers. Polymer Composites 2023, 44, 4719–4733. [Google Scholar] [CrossRef]
- Show, P.; Oladele, K.; Siew, Q.; Aziz Zakry, F.; Lan, J.; Ling, T. Overview of citric acid production from Aspergillus niger. Frontiers in life science 2015, 8, 271–283. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Mucignat, M.; Pegoretti, A.; A, D. Multifunctional xanthan gum/wood fibers based hydrogels as novel topsoil covers for forestry and agricultural applications. Carbohydrate Polymer Technologies and Applications 2024, 7, 100520–100530. [Google Scholar] [CrossRef]
- Behera, B.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Frontiers 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Huang, F.; Tian, Z.; Wang, Y.; Ji, X.; Wang, D.; Fatehi, P. ellulose fiber drainage improvement via citric acid crosslinking. International Journal of Biological Macromolecules 2024, 281, 136338–136347. [Google Scholar] [CrossRef]
- Cui, X.; Ozaki, A.; Asoh, T.; Uyama, H. Cellulose modified by citric acid reinforced Poly (lactic acid) resin as fillers. Polymer Degradation and Stability 2020, 175, 109118–109125. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Smolar, J.; Logar, J.; Pegoretti, A.; Dorigato, A. Effect of different cellulose fillers on the properties of xanthan-based composites for soil conditioning applications. Materials 2023, 16, 7285–7305. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, K.; Wang, Y.; Chen, Y.; Fan, D. Cross-linked peach gum polysaccharide adhesive by citric acid to produce a fully bio-based wood fiber composite with high strength. International Journal of Biological Macromolecules 2023, 253, 127514–127523. [Google Scholar] [CrossRef] [PubMed]
