Submitted:
06 May 2025
Posted:
07 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seed Treatment with Plasma
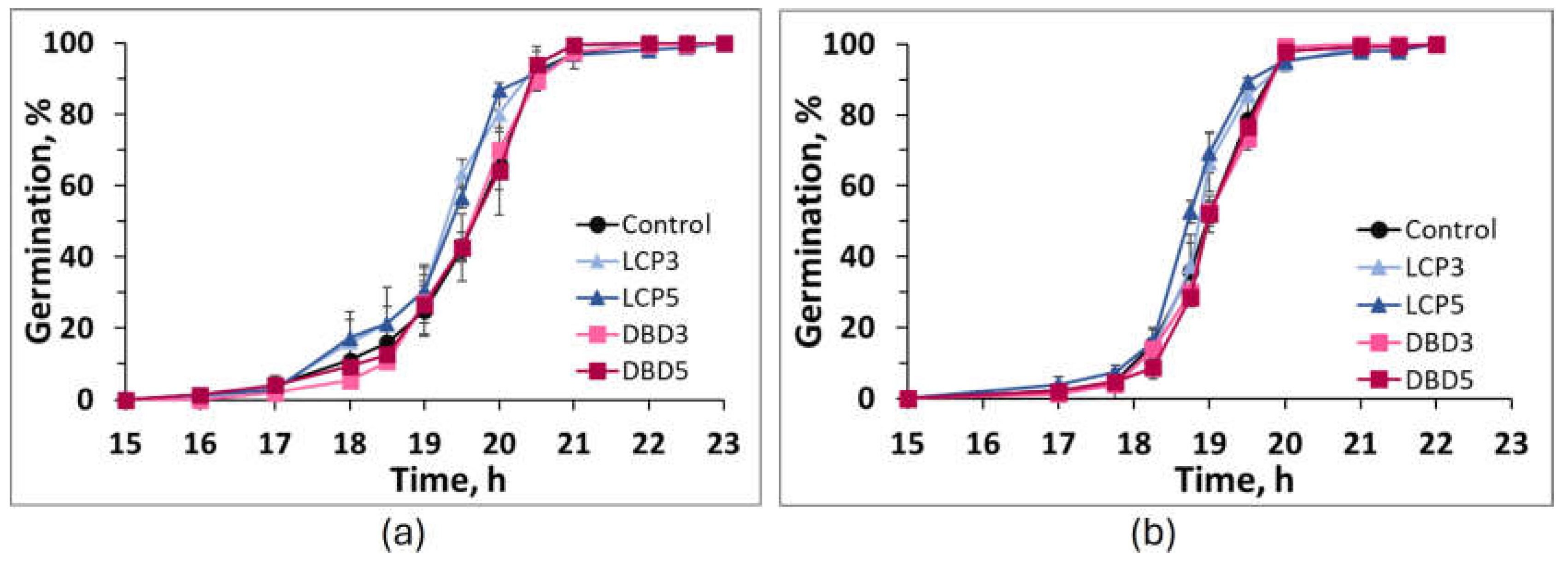
2.3. Seed Germination Test In Vitro
2.4. Cultivation of Seedlings in an Aeroponic System
2.5. Measurement of Photosynthetic Efficiency
2.6. Morphometric Measurements
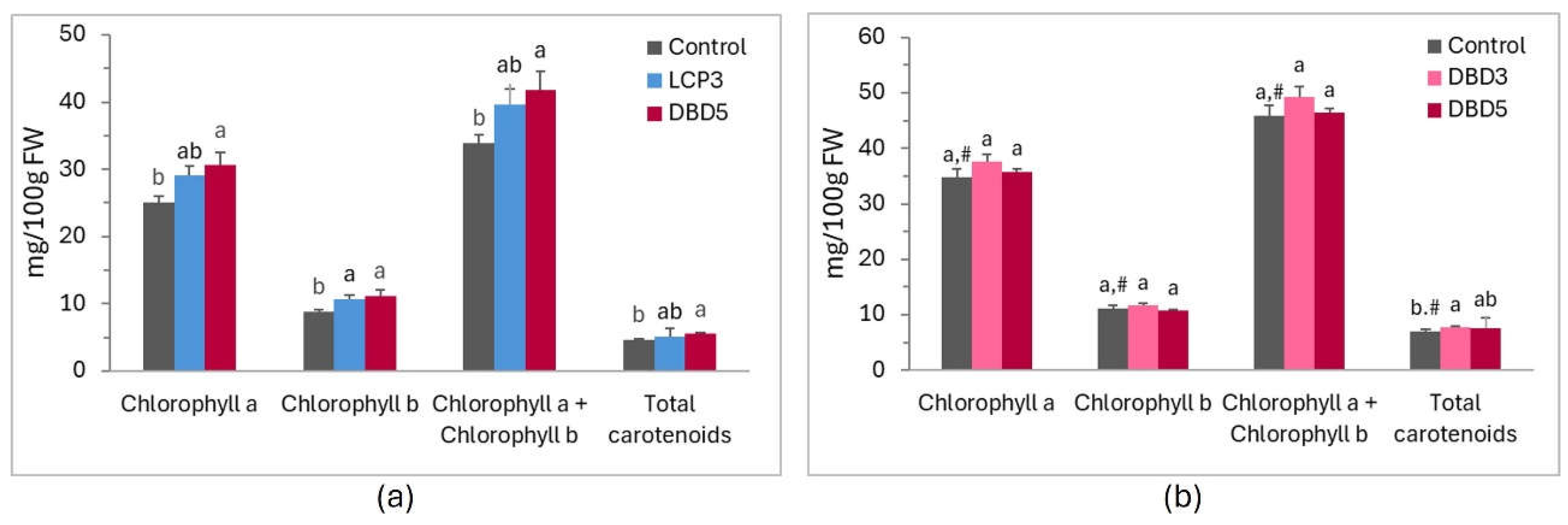
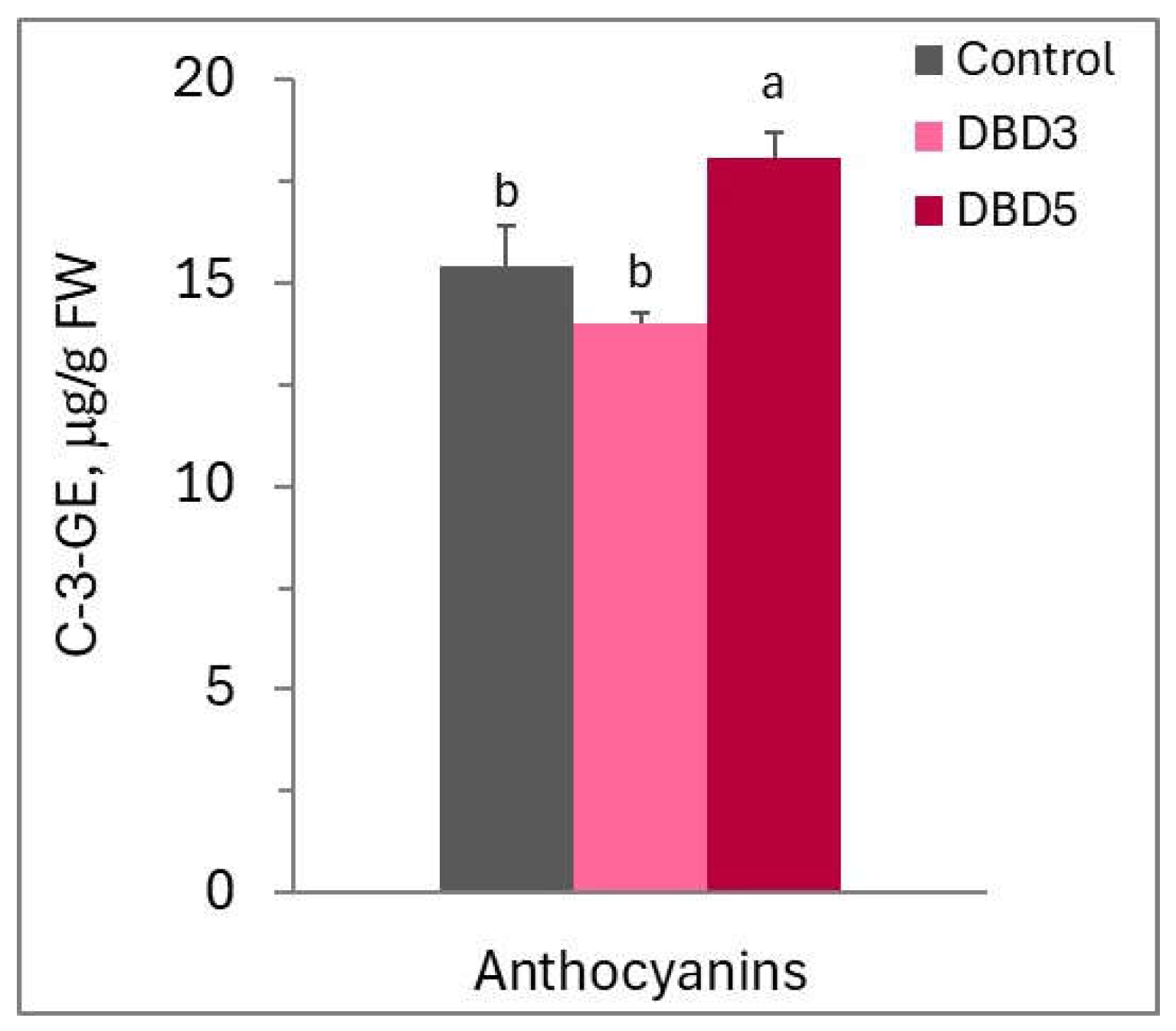
2.7. Analysis of Leaf Biochemical Parameters
2.8. Statistical Analysis
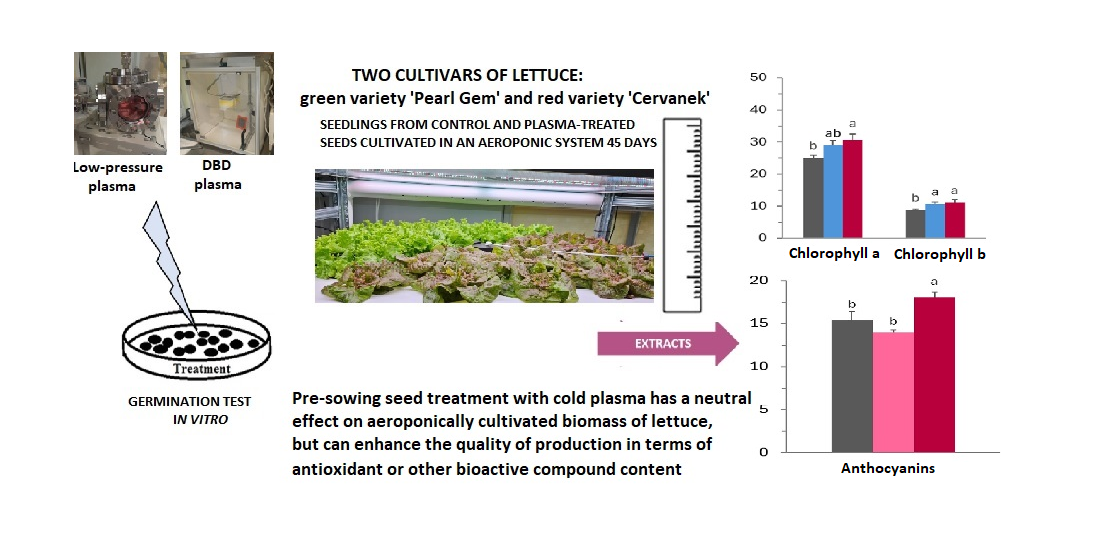
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
Abbreviations
| CP | Cold plasma |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| C-3-GE | Cyanidin-3-glucoside equivalent |
| DBD | Dielectric plasma discharge |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl free radical |
| GAE | Galic acid equivalent |
| FW | Fresh weight |
| LCP | Low-pressure cold plasma |
| LSD | Fisher’s least significant difference |
| Me | Median germination time |
| PAW | Plasma-activated water |
| Qu | Quartile deviation |
| RUE | Rutin equivalent |
| TPC | Total phenolic content |
| Vi | Final germination percentage |
References
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture? A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef]
- Wheaton, E.; Kulshreshtha, S. Environmental Sustainability of Agriculture Stressed by Changing Extremes of Drought and Excess Moisture: A Conceptual Review. Sustainability 2017, 9, 970. [Google Scholar] [CrossRef]
- Siegel, K.R.; Ali, M.K.; Srinivasiah, A.; Nugent, R.A.; Narayan, K.M.V. Do We Produce Enough Fruits and Vegetables to Meet Global Health Need? PLoS ONE 2014, 9, e104059. [Google Scholar] [CrossRef]
- Keatinge, J.D.H.; Yang, RY.; Hughes, J.; Easdown, W.J.; Holmer, R. The importance of vegetables in ensuring both food and nutritional security in attainment of the Millennium Development Goals. Food Sec. 2011, 3, 491–501. [Google Scholar] [CrossRef]
