1. Introduction
Being a pathological hallmark of chronic tissue injury, organ fibrosis is driven by the excessive deposition of extracellular matrix (ECM) components, which results in progressive architectural distortion, functional impairment, and eventual organ failure [1-3]. Accurate staging and biomarker discovery play a crucial role in guiding therapeutic strategies [4-7], while conventional diagnostic approaches, including biopsy-based histopathology and immunofluorescence staining for renal and pulmonary fibrosis, remain constrained by technical variability (e.g., stain consistency, subjective interpretation) and limited sensitivity to early fibrotic changes or dynamic ECM remodeling during the process of treatment [4,7-12]. Therefore, alternative and more informative imaging modalities could be utilized to facilitate accurate and rapid assessment of therapeutic interventions in fibrosis.
In response, nonlinear optical imaging modalities, including Second Harmonic Generation (SHG) and Two-Photon Excitation Fluorescence (TPEF), have emerged as transformative tools for label-free and high-resolution visualization of ECM dynamics [13-15]. Certain studies have identified that label-free SHG imaging provides greater sensitivity in detecting fibrillar collagen deposition within organ fibrosis compared to conventional histological staging [
16,
17].According to James et al., microscopy effectively characterizes altered collagen architecture in 3D idiopathic pulmonary fibrosis spheroid models under crosslinking modulation, while Lee et al. revealed that aged lungs exhibit heightened interstitial collagen density, reduced alveolar expansion, and diminished surfactant secretion through SHG imaging, which collectively underscored SHG's utility in profiling fibrotic pathologies [
18,
19]. Moreover, TPEF employs specific light to deeply excite fluorophores in the tissue, which not only minimizes autofluorescence (AF) but also enhances the detection of endogenous or exogenous markers associated with fibrosis [
13,
16].
Innovative approaches for early detection have been demonstrated by the recent advancements in organ fibrosis research. Notably, the integration of molecular probes with two-photon excitation microscopy has already become a promising strategy, leveraging probe-bound collagen deposition to generate detectable fluorescence signals indicative of incipient fibrotic changes. Fan et al. developed an MAO-B-activated two-photon probe (BiPhAA) for rapid (<10 min) hepatic stellate cell imaging [
20], while Zhou designed a PET-based NO probe (PYSNO) with nanomolar sensitivity to track myocardial fibrosis [
21], both of which risk perturbing native collagen architecture. However, despite these technological breakthroughs, the direct utilization of collagen's AF for early fibrosis detection is still substantially underexplored. In fibrotic tissues, regions of pathological interest are sparsely distributed compared to adjacent normal areas, which leads to attenuated or absent imaging signals and consequent false-negative diagnoses in clinical assessments [
22].
In contrast to the well-characterized role of collagen, elastin is understudied despite its emerging diagnostic relevance. Recent advances demonstrate that elastin quantification promotes reproducible assessment of renal fibrosis progression and longitudinal evaluation of antifibrotic therapeutic efficacy [23-25]. However, persistent technical limitations are faced by the integration of elastin analysis with nonlinear optical imaging modalities. Firstly, the lower physiological abundance of elastin compared to collagen renders its intrinsic contrast often insufficient for label-free histopathological imaging [
24]. Secondly, there is a dearth of studies investigating whether elastin can be used as a biomarker for various stages or types of fibrosis. Although higher-order harmonic generation (SHG/THG) is wavelength-independent in principle, efficient excitation requires specific wavelength ranges. Below 800 nm, the emission spectrum of collagen simultaneously encompasses both SHG peaks and two-photon emission signals [
26]. While its intensity decreases as the excitation wavelength increases, this two-photon fluorescence can interfere with the accurate detection of collagen. The collagen no longer generates two-photon fluorescence above 820 nm, and excitation >820 nm is required to localize the SHG signal from collagen [
27,
28]. However, elastin, a vital component for detection, demonstrates an optimal excitation range of 780-800 nm, encountering signal attenuation at longer wavelengths. Therefore, 820 nm balances collagen SHG specificity and residual elastin detectability.
However, the implementation of nonlinear imaging critically depends on high-performance lasers, which provide intense and tunable light sources essential for exciting nonlinear optical effects [
29]. Due to the advancing tunable ultrafast optical technology, the generation of the required ultrafast imaging light source is enabled. Typically, the nonlinear phase modulation can be employed to realize a nonlinear frequency shift, followed by frequency doubling to provide a tunable ultrafast light source for collagen fiber and elastin imaging [30-34]. By carefully tuning the laser parameters, the corresponding imaging sensitivity of the SHG can be enhanced and therefore ensure imaging accuracy [
34]. The imaging system ultrafast lasers are used to concurrently map collagen fibrils through 780 nm AF and visualize fibrillar organization via 820 nm SHG microscopy at submicron resolution. This integrated approach enables precise collagen localization (early stage) while overcoming SHG signal attenuation, which hence ensures robust detection across heterogeneous tissue structures.
These findings demonstrate the clinical utility of dual-wavelength SHG imaging in fibrosis diagnostics. The 780-nm system enables rapid intraoperative collagen visualization, effectively reducing diagnostic uncertainties in fibrosis when combined with conventional histology. In parallel, the 820-nm imaging modality achieves nanoscale resolution in quantifying collagen ultrastructural features (e.g., fibril orientation, cross-linking density), thereby refining microenvironmental analysis in chronic fibrotic diseases. Together, these complementary methodologies establish a unified diagnostic framework that integrates clinical efficiency with research-grade precision, advancing both pathological practice through real-time decision support and matrix biology studies via mechanistic insights into ECM remodeling dynamics.
2. Materials and Methods
2.1. The EVG (Elastica Van Gieson) Staining
All animal experiments were performed in strict compliance with institutional animal care guidelines and approved by the Biomedical Ethics Committee at Sun Yat-sen University (Guangzhou, China).
The Elastica van Gieson (EVG) staining technique serves as a histochemical method specifically designed to visualize elastic and collagen fibers in tissue sections under microscopy. Concerning histopathological evaluation of fibrosis in mouse models—unilateral ureteral obstruction (UUO), folic acid-induced fibrosis, and bleomycin-induced lung fibrosis—EVG staining was performed as follows: In the UUO model, male C57BL/6 mice were anesthetized and subjected to left ureter ligation, with tissue harvested at 5 and 7 days post-surgery. For the folic acid model, mice received intraperitoneal injections of folic acid (250 mg kg-1 in 0.3 M NaHCO3) and kidneys were collected at 14 days. Moreover, in the bleomycin-induced lung fibrosis model, mice were injected intraperitoneally with bleomycin (500 ng ml-1), and lung tissues were harvested at 28 days.
The tissues were fixed in 4% neutral buffered formalin, dehydrated through an ethanol gradient, cleared in xylene, and embedded within paraffin. Sections (5-8 μm) were cut through employing a microtome, mounted on slides, and then dewaxed. After rehydration, elastin fibers were oxidized with potassium permanganate (KMnO4) and neutralized with oxalic acid. Staining with the reagent (picric acid and fuchsin) of Van Gieson yielded black/dark brown elastin fibers and pink/red collagen fibers, which enabled clear differentiation of extracellular matrix components.
2.2. Identify Regions of Interest by Grid-Targeted Method
Multiphoton microscopy (MPM) imaging of a 5-mm² tissue area (6 µm thickness) requires 1-2 hours, which underscores the necessity of pre-identifying regions of interest (ROIs). In systems lacking brightfield imaging, specimen localization is indispensable for providing morphological context, guiding ROI navigation, and allowing spatial registration of fluorescent signals. Due to the absence of brightfield references, a precise correlation between fluorescent markers and tissue architecture is complicated, especially in specimens with sparse or heterogeneous fluorophore distribution [
35]. Accurate spatial positioning ensures signal specificity validation, optimal z-plane selection, and multi-channel alignment in topographically complex tissues.