- Gebke, S.; Thümmler, K.; Sonnier, R.; Tech, S.; Wagenführ, A.; Fischer, S. Flame retardancy of wood fiber materials using phosphorus-modified wheat starch. Molecules 2020, 25, 335–356. [Google Scholar] [CrossRef]
- Zakaria, R.; Bawon, P.; Lee, S.; Salim, S.; Lum, W.; Al-Edrus, S.; Ibrahim, Z. Properties of particleboard from oil palm biomasses bonded with citric acid and tapioca starch. Polymers 2021, 13, 3494–3509. [Google Scholar] [CrossRef]
- Fu, H.; Dun, M.; Chen, B.; Zhou, Z.; Wang, H.; Wang, W.; Xie, Y.; Wang, Q. Compression rheological behavior of ultrahighly filled wood flour-polyethylene composites. Composites Part B: Engineering 2021, 215, 108766–108781. [Google Scholar] [CrossRef]
- Olonisakin, K.; Lin, H.; Haojin, P.; Aishi, W.; Wang, H.; Li, R.; Xin-Xiang, Z.; W, Y. Fiber treatment impact on toughness and interfacial bonding in epoxidized soya bean oil compatibilized PLA/PBAT bamboo fiber composites. Materials Today Communications 2024, 38, 107790–107803. [Google Scholar] [CrossRef]
- Arman, N.; Chen, R.; Ahmad, S. Review of state-of-the-art studies on the water absorption capacity of agricultural fiber-reinforced polymer composites for sustainable construction. Construction and Building Materials 2021, 302, 124174–124188. [Google Scholar] [CrossRef]
- Sekar, S.; Suresh Kumar, S.; Vigneshwaran, S.; Velmurugan, G. Evaluation of mechanical and water absorption behavior of natural fiber-reinforced hybrid biocomposites. Journal of Natural Fibers 2022, 19, 1772–1782. [Google Scholar] [CrossRef]
- Arul, S.; Adhikary, P.; SiP, J.; Haiter Lenin, A. Moisture diffusion analysis and their effects on the mechanical properties of organic particle