Submitted:
31 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
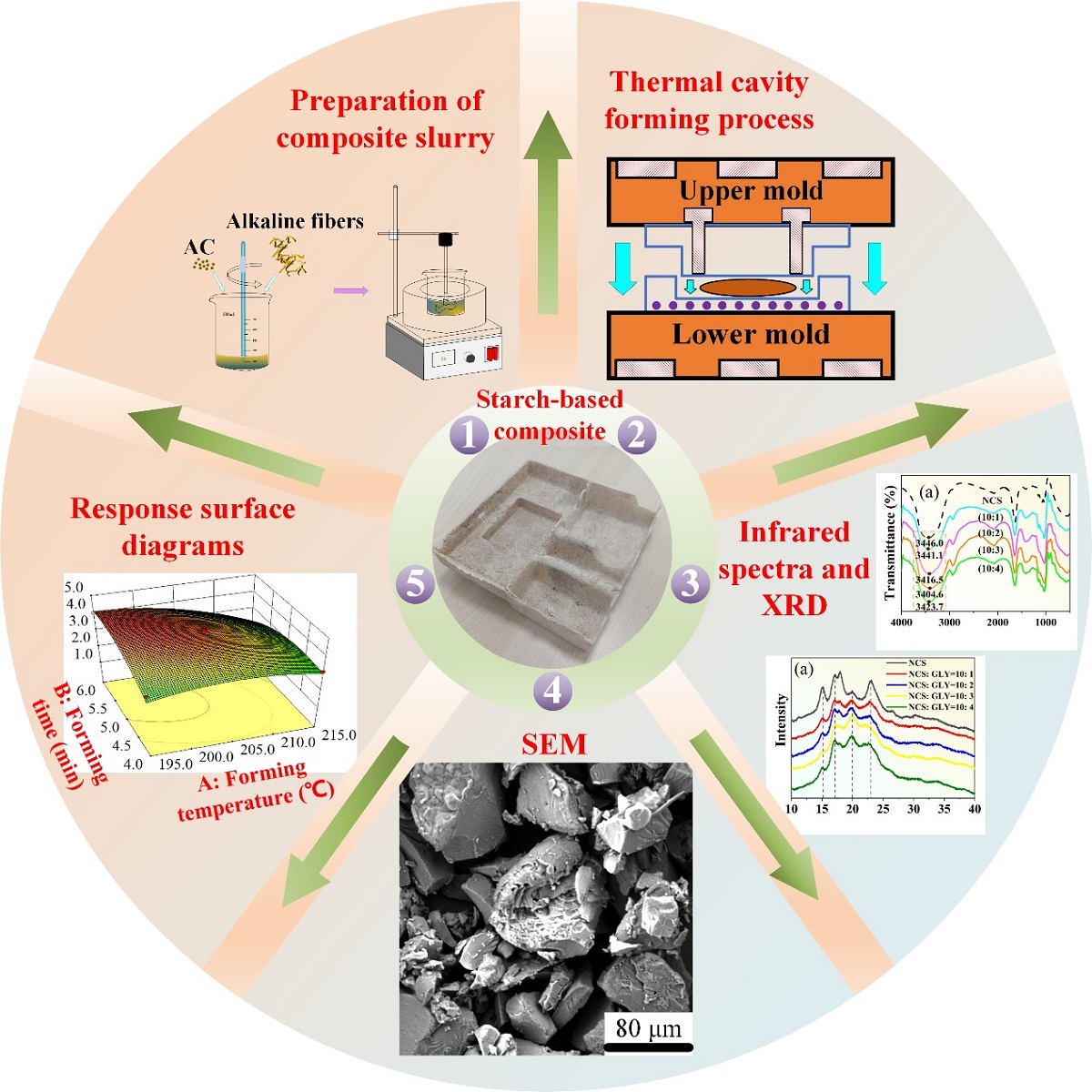
Keywords:
1. Introduction
2. Materials and Experimental Procedures
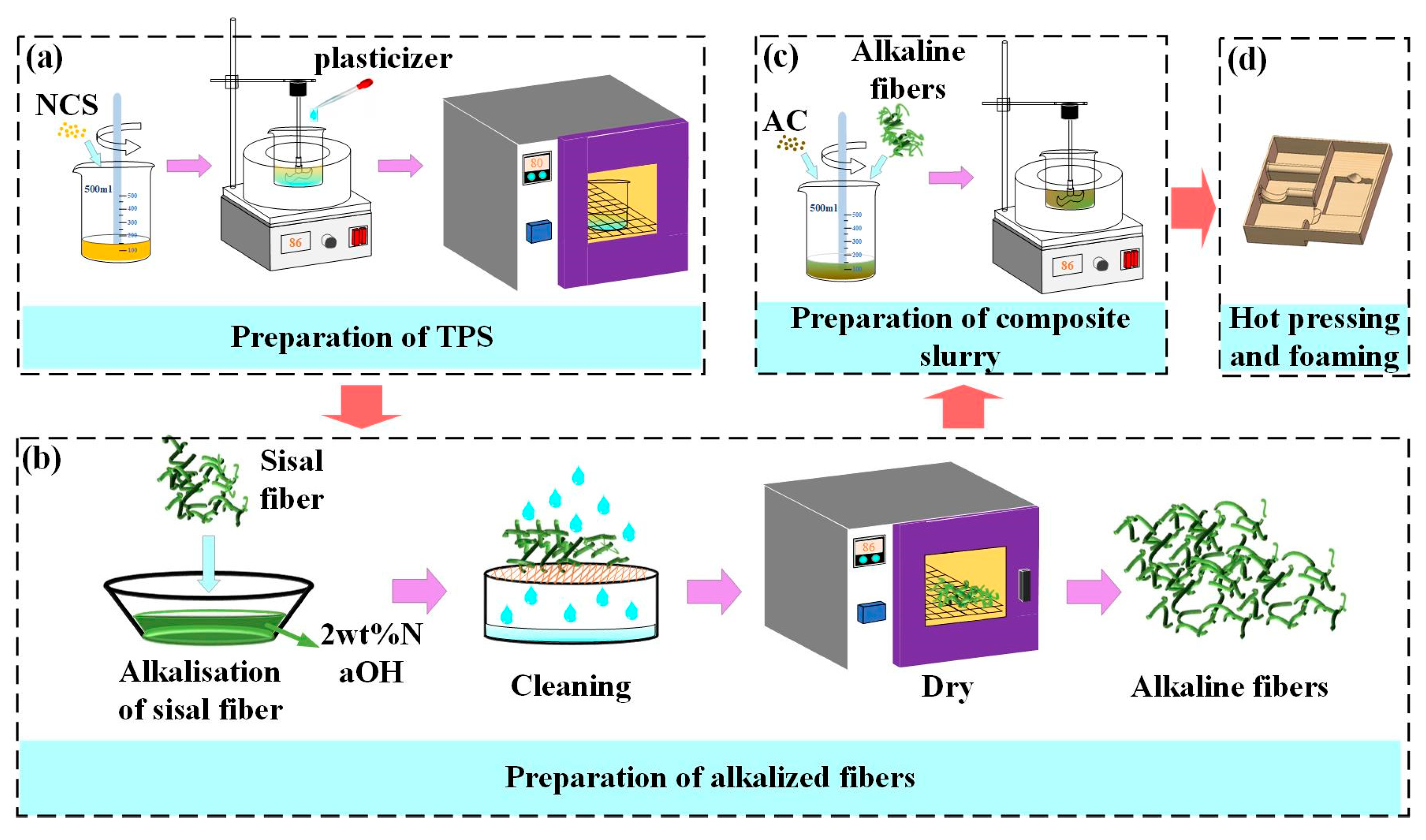
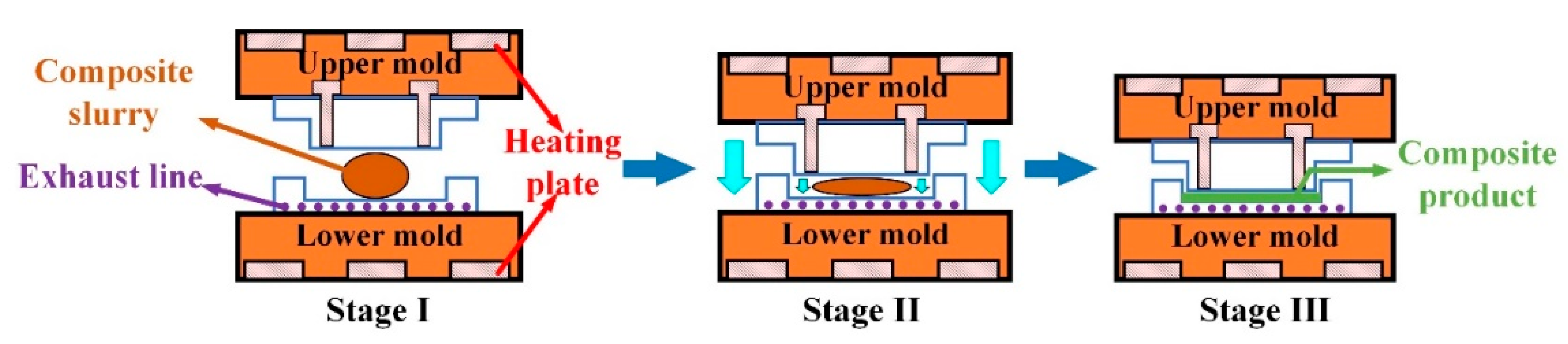
2.1. Materials preparation and experimental set-up
2.2. Material characterizations
2.3. Mechanics Performance Testing
2.4. Process parameter optimization
- Establishing a general mathematical relationship between the input variables and response, which can be expressed as:
- 2.
- Looking for mathematical models. Based on mathematical analysis, the existing McLaughlin or Taylor expansions can meet convergence conditions. According to Samir Charola et al. [34], the experimental data can be fitted into a second-order polynomial model, namely,