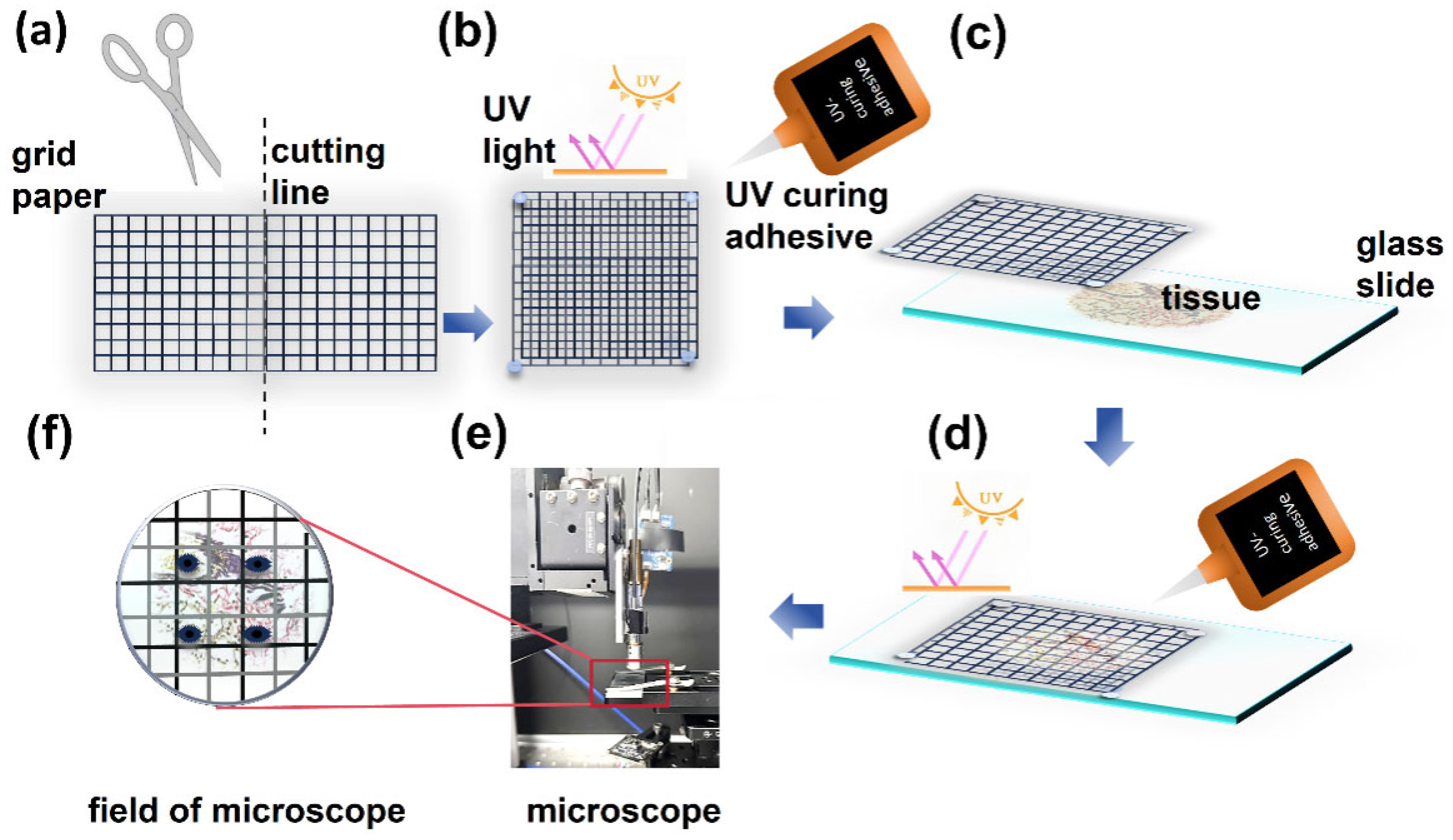
To precisely identify ROIs, a grid-targeted method was implemented. Transparent grid paper was carefully cut into two 1 cm × 1 cm square units (
Figure 1a), each comprising a detailed grid of 100 identical 1 mm × 1 mm subgrids. Through leveraging a specialized ultraviolet-curable adhesive, these units were staggered and bonded and created a composite grid with staggered subgrids of 0.5 mm × 0.5 mm (
Figure 1b). This configuration effectively reduced the observable field of view under optical microscopy and thus enhanced spatial resolution for ROI localization. To ensure stability during imaging, the assembled grid was securely attached beneath the sample slide (
Figure 1c,
Figure 1d). Under optical microscopy, the target position was initially approximated by identifying the corresponding subgrid. The adjacent location was then precisely marked using a fine-tip marker under an optical microscope for further experimental analysis (
Figure 1e).
Following pre-processing, precise alignment between the imaging area and marked ROI was achieved through the coordinated adjustment of the sample stage and imaging probe. Equipped with scan lenses and a Micro-Electromechanical Systems (MEMS) mirror, the probe allowed high-speed planar scanning. Focusing the excitation light onto the sample layer produced a uniform bright red spot on the CCD monitor. The fine positional adjustments of the stage centered this spot within the grid reference markers, ensuring sub-10 μm spatial concordance between the scanning field and fibrotic ROIs. As validated in
Figure 2, this calibration protocol established reproducible targeting accuracy and thereby allowed efficient MPM and quantitative analysis of pathological regions.
2.3. Preparation of Collagen and Elastin Fiber Samples
To assess the accuracy and sensitivity of our imaging system, samples of collagen fibers and elastin fibers for signal detection in both the AF and SHG channels were prepared. Collagen (C8062, Solarbio) was first analyzed to remove salt and then freeze-dried by freeze drier (Venus 2.5L/-90℃, Furuijie), which was subsequently dissolved in 10 mM phosphate-buffered saline at pH 7.4 to a working concentration of 0.01%. Through leveraging a sodium hydroxide solution, the pH of the solution was adjusted to 7. The prepared solution was then coated onto a glass slide and allowed to dry at room temperature (approximately 20°C) for several hours (overnight) until a dry and coated surface was obtained. Once fully dry, a lid was placed over the sample and the gap between the lid and the glass slide was sealed with clear nail polish.
To prepare a 10 mg mL-1 solution, the purchased elastin powder (E920946, Mecklin) was weighed and dissolved in 10 mM phosphate-buffered saline at pH 7.4. This solution was then subjected to heat treatment at 95℃ for ten minutes. The prepared solution was then coated onto a glass slide and allowed to dry at room temperature (approximately 20°C) for several hours (overnight) until a dry and coated surface was obtained. Once fully dry, a lid was placed over the sample and the gap between the lid and the glass slide was sealed with clear nail polish to ensure no leakage or contamination.