- McGreevy, S.R.; Rupprecht, C.D.D.; Niles, D.; Wiek , A.; Carolan, M.; Kallis , G.; Kantamaturapoj , K.; Mangnus, A.; Jehlička, P.; Taherzadeh, O.; Sahakian, M.; Chabay , I.; Colby, A.; Vivero-Pol, J.-L.; Chaudhuri , R.; Spiegelberg, M.; Kobayashi, M.; Balázs, B.; Tsuchiya, K.; Nicholls, C.; Tanaka, K.; Vervoort, J.; Akitsu, M.; Mallee, H.; Ota, K.; Shinkai, R.; Khadse, A.; Tamura, N.; Abe, K.; Altieri, M.; Sato, Y.-I.; Tachikawa, M. Sustainable agrifood systems for a post-growth world. Nat Sustain 2022, 5, 1011–1017. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future Food-Production Systems: Vertical Farming and Controlled-Environment Agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Kumar, T.V.; Verma, R.A. Comprehensive Review on Soilless Cultivation for Sustainable Agriculture. J. Exp. Agric. Int. 2024, 46, 193–207. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A Review on Hydroponics and the Technologies Associated for Medium- and Small-Scale Operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.M.; Syed, T.N.; Chandio, F.A.; Tunio, M.H.; Ahmad, F.; Solangi, K.A. Overview of the Aeroponic Agriculture—An Emerging Technology for Global Food Security. Int. J. Agric. Biol. Eng. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Eldridge, B.M.; Manzoni, L.R.; Graham, C.A.; Rodgers, B.; Farmer, J.R.; Dodd, A.N. Getting to the Roots of Aeroponic Indoor Farming. New Phytol. 2020, 228, 1183–1192. [Google Scholar] [CrossRef]
- Mangaiyarkarasi, R. Aeroponics System for Production of Horticultural Crops. Madras Agric. J. 2020, 107, 1–7. [Google Scholar] [CrossRef]
- Gurley, T.W. Aeroponics; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive compounds in lettuce: highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr Rev Food Sci Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Q.; Wang, Y. Non-Destructive Inspection of Physicochemical Indicators of Lettuce at Rosette Stage Based on Visible/Near-Infrared Spectroscopy. Foods 2024, 13, 1863. [Google Scholar] [CrossRef] [PubMed]
- Popsimonova, G.; Agic, R.; Bogevska, Z.; Davitkovska Jankulovska, M. Lettuce (Lactuca sativa L.) - the neglected vegetable in the Macedonian production and trade. J. Agricult. Food Environ. Sci. 2022, 76, 56–62. [Google Scholar]