- 3.
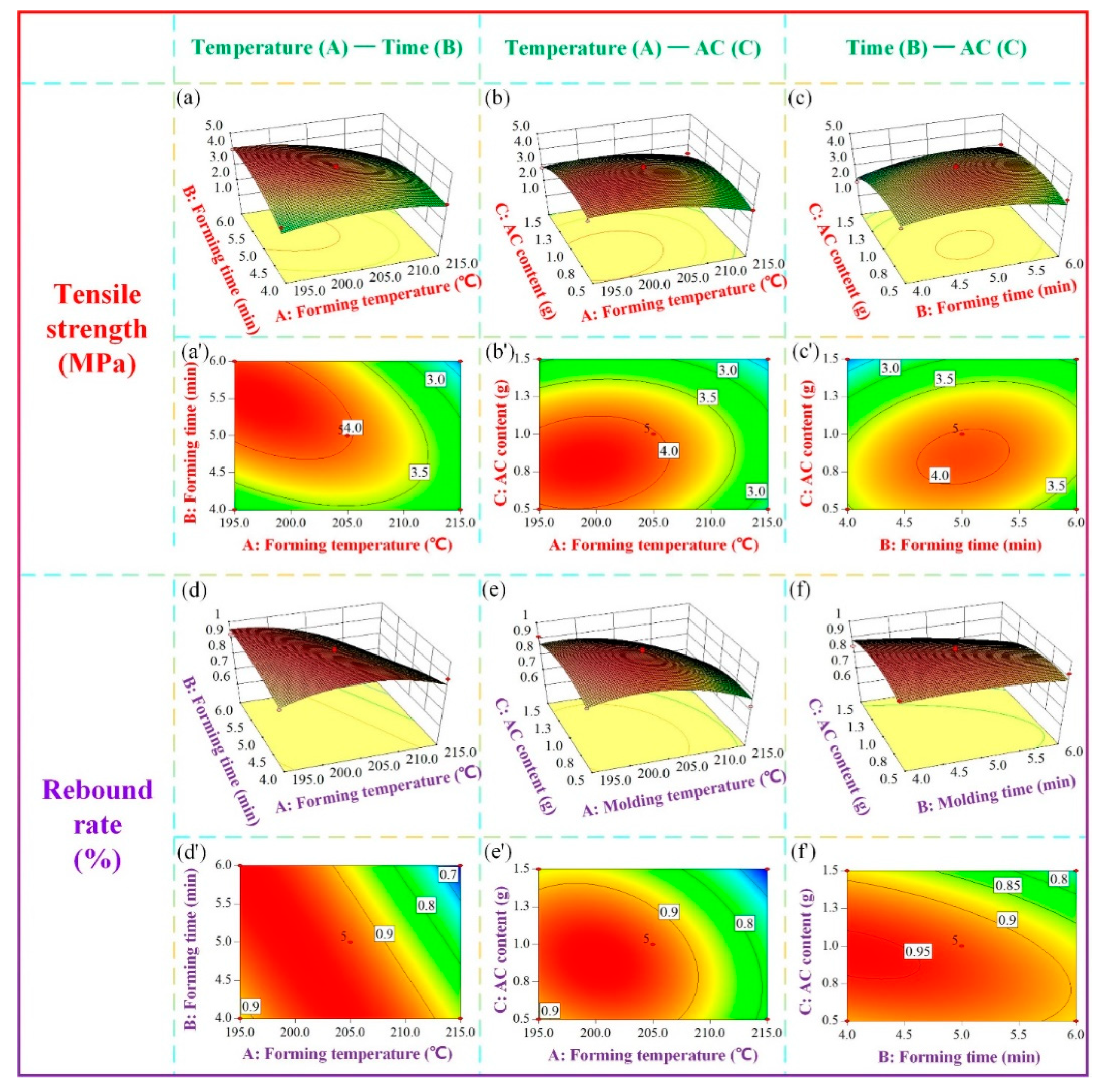
- Using analysis of variance (ANOVA) to evaluate the significance of the prediction model. The reliability of the model can be determined by loss of fit (LOF) and P value. The actual values are the tensile strength and rebound rate measured by experiments. Based on the second-order polynomial model, the response surface and the contour plot which describe the influences of two variables on a single target can be obtained. The third input variable remains unchanged at the center point. Then, the effects of input variables on the response will be analyzed to obtain the highest rebound rate and the tensile strength simultaneously.