filled natural fiber reinforced hybrid polymer composites. International Journal of Polymer Analysis and Characterization 2024, 29, 42–55. [Google Scholar] [CrossRef]
- Khorramnezhad, M.; Akbari, B.; Akbari, M.; Kharaziha, M. Effect of surface modification on physical and cellular properties of PCL thin film. Colloids and Surfaces B: Biointerfaces 2021, 200, 111582–111589. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Martin, J.; Celada, L.; Olsén, P.; Wågberg, L. Strategic functionalization of wood fibers for the circular design of fiber-reinforced hydrogel composites. Cell Reports Physical Science 2025, 1, 1–15. [Google Scholar]
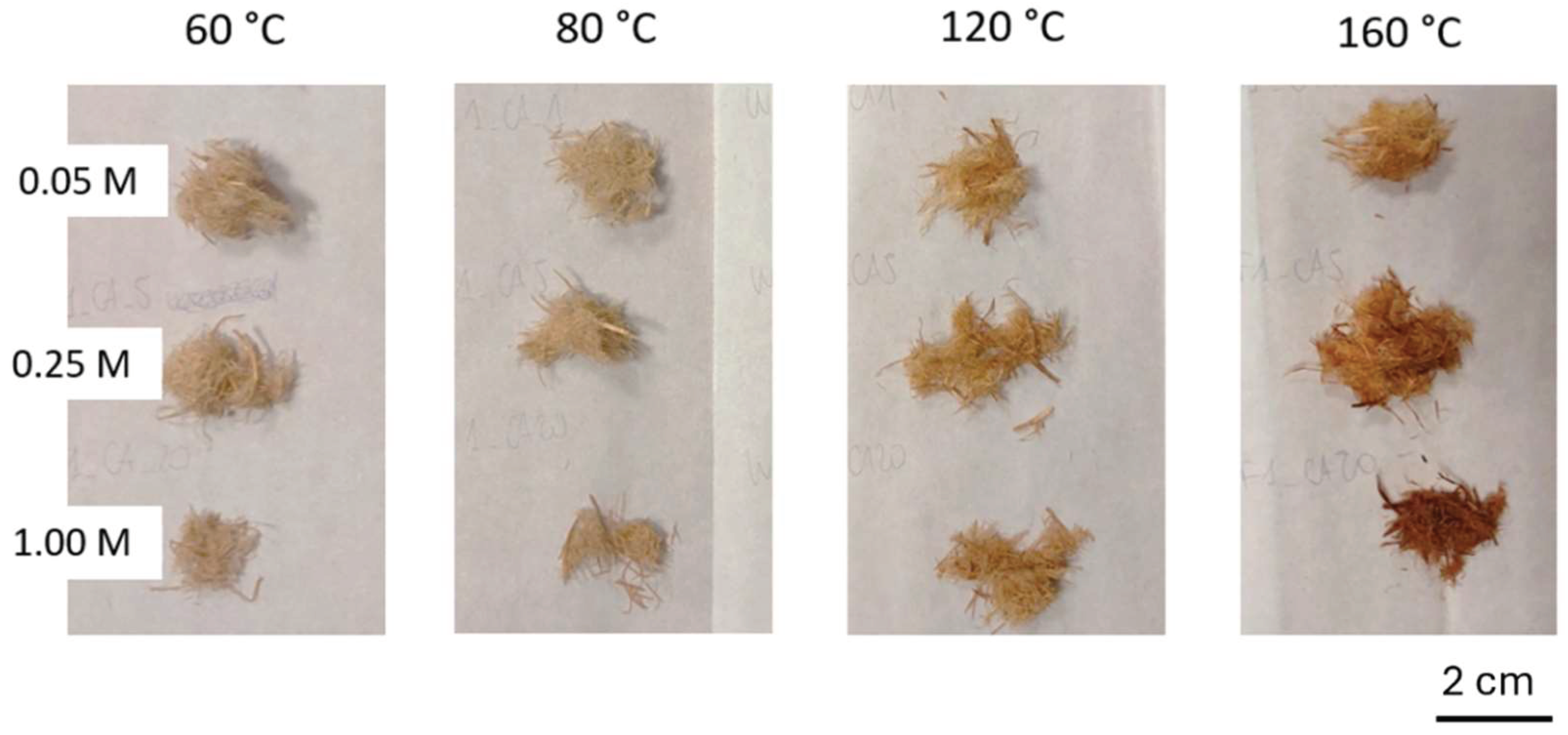
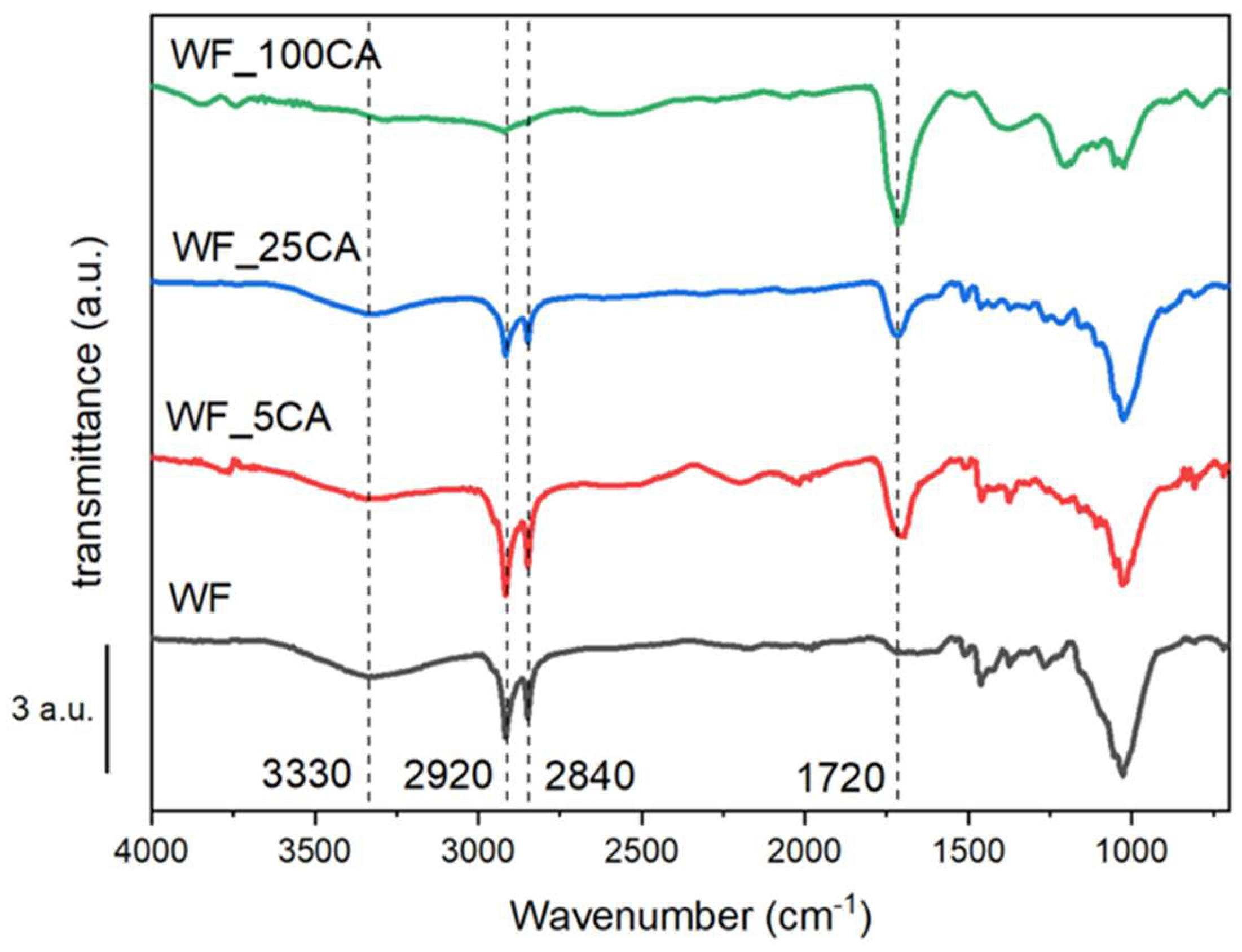
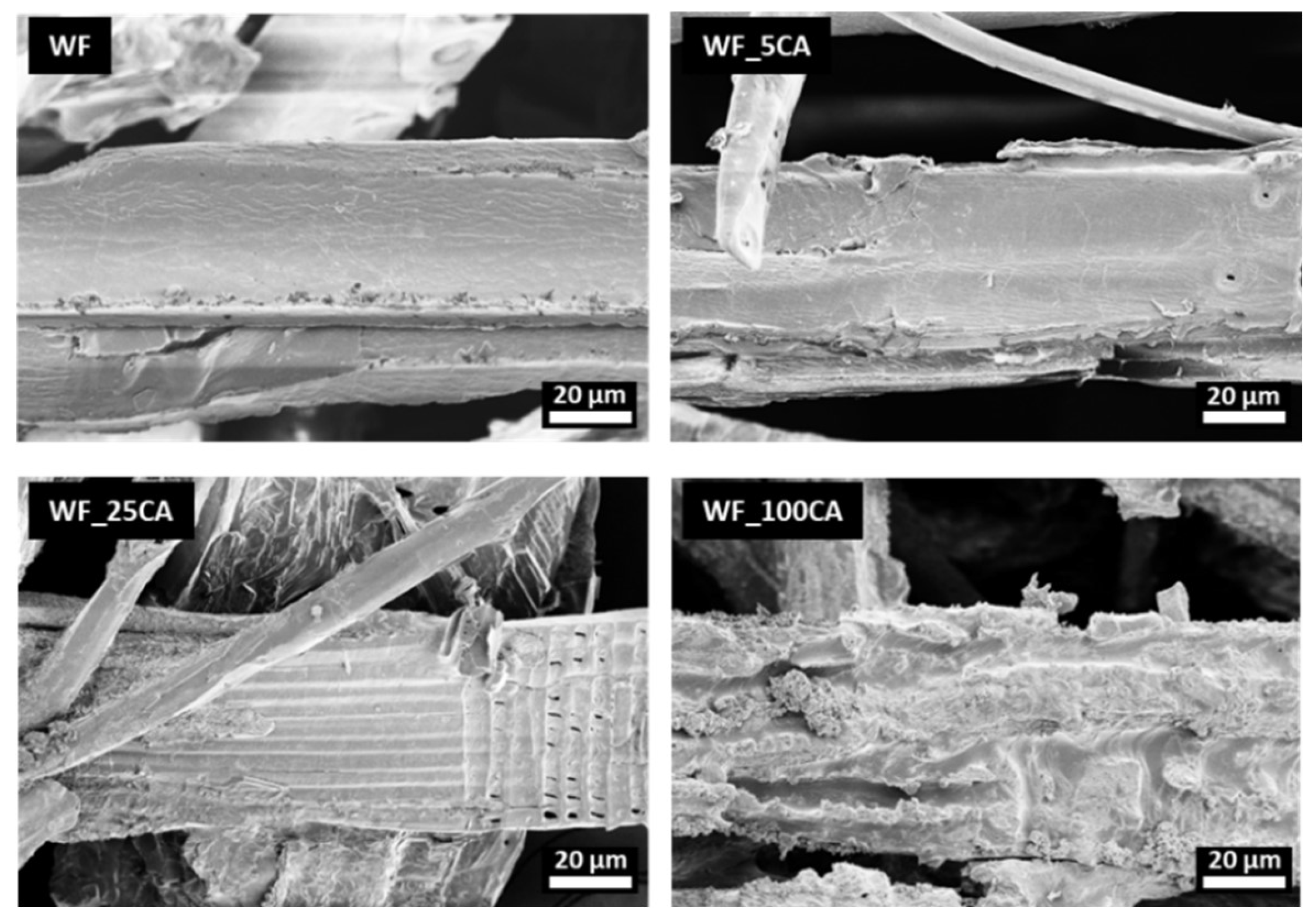
| Sample | PCL content (%wt) |
WF content (%wt) |
CA solution (M) |