- Jie, H.; Kong, L.S. Growth and photosynthetic characteristics of lettuce (Lactuca sativa L.) under fluctuating hot ambient temperatures with the manipulation of cool root-zone temperature. J. Plant Physiol. 1998, 52, 387–391. [Google Scholar] [CrossRef]
- Kacjan-Maršić, N.; Osvald, J. Nitrate content in lettuce (Lactuca sativa L.) grown on aeroponics with different quantities of nitrogen in the nutrient solution. Acta Agronomica Hungarica 2002, 50, 389–397. [Google Scholar] [CrossRef]
- Tan, L.P.; He, J.; Lee, S.K. Effects of Root-Zone Temperature on the Root Development and Nutrient Uptake of Lactuca sativa L “Panama” Grown in an Aeroponic System in the Tropics. J. Plant Nutrition 2002, 25, 297–314. [Google Scholar] [CrossRef]
- He, J.; Austin, P.T.; Lee, S.K. Effects of elevated root zone carbon dioxide and air temperature on photosynthetic gas exchange, nitrate uptake, and total reduced nitrogen content in aeroponically grown lettuce plants. J. Exp. Bot. 2010, 61, 3959–3969. [Google Scholar] [CrossRef]
- Luo, H.Y.; He, J.; Lee, S.K. Interaction between Potassium concentration and Root-Zone Temperature on Growth and Photosynthesis of Temperate Lettuce Grown in the Tropics. J. Plant Nutr. 2012, 35, 1004–1021. [Google Scholar] [CrossRef]
- Albornoz, F.; Lieth, J.H.; González-Fuentes, J.A. Effect of different day and night nutrient solution concentrations on growth, photosynthesis, and leaf NO3-content of aeroponically grown lettuce. Chil. J. Agric. Res. 2014, 74, 240–245. [Google Scholar] [CrossRef]
- Hikosaka, Y.; Kanechi, M.; Sato, M.; Uno, Y. Dry-fog aeroponics affects the root growth of leaf lettuce (Lactuca sativa L. cv. Greenspan) by changing the flow rate of spray fertigation. Environ. Control Biol. 2015, 53, 181–187. [Google Scholar] [CrossRef]
- Mohamed, T.M.K.; Gao, J.M.; Tunio, M. Development and experiment of the intelligent control system for rhizosphere temperature of aeroponic lettuce via the Internet of Things. Int. J. Agric. Biol. Eng. 2022, 15, 225–233. [Google Scholar]