3. Results and Discussion
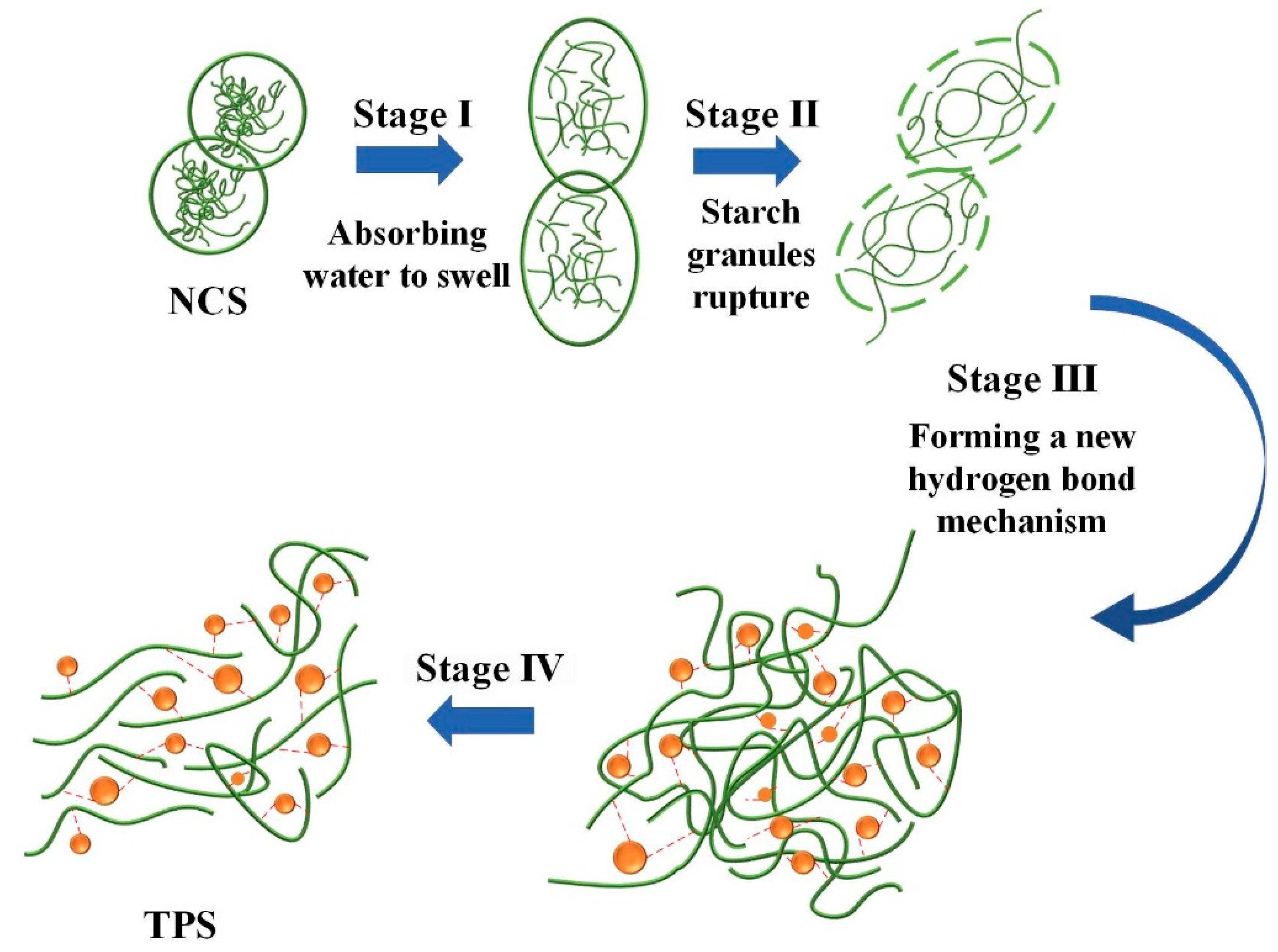
3.1. The plasticization process of starch
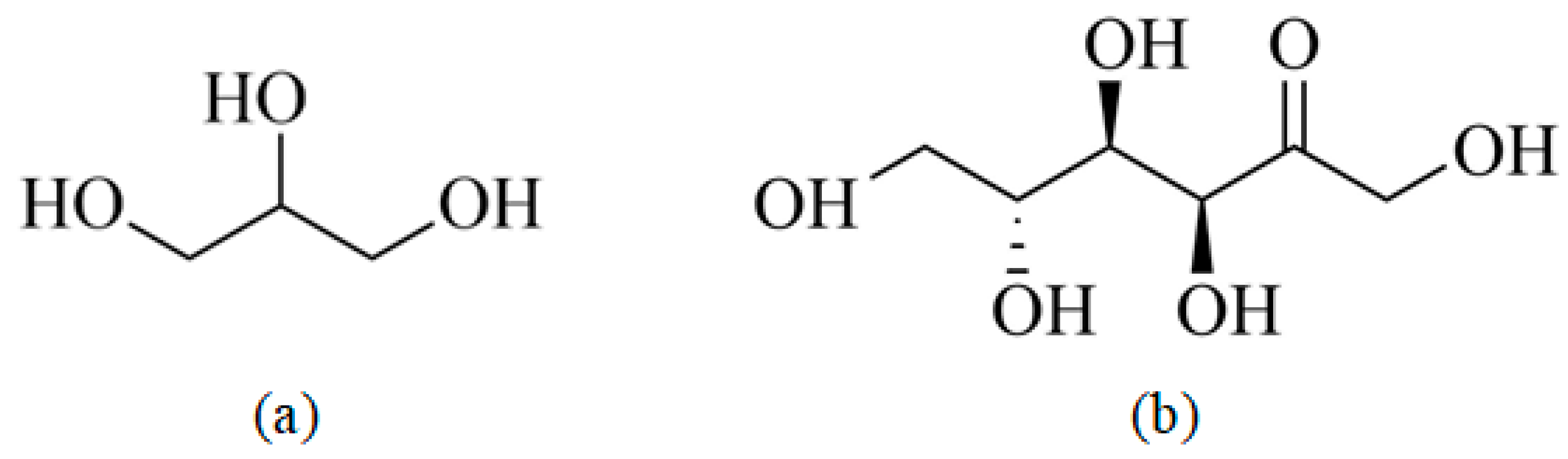
3.2. Effects of plasticizer on TPS
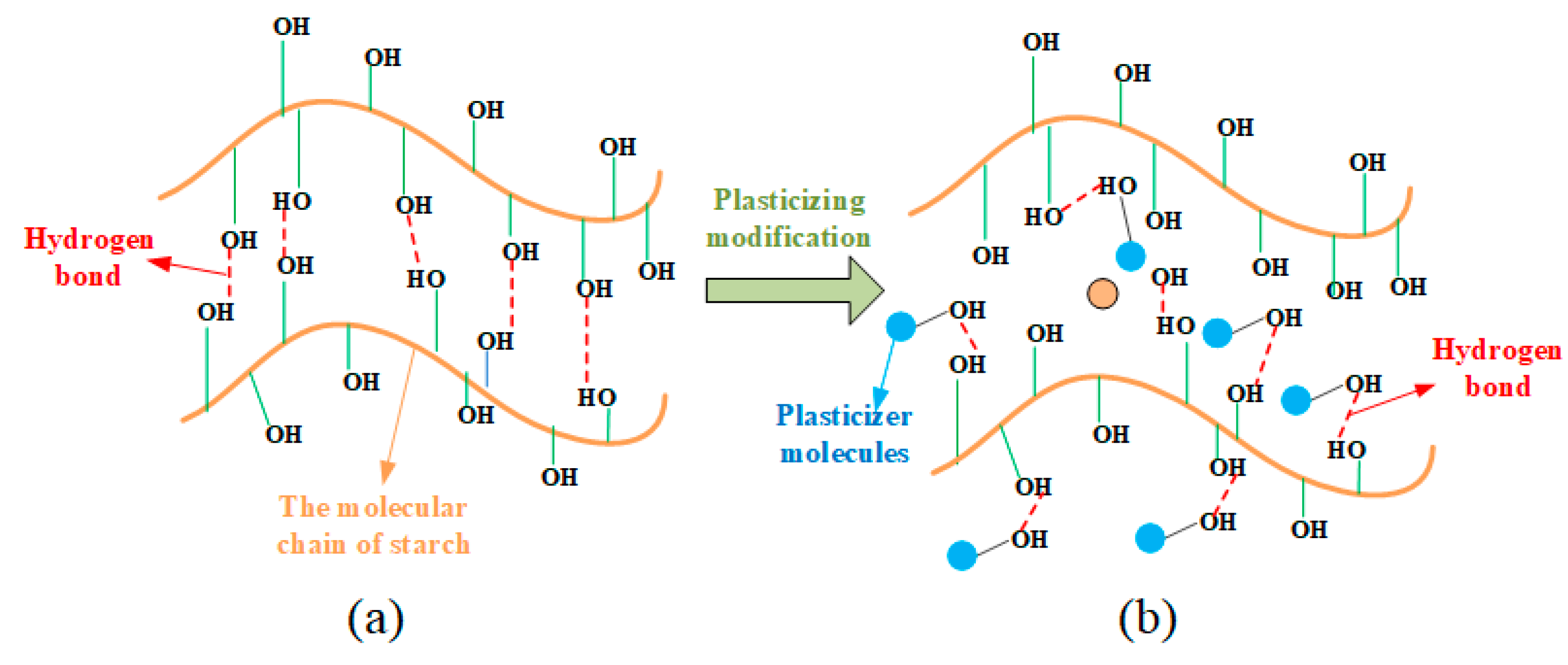
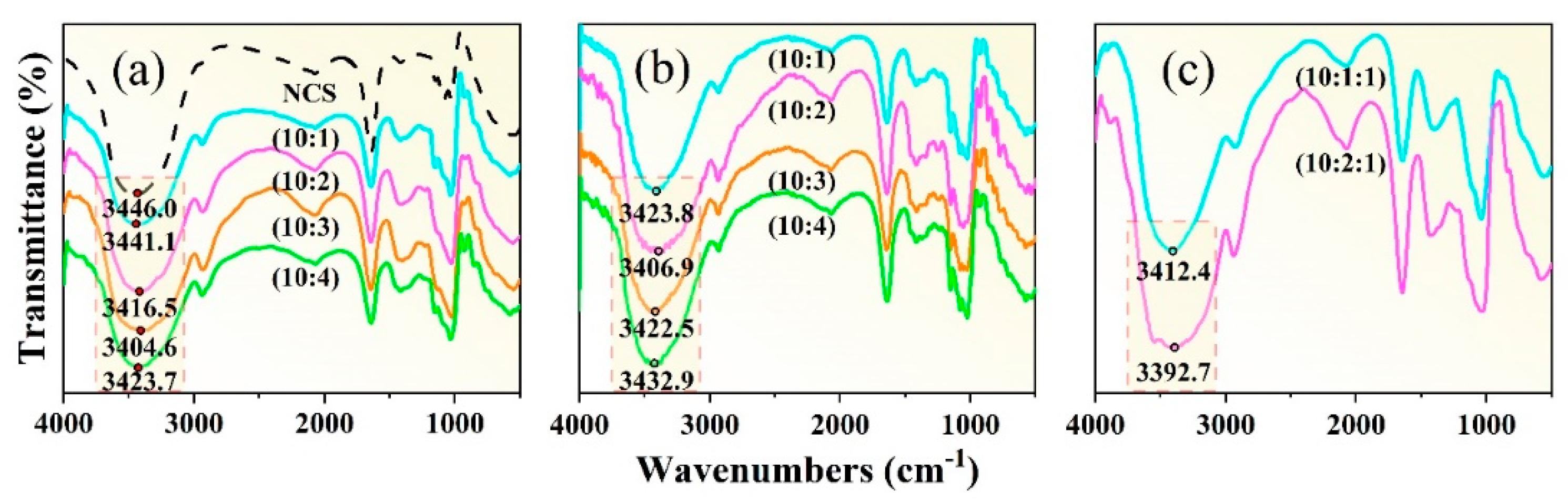
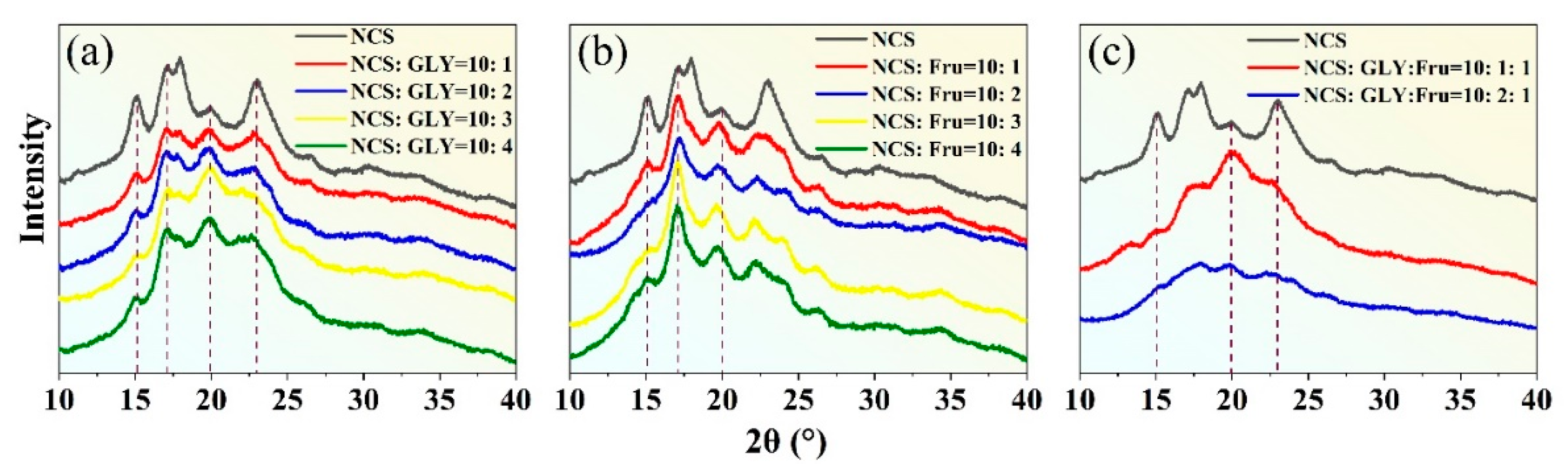
3.2.1. Effects of plasticizer on hydrogen bond
3.2.2. Effects of plasticizer on crystallization type
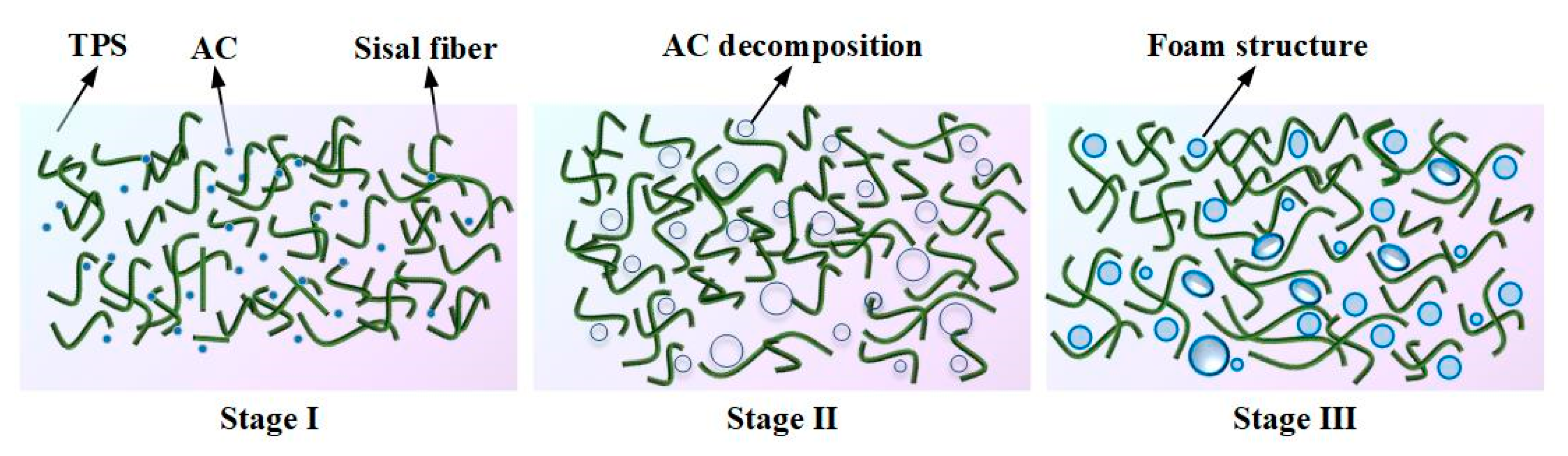
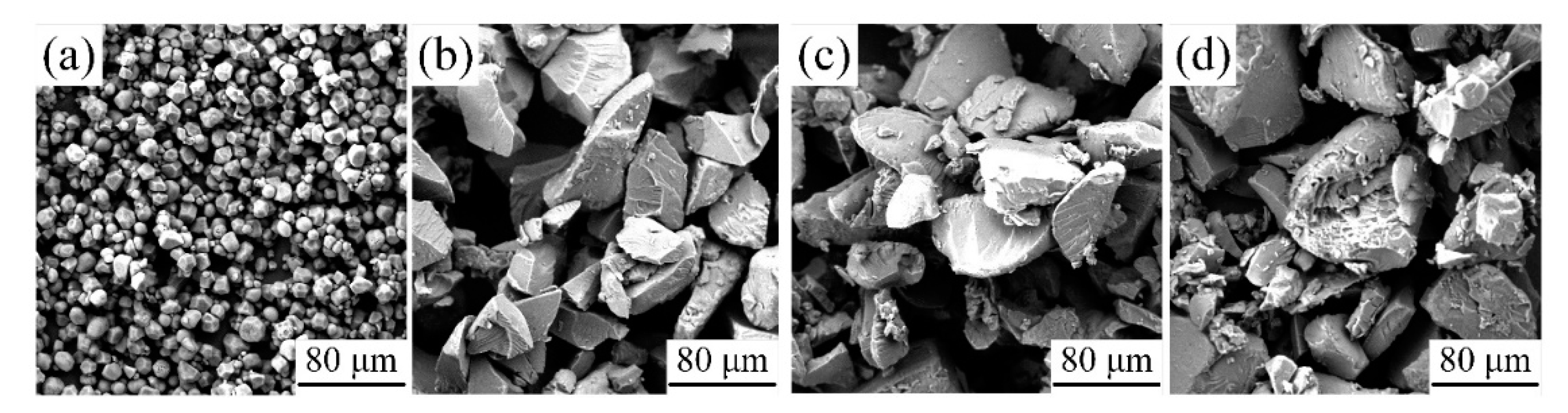
3.2.3. Effects of plasticizer on micromorphology
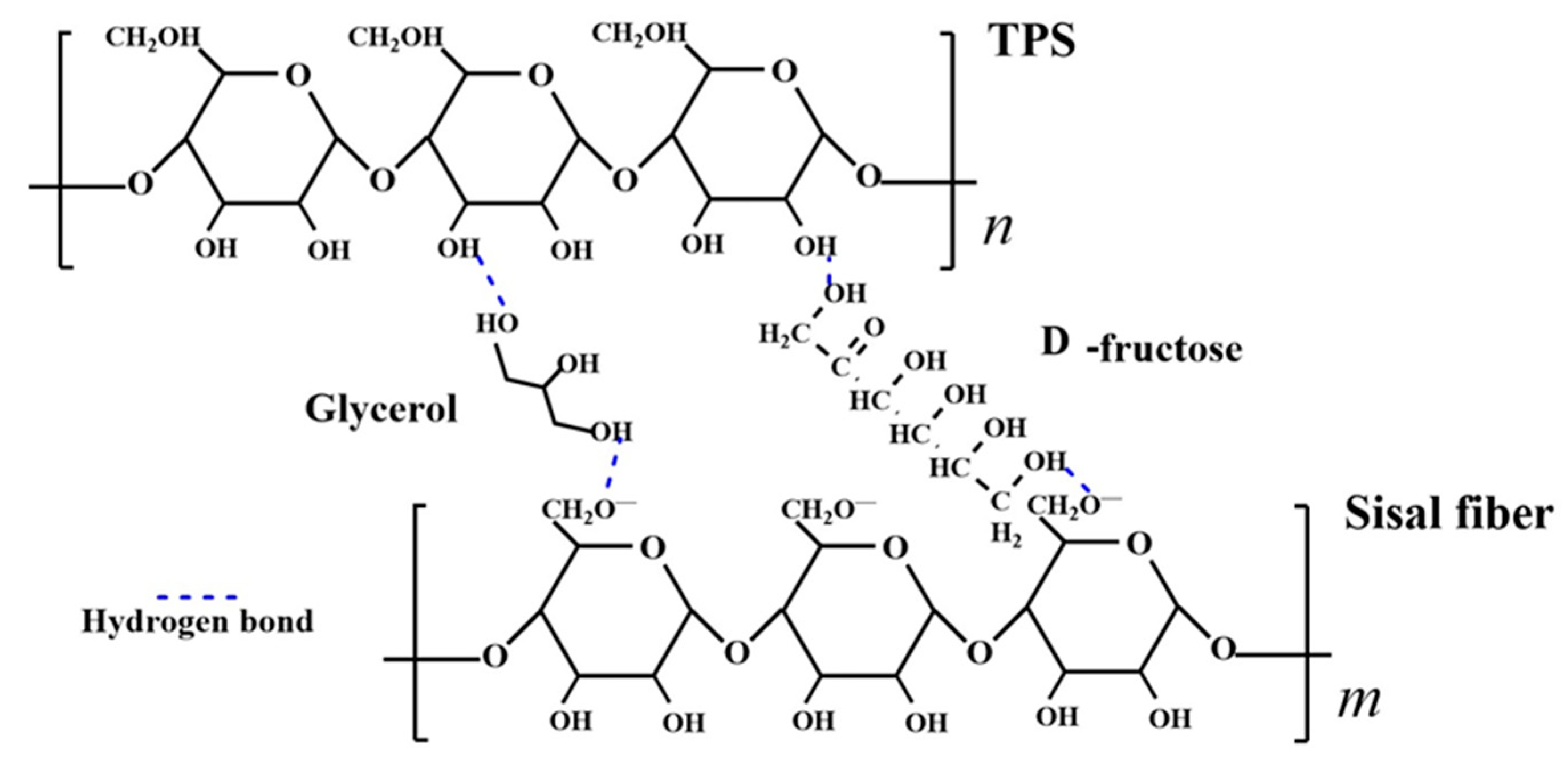
3.3. Molecular linking model of starch-based composites
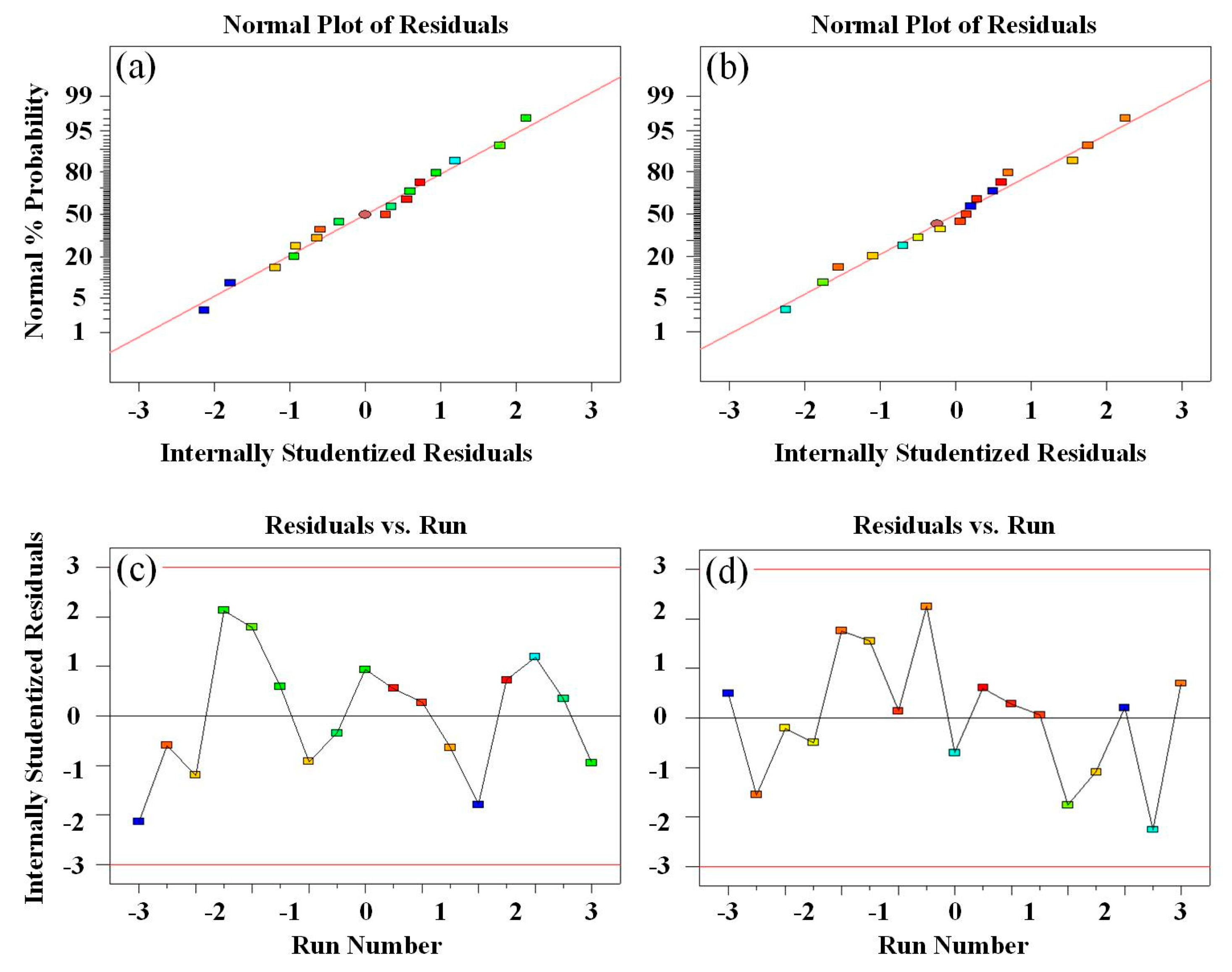
3.4. Parameter optimization of the starch composites
4. Conclusions
Author Contributions
Data Availability
Acknowledgments
Declaration of Competing Interest
References
- Adeniran AA, Shakantu W. The Health and Environmental Impact of Plastic Waste Disposal in South African Townships: A Review. International Journal of Environmental Research and Public Health. 2022, 19, 779. [Google Scholar] [CrossRef]
- Gaston SA, Tulve NS. Urinary phthalate metabolites and metabolic syndrome in U.S. adolescents: Cross-sectional results from the National Health and Nutrition Examination Survey (2003–2014) data. International Journal of Hygiene and Environmental Health. 2019, 222, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. Journal of Hazardous Materials. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Xu R, Chen F, Ding C. [Opportunities, challenges and suggestions for the development of plastic degradation and recycling under the context of circular bioeconomy]. Sheng wu gong cheng xue bao = Chinese journal of biotechnology. 2023, 39, 1867–1882. [Google Scholar] [CrossRef]
- Filho WL, Salvia AL, Bonoli A, Saari UA, Voronova V, Klõga M, et al. An assessment of attitudes towards plastics and bioplastics in Europe. Science of The Total Environment. 2021, 755, 142732. [Google Scholar] [CrossRef]
- Rajvanshi J, Sogani M, Kumar A, Arora S, Syed Z, Sonu K, et al. Perceiving biobased plastics as an alternative and innovative solution to combat plastic pollution for a circular economy. Science of The Total Environment. 2023, 874, 162441. [Google Scholar] [CrossRef]