3. Results
3.1. Imaging System Setup and Configuration
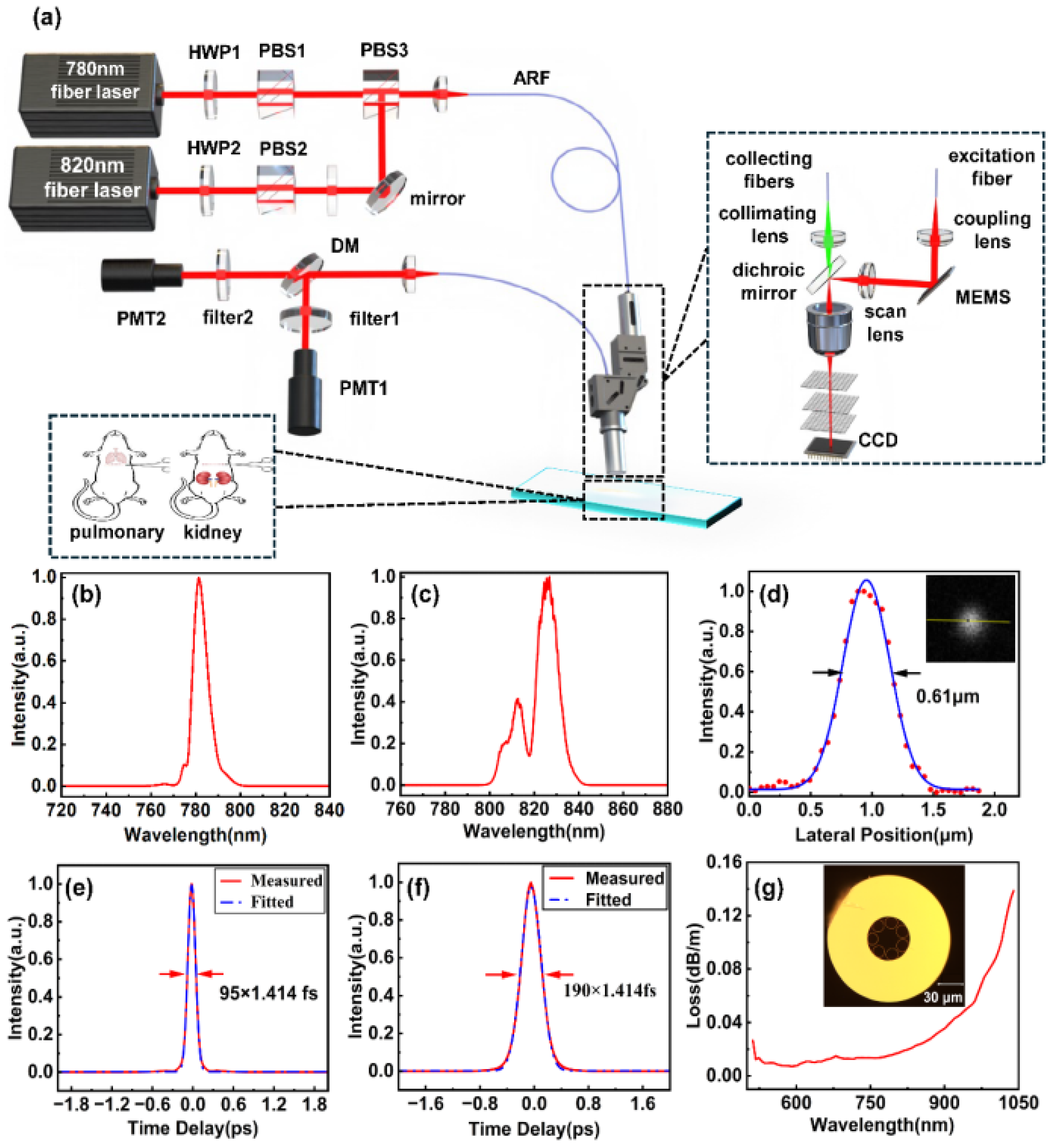
The schematic design of the dual-wavelength imaging system is illustrated in
Figure 2, comprising the fiber femtosecond light source, hollow anti-resonant fiber (ARF), imaging probe, and collection unit. The fiber femtosecond light source outputs two pulses, which are a 780 nm pulse with a 95 fs pulse width and an average power of 50 mW (
Figure 2 b,e), and an 820 nm pulse with a 190 fs pulse width and an average power of 50 mW (
Figure 2 c,f). Using a half-wave plate and a polarization beam splitter (PBS), these two beams are polarized and integrated. The collimated beam is coupled into the probe head via a 1.5-m ARF that has a core diameter of 160 μm and a transmission loss of 50 dB km-1 at both 780 nm and 820 nm.
Figure 2g shows the cross-sectional diagram of the ARF. Since this 1.5-m ARF does not introduce significant optical chirp into the femtosecond pulses, no additional dispersive elements were employed to manage optical dispersion. The waveplate and PBS combination, as shown in the
Figure 2, is utilized to regulate the injection power of the 780–820 nm femtosecond pulses.
The probe head encompasses a Micro-Electromechanical Systems (MEMS) scanner, a scan lens, a dichroic mirror, an objective, and a collecting lens, as shown in
Figure 1a. The MEMS scanner operates at 2400 Hz and yields a frame rate of 9 fps with a frame size of 150 μm × 150 μm. The excitation light is focused by a 9× microscope objective (NA = 0.9) with an average power of approximately 10 mW. When utilizing 820 nm as the excitation wavelength, the resolution, as measured with 100 nm fluorescent beads, is 610 nm (
Figure 1d). The generated harmonic signals are collected by the objective, filtered by a dichroic mirror, and delivered to photomultiplier tubes (PMTs) via a silica fiber. For different detection requirements, two sets of filters were placed before the PMTs. To rapidly locate and detect collagen and elastic fibers, a bandpass filter (F390/40, Jiushi Optics) was installed in the SHG channel, while a longpass filter (LP420, Jiushi Optics) was placed in the AF channel. For precise detection of collagen fibers, a customized ultra-narrow bandpass filter (F410/10, Jiushi Optics) was installed in the SHG channel, and a longpass filter (LP425, Jiushi Optics) was placed in the AF channel.
It is considered that the pre-experiments play a necessary role in ensuring that all three components are coaxial. As exhibited in
Figure 1, a quick localisation method involves the replacement of the original microscope objective and femtosecond laser source with a collimated semiconductor laser source coaxial with the optical axis of the objective. The collimated semiconductor laser projects a bright red spot onto the Charge Coupled Device (CCD) detection surface, which suggests the marked area in the grid (
Figure 1), and the sample grid position is recorded at this point. Along with the sample and grid, the sample stage is moved in 5-millimeter increments, the CCD images are recorded at each step, and these images are subsequently stacked. If the position of the red spot remains unchanged across this series of images, it can be reasonably concluded that the grid paper, sample, CCD, and microscope objective are coaxial. At this stage, the collimated laser source can be replaced with the microscope imaging probe, thus allowing the commencement of sample imaging. Since this study does not examine dynamic tissue changes or require high temporal resolution, multi-frame averaging was harnessed to reduce random noise. For each area, 40 frames were captured and averaged. The field of view size was 150 μm × 150 μm, in which the single frame acquisition time was approximately 4 seconds.
Figure 2.
The experimental setup. (a) The 780-nm and 820-nm femtosecond lasers, with optical power adjusted by a set of PBS and HWP, are coupled into the ARF through polarization beam combining. The miniaturized probe, scanned by a MEMS, performs two-photon and harmonic imaging on stained sections of the mouse lung and kidney tissue. Auto-fluorescence and second-harmonic signals are separated by a dichroic mirror and individually collected by the PMTs. (b,e) 780-nm femtosecond laser spectrum and auto-correlation trace. (c,f) 820-nm femtosecond laser spectrum and auto-correlation trace. (d) The lateral resolution of the 100-nm fluorescent microb
Figure 2.