- Puccinelli, M.; De Padova, A.; Vernieri, P.; Carmassi, G.; Incrocci, L. Response of Aeroponically Cultivated Baby-Leaf Lettuce (Lactuca sativa L. ) Plants with Different Zinc, Copper, Iodine, and Selenium Concentrations. Horticulturae 2024, 10, 726. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef]
- Misra, N.; Schlutter, O.; Cullen, P. Plasma in Food and Agriculture. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Misra, N.N., Schlutter, O., Cullen, P.J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–16. [Google Scholar]
- Bilea, F.; Garcia-Vaquero, M.; Magureanu, M.; Mihaila, I.; Mildažienė, V.; Mozetič, M.; Pawlat, J.; Primc, G.; Puač, N.; Robert, E.; Stancampiano, A.; Topala, I.; Žūkienė, R. Non-Thermal Plasma as Environmentally-Friendly Technology for Agriculture: A Review and Roadmap. Crit. Rev. Plant Sci. 2024, 42, 428–486. [Google Scholar] [CrossRef]
- Ishikawa, K.; Koga, K.; Ohno, N. Plasma-Driven Sciences: Exploring Complex Interactions at Plasma Boundaries. Plasma 2024, 7, 160–177. [Google Scholar] [CrossRef]
- Pánka, D.; Jeske, M.; Łukanowski, A.; Baturo-Ciésniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.D.D.; Szwartc, K. Can Cold Plasma Be Used for Boosting Plant Growth and Plant Protection in Sustainable Plant Production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Naucienė, Z.; Malakauskiene, A.; Zukiene, R.; Januskaitiene, I.; Jakstas, V.; Ivanauskas, L.; Filatova, I.; Lyushkevich, V. Pre-Sowing Seed Treatment with Cold Plasma and Electromagnetic Field Increases Secondary Metabolite Content in Purple Coneflower (Echinacea purpurea) Leaves. Plasma Process. Polym. 2018, 15, 1700059. [Google Scholar] [CrossRef]
- Mildaziene, V.; Paužaitė, G.; Naučienė, Z.; Zukiene, R.; Malakauskienė, A.; Norkeviciene, E.; Slepetiene, A.; Stukonis, V.; Olšauskaite, V.; Padarauskas, A.; Filatova, I.; Lyushkevich, V. Effect of Seed Treatment with Cold Plasma and Electromagnetic Field on Red Clover Germination, Growth and Content of Major Isoflavones. J. Phys. D Appl. Phys. 2020, 