- Cheng M-H, Singh S, Carr Clennon AN, Dien BS, Singh V. Production of Designer Xylose-Acetic Acid Enriched Hydrolysate from Bioenergy Sorghum, Oilcane, and Energycane Bagasses. Bioresource Technology. 2023, 380, 129104. [Google Scholar] [CrossRef] [PubMed]
- Liu W, Zhang X, Ren H, Hu X, Yang X, Liu H. Co-production of spirosiloxane and biochar adsorbent from wheat straw by a low-cost and environment-friendly method. Journal of Environmental Management. 2023, 338, 117851. [Google Scholar] [CrossRef] [PubMed]
- Suvarnna K, Shanjitha S, Selvasekarapandian S, Kirubavathy SJ. Investigation of solid bio-membrane based on corn biomass as a proton-conducting bio-electrolyte. Bulletin of Materials Science. 2023, 46, 112. [Google Scholar] [CrossRef]
- Guo A, Tao X, Kong H, Zhou X, Wang H, Li J, et al. Effects of aluminum hydroxide on mechanical, water resistance, and thermal properties of starch-based fiber-reinforced composites with foam structures. Journal of Materials Research and Technology. 2023, 23, 1570–1583. [Google Scholar] [CrossRef]
- Bangar SP, Purewal SS, Trif M, Maqsood S, Kumar M, Manjunatha V, et al. Functionality and Applicability of Starch-Based Films: An Eco-Friendly Approach. Foods. 2021, 10, 2181. [Google Scholar] [CrossRef]
- Do Val Siqueira L, Arias CILF, Maniglia BC, Tadini CC. Starch-based biodegradable plastics: methods of production, challenges and future perspectives. Current Opinion in Food Science. 2021, 38, 122–130. [Google Scholar] [CrossRef]
- Jane J-L, Robyt JF. Structure studies of amylose-V complexes and retro-graded amylose by action of alpha amylases, and a new method for preparing amylodextrins. Carbohydrate Research. 1984, 132, 105–118. [Google Scholar] [CrossRef]
- Biduski B, Silva WMFD, Colussi R, Halal SLDME, Lim L-T, Dias ÁRG, et al. Starch hydrogels: The influence of the amylose content and gelatinization method. International Journal of Biological Macromolecules. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Zhu F, Liu P. Starch gelatinization, retrogradation, and enzyme susceptibility of retrograded starch: Effect of amylopectin internal molecular structure. Food Chemistry. 2020, 316, 126036. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W. Preparation and properties of lignocellulosic fiber/CaCO3/thermoplastic starch composites. Carbohydrate Polymers. 2019, 211, 204–208. [Google Scholar] [CrossRef]