The experimental setup. (a) The 780-nm and 820-nm femtosecond lasers, with optical power adjusted by a set of PBS and HWP, are coupled into the ARF through polarization beam combining. The miniaturized probe, scanned by a MEMS, performs two-photon and harmonic imaging on stained sections of the mouse lung and kidney tissue. Auto-fluorescence and second-harmonic signals are separated by a dichroic mirror and individually collected by the PMTs. (b,e) 780-nm femtosecond laser spectrum and auto-correlation trace. (c,f) 820-nm femtosecond laser spectrum and auto-correlation trace. (d) The lateral resolution of the 100-nm fluorescent microb
3.2. Label-Free Visualization Of Collagen And Elastin Fibers Utilizing Multiphoton Microscopy
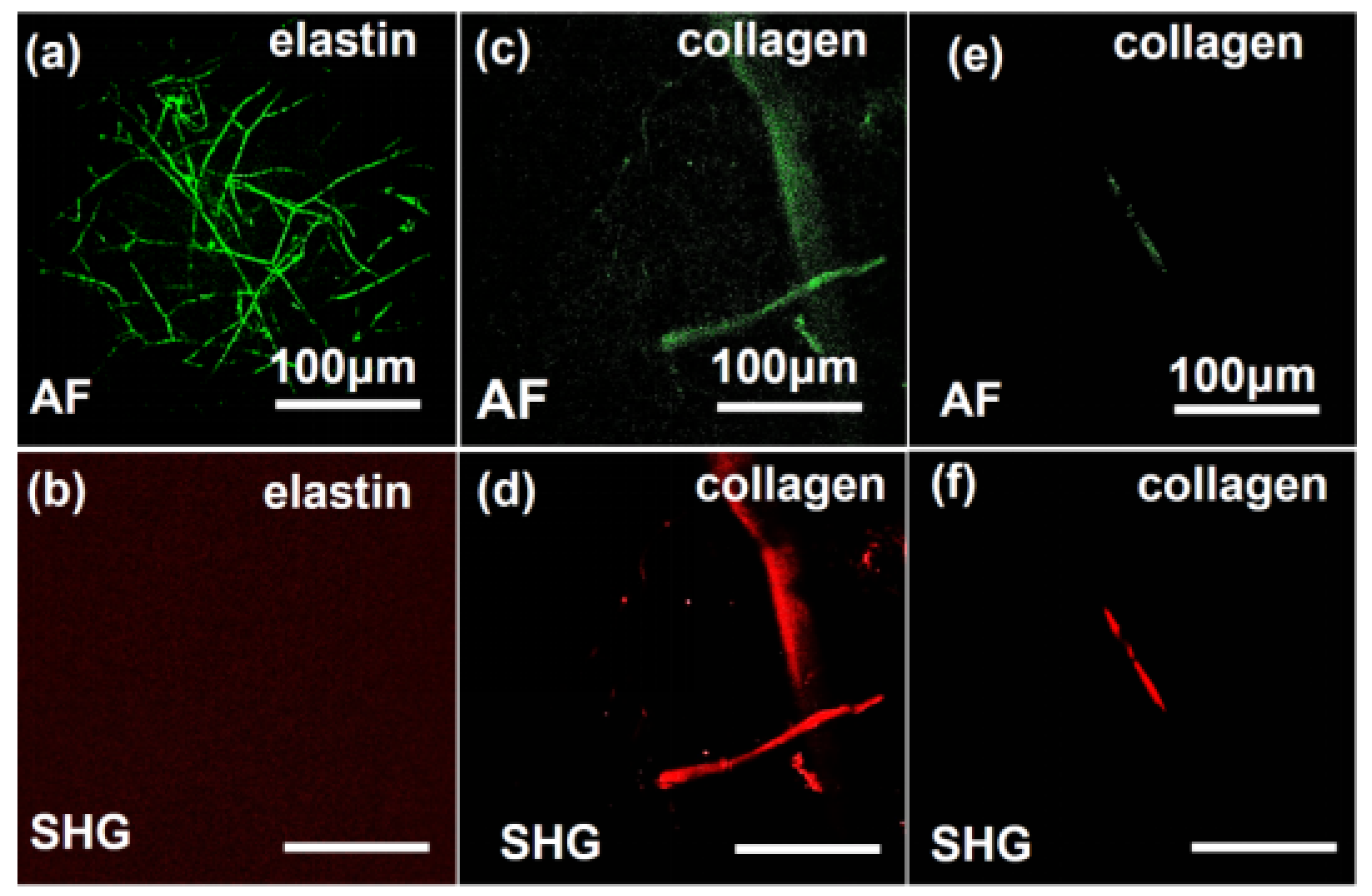
As illustrated in
Figure 3, MPM enables high-fidelity visualization of in situ elastin and collagen fiber architectures. Under 780 nm excitation (Figures 3a,b), elastin and collagen show AF signatures, while SHG signals are exclusively generated from collagen fibers, which verifies the collagen-specific nature of SHG activity. Conversely, collagen fibers maintain intense SHG emission with attenuated AF signal at 820 nm excitation. These wavelength-dependent contrast mechanisms—AF dominance at 780 nm for elastin and collagen profiling and SHG specificity at 820 nm for collagen mapping—enable label-free and artifact-resistant discrimination and quantitative spatiotemporal mapping of collagen/elastin networks.
3.3. Detection of Renal Fibrosis And Pulmonary Fibrosis Utilizing the MPM System
In this study, the MPM system was utilized to acquire high-resolution and label-free images of lung and renal fibrosis tissue sections, and the results were systematically compared with conventional histopathological EVG staining to validate both the accuracy and reliability of MPM-based ECM characterization. All imaging experiments were independently replicated 3 times under identical conditions. Representative images were selected based on consistency with quantitative analyses across biological/technical replicates. The EVG-stained pulmonary tissue sections in
Figure 4 and
Figure 5 reveal distinct histological profiles under light microscopy, which are collagen fibers (pink), elastin (dark purple), and myofibrillar components (yellow) [
36]. Collagen is predominantly localized near vascular structures and constitutes a significant component of alveolar walls, which forms a basement membrane with elastin essential for physiological alveolar expansion and retraction. In control tissue section (
Figure 4a), loosely organized collagen fibers (red arrow) exhibit robust AF signals at 780 nm and significantly exceed elastin-derived AF signals in alveolar walls and perivascular regions. However, SHG signals at 780 nm only partially overlap with EVG and AF profiles, which implies limited sensitivity for full collagen network characterization. In pathological specimens with pulmonary fibrosis section (
Figure 4b), dense fibrotic lesions (red arrow), disrupted alveolar architecture (dotted circle), and thickened septa are demonstrated. While collagen AF signals at 780 nm still play a dominant role in these regions, SHG signals again fail to completely align with EVG/AF markers, which further confirms suboptimal resolution at this wavelength.
At 820 nm (Figures 4c,d), collagen AF signals show a diminishing trend, which presents negligible contrast against alveolar structures, whereas weak AF signals persist in extravascular elastin (red pentagon). Notably, SHG imaging at 820 nm achieves near-complete spatial overlap with EVG/AF-defined collagen fibers, especially in perivascular regions with the dense aggregation of pathological collagen. These results demonstrate wavelength-dependent collagen detection. The 780-nm excitation wavelength enables precise AF-guided localization of collagen microarchitecture. At 820 nm, SHG achieves fiber-level specificity for resolving pathological fibrotic networks. Owing to this dual-wavelength approach, comprehensive visualization of collagen dynamics in pulmonary fibrosis sections is facilitated, balancing sensitivity (780 nm AF) and precision (820 nm SHG) for advanced pathological analysis.