53, 264001. [Google Scholar] [CrossRef]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.O.; Ardebili, Z. Seed Priming with Cold Plasma Improved Seedling Performance, Secondary Metabolism, and Expression of Deacetylvindoline O-Acetyltransferase Gene in Catharanthus roseus. Contrib. Plasma Phys. 2020, 60, e201900159. [Google Scholar] [CrossRef]
- Judickaitė, A.; Lyushkevich, V.; Filatova, I.; Mildažienė, V.; Žūkienė. The Potential of Cold Plasma and Electromagnetic Field as Stimulators of Natural Sweeteners Biosynthesis in Stevia rebaudiana Bertoni. Plants 2022, 11, 611. [Google Scholar] [CrossRef]
- Lyu, X.; Chen, Y.; Gao, S.; Cao, W.; Fan, D.; Duan, Z.; Xia, Z. Metabolomic and transcriptomic analysis of cold plasma promoting biosynthesis of active substances in broccoli sprouts. Phytochem. Anal. 2023, 34, 925–937. [Google Scholar] [CrossRef]
- Ivankov, A.; Naučienė, Z.; Degutytė-Fomins, L.; Žūkienė, R.; Januškaitienė, I.; Malakauskienė, A.; Jakštas, V.; Ivanauskas, L.; Romanovskaja, D.; Šlepetiene, A.; Filatova, I.; Lyushkevich, V.; Mildažienė, V. Changes in Agricultural Performance of Common Buckwheat Induced by Seed Treatment with Cold Plasma and Electromagnetic Field. Appl. Sci. 2021, 11, 4391. [Google Scholar] [CrossRef]
- Čėsniene, I.; Čėsna, V.; Miškelytė, D.; Novickij, V.; Mildažienė, V.; Sirgedaitė-Šežienė, V. Seed Treatment with Cold Plasma and Electromagnetic Field: Changes in Antioxidant Capacity of Seedlings in Different Picea abies (L.) H. Karst Half-Sib Families. Plants 2024, 13, 2021. [Google Scholar] [CrossRef]
- Asefi, N.; Singh, R.K. The Impact of Cold Plasma and Plasma-Activated Water on Germination of Grains and Legumes for Enhanced Nutritional Value. Curr. Nutr. Rep. 2025, 14, 57. [Google Scholar] [CrossRef]
- Okumura, T.; Attri, P.; Kamataki, K.; Yamashita, N.; Tsukada, Y.; Itagaki, N.; Shiratani, M.; Ishibashi, Y.; Kuchitsu, K.; Koga, K. Detection of NO3− introduced in plasma-irradiated dry lettuce seeds using liquid chromatography-electrospray ionization quantum mass spectrometry (LC-ESI QMS). Sci. Rep. 2022, 12, 12525. [Google Scholar] [CrossRef]