- Zhang B, Xie F, Zhang T, Chen L, Li X, Truss RW, et al. Different characteristic effects of ageing on starch-based films plasticised by 1-ethyl-3-methylimidazolium acetate and by glycerol. Carbohydrate Polymers. 2016, 146, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Kahvand F, Fasihi M. Plasticizing and anti-plasticizing effects of polyvinyl alcohol in blend with thermoplastic starch. International Journal of Biological Macromolecules. 2019, 140, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Martín L, Beltrán-Osuna ÁA, Perilla JE. Effect of the Addition of Citric Acid and Whey Protein Isolate in Canna indica L. Starch Films Obtained by Solvent Casting. Journal of Polymers and the Environment. 2020, 28, 871–883. [Google Scholar] [CrossRef]
- Zeng G-S, Lin R-Z, Zheng L-J, Chen L, Meng C. Preparation and performance study of waste paper pulp reinforced cornstarch-based composites. Journal of Functional Materials. 2012, 43, 2218–2221. [Google Scholar]
- De Graaf RA, Karman AP, Janssen LPBM. Material Properties and Glass Transition Temperatures of Different Thermoplastic Starches After Extrusion Processing. Starch - Stärke. 2003, 55, 80–86. [Google Scholar] [CrossRef]
- Enrione J, Osorio F, Pedreschi F, Hill S. Prediction of the Glass Transition Temperature on Extruded Waxy Maize and Rice Starches in Presence of Glycerol. Food and Bioprocess Technology. 2010, 3, 791–796. [Google Scholar] [CrossRef]
- Schmitt H, Guidez A, Prashantha K, Soulestin J, Lacrampe MF, Krawczak P. Studies on the effect of storage time and plasticizers on the structural variations in thermoplastic starch. Carbohydrate Polymers. 2015, 115, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz M, Staciwa P, Spychaj T. Low Transition Temperature Mixtures (LTTM) Containing Sugars as Potato Starch Plasticizers. Starch - Stärke. 2019, 71, 1900004. [Google Scholar] [CrossRef]
- Saberi B, Chockchaisawasdee S, Golding JB, Scarlett CJ, Stathopoulos CE. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. International Journal of Biological Macromolecules. 2017, 104, 345–359. [Google Scholar] [CrossRef]
- Müller P, Renner K, Móczó J, Fekete E, Pukánszky B. Thermoplastic starch/wood composites: Interfacial interactions and functional properties. Carbohydrate Polymers. 2014, 102, 821–829. [Google Scholar] [CrossRef]