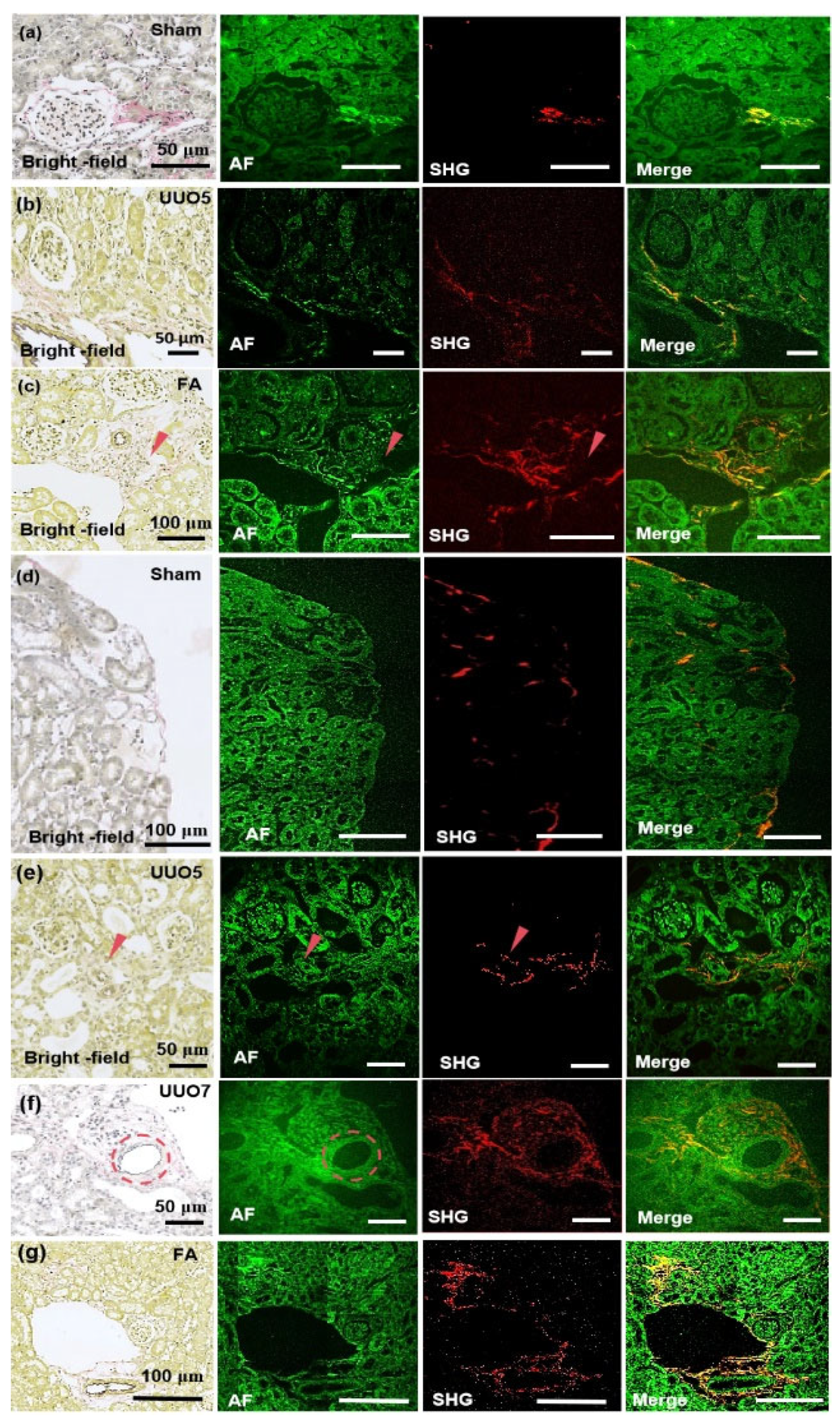
Figure 5a presents the imaging of renal tissue section from the sham-operation group at 780 nm. The glomeruli exhibit normal size, in which minimal collagen fibers are presented at the glomerular capsule, and the renal tubules appear normal. The AF signal at the collagen site is particularly intense under 780-nm excitation, which correlates well with SHG and EVG staining (pink). In
Figure 5b, the imaging of renal fibrosis tissue section from the unilateral ureteral obstruction (UUO) 5-day group under 780nm excitation is illustrated. The glomeruli are markedly atrophied, the renal tubules demonstrate slight dilation and edema, and interstitial collagen is evident from EVG staining (pink) and SHG. In addition, a small amount of collagen is significantly detectable under 780nm excitation AF.
Figure 5c presents the imaging of renal fibrosis tissue section induced by folic acid at 780. Folic acid induction reveals atrophied renal tubules, glomeruli nearly filling the entire renal capsule, and increased interstitial collagen fibers beside the renal tubules, accompanied by diffuse collagen deposition. Under 780-nm excitation, the AF signal of collagen protein fibers is visible, which is more distinct than the collagen fibers represented by pink EVG staining, and its SHG signal is also detectable.
The imaging of renal tissue sections from the sham-operation group under 820-nm excitation is shown in
Figure 5d. A minimal amount of collagen is present at the cortical edge, the renal tubules are not dilated, and there are hardly any collagen fibers in the renal interstitium. These features can be detected by both SHG and AF.
Figure 5e presents the imaging of renal fibrosis tissue section from the UUO 5-day group at 820 nm. According to EVG staining, atrophied glomeruli and slightly dilated renal tubules exist in the UUO 5-day group. The collagen distribution is unidentifiable, due to the pink EVG staining at the red arrow is rather indistinct. The AF signal intensity at 820 nm is not particularly prominent and does not detect a strong fluorescence AF signal as observed with 780-nm excitation. However, a clear SHG signal is detectable and shows the distinct arrangement of collagen.
Figure 5f displays the imaging of renal fibrosis tissue section from the UUO 7-day group at 820 nm. Prominent sawtooth-shaped elastic fibers are observed around the blood vessels marked by the dashed circle, with collagen protein fibers accumulating around the vessels and significant dilation of the renal tubules. In the AF channel, corresponding detection of sawtooth-shaped elastic fibers is possible and indicates the potential of this system to detect trace levels of elastic fibers. Moreover, SHG reaffirms the sensitivity of the 820-nm system in SHG detection as it detects the distribution of collagen fibers at the sub-micron level.
Figure 5g shows the imaging of renal fibrosis tissue section induced by folic acid at 820 nm. The glomeruli are significantly atrophied, the renal tubules are remarkably dilated, and deposited collagen accumulates around the elastic vessels and diffuses into the interstitium. Despite the absence of a detectable differential signal in the AF channel, SHG accurately detects the distribution of collagen fibers. This translation maintains scientific rigor, emphasizes modality-specific findings (AF versus SHG versus EVG), and underscores wavelength-dependent performance differences, which guarantees both logical continuity and technical precision.
4. Discussion
Although fibrosis imaging witnesses significant advancements, research on early-stage pathological characterization is still limited. Most studies have focused on tissue-level imaging, primarily because fibrotic markers in bulk tissues generate stronger signals, which facilitates easier detection [37-40]. However, this approach overlooks the critical need for early diagnosis, where fibrotic sections typically exhibit low marker content, resulting in weak imaging signals that compromise diagnostic accuracy. Furthermore, comprehensive assessment of disease progression is restricted by the limited availability of specific fibrosis markers. In clinical practice, particularly during intraoperative frozen section analysis, the demand for rapid and precise detection is paramount. While AF imaging, SHG, and other multiphoton microscopy techniques theoretically detect collagen deposition in fibrosis [
14,
16,
41], their clinical implementation confronts critical limitations. Signal attenuation or absence caused by low biomarker density, inadequate tissue processing protocols, or subcellular localization failure compromises diagnostic reliability in heterogeneous pathological specimens. All these challenges need to be better addressed to bridge the gap between theoretical capabilities and clinical utility. While the integration of TPEF and SHG imaging techniques can serve as complementary methods for detecting tissue fibrosis, rapid clinical detection necessitates an initial comprehensive tissue imaging followed by precise SHG imaging to underscore fibrosis distribution. However, subsequent rapid detection can be significantly expedited if the approximate location of the target area can be preliminarily identified during the initial overall imaging.
To overcome these challenges, a custom laser imaging system with 780-nm and 820-nm excitation wavelengths was developed, coupled with a novel localization method for precise region-of-interest targeting. For balancing the detection wavelength requirements for both collagen and elastin, we selected the shortest possible excitation wavelength while ensuring sufficient excitation intensity. Compared to traditional near-infrared second harmonic microscopy, this configuration allows the simultaneous achievement of high two-photon and second harmonic excitation intensities while maximizing resolution, which enables precise localization of even the smallest collagen and elastin fibers. Through the integration of a cost-effective CCD camera, our system achieves accurate marker identification despite the differences between bright-field and fluorescence imaging. The experimental results demonstrate precise localization of collagen regions, with SHG imaging significantly visualizing fibrous structures. This approach not only addresses the limitations of commercial devices that lack bright-field modes but also offers a cost-effective solution for fibrosis detection. The presented design aim to both reduce system size and enhance convenience, which tackles the growing demand for kidney disease monitoring. To achieve submicron resolution, a miniaturized 9× objective lens with a resolution of 0.61 μm at 820 nm is employed, which allows the distinction of even the smallest collagen structures.