- Okumura, T.; Anan, T.; Shi, H.; Attri, P.; Kamataki, K.; Yamashita, N.; Itagaki, N.; Shiratani, M.; Ishibashi, Y.; Koga, K.; Mildažienė, V. Response of lettuce seeds undergoing dormancy break and early senescence to plasma irradiation. Appl. Phys. Express 2024, 17, 057001. [Google Scholar] [CrossRef]
- Ivankov, A.; Naučienė, Z.; Žūkienė, R.; Degutytė-Fomins, L.; Malakauskienė, A.; Kraujalis, P.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V.; Mildažienė, V. Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field. Appl. Sci. 2020, 10, 8519. [Google Scholar] [CrossRef]
- Judickaitė, A.; Jankaitytė, E.; Ramanciuškas, E.; Degutytė-Fomins, L.; Naučienė, Z.; Kudirka, G.; Okumura, T.; Koga, K.; Shiratani, M.; Mildažienė, V.; Žūkienė, R. Effects of Seed Processing with Cold Plasma on Growth and Biochemical Traits of Stevia rebaudiana Bertoni Under Different Cultivation Conditions: In Soil Versus Aeroponics. Plants 2025, 14, 271. [Google Scholar] [CrossRef]
- Judickaitė, A.; Venckus, J.; Koga, K.; Shiratani, M.; Mildažienė, V.; Žūkienė, R. Cold Plasma-Induced Changes in Stevia rebaudiana Morphometric and Biochemical Parameter Correlations. Plants 2023, 12, 1585. [Google Scholar] [CrossRef] [PubMed]
- Richards, F.J. A Flexible Growth Function for Empirical Use. J. Exp. Bot. 1959, 10, 290–300. [Google Scholar] [CrossRef]
- Hara, Y. Calculation of Population Parameters Using Richards Function and Application of Indices of Growth and Seed Vigor to Rice Plants. Plant Prod. Sci. 1999, 2, 129–135. [Google Scholar] [CrossRef]
- Blinstrubienė, A.; Burbulis, N.; Juškevičiūtė, N.; Vaitkevičienė, N.; Žūkienė, R. Effect of Growth Regulators on Stevia rebaudiana Bertoni Callus Genesis and Influence of Auxin and Proline to Steviol Glycosides, Phenols, Flavonoids Accumulation, and Antioxidant Activity in Vitro. Molecules 2020, 25, 2759. [Google Scholar] [CrossRef]