- Mohammed AABA, Hasan Z, Omran AAB, Kumar VV, Elfaghi AM, Ilyas RA, et al. Corn: Its Structure, Polymer, Fiber, Composite, Properties, and Applications. Polymers. 2022, 14, 4396. [Google Scholar] [CrossRef]
- Jenkins PJ, Donald AM. The influence of amylose on starch granule structure. International Journal of Biological Macromolecules. 1995, 17, 315–321. [Google Scholar] [CrossRef]
- Zhang C-W, Li F-Y, Li J-F, Xie Q, Xu J, Guo A-F, et al. Effect of Crystal Structure and Hydrogen Bond of Thermoplastic Oxidized Starch on Manufacturing of Starch-Based Biomass Composite. International Journal of Precision Engineering and Manufacturing-Green Technology. 2018, 5, 435–440. [Google Scholar] [CrossRef]
- Chen S, Li F-Y, Li J-F, Sun X, Cui J-F, Zhang C-W, et al. Effects of single-modification/cross-modification of starch on the mechanical properties of new biodegradable composites. RSC advances. 2018, 8, 12400–12408. [Google Scholar] [CrossRef]
- Xu Q, Kennedy JF, Liu L. An ionic liquid as reaction media in the ring opening graft polymerization of ε-caprolactone onto starch granules. Carbohydrate Polymers. 2008, 72, 113–121. [Google Scholar] [CrossRef]
- Zha X, Sadi MS, Yang Y, Luo T, Huang N. Introduction of poly(acrylic acid) branch onto acetate starch for polyester warp sizing. The Journal of The Textile Institute. 2021, 112, 273–285. [Google Scholar] [CrossRef]
- Pérez-Chávez R, Sánchez-Aguilar J, Calderas F, Maddalena L, Carosio F, Sanchez-Olivares G. A statistical approach to the development of flame retardant and mechanically strong natural fibers biocomposites. Polymer Degradation and Stability. 2022, 201, 109991. [Google Scholar] [CrossRef]
- Singh S, Chakraborty JP, Mondal MK. Pyrolysis of torrefied biomass: Optimization of process parameters using response surface methodology, characterization, and comparison of properties of pyrolysis oil from raw biomass. Journal of Cleaner Production. 2020, 272, 122517. [Google Scholar] [CrossRef]
- Xu Y-Z, Soloway RD, Lin X-F, Zhi X, Weng S-F, Wu Q-G, et al. Fourier transform infrared (FT-IR) mid-IR spectroscopy separates normal and malignant tissue from the colon and stomach. Gastroenterology. 2000, 118, A1417. [Google Scholar] [CrossRef]
- Ishkhanyan AM, Krainov VP. Van der Waals Attraction of Hydrogen Atoms. Journal of Experimental and Theoretical Physics. 2021, 132, 892–896. [Google Scholar] [CrossRef]