Collagen fibers exhibit the strongest AF signals and allow direct localization at 780 nm, while elastin shows weaker fluorescence. SHG at 780 nm detects collagen in early UUO models. At 820 nm, all components display strong AF signals, with collagen playing the dominant role, followed by myofibrils and elastin. SHG signals at 820 nm are more robust and enable sub-micron collagen detection in renal fibrosis. Early fibrosis shows minimal collagen deposition, with rare elastin emerging. In lung fibrosis models, collagen deposition is observed after 14 days, primarily in peripheral vessels and alveolar walls, with cross-linked elastin indicating developmental relevance. According to the experimental results, our equipment not only achieves sub-micron resolution but also exhibits excellent sensitivity. Current diagnostic approaches predominantly employ collagen-targeted fluorescent probes for early fibrotic pathology detection [
20,
21]. Label-free AF imaging remains underexplored in this context, despite its potential to avoid exogenous labeling artifacts and maintain native extracellular matrix integrity. Our system effectively captures collagen AF signals, allowing for rapid preliminary localization of collagen. Additionally, it was also observed that the SHG signal at 820-nm wavelength is significantly stronger than that at 780 nm. Although SHG signal intensity is theoretically independent of excitation wavelength, the longer wavelength reduces light scattering and thereby improves the signal-to-noise ratio and enhances SHG signal strength. However, under both 780-nm and 820-nm excitation conditions, the SHG signal does not fully correspond to the collagen signal obtained at 780 nm, which discrepancy is attributed to the polarization properties of SHG, not fully representing the overall tissue structure. Future investigations hence include SHG polarization analysis. Moreover, elastin detection appears feasible but requires further validation through experiments at different stages of fibrosis development.
5. Conclusions
In conclusion, a multiphoton microscopy system driven by a fiber-based ultrafast laser was developed, enabling excitation wavelengths at 780 nm and 820 nm for high-sensitivity nonlinear optical imaging. Through simultaneously capturing SHG and two-photon AF signals, this system allows for the precise identification and detection of collagen and elastin fibers. To enhance sample localization and imaging efficiency under non-fluorescence microscopy conditions, we incorporated a grid labeling technique. Using the grid labeling method, the tissue sections from mouse models of renal and pulmonary fibrosis were analyzed. The results revealed that the collagen signal was the most prominently detected in the 780 nm AF channel and enabled rapid and accurate identification of abnormally deposited collagen within the samples. Additionally, the 820 nm imaging system successfully resolved sub-micrometer-scale collagen signals through SHG, which allowed abnormally deposited collagen in early-stage fibrosis could be easily detected. Elastin deposition was observed in pulmonary fibrosis but not within renal fibrosis, suggesting that elastin accumulation is minimal or absent in the early stages of renal fibrosis.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, D.W.L., Y.Z.L., L.S.F., A.M.W, and X.J.; methodology, B.S.Y. and Q.D.C.; validation, B.S.Y., Q.D.C and R.Z.; formal analysis, D.W.L. and Y.Z.L. investigation, R.Z.; resources, Y.Z.L.; data curation, B.S.Y. and Q.D.C.; writing—original draft preparation, B.S.Y. and Q.D.C.; writing—review and editing, Y.Z.L., L.S.F., A.M.W, and X.J.; visualization, B.S.Y. and Q.D.C.; supervision, Y.Z.L.; project administration, Y.Z.L., L.S.F., A.M.W, and X.J; funding acquisition, Y.Z.L., L.S.F., A.M.W, and X.J. All authors have read and agreed to the published version of the manuscript.” B.S.Y. and Q.D.C. contributed equally to this work. L.S.F., and X.J. designed this study.
Funding
This research was funded by National Key R&D Program of China (No.2023YFF0718400); National Natural Science Foundation of China (No.62475008, No.62305186); Zhongshan Municipal Bureau of Science and Technology (No.CXTD2024007); High-level Innovative Research Institute (No.2021B0909050003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
The authors thank Si-Ying Wang for assistance with laser system setup; Qun Zhang; Shuo-Ru Liu and Shuo Lu for support with biological sample processing; Cong-Hao Wang and Hong-Hao Ma for help with image analysis; and Yuan-Yu Zhang for assistance with manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF |
Autofluorescence |
| ARF |
Anti-resonant Fiber |
| CCD |
Charge Coupled Device |
| ECM |
Extracellular Matrix |
| EVG |
Elastica van Gieson |
| MEMS |
Micro-Electromechanical Systems |
| MPM |
Multiphoton microscopy |
| NA |
Numerical Aperture |
| PBS |
Polarization Beam Splitter |
| PMT |
Photomultiplier Tube |
| ROI |
Regions Of Interest |
| SHG |
Second Harmonic Generation |
| THG |
Third-Harmonic Generation |
| TIFF |
Tagged Image File Format |
| TPEF |
Two-Photon Excitation Fluorescence |
| UUO |
Unilateral Ureteral Obstruction |
| UV |
Ultraviolet |
References
- Jun, J. I.; L. Lau, F. Resolution of organ fibrosis. J. Clin. Invest. 2018, 128, 97-107. [CrossRef]
- Lurje, I.; Gaisa, N.T. Weiskirchen, R.; Tacke, F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol. Aspects Med. 2023, 92, 101191. [CrossRef]
- Selman, M.; Pardo, A. From pulmonary fibrosis to progressive pulmonary fibrosis: a lethal pathobiological jump. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L600-L607.
- Farris, A.B.; Vizcarra, J.; Amgad, M.; Donald Cooper, L.A.; Gutman, D., et al. Image Analysis pipeline for renal allograft evaluation and fibrosis quantification., Kidney Int. Rep. 2021, 6, 1878-1887. [CrossRef]
- Siddiqui, M.S.; Yamada, G.; Vuppalanchi, R.; Van Natta, M.; Loomba, R., et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin. Gastroenterol. Hepatol. 2019, 17, 1877-1885. [CrossRef]
- Collins, B.F.; Luppi, F. Diagnosis and management of fibrotic interstitial lung diseases. Clin. Chest Med. 2021, 42, 321-335. [CrossRef]
- Farris, A.B.; Chan, S.; Climenhaga, J.; Adam, B.; Bellamy, C.O., et al. Banff fibrosis study: multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am. J. Transplant. 2014, 14, 897-907. [CrossRef]
- Chen, Y.; He, H.; Luo, L.; Liu, K.; Jiang, M., et al. Studying pulmonary fibrosis due to microbial infection via automated microscopic image analysis. Front. Microbiol. 2023, 14, 1176339. [CrossRef]
- Agrawal, G.P., Nonlinear fiber optics: Its history and recent progress [Invited]. J. Opt. Soc. Am. B. 2011, 28, A1-A10. [CrossRef]
- Baues, M.; Dasgupta, A.; Ehling, J.; Prakash, J.; Boor, P., et al. Fibrosis imaging: current concepts and future directions. Adv. Drug Deliv. Rev. 2017, 121, 9-26.
- Hermsen, M.; Bel, T.D.; Boer, M.D. Steenbergen, E.J.; Laak, J.A.W.M.v.d. Deep learning–Based histopathologic assessment of kidney tissue. J. Am. Soc. Nephrol. 2019, 30, 1968-1979.
- Jiang, B.; Liu, F.; Fu, H.; Mao, J. Advances in imaging techniques to assess kidney fibrosis. Ren. Fail. 2023, 45, 2171887. [CrossRef]
- Ranjit, S.; Dobrinskikh, E.; Montford, J.; Dvornikov, A.; Lehman, A., et al. Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int. 2016, 90, 1123-1128.
- Bhuiyan, S.; Widdop, R.E.; Samuel, C.S. Determination of interstitial collagen deposition and related topography using the second harmonic generation-based histoIndex platform. Methods Mol. Biol. 2023, 2664, 173-184.
- Bhuiyan, S.; Shen, M.; Chelvaretnam, S.; Tan, A.Y.; Ho, G.; et al., Assessment of renal fibrosis and anti-fibrotic agents using a novel diagnostic and stain-free second-harmonic generation platform. FASEB. J. 2021, 35, e21595. [CrossRef]
- Saitou, T.; Takanezawa, S.; Ninomiya, H.; Watanabe, T.; Yamamoto, S., et al. Tissue intrinsic fluorescence spectra-based digital pathology of liver fibrosis by marker-controlled segmentation. Front. Med. (Lausanne) 2018, 5, 00350.
- Durgam, S.; Singh, B.; Cole, S.L.; Brokken, M.T.; Stewart, M. Quantitative assessment of tendon hierarchical structure by combined second harmonic generation and immunofluorescence microscopy. Tissue Eng. Part C Methods 2020, 26, 253-262. [CrossRef]
- Lee, S.; Islam, M. N.; Boostanpour, K.; Aran, D.; Jin, G.; Christenson S.; Matthay, M. A.; Eckalbar, W. L.; DePianto, D. J.; Arron, J. R.; et al. Molecular programs of fibrotic change in aging human lung. Nat. Commun. 2021, 12, 6309.