- Perez-Lopez, U.; Pinzino, C.; Quartacci, M.F.; Ranieri, A.; Sgherri, C. Phenolic composition and related antioxidant properties in differently colored lettuces: A study by electron paramagnetic resonance (EPR) kinetics. J. Agric. Food Chem. 2014, 62, 12001–12007. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3–1. [Google Scholar] [CrossRef]
- Veerana, M.; Ketya, W.; Choi, E.-H.; Park, G. Non-thermal plasma enhances growth and salinity tolerance of bok choy (Brassica rapa subsp. chinensis) in hydroponic culture. Front. Plant Sci. 2024, 15, 1445791. [Google Scholar] [CrossRef]
- Lukacova, Z.; Svubova, R.; Selvekova, P.; Hensel, K. The Effect of Plasma Activated Water on Maize (Zea mays L.) under Arsenic Stress. Plants 2021, 10, 1899. [Google Scholar] [CrossRef]
- Ruamrungsri, S.; Sawangrat, C.; Panjama, K.; Sojithamporn, P.; Jaipinta, S.; Srisuwan, W.; Intanoo, M.; Inkham, C.; Thanapornpoonpong, S.-N. Effects of Using Plasma-Activated Water as a Nitrate Source on the Growth and Nutritional Quality of Hydroponically Grown Green Oak Lettuces. Horticulturae 2023, 9, 248. [Google Scholar] [CrossRef]
- Gao, H.; Wang, G.; Huang, Z.; Nie, L.; Liu, D.; Lu, X.; He, G.; Ostrikov, K.K. Plasma-Activated Mist: Continuous-Flow, Scalable Nitrogen Fixation, and Aeroponics. CS Sustain. Chem. Eng. 2023, 11, 4420–4429. [Google Scholar] [CrossRef]
- Lakshmikanthan, M.; Muthu, S.; Krishnan, K.; Altemimi, A.B.; Haider, N.N.; Govindan, L.; Selvakumari, J.; Alkanan, Z.T.; Cacciola, F.; Francis, Y.M. A comprehensive review on anthocyanin-rich foods: Insights into extraction, medicinal potential, and sustainable applications. J. Agric. Food Res. 2024, 17, 101245. [Google Scholar] [CrossRef]

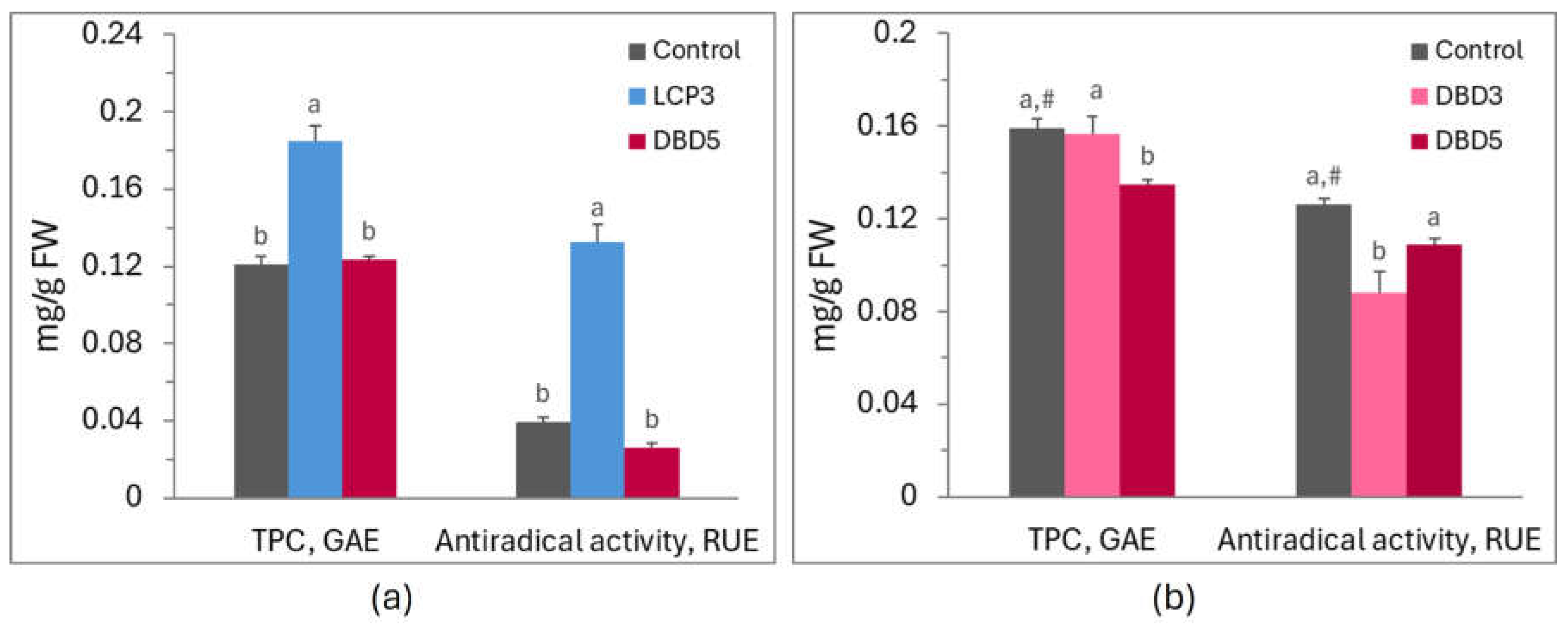
| Cultivar | Indice | Control | LCP3 | LCP5 | DBD3 | DBD5 |
|---|---|---|---|---|---|---|
| ‘Pearl Gem’ | Vi, % | 99.3±0.7 | 99.3±0.7 | 100.0±0.0 | 100.0±0.0 | 100.0±1.0 |
| Me, h | 19.6±0.2a | 19.2±0.1b | 19.3±0.1b | 19.6±0.1a | 19.7±0.1a | |
| Qu, h | 0.6±0.0a | 0.6±0.1a | 0.6±0.0a | 0.5±0.0a | 0.6±0.0a | |
| ‘Cervanek’ | Vi, % | 100.0±0.0a | 100.0±0.0a | 100.0±0.0a | 100.0±0.1a | 100.0±0.1a |
| Me, h | 19.0±0.1a,b,# | 18.9±0.0b | 18.7±0.1c | 19.0±0.0a | 19.1±0.0a | |