|---|---|---|---|
| PCL | 100 | 0 | - |
| PCL_5WF | 95 | 5 | - |
| PCL_10WF | 90 | 10 | - |
| PCL_20WF | 80 | 20 | - |
| PCL_10WF_5CA | 90 | 10 | 0.05 |
| PCL_10WF_25CA | 90 | 10 | 0.25 |
| PCL_10WF_100CA | 90 | 10 | 1.00 |
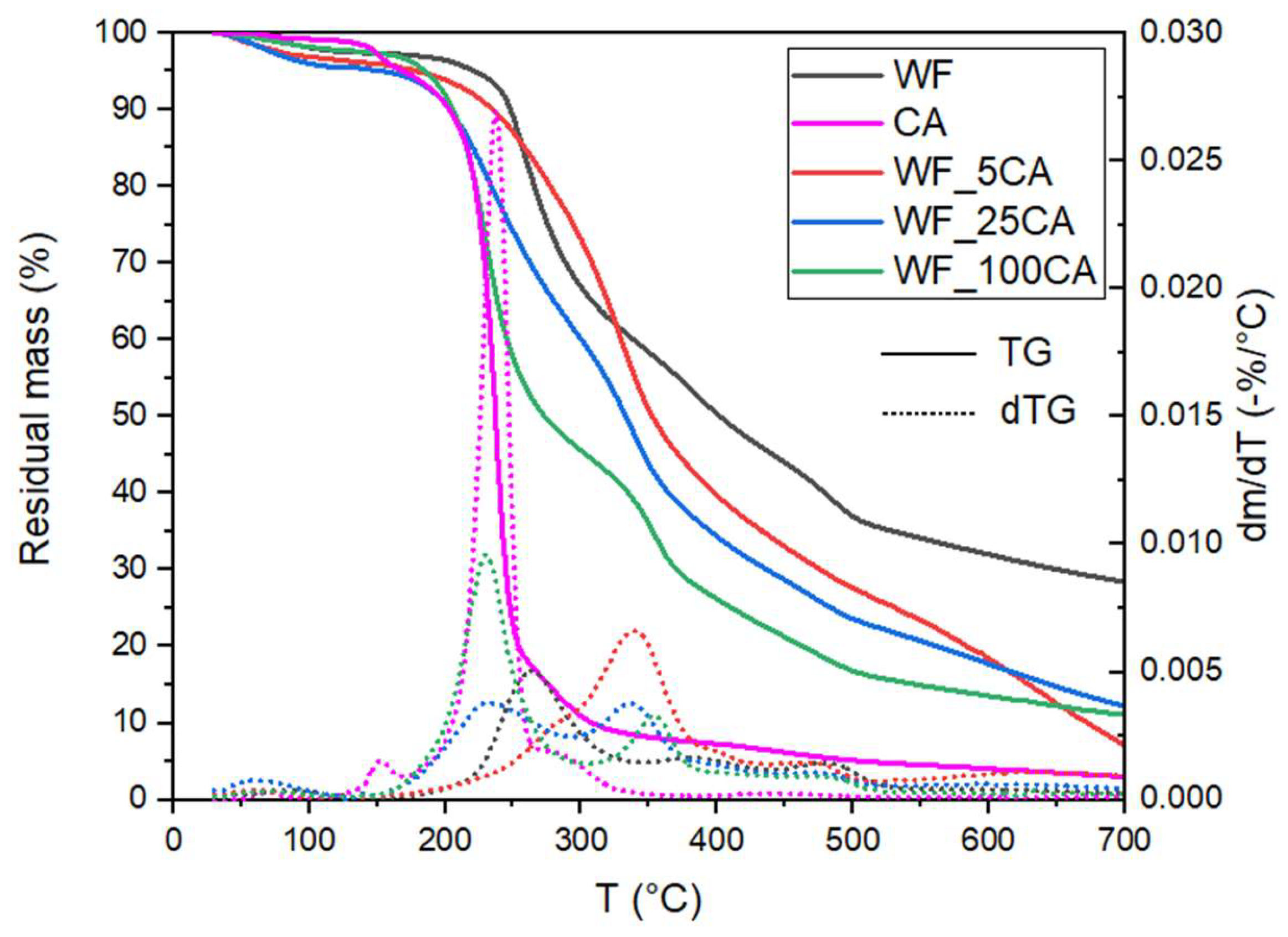
| Samples | T5% (°C) | Td,CA (°C) | Td,WF_1 (°C) | Td,WF_2 (°C) | Td,WF_3 (°C) | m700 (%) |
|---|---|---|---|---|---|---|
| WF | 227.3 | - | 264.6 | 391.3 | 482.8 | 27.8 |
| WF_5CA | 190.4 | - | 256.3 | 338.3 | 479.3 | 7.5 |
| WF_25CA | 175.2 | 235.7 | - | 336.8 | 476.8 | 12.4 |
| WF_100CA | 182.3 | 225.5 | - | 355.5 | 477.2 | 11.2 |
| CA | 170.2 | 235.3 | - | - | - | 0.0 |
| Samples | T5% (°C) | Td,WF (°C) | Td,PCL (°C) | m700 (%) |
|---|---|---|---|---|
| PCL | 398.2 | - | 433.5 | 0.0 |
| PCL_5WF | 386.5 | 259.0 | 433.3 | 0.0 |
| PCL_10WF | 321.2 | 258.0 | 432.3 | 2.5 |