- James, D. S.; Brereton, C. J.; Davies, D. E.; Jones, M. G.; Campagnola, P. J. Examining lysyl oxidase-like modulation of collagen architecture in 3D spheroid models of idiopathic pulmonary fibrosis via second-harmonic generation microscopy. J. Biomed. Opt. 2021, 26, 066501. [CrossRef]
- Fan, N.; Wu, C.; Zhou, Y.; Wang, X.; Li, P., et al. Rapid two-photon fluorescence imaging of monoamine oxidase b for diagnosis of early-stage liver fibrosis in mice. Anal. Chem. 2021, 93, 7110-7117.
- Zhou, T.; Wang, J.; Xu, J.; Zheng, C.; Niu, Y.; et al. A Smart Fluorescent Probe for NO Detection and Application in Myocardial Fibrosis Imaging. Analytical chemistry. Anal .Chem. 2020, 92, 5064-5072.
- Pantazis, P.; Maloney, J.; Wu, D.; Fraser, S.E. Second harmonic generating (SHG) nanoprobes for in vivo imaging. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 14535-14540.
- Scheinfeld, N. Nephrogenic fibrosing dermopathy: a comprehensive review for the dermatologist. Am. J. Clin. Dermatol. 2006, 7, 237-247.
- Sun, Q.; Baues, M.; Klinkhammer, B.M.; Ehling, J.; Djudjaj, S.; et al. Elastin imaging enables noninvasive staging and treatment monitoring of kidney fibrosis. Sci. Transl. Med. 2019, 11, aat4865. [CrossRef]
- Chen, W.; Yan, X.; Xu, A.; Sun, Y.; Wang, B.; et al. Dynamics of elastin in liver fibrosis: Accumulates late during progression and degrades slowly in regression. J. Cell. Physiol. 2019, 234, 22613-22622.
- Peyrot, D. A.; Lefort, C.; Steffenhagen, M.; Mansuryan, T.; Ducourthial, G.; et al. Development of a nonlinear fiber-optic spectrometer for human lung tissue exploration. Biomed. Opt. Express. 2012, 3, 840-853. [CrossRef]
- Thornhill; P, D. Separation of a series of chromophores and fluorophores present in elastin. Biochem. J. 1975, 147, 215-219.
- Wr., Z.; Rm., W.; Christie, R.; Ay., N.; Bt., H.; et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 7075-7080.
- Cheng, Q.D.; Chung, H.Y.; Schubert, R.; Chia, S.H.; Falke, S.; et al. Protein-crystal detection with a compact multimodal multiphoton microscope. Commun. Biol. 2021, 3, 569. [CrossRef]
- Mikami, H.; Gao, L.; Goda, K. Ultrafast optical imaging technology: principles and applications of emerging methods. Nanophotonics 2016, 5, 497-507.
- Aghigh, A.; Bancelin, S.; Rivard, M.; Pinsard, M.; Ibrahim, H.; et al. Second harmonic generation microscopy: a powerful tool for bio-imaging. Biophys. Rev. 2023, 15, 43-70. [CrossRef]
- Zach, A.; Mohseni, M.; Polzer, C.; Nicholson, J.W.; Hellerer, T. All-fiber widely tunable ultrafast laser source for multimodal imaging in nonlinear microscopy. Opt. Lett. 2019, 44, 5218-5221.
- Qin, Y.; Batjargal, O.; Cromey, B.; Kieu, K. All-fiber high-power 1700 nm femtosecond laser based on optical parametric chirped-pulse amplification. Opt. Express 2020, 28, 2317-2325.
- Kiriyama, H.; Mori, M.; Nakai, Y.; Yamamoto, Y.; Tanoue, M.; et al. High-energy, high-contrast, multiterawatt laser pulses by optical parametric chirped-pulse amplification. Opt. Lett. 2007, 32, 2315-2317.
- Gade, P.S.; Robertson, A.M.; Chuang, C.Y. Multiphoton imaging of collagen, elastin, and calcification in intact soft-tissue samples. Curr. Protoc. Cytom. 2018, 128, e51.
- Kazlouskaya, V.; Malhotra, S.; Lambe, J.; Idriss, M.H.; Elston, D.; et al. The utility of elastic Verhoeff-Van Gieson staining in dermatopathology. Pathol. 2012, 40, 211-225. [CrossRef]
- ark, C.Y.; Marando, C.M.; Liao, J.A.; Lee, J.K.; Kwon, J.; et al. Details of the collagen and elastin architecture in the human limbal conjunctiva, tenon's capsule and sclera revealed by two-photon excited fluorescence microscopy. Invest. Ophthalmol. Vis. Sci. 2016, 57, 5602-5610.
- Safaee, M.M.; Nishitani, S.; Mcfarlane, I.R.; Yang, S.J.; Sun, E.; et al. Dual infrared 2-photon microscopy achieves minimal background deep tissue imaging in brain and plant tissues. Adv. Funct. Mater. 2024, 34, 202404709. [CrossRef]
- Lan, M.; Zhao, S.; Niu, G.; Wu, F.; Yan, L.; et al. Lysosome-targetable polythiophene nanoparticles for two-photon excitation photodynamic therapy and deep tissue imaging. J. Mater. Chem. B. 2017, 5, 3651-3657.
- Stringari, C.; Abdeladim, L.; Malkinson, G.; Mahou, P.; Beaurepaire, E., Multicolor two-photon imaging of endogenous fluorophores in living tissues by wavelength mixing. Sci. Rep. 2017, 7, 3792. [CrossRef]
- Alberini, R.; Spagnoli, A.; Sadeghinia, M.J.; Skallerud, B.; Terzano, M.; et al. Second harmonic generation microscopy, biaxial mechanical tests and fiber dispersion models in human skin biomechanics.Acta Biomater. 2024, 185, 266-280.
Figure 1.
Fabrication of a translucent grid-based marker for precision spatial localization in MPM. (a) fabrication of primary grid units. (b) UV-adhesive assembly of the staggered composite grid. (c) alignment for composite grid-tissue integration. (d) fixed composite grid on the tissue section. (e) composite-guided ROI localization. (f) magnified view of precision marking on composite grid.
Figure 1.
Fabrication of a translucent grid-based marker for precision spatial localization in MPM. (a) fabrication of primary grid units. (b) UV-adhesive assembly of the staggered composite grid. (c) alignment for composite grid-tissue integration. (d) fixed composite grid on the tissue section. (e) composite-guided ROI localization. (f) magnified view of precision marking on composite grid.
Figure 3.
Images of collagen and elastin fibers utilizing the MPM technique system. The method of detection and imaging is indicated in the lower left of each image. (a,d) MPM imaging of elastin at 780-nm excitation wavelength. (b,e) MPM imaging of collagen at 780-nm excitation wavelength. (c,f) MPM imaging methods for collagen at 820-nm excitation wavelength.
Figure 3.
Images of collagen and elastin fibers utilizing the MPM technique system. The method of detection and imaging is indicated in the lower left of each image. (a,d) MPM imaging of elastin at 780-nm excitation wavelength. (b,e) MPM imaging of collagen at 780-nm excitation wavelength. (c,f) MPM imaging methods for collagen at 820-nm excitation wavelength.
Figure 4.