- Khatun A, Waters DLE, Liu L. A Review of Rice Starch Digestibility: Effect of Composition and Heat-Moisture Processing. Starch - Stärke. 2019, 71, 1900090. [Google Scholar] [CrossRef]
- Shahbazi M, Majzoobi M, Farahnaky A. Impact of shear force on functional properties of native starch and resulting gel and film. Journal of Food Engineering. 2018, 223, 10–21. [Google Scholar] [CrossRef]
- Onyishi HO, Oluah CK. Effect of stretch ratio on the induced crystallinity and mechanical properties of biaxially stretched PET. Phase Transitions. 2020, 93, 924–934. [Google Scholar] [CrossRef]
- He X, Wu S, Fu D, Ni J. Preparation of sodium carboxymethyl cellulose from paper sludge. Journal of Chemical Technology & Biotechnology. 2009, 84, 427–434. [Google Scholar] [CrossRef]
- Agrawal R, Saxena NS, Sharma KB, Thomas S, Sreekala MS. Activation energy and crystallization kinetics of untreated and treated oil palm fibre reinforced phenol formaldehyde composites. Materials Science and Engineering: A. 2000, 277, 77–82. [Google Scholar] [CrossRef]
- Idris A, Kormin F, Noordin M. Application of response surface methodology in describing the performance of thin film composite membrane. Separation and Purification Technology. 2006, 49, 271–280. [Google Scholar] [CrossRef]
- Atapour M, Kariminia H-R, Moslehabadi PM. Optimization of biodiesel production by alkali-catalyzed transesterification of used frying oil. Process Safety and Environmental Protection. 2014, 92, 179–185. [Google Scholar] [CrossRef]
- Azizi Namaghi H, Mousavi SM. Factorial experimental design for treatment of an industrial wastewater using micellar-enhanced ultrafiltration. Desalination and Water Treatment. 2016, 57, 5416–5424. [Google Scholar] [CrossRef]
- Xiarchos I, Jaworska A, Zakrzewska-Trznadel G. Response surface methodology for the modelling of copper removal from aqueous solutions using micellar-enhanced ultrafiltration. Journal of Membrane Science. 2008, 321, 222–231. [Google Scholar] [CrossRef]
- Lesch, SM. Sensor-directed response surface sampling designs for characterizing spatial variation in soil properties. Computers and Electronics in Agriculture. 2005, 46, 153–179. [Google Scholar] [CrossRef]
| NCS: GLY | NCS: D-fructose | NCS: GLY: D-fructose |
|---|---|---|
| 10: 1 | 10: 1 | 10: 1: 1 |
| 10: 2 | 10: 2 | 10: 2: 1 |
| 10: 3 | 10: 3 | |
| 10: 4 | 10: 4 |