| PCL_20WF | 259.2 | 260.5 | 431.2 | 6.0 |
| PCL_10WF_5CA | 334.3 | 284.7 | 438.5 | 1.1 |
| PCL_10WF_25CA | 314.1 | 296.2 | 437.5 | 1.9 |
| PCL_10WF_100CA | 321.2 | 298.7 | 437.7 | 2.0 |
| Sample | 0 °C | 25 °C | |||||
|---|---|---|---|---|---|---|---|
| E (MPa) |
σy (MPa) | ϵb (%) |
E (MPa) |
σy (MPa) | ϵb (%) |
VST (°C) |
|
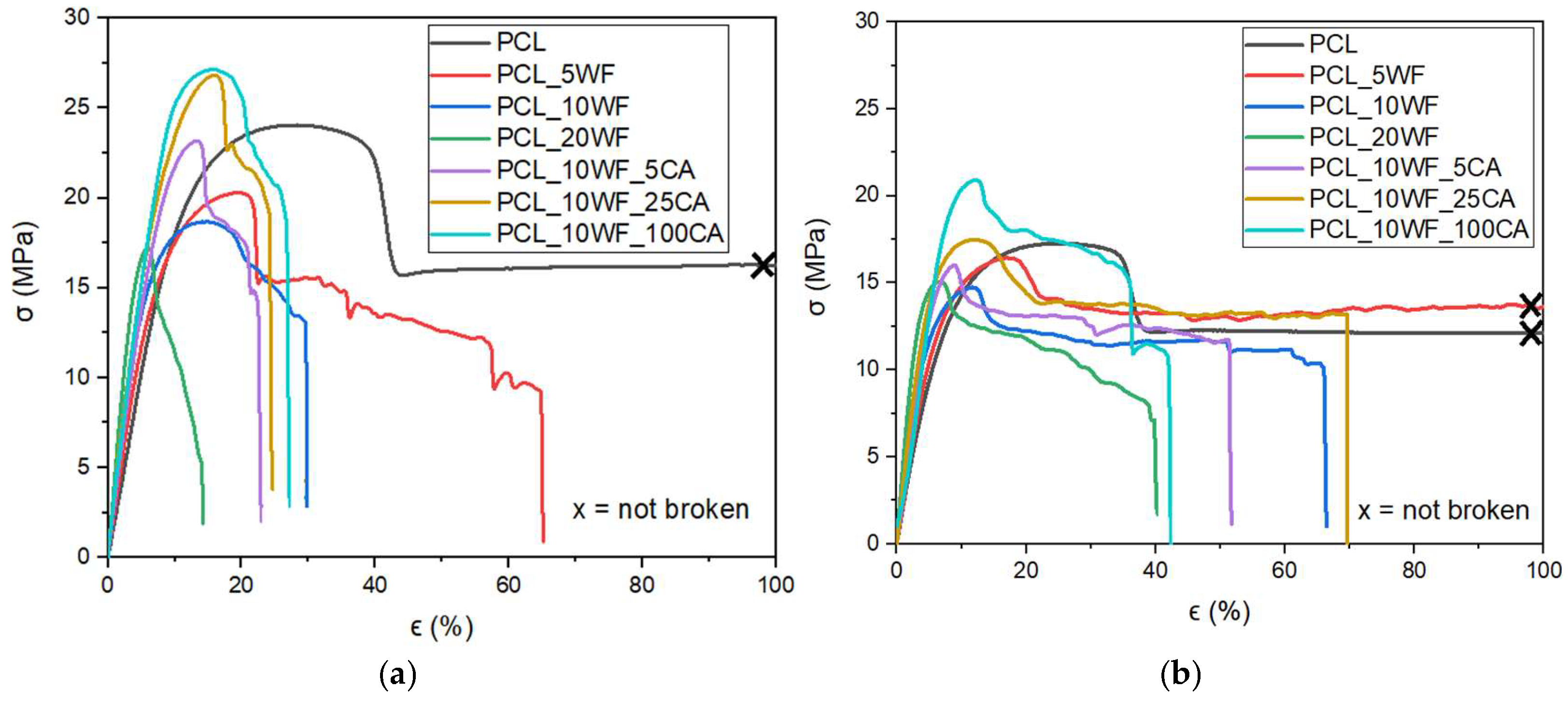
| PCL | 228 ± 14 | 23 ± 1 | 358 ± 83 | 202 ± 5 | 17 ± 1 | 1301 ± 4 | 52.7 ± 0.6 |
| PCL_5WF | 259 ± 16 | 19 ± 1 | 46 ± 19 | 271 ± 15 | 14 ± 3 | 1016 ± 50 | 54.2 ± 0.5 |
| PCL_10WF | 327 ± 14 | 19 ± 1 | 27 ± 10 | 333 ± 3 | 15 ± 1 | 61 ± 21 | 56.1 ± 0.5 |
| PCL_20WF | 458 ± 15 | 17 ± 1 | 14 ± 1 | 467 ± 69 | 14 ± 1 | 36 ± 11 | 58.8 ± 0.7 |
| PCL_10WF_5CA | 337 ± 30 | 23 ± 1 | 23 ± 2 | 337 ± 37 | 14 ± 2 | 49 ± 10 | 56.5 ± 0.3 |
| PCL_10WF_25CA | 354 ± 27 | 27 ± 2 | 33 ± 7 | 348 ± 31 | 15 ± 2 | 54 ± 19 | 57.7 ± 0.5 |