| Qu, h | 0.5±0.0a | 0.5±0.0a | 0.5±0.0a | 0.5±0.0a | 0.5±0.1a |
| Cultivar | Parameter | Control | LCP3 | LCP5 | DBD3 | DBD5 |
|---|---|---|---|---|---|---|
| ‘Pearl Gem’ | Seedling length, mm | 93.3.±1.7b | 105.0±1.7a | 101.0±1.7a | 89.9±1.6c | 102.8±1.3a |
| Root length, mm | 62.6±1.5b | 73.7±1.5a | 70.7±1.5a | 58.5±1.4c | 72.2±1.8a | |
| Shoot length, mm | 31.4±0.5a | 31.3±0.5a | 30.1±0.4a | 31.5±0.3a | 30.6±1.0a | |
| Seedling weight, mg | 17.6±0.4b | 19.4±0.2a | 18.8±0.6a,b | 18.4±0.4a,b | 19.2±0.2a | |
| ‘Cervanek’ | Seedling length, mm | 78.0±1.9a# | 78.8±1.7a | 82.2±1.9a | 80.2±3.2a | 80.0±1.2a |
| Root length, mm | 56.6±1.9b# | 58.9±1.9a,b | 65.3±1.8a | 56.8±3.2b | 56.8±1.3b | |
| Shoot length, mm | 21.5±0.3b# | 19.8±0.3c | 19.9±0.2c | 23.4±0.3a | 23.2±0.4a | |
| Seedling weight, mg | 19.2±0.4b | 19.1±0.3b | 19.2±0.7b | 19.9±0.2a | 20.7±0.3a |
| Cultivar | ‘Pearl Gem’ | ‘Cervanek’ | ||||
|---|---|---|---|---|---|---|
| Parameter | Control | LCP3 | DBD5 | Control | DBD3 | DBD5 |
| Plant length,cm | 52.1±2.3 | 47.3±2.7 | 51.4±2.6 | 49.8±1.6 | 52.8±1.4 | 49.5±0.4 |
| Root length, cm | 37.3±2.8 | 34.1±2.7 | 37.8±2.5 | 37.8±1.8 | 40.6±1.4 | 37.6±0.8 |
| Head length, cm | 13.3±1.2 | 13.1±0.5 | 13.1±0.4 | 11.1±0.7 | 10.6±1.4 | 11.9±0.5 |
| Head width, cm | 26.5±1.7 | 24.7±1.0 | 26.5±1.7 | 23.6±1.0 | 25.7±0.9 | 24.4±0.7 |
| Plant weight, g | 40.3±4.6 | 36.2±5.3 | 36.3±3.6 | 29.0±3.7# | 29.1±2.3 | 28.3±2.8 |
| Root weight, g | 6.6±0.7 | 6.1±0.7 | 6.1±0.5 | 4.4±0.5# | 4.2±0.4 | 4.4±0.5 |
| Head weight, g | 33.8±4.0 | 30.1±4.7 | 30.3±3.2 | 24.7±3.3# | 24.9±2.0 | 23.9±2.4 |
| Leaf number | 9.5±0.7 | 9.7±0.6 | 9.6±0.3 | 16.3±0.7# | 15.8±0.4 | 15.8±0.8 |
| Cultivar | ‘Pearl Gem’ | ‘Cervanek’ | ||||
|---|---|---|---|---|---|---|
| Indice | Control | LCP3 | DBD3 | Control | DBD3 | DBD5 |
| Fv/Fm | 0.86±0.01a | 0.85±0.01a | 0.86± 0.00a | 0.84± 0.01a,# | 0.85± 0.00a | 0.84± 0.00a |
| PIABS | 2.38±0.17b | 3.04±0.33a | 3.26 ± 0.28a | 1.21±0.16b# | 1.90 ± 0.16a | 1.76 ± 0.09a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