| level | Forming temperature (°C) | Forming time (min) | AC content (g) |
|---|---|---|---|
| -1 | 195 °C | 4 | 0.5 |
| 0 | 205 °C | 5 | 1 |
| 1 | 215 °C | 6 | 1.5 |
| Experiment number | Forming temperature (°C) | Forming time (min) |
AC content (g) | Tensile strength (MPa) | Rebound rate (%) |
|---|---|---|---|---|---|
| 1 | 215 | 6.00 | 1.00 | 1.90 | 64.4 |
| 2 | 195 | 6.00 | 1.00 | 4.02 | 92.5 |
| 3 | 195 | 5.00 | 0.50 | 3.76 | 88.5 |
| 4 | 195 | 4.00 | 1.00 | 3.33 | 87.2 |
| 5 | 205 | 6.00 | 0.50 | 3.4 | 92.4 |
| 6 | 215 | 4.00 | 1.00 | 3.11 | 89.7 |
| 7 | 205 | 5.00 | 1.00 | 3.76 | 94.0 |
| 8 | 195 | 5.00 | 1.50 | 2.88 | 91.6 |
| 9 | 205 | 6.00 | 1.50 | 3.06 | 73.3 |
| 10 | 205 | 5.00 | 1.00 | 4.18 | 95.7 |
| 11 | 205 | 5.00 | 1.00 | 4.10 | 94.5 |
| 12 | 205 | 5.00 | 1.00 | 3.84 | 93.7 |
| 13 | 205 | 4.00 | 1.50 | 1.93 | 84.8 |
| 14 | 205 | 5.00 | 1.00 | 4.23 | 89.4 |
| 15 | 215 | 5.00 | 1.50 | 2.49 | 64.2 |
| 16 | 215 | 5.00 | 0.50 | 2.79 | 73.3 |
| 17 | 205 | 4.00 | 0.50 | 3.31 | 92.1 |
| Coefficient source | Sum of squares | Degree of freedom | variance | p-value |
|---|---|---|---|---|
| model | 7.84 | 9 | 0.87 | 0.0049 |
| A- Forming temperature | 1.71 | 1 | 1.71 | 0.0045 |
| B- Forming time | 0.061 | 1 | 0.061 | 0.4629 |
| C-AC content | 1.05 | 1 | 1.05 | 0.0147 |
| AB | 0.90 | 1 | 0.90 | 0.0205 |
| AC | 0.084 | 1 | 0.084 | 0.3931 |
| BC | 0.27 | 1 | 0.27 | 0.1468 |
| A2 | 0.81 | 1 | 0.81 | 0.0257 |
| B2 | 1.03 | 1 | 1.03 | 0.0155 |
| C2 | 1.53 | 1 | 1.53 | 0.0060 |
| Residual error | 0.71 | 7 | 0.10 | 0.1050 |
| LOF | 0.53 | 3 | 0.18 | |
| Error term | 0.18 | 4 | 0.044 | |
| Total variation | 8.55 | 16 |
| Coefficient source | Sum of squares | Degree of freedom | variance | p-value |
|---|---|---|---|---|
| model | 0.16 | 9 | 0.018 | 0.0027 |
| A- Forming temperature | 0.058 | 1 | 0.058 | 0.0007 |
| B- Forming time | 0.012 | 1 | 0.012 | 0.0327 |
| C-AC content | 0.013 | 1 | 0.013 | 0.0282 |
| AB | 0.024 | 1 | 0.024 | 0.0078 |
| AC | 0.0037 | 1 | 0.0037 | 0.1854 |
| BC | 0.0035 | 1 | 0.0035 | 0.1984 |
| A2 | 0.028 | 1 | 0.028 | 0.0051 |
| B2 | 0.0015 | 1 | 0.0015 | 0.3839 |
| C2 | 0.015 | 1 | 0.015 | 0.0220 |
| Residual error | 0.012 | 7 | 0.0017 | |
| LOF | 0.0098 | 3 | 0.0033 | 0.0632 |
| Error term | 0.0022 | 4 | 0.0057 | |
| Total variation | 0.17 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