| PCL_10WF_100CA | 354 ± 31 | 26 ± 3 | 27 ± 2 | 348 ± 21 | 19 ± 3 | 53 ± 22 | 58.8 ± 0.5 |
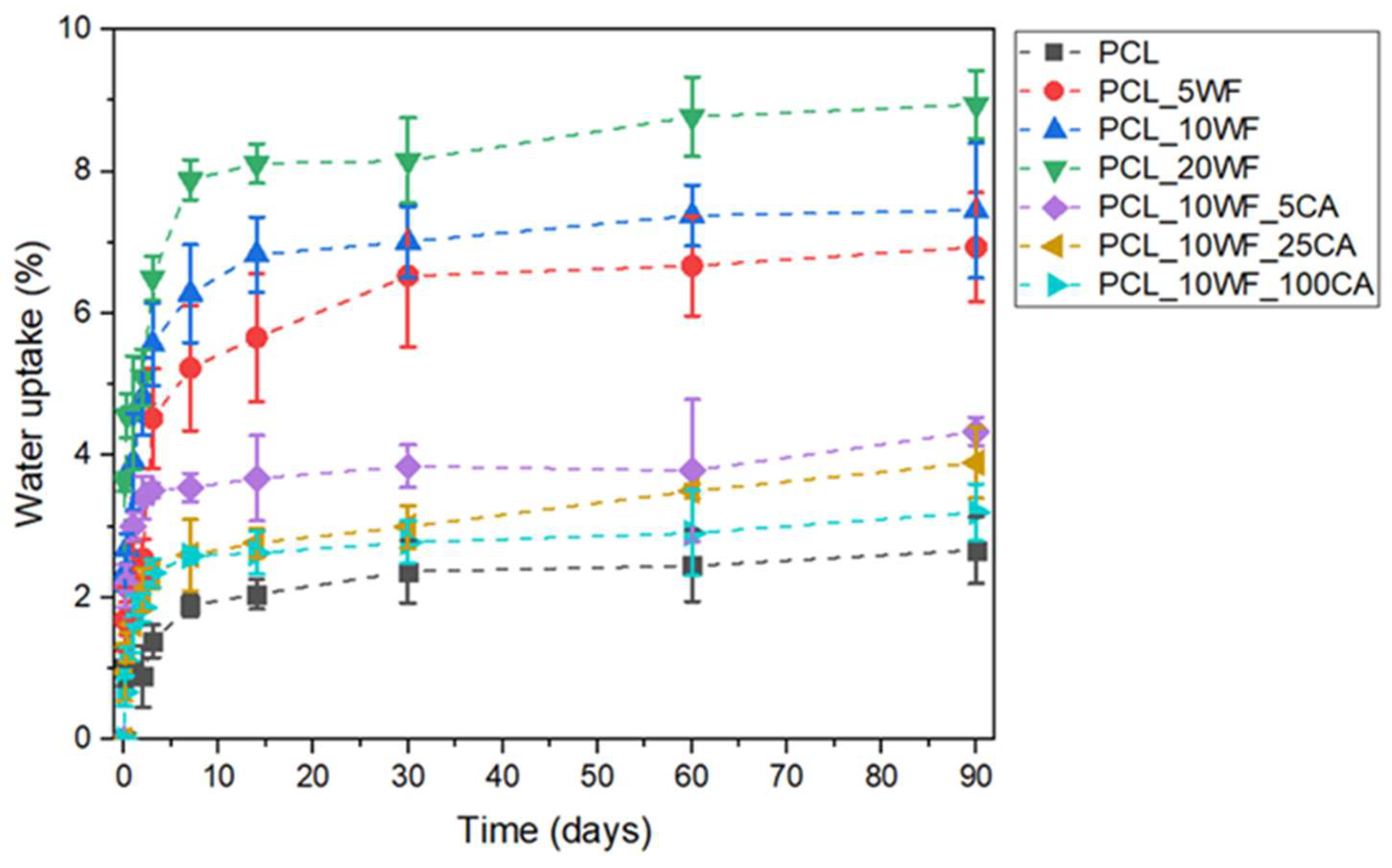
| Samples | D (10-13 × m2/s) | WU90 (%) |
|---|---|---|
| PCL | 1.06 ± 0.36 | 2.67 ± 0.47 |
| PCL_5WF | 1.31 ± 0.46 | 6.93 ± 0.77 |
| PCL_10WF | 1.27 ± 0.52 | 7.44 ± 0.95 |
| PCL_20WF | 1.27 ± 0.64 | 8.94 ± 0.48 |
| PCL_10WF_5CA | 1.22 ± 0.43 | 4.34 ± 0.20 |
| PCL_10WF_25CA | 1.18 ± 0.36 | 3.90 ± 0.51 |
| PCL_10WF_100CA | 1.19 ± 0.62 | 3.22 ± 0.42 |
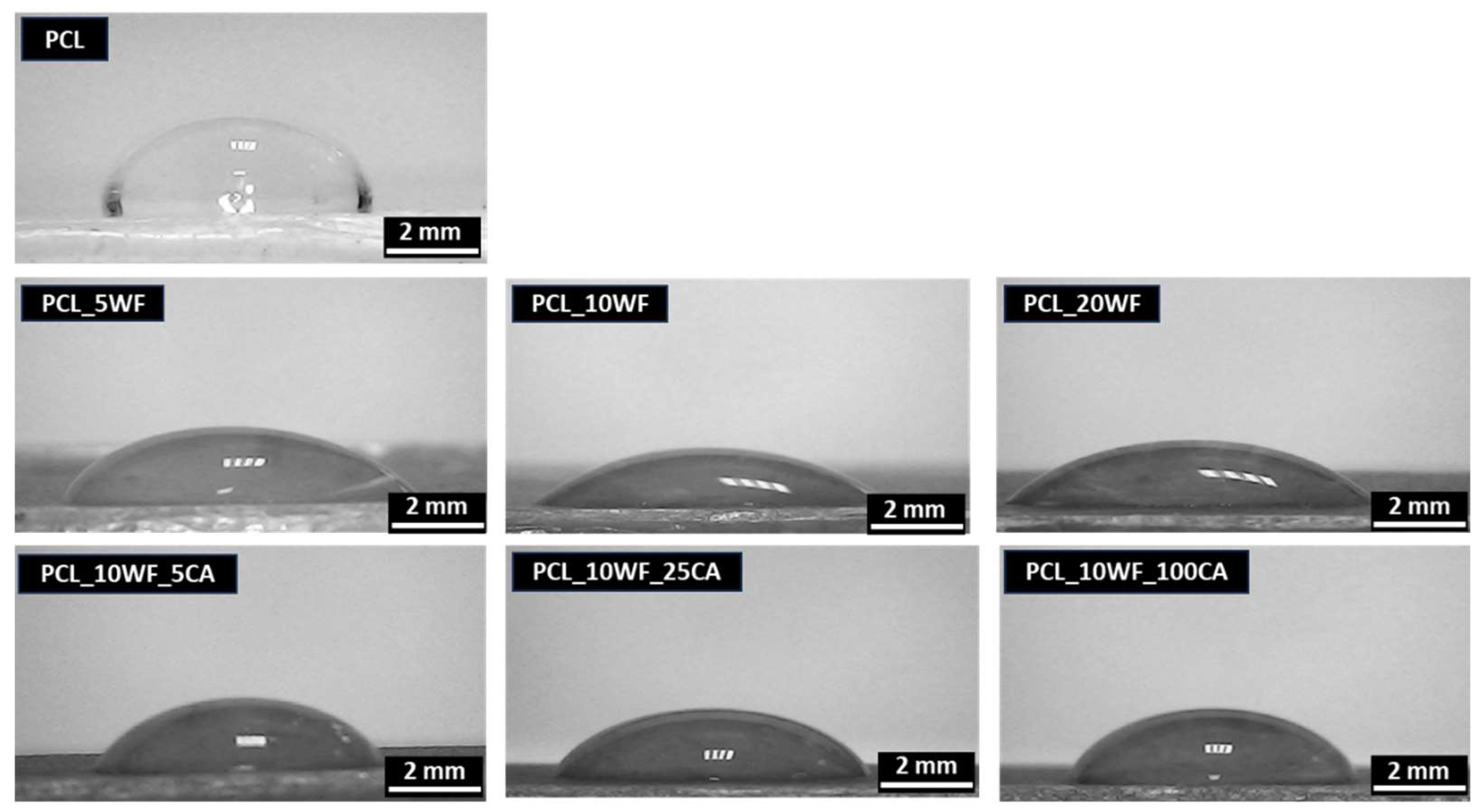
| Samples | θc (°) |
|---|---|
| PCL | 84.2 ± 3.1 |
| PCL_5WF | 47.8 ± 2.9 |
| PCL_10WF | 51.2 ± 2.4 |
| PCL_20WF | 45.4 ± 4.5 |
| PCL_10WF_5CA | 55.2 ± 3.4 |
| PCL_10WF_25CA | 56.5 ± 1.3 |
| PCL_10WF_100CA | 61.2 ± 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