Images of pulmonary tissue specimens selected for the imaging experiments. Imaging modality is noted in the lower left of each panel, with the experimental model labeled in the upper right. (a) MPM imaging of pulmonary tissue section in the sham-operated group at 780 nm. (b) MPM imaging of pulmonary tissue section in the fibrosis group at 780 nm. (c) MPM imaging of pulmonary tissue sections in the sham-operated group at 820 nm. (d) MPM imaging of pulmonary tissue section in the fibrosis group at 820 nm. Con: control group, BF: Bleomycin induced pulmonary fibrosis group, the corresponding EVG-stained pulmonary tissues reveal distinct histological features under light microscopy, with collagen fibers stained pink, elastin fibers exhibiting dark purple, and myofibrillar components demonstrating yellow. In the images, the red arrows indicate collagen fibers, red dashed circles highlight alveolar structures, and red stars denote elastin fibers surrounding blood vessels. For multi-channel image analysis, individual channels (e.g., SHG, TPEF) were imported into ImageJ and assigned to specific colors (e.g., red, green) by leveraging the "Merge Channels" function. The final composite images were generated by precisely aligning and integrating the channels, which guaranteed accurate colocalization and signal representation. These high-resolution merged images, saved in TIFF format, enabling detailed visualization and quantitative analysis of complex biological structures and facilitate robust interpretation of ECM.
Figure 4.
Images of pulmonary tissue specimens selected for the imaging experiments. Imaging modality is noted in the lower left of each panel, with the experimental model labeled in the upper right. (a) MPM imaging of pulmonary tissue section in the sham-operated group at 780 nm. (b) MPM imaging of pulmonary tissue section in the fibrosis group at 780 nm. (c) MPM imaging of pulmonary tissue sections in the sham-operated group at 820 nm. (d) MPM imaging of pulmonary tissue section in the fibrosis group at 820 nm. Con: control group, BF: Bleomycin induced pulmonary fibrosis group, the corresponding EVG-stained pulmonary tissues reveal distinct histological features under light microscopy, with collagen fibers stained pink, elastin fibers exhibiting dark purple, and myofibrillar components demonstrating yellow. In the images, the red arrows indicate collagen fibers, red dashed circles highlight alveolar structures, and red stars denote elastin fibers surrounding blood vessels. For multi-channel image analysis, individual channels (e.g., SHG, TPEF) were imported into ImageJ and assigned to specific colors (e.g., red, green) by leveraging the "Merge Channels" function. The final composite images were generated by precisely aligning and integrating the channels, which guaranteed accurate colocalization and signal representation. These high-resolution merged images, saved in TIFF format, enabling detailed visualization and quantitative analysis of complex biological structures and facilitate robust interpretation of ECM.
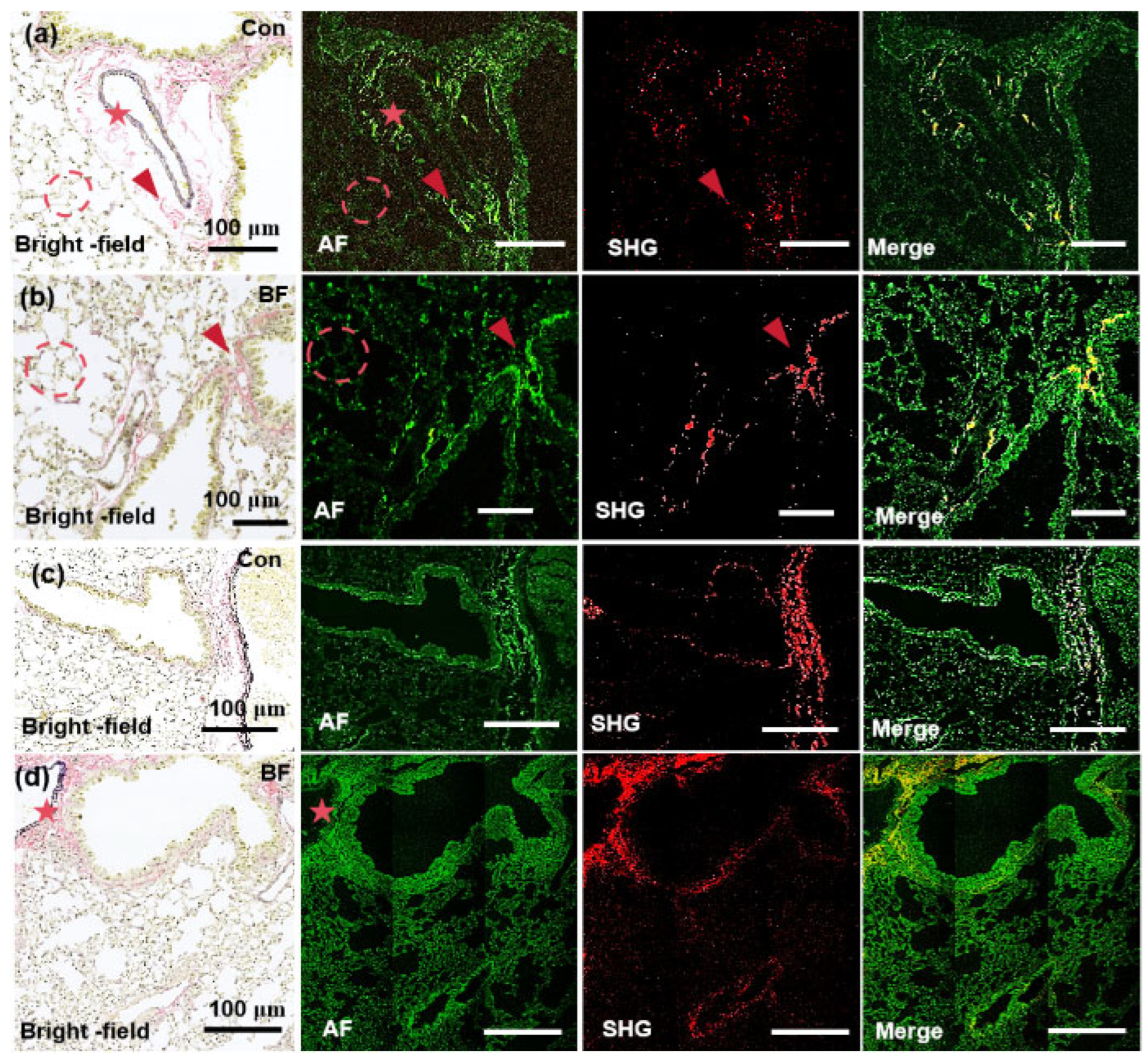
Figure 5.
Images of renal tissue specimens selected for the imaging experiments. Imaging modality is noted in the lower left of each panel, with the experimental model labeled in the upper right. (a-c) MPM imaging of kidney tissue section in the sham-operated group, renal tissue sections in the 5-day UUO model, and renal tissue sections in the folic acid model at 780 nm. (d-g) MPM imaging of kidney tissue in the sham-operated group, renal tissue sections in the 5-day UUO model, renal tissue in the 7-day UUO model, and renal tissue in the folic acid model at 820-nm excitation wavelength. Sham: sham-operation group; UUO5: UUO5-day renal fibrosis group. UUO7: UUO7-day renal fibrosis group; FA: folic acid induced renal fibrosis group. The red arrows represent collagen deposits and areas, and the red dotted circles indicate elastin fibers. For multi-channel analysis, channels were imported into ImageJ, assigned colors, and merged by leveraging "Merge Channels." The high-resolution composite images, saved as TIFFs, enable detailed visualization and analysis of biological structures, which contributes to ECM interpretation.
Figure 5.
Images of renal tissue specimens selected for the imaging experiments. Imaging modality is noted in the lower left of each panel, with the experimental model labeled in the upper right. (a-c) MPM imaging of kidney tissue section in the sham-operated group, renal tissue sections in the 5-day UUO model, and renal tissue sections in the folic acid model at 780 nm. (d-g) MPM imaging of kidney tissue in the sham-operated group, renal tissue sections in the 5-day UUO model, renal tissue in the 7-day UUO model, and renal tissue in the folic acid model at 820-nm excitation wavelength. Sham: sham-operation group; UUO5: UUO5-day renal fibrosis group. UUO7: UUO7-day renal fibrosis group; FA: folic acid induced renal fibrosis group. The red arrows represent collagen deposits and areas, and the red dotted circles indicate elastin fibers. For multi-channel analysis, channels were imported into ImageJ, assigned colors, and merged by leveraging "Merge Channels." The high-resolution composite images, saved as TIFFs, enable detailed visualization and analysis of biological structures, which contributes to ECM interpretation.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).